Published online Nov 21, 2015. doi: 10.3748/wjg.v21.i43.12322
Peer-review started: July 3, 2015
First decision: August 31, 2015
Revised: September 3, 2015
Accepted: October 17, 2015
Article in press: October 20, 2015
Published online: November 21, 2015
Processing time: 142 Days and 11.7 Hours
Liver cirrhosis is a paradigm of intestinal dysbiosis. The qualitative and quantitative derangement of intestinal microbial community reported in cirrhotic patients seems to be strictly related with the impairment of liver function. A kind of gut microbial “fingerprint”, characterized by the reduced ratio of “good” to “potentially pathogenic” bacteria has recently been outlined, and is associated with the increase in Model for End-Stage Liver Disease and Child Pugh scores. Moreover, in patients presenting with cirrhosis complications such as spontaneous bacterial peritonitis (SBP), hepatic encephalopathy (HE), and, portal hypertension intestinal microbiota modifications or the isolation of bacteria deriving from the gut are commonly reported. Rifaximin is a non-absorbable antibiotic used in the management of several gastrointestinal diseases. Beyond bactericidal/bacteriostatic, immune-modulating and anti-inflammatory activity, a little is known about its interaction with gut microbial environment. Rifaximin has been demonstrated to exert beneficial effects on cognitive function in patients with HE, and also to prevent the development of SBP, to reduce endotoxemia and to improve hemodynamics in cirrhotics. These results are linked to a shift in gut microbes functionality, triggering the production of favorable metabolites. The low incidence of drug-related adverse events due to the small amount of circulating drug makes rifaximin a relatively safe antibiotic for the modulation of gut microbiota in advanced liver disease.
Core tip: Advanced liver disease is characterized by intestinal dysbiosis, which has been involved in the pathogenesis of complications. Rifaximin is able to improve cognitive tests and practical abilities, to reduce the risk of hepatic encephalopathy (HE) recurrence and the number of HE-related hospitalizations. Rifaximin efficacy seems not associated with major changes in gut bacteria composition but rather with a shift in the microbiome functionality. Rifaximin is useful in the prevention of spontaneous bacterial peritonitis in patients with ascites. Rifaximin reduces endotoxemia and has beneficial effects on cirrhotic patients hemodynamics, reducing the incidence of complications related to portal hypertension.
- Citation: Ponziani FR, Gerardi V, Pecere S, D’Aversa F, Lopetuso L, Zocco MA, Pompili M, Gasbarrini A. Effect of rifaximin on gut microbiota composition in advanced liver disease and its complications. World J Gastroenterol 2015; 21(43): 12322-12333
- URL: https://www.wjgnet.com/1007-9327/full/v21/i43/12322.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i43.12322
Rifaximin is a non-systemic antibiotic approved for the treatment of traveler’s diarrhea, irritable bowel syndrome (IBS) with diarrhea and overt hepatic encephalopathy (HE)[1]. It has in vitro bactericidal and bacteriostatic activity against aerobic and anaerobic Gram-positive and Gram-negative species, being also able to reduce bacterial virulence and translocation, and to inhibit bacterial adherence to gut mucosa[2-7].
Due to the low systemic absorption (only 0.4% of the oral administered dose), rifaximin has an optimal tolerability profile, and side effects as well as the induction of bacterial resistance are nearly lacking[1,8,9].
Beyond that, rifaximin has particular features which are not typical of a common antibiotic molecule. In vitro and in vivo models and preliminary experiences in humans[10-14] have demonstrated that rifaximin does not change the overall composition of the gut microbiota while it is able to provide minimal changes, such as promoting the growth of bacteria beneficial to the gut. Nevertheless, rifaximin modulates the release of inflammatory cytokines[15,16] and increases NF-κB expression, exerting anti-inflammatory effects that could counteract the pro-inflammatory response observed in conditions of gut microbiota derangement[17].
Based on these evidences, rifaximin use has been extended to the management of pathologies associated with gut microbiota deregulation such as irritable bowel syndrome[11,18-21], inflammatory bowel diseases[10,13,22-30], diverticular disease[31-36] and liver cirrhosis and its complications.
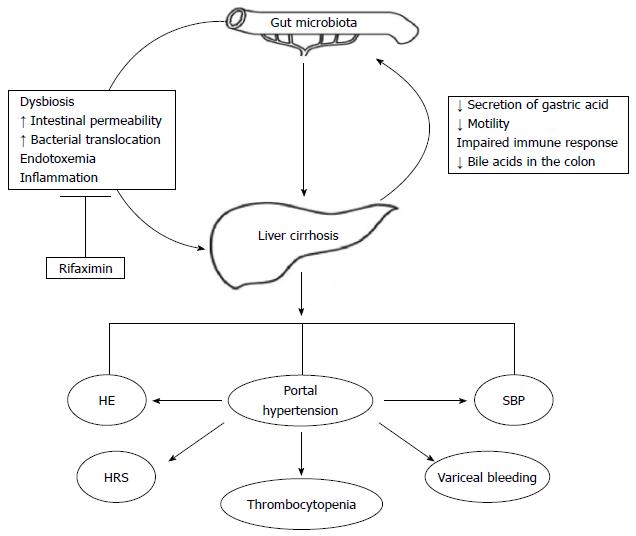
Liver cirrhosis is a paradigm of intestinal dysbiosis. Indeed, the physiological partitioning of the gastrointestinal tract is deranged in cirrhotic patients, due to the decreased secretion of gastric acid (often favored by medications[37]), to the reduced gastrointestinal motility, to the impaired systemic and mucosal immune response and to the low concentration of bile acids in the colon[38]. The epiphenomenon of this chronic dysfunction is a profound alteration of the gut microbiota composition, which is both quantitative (Small Intestinal Bacterial Overgrowth, SIBO) and more pronounced in the advanced stages of the disease and in case of decompensation (Figure 1).
This is the rationale for gut microbiota modulation in patients with liver cirrhosis, especially in those with severe impairment of liver function presenting with complications.
The introduction of metagenomic techniques such as 16S rRNA-based pyrosequencing has recently allowed to identify which modifications of the gut microenvironment are the most frequently observed in liver cirrhotic patients[39]. The human gut hosts a bacterial core community involved in maintaining gastrointestinal health and mainly composed of the phyla Bacteroidetes and Firmicutes, which include the genera Bacteroides, Clostridium clusters XIVa and IVa, Eubacterium, Faecalibacterium, Lactobacillus, and Roseburia. In patients affected by liver cirrhosis, at the phylum level, Bacteroidetes are decreased in favor of Fusobacteria and Proteobacteria, such as Enterobacteriaceae and Pasteurellaceae[40-42]. Looking at family, genus and species division, the increase in Enterobacteriaceae, Streptococcaceae and Veillonellaceae abundance has been reported in cirrhotic patients compared with healthy controls, whereas Lachnospiraceae, Ruminococcaceae, Clostridium clusters XI and XIVab, lactic acid bacteria, Bifidobacteria and Faecalibacterium prausnitzii seem to be reduced[40-46]. Notably, Enterobacteriaceae family includes Escherichia coli and Klebsiella spp., key bacteria in the pathogenesis of spontaneous bacterial peritonitis (SBP). In addition to the unbalance between potentially pathogenic and beneficial bacteria, the major part of the metagenomic species enriched in cirrhotics’ fecal samples belong to Veillonella or Streptococcus taxa, which usually derive from the mouth or the small intestine[47]. Although this may apparently confirm the subversion of the gastrointestinal physiology occurring during the course of liver disease, when cirrhotics’ salivary microbiota is specifically analyzed and compared with the fecal one, they seem significantly different rather than similar[46]. More in detail, Streptococcaceae are prevalent in the saliva, whereas stools are characterized by a reduction in the autochthonous taxa Lachnospiraceae, Ruminococcaceae, and Clostridiales XIV. However, about half of samples analyzed in this study belonged to patients who have had previous episodes of HE and were on lactulose, with the addition of rifaximin in two cases. Further analyses to discriminate conditions predisposing to the “mixing-up” of bacteria from different sites of the gastrointestinal tract are needed to quell this debate.
Interestingly, the alteration of gut microbiota composition seems to have a prognostic significance, or at least to follow the evolution of liver disease. Generally speaking, Qin et al[47] demonstrated that metagenomic species enriched in cirrhotic patients correlate with the severity of the disease, in a proportion dependent on bacterial load. In other studies, the reduction in Clostridiaceae as well as in Veillonellaceae and in Porphyromonadaceae has been associated with inflammation and with the progression of liver disease and Streptococcaceae have been reported to correlate positively with Child Pugh score in contrast to Lachnospiraceae which correlated negatively[41,43,48].
Taking together these findings, cirrhotic patients’ microbiota is characterized by a higher proportion of potentially pathogenic bacteria, lacking of those species recognized as beneficial to intestinal health and homeostasis. Notwithstanding, the reduction in the ratio between “good” (e.g., Lachnospiraceae, Ruminococcaceae and Clostridia cluster XIV) and potentially “bad” bacteria (e.g., Staphylococcaeae, Enterobacteriaeceae and Enterococcaceae) - namely “cirrhosis dysbiosis ratio” or CDR - is characteristic of the individuals with a more severe disease, such as cirrhotic outpatients and inpatients[48].
Given the evidence that the progression of liver disease is associated with a change in the gut microenvironment, liver cirrhosis complications consequently grow in the soil of intestinal dysbiosis.
Several differences have been reported in the gut microbiota of cirrhotic patients with or without HE. In patients with minimal HE, Streptococcaceae represent the prevalent bacterial family, and the abundance of Streptococcus salivarius, which is involved the production of ammonia, is increased[45]. Alcaligeneceae, Porphyromonadaceae and Enterobacteriaceae have also been associated with HE in cirrhotics; in particular, Alcaligeneceae and Porphyromonadaceae are significantly linked with poor cognitive performance, and Enterobacteriaceae with a worse MELD score[49]. In addition, a decreased CDR has been reported in cirrhotic patients with HE[48]. Similar results have been obtained by the analysis of mucosal microbiome from sigmoid biopsies: Enterococcus, Veillonella, Megasphaera, Bifidobacterium, and Burkholderia were predominant in patients with HE, whereas cirrhotics without HE presented an increased abundance of the “good” genus Roseburia, and the healthy controls an increased abundance of Dorea, Subdoligranulum, Incertae Sedis XIV, Blautia, Roseburia, Faecalibacterium and a few pathogenic genera[50]. Since the intestinal microenvironment of cirrhotics without HE has been demonstrated to be closer to healthy people's one[46], it is not surprising that, in patients with HE, the more the mucosal microbiota resembled that of controls, the better was the cognitive performance and the lower were the serum markers of inflammation[50].
Studies focused on clinical outcomes reported a high efficacy of rifaximin in cirrhotics with HE and a mild/moderate stage of disease. A randomized, double-blind, placebo-controlled trial including 299 patients has proved that rifaximin with or without lactulose is able to reduce the risk of HE recurrence and the rate of HE-related hospitalization, especially in patients with MELD score < 18[51]. Similar results were also obtained in other studies including patients in different stages of liver disease, receiving various treatment schedules (Table 1)[52-58].
| Study | Study design | No. patients | Disease severity | HE type | Treatment schedule | Results | Safety |
| Mas et al[56] 2003 | Prospective randomized, double-blind, double-dummy, controlled trial | 103 | Not reported | Overt HE | 50 pts rifaximin 1200 mg/d for 5-10 d | Improved neuropsychiatric and psychometric parameters in both groups | Abdominal pain: 4% rifaximin |
| 53 pts lactitol 60 g/d for 5-10 d | Reduced blood ammonia levels in both groups | Mild diarrhea: 2% lactitol | |||||
| No significant differences in efficacy (resolution/improvement 81.6% rifaximin vs 80.4% lactitol; unchanged/failure 18.4% rifaximin vs 19.6% lactitol) | Vomiting: 2% lactitol | ||||||
| HE complete resolution: 53.1% rifaximin vs 37.2% lactitol | |||||||
| Paik et al[57] 2005 | Prospective randomized | CTP: | Overt HE | 32 pts rifaximin 400 mg TID for 7 d | Reduction in blood ammonia levels similar in both groups | Abdominal pain: 3% rifaximin | |
| rifaximin A: 0%, B: 50%, C: 50% | 22 pts lactulose 90 mL/d for 7 d | Improvement in HE grade and index similar in both groups | Severe diarrhea: 4.5% lactulose | ||||
| lactulose A: 0%, B: 64%, C: 36% | Improvement in HE grade similar in both groups | ||||||
| Leevy et al[58] 2007 | Retrospective | 145 | Not reported | Overt HE | Lactulose 30 cc BID for ≥ 6 mo followed by rifaximin 400 mg TID for ≥ 6 mo | HE grade III or IV: 6% after rifaximin 25% after lactulose (P < 0.001) | Hospitalizations (mean number): 0.5 rifaximin period vs 1.6 lactulose period (P = 0.001) |
| Asterixis: 63% after rifaximin vs 93% after lactulose (P < 0.001) | Hospitalizations days (mean): 2.5 rifaximin period vs 7.3 lactulose period (P = 0.001) | ||||||
| Diarrhea: 89% during lactulose, 99% during rifaximin | |||||||
| Flatulence: 100% during lactulose, 100% during rifaximin | |||||||
| Abdominal pain: 100% during lactulose, 100% during rifaximin | |||||||
| Headache: 100% during lactulose, 99% during rifaximin | |||||||
| However, severe adverse events were more common in the lactulose period (P < 0.001) | |||||||
| Bass et al[51] 2010 | Prospective, randomized, double-blind, placebo-controlled | 299 | MELD score (%): | Overt HE | 140 pts 550 mg BID for 6 mo | Rifaximin is more effective than placebo in maintaining HE remission (P < 0.001) | Incidence of adverse events was similar in the two groups; most frequently reported: nausea diarrhea, fatigue |
| rifaximin | 159 pts placebo | Breakthrough episodes rate: 22.1% rifaximin vs 45.9% placebo | Bacterial peritonitis: 1.4% rifaximin vs 2.5% placebo | ||||
| ≤ 10: 24.3% | 90% of pts also received lactulose | Risk of HE-related hospitalization: 13.6% rifaximin vs 22.6% placebo (P = 0.01) | Bacteremia: 0.7% rifaximin vs 1.3% placebo | ||||
| 11-18: 67.1% | C. difficile infection: 1.4% rifaximin vs 0% placebo | ||||||
| 19-24: 8.6% | Sepsis: 0% rifaximin vs 1.3% placebo | ||||||
| placebo: | |||||||
| ≤ 10: 30.2% | |||||||
| 11-18: 60.4% | |||||||
| 19-24: 8.8% | |||||||
| Bajaj et al[59] 2011 | Prospective, randomized, double-blind, placebo-controlled | 42 | MELD score (mean) | Minimal HE | 21 pts rifaximin 550 mg BID | Total driving errors improvement: 76% rifaximin vs 31% placebo (P = 0.013), with a significant reduction of speeding tickets (P = 0.005) and illegal turns on navigation (P = 0.01) | Infections rate: 0% |
| rifaximin: 9 | 21 pts placebo for 8-wk | Hospitalization rate: 0% | |||||
| placebo: 9 | Nausea: 14% rifaximin vs 14% placebo | ||||||
| Cognitive performance improvement: 91% rifaximin vs 61% placebo (P = 0.01) | Self-limited vomiting: 5% rifaximin vs 5% placebo | ||||||
| Improved psycho-social dimension (quality of life assessment by Sickness Impact Profile questionnaire) in the rifaximin group compared with the placebo group (P = 0.04) | Abdominal pain: 24% rifaximin vs 24% placebo | ||||||
| Flatulence: 19% rifaximin vs 43% placebo | |||||||
| Headache: 19% rifaximin vs 33% placebo | |||||||
| Flu-like symptoms: 5% rifaximin | |||||||
| Constipation: 5% rifaximin | |||||||
| Self-limited diarrhea: 5% rifaximin vs 5% placebo | |||||||
| Hitching: 5% placebo | |||||||
| Anorexia and dry mouth: 5% placebo | |||||||
| Neff et al[52] 2012 | Retrospective | 203 | MELD score (mean, range): | Overt HE | 149 pts rifaximin monotherapy (400-1600 mg/d) | 1-yr HE remission rate: 81% rifaximin vs 67% rifaximin + lactulose | Incidence of gastrointestinal bleeding, infection, hospitalization for dehydration/overt HE similar in both groups |
| rifaximin 12 (8-27) | 54 pts rifaximin (600-1200 mg/d) + lactulose (90 mL/d) dual therapy | Lower incidence of overt HE episodes in pts with mean MELD score ≤ 20 | |||||
| rifaximin + lactulose 13 (11-26) | |||||||
| Bajaj et al[62] 2013 | Prospective | 20 | MELD score (mean ± SD): 9.8 ± 3.3 | Minimal HE | 550 mg BID for 8 wk | Significant improvement in cognitive performance on all tests apart from the block design test | Not reported |
| Significant improvement in serum bilirubin but not the other MELD score components | |||||||
| No significant microbial change (modest reduction in Veillonellaceae and increase in Eubacteriaceae) | |||||||
| Significant increase in serum saturated (myristic, caprylic, palmitic, palmitoleic, oleic and eicosanoic) and unsaturated (linoleic, linolenic, gamma-linolenic and arachnidonic) fatty acids, serum fructose, succinic acid and citramalic acid | |||||||
| Change in correlation networks involving several bacteria (Enterobacteriaceae, Bacteroidaceae, Veillonellaceae, Porphyromonadaceae and Rikenellaceae) reflecting a functional shift in the gut microbiome | |||||||
| Sharma et al[53] 2013 | Prospective, randomized, double-blind, placebo-controlled | 120 | CTP score (mean ± SD): | Overt HE | group A (63 pts): lactulose + rifaximin 1200 mg/d | HE remission rate: 76% group A vs 50.8% group B (P < 0.004) | Diarrhea: 13% group A vs 10% group B (P > 0.05) |
| group A 9.9 ± 2.8 | group B (57 pts): lactulose + placebo | Mortality: 23.8% group A vs 49.1% group B (P < 0.05). Death was mainly due to sepsis | Abdominal pain: 6% group A vs 7% group B (P > 0.05) | ||||
| group B 9.4 ± 2.5 | Hospital stay (mean ± SD): 5.8 ± 3.4 in group A vs 8.2 ± 4.6 group B (P = 0.001) | ||||||
| MELD score (mean ± SD): | |||||||
| group A 24.9 ± 6.6 | |||||||
| group B 23.8 ± 5.18 | |||||||
| Maharshi et al[54] 2014 | Prospective, randomized, controlled | 120 pts with acute variceal bleeding and no HE | CTP and MELD scores comparable between groups but not reported | Overt HE | 60 pts lactulose 30 mL QID | Incidence of HE: 15% rifaximin vs 17% lactulose (P = 1) | Rifaximn group: 5% abdominal pain and nausea |
| 60 pts rifaximin 400 mg TID | Mortality: 17% rifaximin vs 13% lactulose (P = 1) | Lactulose group: 26.6% diarrhea, 15% abdominal bloating | |||||
| for 5 d | Hospital stay (mean ± SD): 10.6 ± 3.1 d rifaximin vs 12.4 ± 3.5 lactulose (pts with HE, P = 0.35); 6.3 ± 1.6 rifaximin vs 6.9 ± 1.9 lactulose (pts without HE, P = 0.18) | ||||||
| Sharma et al[55] 2014 | Prospective, randomized, controlled | 124 | CTP | Minimal HE | 31 pts LOLA 3 g TID for 2 mo | LOLA, rifaximin, and probiotics are superior to placebo in improving critical flicker frequency score | Not reported |
| LOLA | 31 pts rifaximin 400 mg TID for 2 mo | LOLA, rifaximin, and probiotics are superior to placebo in improving neuropsychometric tests | |||||
| A: 22.5%, B: 42%, C: 35.5% | 32 pts probiotics BID for 2 mo | ||||||
| rifaximn | 30 pts placebo | ||||||
| A: 39%, B: 32%, C: 29% | |||||||
| probiotics | |||||||
| A: 19%, B: 66%, C: 16% | |||||||
| placebo | |||||||
| A: 33%, B: 27%, C: 40% |
In addition to the roughly evident benefits on overt HE, rifaximin has also been reported to improve operational abilities and input integration capacity in patients with minimal HE, as demonstrated by the amelioration of driving simulator performance[59]. This positive shift in cognitive tests and practical abilities is undoubtedly accompanied by a significant improvement in health-related quality of life[60,61].
At the microscopic level, rifaximin does not seem to change stool microbiota composition in patients with minimal HE, and only a reduction in Veillonellaceae and an increase in Eubacteraceae have been observed[62]. Reasonably, the improvement in cognitive function and the reduced endotoxemia associated with rifaximin treatment derive from a beneficial modulation of gut microbiota metabolic profile rather than from a major rearrangement of the intestinal microbial community. Indeed, the Authors reported an increase in saturated and unsaturated fatty acids and in serum fructose, succinic acid and citramalic acid production after rifaximin treatment, but the most relevant finding was the modification of correlation networks involving several bacteria (Enterobacteriaceae, Bacteroidaceae, Veillonellaceae, Porphyromonadaceae and Rikenellaceae), metabolites and clinical outcomes, suggesting a functional change in the gut microbiome. Although only patients with minimal HE have been included and some selection biases could be identified, the study by Bajaj et al[62] is to date the only published experience reporting the metagenomic and metabolomic changes produced by rifaximin treatment in cirrhotics with minimal HE. Nevertheless, despite the good results in terms of efficacy, rifaximin role in the treatment of cirrhotics at high risk of developing HE, such as patients with high MELD scores or with transjugular intrahepatic portosystemic shunts or surgical portosystemic shunts or those with a recent episode of acute variceal bleeding, needs to be further investigated[63-65].
Ascites and SBP are typical manifestations of decompensated liver disease. SIBO and bacterial translocation are the mainstay of SBP. Indeed, SIBO prevalence among cirrhotics is high, ranging between 30% and 70%[38] and it has been associated with the development of SBP due to the translocation of intestinal bacteria to the systemic circulation and the ascitic fluid[66]. Gram-negative bacteria such as Escherichia coli and Klebsiella spp. as well as Pneumococci, Streptococci and other Gram-positive and Gram-negative bacteria have been identified in 50% of cases by culture-based analysis of ascitic fluid[67]. However, bacterial DNA can be recognized in the ascites of half of cirrhotics even in absence of SBP and with negative cultures[44], and several studies identified microbes usually present within the gut[41,43,68]. Ascites microbial composition is linked with the stage of liver disease; indeed, Child-Pugh score is correlated with ascitic bacteria similarity and ascitic neutrophil count, further strengthening the connection between gut microbiota and liver cirrhosis progression[68].
Therefore, it has been hypothesized that rifaximin, being effective on SIBO, could be useful in preventing SBP. In the retrospective study by Hanouneh et al[66] a 72% reduction in SBP occurrence and a transplant free survival of 72% were observed in the 49 cirrhotic patients with ascites who received rifaximin (Table 2).
| Study | Study design | No. patients | Disease severity | Disease complication | Treatment schedule | Results | Safety |
| Hanouneh et al[66] 2012 | Retrospective | 404 | MELD score (mean ± SD): | SBP | 49 pts received rifaximin | SBP incidence: 11% in pts on rifaximin vs 32% in controls (P = 0.002) | Not reported |
| rifaximin: 17.6 ± 7.7 | 400 mg TID mainly for HE | ||||||
| no rifaximin 17.7 ± 7.5 | (recurrent HE or intolerance to lactulose) | 72% SBP reduction rate in rifaximin group after adjusting for MELD score, CTP score, serum sodium, and ascitic fluid total proteins (P = 0.007) | |||||
| CTP score | |||||||
| rifaximin B: 6.1%, C: 93.9% | |||||||
| no rifaximin B: 33%, C: 67% | |||||||
| 72% transplant-free survival for pts on rifaximin vs 57% for controls (P = 0.045) | |||||||
| Lutz et al[69] 2014 | Prospective, observational | 152 | CTP score: | SBP | Group 1 (108 pts): no prophylaxis | SBP occurrence rate: 32/152 (21%) overall, 22.2% group 1, 29.6% group 2 and 0% group 3 (P = 0.02 group 2 vs group 3 and P = 0.04 group 1 vs group 3) | Data available for SBP pts only |
| no prophylaxis: | Nosocomial infections: 38% rifaximin vs 54% no rifaximin (P = 0.690) | ||||||
| A: 1%, B: 57%, C: 43% | Group 2 (27 pts): rifaximin 400 mg TID | ||||||
| rifaximin: | Isolation of bacteria resistant to III generation cephalosporin: 25% rifaximin vs 46% no rifaximin | ||||||
| A: 0%, B: 33%, C: 67% | Group 3 (17 pts): systemically absorbed antibiotic prophylaxis | ||||||
| systemically absorbed antibiotics: | |||||||
| A:12%, B: 47%, C: 41% | Isolation of multidrug resistant bacteria: 25% rifaximin vs 9% no rifaximin |
Another prospective observational study reported that different bacterial species could be identified in the ascitic fluid of patients receiving rifaximin compared to those who did not receive SBP prophylaxis[69]. Indeed, Enterococci and Escherichia coli were isolated from the ascites of patients without prophylaxis and Klebsiella spp. were isolated in those on rifaximin. However, this finding had no predictive value, since the incidence of SBP was similar between the two groups.
Intestinal decontamination improves hemodynamics in animal models of cirrhosis by reducing endotoxemia related to bacterial translocation[70]. Similar results have also been obtained in humans[71], and have been associated with a lower incidence of complications (Table 3).
| Study | Study design | No. patients | Disease severity | Treatment schedule | Results | Safety |
| Vlachogiannakos et al[71] 2009 | Prospective | 30 | welve patients (40%) were Child-Pugh B and 18 (60%) Child-Pugh C | Rifaximin 1200 mg/d for 28 d | Median (range) plasma endotoxin levels decreased significantly after rifaximin administration both in systemic [1.45 (0-3.1) vs 0.7 (0-2.7), P < 0.0001] and splanchnic circulation [1.8 (0-3.4) vs 0.8 (0-2.1), P < 0.0001]. Meanwhile, the difference seen in endotoxin levels between the splanchnic and systemic circulation at day 0 (P = 0.001) was not noted at day 29 (P = 0.137) | Abdominal pain: 3% |
| CTP score: A: 0%, B: 40%, C: 60% | Self-limited diarrhea: 3% | |||||
| MELD score (mean, range): 17 (11-27) | Reduction in endotoxin levels in both systemic and splanchnic circulation compared to baseline (P < 0.0001) | |||||
| B: 40%, C: 60% | Reduction in HVPG compared to baseline (P < 0.0001) | |||||
| Reduction in HVPG correlated with hepatic vein endotoxin values (P = 0.023) | ||||||
| Kalambokis et al[73] 2012 | Prospective | 9 | CTP score: B: 56%, C: 44% | 8-wk course of rifaximin (1200 mg/d) | Rifaximin significantly reduced plasma endotoxin levels | Not reported |
| Rifaximin 1200 mg/d for 8 wk | Reduction in plasma endotoxin levels compared to baseline (P < 0.01) | |||||
| Vlachogiannakos et al[72] 2012 | Prospective | 69 | welve patients (40%) were Child-Pugh B and 18 (60%) Child-Pugh C | 23 pts who achieved a decrease in HVPG after 28-d rifaximin treatment[71] | Median (range) plasma endotoxin levels decreased significantly after rifaximin administration both in systemic [1.45 (0-3.1) vs 0.7 (0-2.7), P < 0.0001] and splanchnic circulation [1.8 (0-3.4) vs 0.8 (0-2.1), P < 0.0001]. Meanwhile, the difference seen in endotoxin levels between the splanchnic and systemic circulation at day 0 (P = 0.001) was not noted at day 29 (P = 0.137) | Nausea: 9% |
| Self-limited rash in the extremities: 4% | ||||||
| Persistent diarrhea: 4% | ||||||
| CTP score | 46 cirrhotic controls | |||||
| rifaximin | Reduction in plasma endotoxin levels in both systemic and splanchnic circulation compared to baseline (P < 0.0001) | |||||
| A: 0%, B: 48%, C: 52% | ||||||
| controls: | Risk of developing variceal bleeding: 35% rifaximin vs 59.5% controls (P = 0.011) | |||||
| A: 0%, B: 48%, C: 52% | Incidence of HE: 31.5% rifaximin vs 47% controls (P = 0.034) | |||||
| MELD score (mean ± SD) | Incidence of SBP: 4.5% rifaximin vs 46% controls (P = 0.027) | |||||
| rifaximin: 17.2 ± 3.6 | Incidence of HRS: 4.5% rifaximin vs 51% controls (P = 0.037) | |||||
| controls: 16.6 ± 3.5 | ||||||
| Kalambokis et al[75] 2012 | Prospective, randomized, placebo-controlled | 23 | CTP score | 13 pts: rifaximin 1200 mg/d for 4 wk | Reduction in endotoxin levels compared to control group (P = 0.005) | Not reported |
| rifaximin: | Increase in mean platelets count in rifaximin group compared to controls (P = 0.006) | |||||
| A: 0%, B: 46%, C: 54% | ||||||
| placebo: | 10 cirrhotic pts: placebo | |||||
| A: 0%, B: 40%, C: 60% | ||||||
| Bajaj et al[62] 2013 | Prospective | 20 | MELD score (mean ± SD): 9.8 ± 3.3 | Rifaximin 550 mg BID for 8 wk | Reduction in plasma endotoxin levels compared to baseline (P = 0.02) | Not reported |
Twenty-three patients with decompensated alcoholic cirrhosis who achieved a reduction of hepatic venous pressure gradient (HVPG) after 28 d of rifaximin treatment were then followed-up for 5 years[72]. Compared to matched controls, rifaximin group showed a lower incidence of complications related to portal hypertension, such as variceal bleeding, HE, SBP and hepatorenal syndrome, and a better survival compared to controls. Other studies confirmed a reduction in endotoxemia, serum bilirubin, Child-Pugh and MELD scores, together with an increase in serum albumin levels after rifaximin treatment[62,73].
Rifaximin has also been demonstrated to have beneficial effects in the treatment of thrombocytopenia, the pathogenesis of which has not been completely clarified yet in cirrhotics. Endotoxemia has been advocated to contribute, together with portal hypertension, in the development of thrombocytopenia in these patients[74]; indeed, a small prelimiary study demonstrated an increase in platelets count and a decrease in endotoxin levels in 13 patients with alcoholic cirrhosis receiving rifaximin for a 4-wk course, compared to 10 controls[75]. Even if these results may encourage the use of rifaximin to minimize the complications of endotoxemia due to portal hypertension, larger, randomized, controlled studies extended also to non alcoholic liver disease are required to confirm any clinical efficacy.
Rifaximin benefits are generally paralleled by a good safety profile, since the reported rate of adverse events between treated cirrhotics and those who did not receive the drug is similar, and toxicity mainly involves the gastrointestinal tract (e.g., abdominal pain or diarrhea) (Tables 1-3). In particular, nor increase in the rate of infections neither development of antibiotic resistance are common in cirrhotics treated with rifaximin[76,77]. Although some cases of Clostridium difficile infection have been reported[51,78], the incidence is comparable to that observed in patients with advanced liver disease and is affected by confounding factors, such as age, repeated hospitalizations, ongoing therapy with proton pump inhibitors and previous courses of antibiotics[78]. Candida albicans has also been isolated in fecal samples of about 20% of cirrhotics during rifaximin treatment[65]. Probably, this finding should not be considered unequivocally harmful, since Candida organisms commonly colonize the human gastrointestinal tract as a component of the resident mycobiota[79].
Even if the limited incidence of adverse events has to be attributed to the small amount of rifaximin reaching the systemic circulation, a special consideration regarding its absorption in patients with advanced liver disease is mandatory. Indeed, due to the increased intestinal permeability, higher systemic rifaximin concentrations have been observed in cirrhotics compared to healthy subjects[80]. For this reason, although it may not represent a major problem in the short-term drug administration, the effects of a possible increase in systemic absorption should be cautiously taken into account in cases of prolonged rifaximin administration.
P- Reviewer: Bashashati M, Kristensen K, Miura K, Singh N S- Editor: Ma YJ L- Editor: A E- Editor: Ma S
| 1. | Calanni F, Renzulli C, Barbanti M, Viscomi GC. Rifaximin: beyond the traditional antibiotic activity. J Antibiot (Tokyo). 2014;67:667-670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 78] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 2. | Marchese A, Salerno A, Pesce A, Debbia EA, Schito GC. In vitro activity of rifaximin, metronidazole and vancomycin against Clostridium difficile and the rate of selection of spontaneously resistant mutants against representative anaerobic and aerobic bacteria, including ammonia-producing species. Chemotherapy. 2000;46:253-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 85] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 3. | Scarpignato C, Pelosini I. Rifaximin, a poorly absorbed antibiotic: pharmacology and clinical potential. Chemotherapy. 2005;51 Suppl 1:36-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 174] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 4. | DuPont HL, Jiang ZD, Ericsson CD, Adachi JA, Mathewson JJ, DuPont MW, Palazzini E, Riopel LM, Ashley D, Martinez-Sandoval F. Rifaximin versus ciprofloxacin for the treatment of traveler’s diarrhea: a randomized, double-blind clinical trial. Clin Infect Dis. 2001;33:1807-1815. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 120] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 5. | Taylor DN, Bourgeois AL, Ericsson CD, Steffen R, Jiang ZD, Halpern J, Haake R, Dupont HL. A randomized, double-blind, multicenter study of rifaximin compared with placebo and with ciprofloxacin in the treatment of travelers’ diarrhea. Am J Trop Med Hyg. 2006;74:1060-1066. [PubMed] |
| 6. | Bielaszewska M, Idelevich EA, Zhang W, Bauwens A, Schaumburg F, Mellmann A, Peters G, Karch H. Effects of antibiotics on Shiga toxin 2 production and bacteriophage induction by epidemic Escherichia coli O104: H4 strain. Antimicrob Agents Chemother. 2012;56:3277-3282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 152] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 7. | Jiang ZD, DuPont HL, La Rocco M, Garey KW. In vitro susceptibility of Clostridium difficile to rifaximin and rifampin in 359 consecutive isolates at a university hospital in Houston, Texas. J Clin Pathol. 2010;63:355-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 34] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 8. | Blandizzi C, Viscomi GC, Marzo A, Scarpignato C. Is generic rifaximin still a poorly absorbed antibiotic? A comparison of branded and generic formulations in healthy volunteers. Pharmacol Res. 2014;85:39-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 9. | Tandon P, Delisle A, Topal JE, Garcia-Tsao G. High prevalence of antibiotic-resistant bacterial infections among patients with cirrhosis at a US liver center. Clin Gastroenterol Hepatol. 2012;10:1291-1298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 126] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 10. | Xu D, Gao J, Gillilland M, Wu X, Song I, Kao JY, Owyang C. Rifaximin alters intestinal bacteria and prevents stress-induced gut inflammation and visceral hyperalgesia in rats. Gastroenterology. 2014;146:484-496.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 205] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 11. | Gao J, Gillilland MG, Owyang C. Rifaximin, gut microbes and mucosal inflammation: unraveling a complex relationship. Gut Microbes. 2014;5:571-575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 31] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 12. | Ridlon JM, Alves JM, Hylemon PB, Bajaj JS. Cirrhosis, bile acids and gut microbiota: unraveling a complex relationship. Gut Microbes. 2013;4:382-387. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 227] [Cited by in RCA: 271] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 13. | Maccaferri S, Vitali B, Klinder A, Kolida S, Ndagijimana M, Laghi L, Calanni F, Brigidi P, Gibson GR, Costabile A. Rifaximin modulates the colonic microbiota of patients with Crohn’s disease: an in vitro approach using a continuous culture colonic model system. J Antimicrob Chemother. 2010;65:2556-2565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 143] [Article Influence: 9.5] [Reference Citation Analysis (1)] |
| 14. | Brigidi P, Swennen E, Rizzello F, Bozzolasco M, Matteuzzi D. Effects of rifaximin administration on the intestinal microbiota in patients with ulcerative colitis. J Chemother. 2002;14:290-295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 64] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 15. | Brown EL, Xue Q, Jiang ZD, Xu Y, Dupont HL. Pretreatment of epithelial cells with rifaximin alters bacterial attachment and internalization profiles. Antimicrob Agents Chemother. 2010;54:388-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 79] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 16. | Vitali B, Perna F, Lammers K, Turroni S, Gionchetti P, Brigidi P. Immunoregulatory activity of rifaximin associated with a resistant mutant of Bifidobacterium infantis. Int J Antimicrob Agents. 2009;33:387-389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 17. | Mencarelli A, Renga B, Palladino G, Claudio D, Ricci P, Distrutti E, Barbanti M, Baldelli F, Fiorucci S. Inhibition of NF-κB by a PXR-dependent pathway mediates counter-regulatory activities of rifaximin on innate immunity in intestinal epithelial cells. Eur J Pharmacol. 2011;668:317-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 98] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 18. | Laterza L, Ianiro G, Scoleri I, Landi R, Bruno G, Scaldaferri F, Gaetani E, Campanale M, Gasbarrini A. Rifaximin for the treatment of diarrhoea-predominant irritable bowel syndrome. Expert Opin Pharmacother. 2015;16:607-615. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 19. | Pimentel M, Lembo A, Chey WD, Zakko S, Ringel Y, Yu J, Mareya SM, Shaw AL, Bortey E, Forbes WP. Rifaximin therapy for patients with irritable bowel syndrome without constipation. N Engl J Med. 2011;364:22-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 690] [Cited by in RCA: 691] [Article Influence: 49.4] [Reference Citation Analysis (0)] |
| 20. | Schmulson M, Bielsa MV, Carmona-Sánchez R, Hernández A, López-Colombo A, López Vidal Y, Peláez-Luna M, Remes-Troche JM, Tamayo JL, Valdovinos MA. Microbiota, gastrointestinal infections, low-grade inflammation, and antibiotic therapy in irritable bowel syndrome: an evidence-based review. Rev Gastroenterol Mex. 2014;79:96-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 21. | Cremonini F, Lembo A. Rifaximin for the treatment of irritable bowel syndrome. Expert Opin Pharmacother. 2012;13:433-440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 22. | Jigaranu AO, Nedelciuc O, Blaj A, Badea M, Mihai C, Diculescu M, Cijevschi-Prelipcean C. Is rifaximin effective in maintaining remission in Crohn’s disease? Dig Dis. 2014;32:378-383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 23. | Guslandi M. Rifaximin in the treatment of inflammatory bowel disease. World J Gastroenterol. 2011;17:4643-4646. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 31] [Cited by in RCA: 37] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 24. | Prantera C, Lochs H, Campieri M, Scribano ML, Sturniolo GC, Castiglione F, Cottone M. Antibiotic treatment of Crohn’s disease: results of a multicentre, double blind, randomized, placebo-controlled trial with rifaximin. Aliment Pharmacol Ther. 2006;23:1117-1125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 121] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 25. | Shafran I, Johnson LK. An open-label evaluation of rifaximin in the treatment of active Crohn’s disease. Curr Med Res Opin. 2005;21:1165-1169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 49] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 26. | Shafran I, Burgunder P. Rifaximin for the treatment of newly diagnosed Crohn’s disease: a case series. Am J Gastroenterol. 2008;103:2158-2160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 27. | Campieri M, Rizzello F, Venturi A. Combination of antibiotic and probiotic treatment is efficacious in prophylaxis of post-operative recurrence of Crohn’s disease: a randomized controlled study vs mesalamine. Gastroenterology. 2000;118:A781. |
| 28. | Prantera C, Lochs H, Grimaldi M, Danese S, Scribano ML, Gionchetti P. Rifaximin-extended intestinal release induces remission in patients with moderately active Crohn’s disease. Gastroenterology. 2012;142:473-481.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 136] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 29. | Guslandi M, Petrone MC, Testoni PA. Rifaximin for active ulcerative colitis. Inflamm Bowel Dis. 2006;12:335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 33] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 30. | Gionchetti P, Rizzello F, Ferrieri A, Venturi A, Brignola C, Ferretti M, Peruzzo S, Miglioli M, Campieri M. Rifaximin in patients with moderate or severe ulcerative colitis refractory to steroid-treatment: a double-blind, placebo-controlled trial. Dig Dis Sci. 1999;44:1220-1221. [PubMed] |
| 31. | Cianci R, Frosali S, Pagliari D, Cesaro P, Petruzziello L, Casciano F, Landolfi R, Costamagna G, Pandolfi F. Uncomplicated diverticular disease: innate and adaptive immunity in human gut mucosa before and after rifaximin. J Immunol Res. 2014;2014:696812. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 32. | Boynton W, Floch M. New strategies for the management of diverticular disease: insights for the clinician. Therap Adv Gastroenterol. 2013;6:205-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 30] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 33. | Moretti A, Spagnolo A, Mangone M, Chiesara F, Aratari A, Papi C, Koch M. [Role of rifaximin in the treatment of colonic diverticular disease]. Clin Ter. 2012;163:33-38. [PubMed] |
| 34. | Papi C, Ciaco A, Koch M, Capurso L. Efficacy of rifaximin in the treatment of symptomatic diverticular disease of the colon. A multicentre double-blind placebo-controlled trial. Aliment Pharmacol Ther. 1995;9:33-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 106] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 35. | Latella G, Pimpo MT, Sottili S, Zippi M, Viscido A, Chiaramonte M, Frieri G. Rifaximin improves symptoms of acquired uncomplicated diverticular disease of the colon. Int J Colorectal Dis. 2003;18:55-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 106] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 36. | Colecchia A, Vestito A, Pasqui F, Mazzella G, Roda E, Pistoia F, Brandimarte G, Festi D. Efficacy of long term cyclic administration of the poorly absorbed antibiotic Rifaximin in symptomatic, uncomplicated colonic diverticular disease. World J Gastroenterol. 2007;13:264-269. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 73] [Cited by in RCA: 74] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 37. | Bajaj JS, Cox IJ, Betrapally NS, Heuman DM, Schubert ML, Ratneswaran M, Hylemon PB, White MB, Daita K, Noble NA. Systems biology analysis of omeprazole therapy in cirrhosis demonstrates significant shifts in gut microbiota composition and function. Am J Physiol Gastrointest Liver Physiol. 2014;307:G951-G957. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 120] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 38. | Giannelli V, Di Gregorio V, Iebba V, Giusto M, Schippa S, Merli M, Thalheimer U. Microbiota and the gut-liver axis: bacterial translocation, inflammation and infection in cirrhosis. World J Gastroenterol. 2014;20:16795-16810. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 156] [Cited by in RCA: 169] [Article Influence: 15.4] [Reference Citation Analysis (1)] |
| 39. | Morgan XC, Huttenhower C. Meta’omic analytic techniques for studying the intestinal microbiome. Gastroenterology. 2014;146:1437-1448.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 200] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 40. | Lu H, Wu Z, Xu W, Yang J, Chen Y, Li L. Intestinal microbiota was assessed in cirrhotic patients with hepatitis B virus infection. Intestinal microbiota of HBV cirrhotic patients. Microb Ecol. 2011;61:693-703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 179] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 41. | Wiest R, Lawson M, Geuking M. Pathological bacterial translocation in liver cirrhosis. J Hepatol. 2014;60:197-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 494] [Cited by in RCA: 562] [Article Influence: 51.1] [Reference Citation Analysis (0)] |
| 42. | Wei X, Yan X, Zou D, Yang Z, Wang X, Liu W, Wang S, Li X, Han J, Huang L. Abnormal fecal microbiota community and functions in patients with hepatitis B liver cirrhosis as revealed by a metagenomic approach. BMC Gastroenterol. 2013;13:175. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 88] [Cited by in RCA: 106] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 43. | Chen Y, Yang F, Lu H, Wang B, Chen Y, Lei D, Wang Y, Zhu B, Li L. Characterization of fecal microbial communities in patients with liver cirrhosis. Hepatology. 2011;54:562-572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 674] [Cited by in RCA: 797] [Article Influence: 56.9] [Reference Citation Analysis (3)] |
| 44. | Tuomisto S, Pessi T, Collin P, Vuento R, Aittoniemi J, Karhunen PJ. Changes in gut bacterial populations and their translocation into liver and ascites in alcoholic liver cirrhotics. BMC Gastroenterol. 2014;14:40. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 89] [Cited by in RCA: 109] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 45. | Zhang Z, Zhai H, Geng J, Yu R, Ren H, Fan H, Shi P. Large-scale survey of gut microbiota associated with MHE Via 16S rRNA-based pyrosequencing. Am J Gastroenterol. 2013;108:1601-1611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 146] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 46. | Bajaj JS, Betrapally NS, Hylemon PB, Heuman DM, Daita K, White MB, Unser A, Thacker LR, Sanyal AJ, Kang DJ. Salivary microbiota reflects changes in gut microbiota in cirrhosis with hepatic encephalopathy. Hepatology. 2015;62:1260-1271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 249] [Article Influence: 24.9] [Reference Citation Analysis (0)] |
| 47. | Qin N, Yang F, Li A, Prifti E, Chen Y, Shao L, Guo J, Le Chatelier E, Yao J, Wu L. Alterations of the human gut microbiome in liver cirrhosis. Nature. 2014;513:59-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1230] [Cited by in RCA: 1537] [Article Influence: 139.7] [Reference Citation Analysis (40)] |
| 48. | Bajaj JS, Heuman DM, Hylemon PB, Sanyal AJ, White MB, Monteith P, Noble NA, Unser AB, Daita K, Fisher AR. Altered profile of human gut microbiome is associated with cirrhosis and its complications. J Hepatol. 2014;60:940-947. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 659] [Cited by in RCA: 837] [Article Influence: 76.1] [Reference Citation Analysis (0)] |
| 49. | Bajaj JS, Ridlon JM, Hylemon PB, Thacker LR, Heuman DM, Smith S, Sikaroodi M, Gillevet PM. Linkage of gut microbiome with cognition in hepatic encephalopathy. Am J Physiol Gastrointest Liver Physiol. 2012;302:G168-G175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 347] [Cited by in RCA: 423] [Article Influence: 32.5] [Reference Citation Analysis (0)] |
| 50. | Bajaj JS, Hylemon PB, Ridlon JM, Heuman DM, Daita K, White MB, Monteith P, Noble NA, Sikaroodi M, Gillevet PM. Colonic mucosal microbiome differs from stool microbiome in cirrhosis and hepatic encephalopathy and is linked to cognition and inflammation. Am J Physiol Gastrointest Liver Physiol. 2012;303:G675-G685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 354] [Cited by in RCA: 429] [Article Influence: 33.0] [Reference Citation Analysis (0)] |
| 51. | Bass NM, Mullen KD, Sanyal A, Poordad F, Neff G, Leevy CB, Sigal S, Sheikh MY, Beavers K, Frederick T. Rifaximin treatment in hepatic encephalopathy. N Engl J Med. 2010;362:1071-1081. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 868] [Cited by in RCA: 868] [Article Influence: 57.9] [Reference Citation Analysis (0)] |
| 52. | Neff GW, Jones M, Broda T, Jonas M, Ravi R, Novick D, Kaiser TE, Kemmer N. Durability of rifaximin response in hepatic encephalopathy. J Clin Gastroenterol. 2012;46:168-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 53. | Sharma BC, Sharma P, Lunia MK, Srivastava S, Goyal R, Sarin SK. A randomized, double-blind, controlled trial comparing rifaximin plus lactulose with lactulose alone in treatment of overt hepatic encephalopathy. Am J Gastroenterol. 2013;108:1458-1463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 231] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 54. | Maharshi S, Sharma BC, Srivastava S, Jindal A. Randomised controlled trial of lactulose versus rifaximin for prophylaxis of hepatic encephalopathy in patients with acute variceal bleed. Gut. 2015;64:1341-1342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 55. | Sharma K, Pant S, Misra S, Dwivedi M, Misra A, Narang S, Tewari R, Bhadoria AS. Effect of rifaximin, probiotics, and l-ornithine l-aspartate on minimal hepatic encephalopathy: a randomized controlled trial. Saudi J Gastroenterol. 2014;20:225-232. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 64] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 56. | Mas A, Rodés J, Sunyer L, Rodrigo L, Planas R, Vargas V, Castells L, Rodríguez-Martínez D, Fernández-Rodríguez C, Coll I. Comparison of rifaximin and lactitol in the treatment of acute hepatic encephalopathy: results of a randomized, double-blind, double-dummy, controlled clinical trial. J Hepatol. 2003;38:51-58. [PubMed] |
| 57. | Paik YH, Lee KS, Han KH, Song KH, Kim MH, Moon BS, Ahn SH, Lee SJ, Park HJ, Lee DK. Comparison of rifaximin and lactulose for the treatment of hepatic encephalopathy: a prospective randomized study. Yonsei Med J. 2005;46:399-407. [PubMed] |
| 58. | Leevy CB, Phillips JA. Hospitalizations during the use of rifaximin versus lactulose for the treatment of hepatic encephalopathy. Dig Dis Sci. 2007;52:737-741. [PubMed] |
| 59. | Bajaj JS, Heuman DM, Wade JB, Gibson DP, Saeian K, Wegelin JA, Hafeezullah M, Bell DE, Sterling RK, Stravitz RT. Rifaximin improves driving simulator performance in a randomized trial of patients with minimal hepatic encephalopathy. Gastroenterology. 2011;140:478-487.e1. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 187] [Cited by in RCA: 178] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 60. | Sanyal A, Younossi ZM, Bass NM, Mullen KD, Poordad F, Brown RS, Vemuru RP, Mazen Jamal M, Huang S, Merchant K. Randomised clinical trial: rifaximin improves health-related quality of life in cirrhotic patients with hepatic encephalopathy - a double-blind placebo-controlled study. Aliment Pharmacol Ther. 2011;34:853-861. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 109] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 61. | Sidhu SS, Goyal O, Mishra BP, Sood A, Chhina RS, Soni RK. Rifaximin improves psychometric performance and health-related quality of life in patients with minimal hepatic encephalopathy (the RIME Trial). Am J Gastroenterol. 2011;106:307-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 184] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 62. | Bajaj JS, Heuman DM, Sanyal AJ, Hylemon PB, Sterling RK, Stravitz RT, Fuchs M, Ridlon JM, Daita K, Monteith P. Modulation of the metabiome by rifaximin in patients with cirrhosis and minimal hepatic encephalopathy. PLoS One. 2013;8:e60042. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 254] [Cited by in RCA: 339] [Article Influence: 28.3] [Reference Citation Analysis (0)] |
| 63. | Wang CC, Kao JH. Rifaximin treatment in hepatic encephalopathy. N Engl J Med. 2010;362:2423; author reply 2424-2425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 64. | Zullo A, Hassan C, Lorenzetti R. Rifaximin therapy in minimal hepatic encephalopathy cirrhotics. Am J Gastroenterol. 2011;106:2041; author reply 2041-2042. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 65. | Zullo A, Hassan C, Ridola L, Lorenzetti R, Campo SM, Riggio O. Rifaximin therapy and hepatic encephalopathy: Pros and cons. World J Gastrointest Pharmacol Ther. 2012;3:62-67. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 26] [Cited by in RCA: 21] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 66. | Hanouneh MA, Hanouneh IA, Hashash JG, Law R, Esfeh JM, Lopez R, Hazratjee N, Smith T, Zein NN. The role of rifaximin in the primary prophylaxis of spontaneous bacterial peritonitis in patients with liver cirrhosis. J Clin Gastroenterol. 2012;46:709-715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 74] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 67. | Rogers GB, Russell LE, Preston PG, Marsh P, Collins JE, Saunders J, Sutton J, Fine D, Bruce KD, Wright M. Characterisation of bacteria in ascites--reporting the potential of culture-independent, molecular analysis. Eur J Clin Microbiol Infect Dis. 2010;29:533-541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 68. | Rogers GB, van der Gast CJ, Bruce KD, Marsh P, Collins JE, Sutton J, Wright M. Ascitic microbiota composition is correlated with clinical severity in cirrhosis with portal hypertension. PLoS One. 2013;8:e74884. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 69. | Lutz P, Parcina M, Bekeredjian-Ding I, Nischalke HD, Nattermann J, Sauerbruch T, Hoerauf A, Strassburg CP, Spengler U. Impact of rifaximin on the frequency and characteristics of spontaneous bacterial peritonitis in patients with liver cirrhosis and ascites. PLoS One. 2014;9:e93909. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 43] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 70. | Guarner C, Runyon BA, Heck M, Young S, Sheikh MY. Effect of long-term trimethoprim-sulfamethoxazole prophylaxis on ascites formation, bacterial translocation, spontaneous bacterial peritonitis, and survival in cirrhotic rats. Dig Dis Sci. 1999;44:1957-1962. [PubMed] |
| 71. | Vlachogiannakos J, Saveriadis AS, Viazis N, Theodoropoulos I, Foudoulis K, Manolakopoulos S, Raptis S, Karamanolis DG. Intestinal decontamination improves liver haemodynamics in patients with alcohol-related decompensated cirrhosis. Aliment Pharmacol Ther. 2009;29:992-999. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 138] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 72. | Vlachogiannakos J, Viazis N, Vasianopoulou P, Vafiadis I, Karamanolis DG, Ladas SD. Long-term administration of rifaximin improves the prognosis of patients with decompensated alcoholic cirrhosis. J Gastroenterol Hepatol. 2013;28:450-455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 173] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 73. | Kalambokis GN, Tsianos EV. Rifaximin reduces endotoxemia and improves liver function and disease severity in patients with decompensated cirrhosis. Hepatology. 2012;55:655-656. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 53] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 74. | Kalambokis G, Tsianos EV. Endotoxaemia in the pathogenesis of cytopenias in liver cirrhosis. Could oral antibiotics raise blood counts? Med Hypotheses. 2011;76:105-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 75. | Kalambokis GN, Mouzaki A, Rodi M, Tsianos EV. Rifaximin improves thrombocytopenia in patients with alcoholic cirrhosis in association with reduction of endotoxaemia. Liver Int. 2012;32:467-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 45] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 76. | Mullen KD, Sanyal AJ, Bass NM, Poordad FF, Sheikh MY, Frederick RT, Bortey E, Forbes WP. Rifaximin is safe and well tolerated for long-term maintenance of remission from overt hepatic encephalopathy. Clin Gastroenterol Hepatol. 2014;12:1390-7.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 151] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 77. | Bajaj JS, Barrett AC, Bortey E, Paterson C, Forbes WP. Prolonged remission from hepatic encephalopathy with rifaximin: results of a placebo crossover analysis. Aliment Pharmacol Ther. 2015;41:39-45. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 44] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 78. | Vanjak D, Girault G, Branger C, Rufat P, Valla DC, Fantin B. Risk factors for Clostridium difficile infection in a hepatology ward. Infect Control Hosp Epidemiol. 2007;28:202-204. [PubMed] |
| 79. | Kumamoto CA. Inflammation and gastrointestinal Candida colonization. Curr Opin Microbiol. 2011;14:386-391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 173] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 80. | Bajaj JS, Riggio O. Drug therapy: rifaximin. Hepatology. 2010;52:1484-1488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 34] [Article Influence: 2.3] [Reference Citation Analysis (0)] |