Published online Nov 21, 2015. doi: 10.3748/wjg.v21.i43.12311
Peer-review started: April 24, 2015
First decision: August 2, 2015
Revised: August 4, 2015
Accepted: October 23, 2015
Article in press: October 26, 2015
Published online: November 21, 2015
Processing time: 208 Days and 8.5 Hours
End-stage liver disease (ESLD) is a leading cause of morbidity and mortality amongst human immunodeficiency virus (HIV)-positive individuals. Chronic hepatitis B and hepatitis C virus (HCV) infection, drug-induced hepatotoxicity related to combined anti-retro-viral therapy, alcohol related liver disease and non-alcohol related fatty liver disease appear to be the leading causes. It is therefore, anticipated that more HIV-positive patients with ESLD will present as potential transplant candidates. HIV infection is no longer a contraindication to liver transplantation. Key transplantation outcomes such as rejection and infection rates as well as medium term graft and patient survival match those seen in the non-HIV infected patients in the absence of co-existing HCV infection. HIV disease does not seem to be negatively impacted by transplantation. However, HIV-HCV co-infection transplant outcomes remain suboptimal due to recurrence. In this article, we review the key challenges faced by this patient cohort in the pre- and post-transplant period.
Core tip: Liver disease is a major cause of mortality and morbidity in human immunodeficiency virus (HIV) positive patients. It is therefore increasingly likely that HIV positive patients with chronic liver disease are likely to present as potential liver transplant candidates. We therefore review the current data with regards to liver transplantation in HIV positive patients.
- Citation: Joshi D, Agarwal K. Role of liver transplantation in human immunodeficiency virus positive patients. World J Gastroenterol 2015; 21(43): 12311-12321
- URL: https://www.wjgnet.com/1007-9327/full/v21/i43/12311.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i43.12311
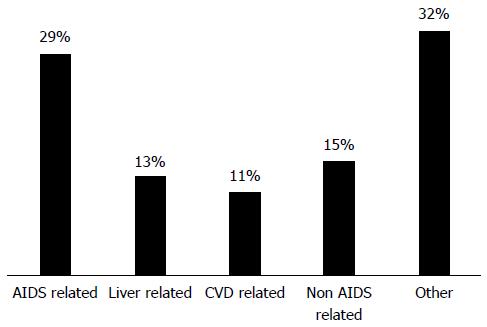
The management and treatment of human immunodeficiency virus (HIV)-1 infection was revolutionized in 1996 in Western Europe and North America following the introduction of combined anti-viral therapy (cART), resulting in the reduction of acquired immune deficiency syndrome (AIDS) and AIDS-related deaths[1]. Such was the success of cART, that now more than 50% of deaths in HIV-positive patients on cART are not directly related to HIV infection or AIDS[1-3]. The increase in non-AIDS related morbidities compared to the general population appears to be multifactorial: HIV infection leads to a state of immune dysregulation and inflammation whilst cART predisposes to dyslipidaemia and diabetes[4]. The most recent D:A:D (Data collection on adverse events of anti-HIV drugs) study demonstrated that liver disease is now the second commonest cause of a non-AIDS related death, having been overtaken by non-AIDS defining cancers (Figure 1)[5].
Liver related mortality however, remains high. Given the similar transmission routes, co-infection with chronic hepatitis C virus (HCV) and chronic hepatitis B virus (HBV) is common[6]. Other liver-related aetiologies amongst HIV-positive individuals include cART related liver toxicity, alcohol, non-alcohol related liver disease (NAFLD) and hepatocellular carcinoma (HCC). HIV positive patients present with the same clinical sequelae of chronic liver disease as HIV negative patients but tend to present at a younger age and a markedly reduced survival rate after the first episode of decompensation[7]. In HIV-positive patients with compensated cirrhosis an increased mortality rate is associated with age > 50 years, model for end-stage liver disease (MELD) score > 11 and poor control of HIV disease[8].
One third of patients with HIV infection are co-infected with chronic HCV, and the majority of deaths in HIV-positive patients with end-stage liver disease (ESLD) can be directly attributable to HCV infection[8]. At-risk groups include intravenous drug users, haemophiliacs who were exposed to contaminated infusions of plasma derived factor VIII or X concentrate and men who have sex with men (MSM)[9]. Recent data has demonstrated an increasing incidence of acute HCV cases in MSM who are HIV-positive acquired through sexual transmission[10,11].
HCV infection in a HIV positive individual is associated with a reduced rate of spontaneous HCV RNA clearance and therefore, more likely to result in chronic HCV infection[12]. HIV/HCV co-infection results in a more aggressive, rapid fibrosis progression rate compared HCV mono-infected patients[13-15]. In a study of 135 patients with HIV/HCV followed over a median of 3 years, 44% had evidence of fibrosis progression whilst 13% developed cirrhosis[16]. Mechanisms for the accelerated fibrosis rates include up-regulation of pro-fibrotic pathways, enhanced microbial translocation, increased numbers of pro-fibrogenic CD8+ cells, reduction in CD4+ cells and reduction of interleukin-10 expression[12,17-20]. Clinical factors predictive of fibrosis progression include detectable HIV viraemia, low CD4+ counts, baseline necro-inflammatory activity on liver biopsy and increased alcohol consumption (> 50 g per day)[16,21]. Survival after the first episode of hepatic decompensation is inferior amongst HIV/HCV co-infected patients compared to HCV mono-infected patients; median estimated survival of only 13 mo[22].
Treatment of HCV in HIV-positive patients with pegylated-interferon (PEG) and ribavirin was associated with an increased side-effect profile and inferior sustained virological response rates (SVR) of 17%-35%[23-25]. The advent of the new directly acting antiviral (DAAs) agents have dramatically improved outcomes amongst HIV/HCV co-infected patients. The first generation of the new DAAS were boceprevir and telaprevir, both NS3A/4 protease inhibitors (PI). In phase II studies, triple therapy with boceprevir or telaprevir in conjunction with PEG and ribavirin resulted in SVR rates of 63% and 74% respectively in HIV-positive HCV genotype 1 infected patients[26,27]. Simeprevir, a second generation NS3/4 PI, plus PEG and ribavirin has also been evaluated in a Phase III trial demonstrating SVR12 rates of 74%[28]. Sofosbuvir, a NS5B polymerase inhibitor, has pan-genotypic activity and a high genetic barrier to resistance with no significant drug interactions with cART. Sofosbuvir plus weight-based ribavirin has been evaluated in the PHOTON-1 study in genotypes (G) 1-3[29]. Results demonstrated SVR12 rates similar to HCV mono-infected patients (G1 76%, G2 88%, G3 67%) with no evidence of HIV breakthrough[29]. SVR rates of 98% were recently published using the combination of ledipasvir and sofosbuvir for 12 wk[30]. TURQUOISE-I was a randomised, open label study assessing the all-oral 3 direct-acting antiviral (3D) regimen of ombitasvir, paritaprevir (co-dosed with ritonavir), dasabavir and ribavirin in HIV-HCV (genotype 1) co-infected patients[31]. SVR rates for 12 wk and 24 wk of this all oral treatment regimen were 94% and 91% respectively with minimal side-effects[31]. Drug-drug interactions between the anti-HCV DAAs and cART medications are concerning. Table 1 summarises possible interactions and recommended dose adjustments where appropriate.
| Anti-HCV DAA | cART not recommended | Dose changes |
| Boceprevir | Atazanavir | |
| Darunavir/ritonavir | ||
| Efavirenz | ||
| Ritonavir | ||
| Telaprevir | Darunavir | Efavirenz - dose increased |
| Lopinavir/ritonavir | to 1125 mg TDS | |
| Simeprevir | Atazanavir | |
| Efavirenz | ||
| Darunavir/ritonavir | ||
| Nevirapine | ||
| Daclatasvir | - | Efavirenz - dose increased to 90 mg OD |
| Atazanavir and protease inhibitors - dose reduced to 30 mg OD | ||
| Sofosbuvir | Didanosine | |
| Zidovudine |
All HIV/HCV positive patients listed for liver transplantation should be considered for anti-viral therapy. HIV/HCV co-infected patients with decompensated cirrhosis, where the treatment aim is viral suppression prior to liver transplantation, should be treated with sofosbuvir and weight based ribavirin for up to 48 wk[32].
Ten percent of the HIV-positive population have evidence of chronic HBV infection[33]. Vertical transmission of HBV remains the most common route of infection worldwide, whilst sexual transmission and percutaneous transmission is more common in Europe and North America[34]. Chronic HBV infection is more common in HIV-positive patients, especially individuals with low CD4+ cell counts[35]. In addition, HIV/HBV co-infected patients have higher HBV DNA titres compared to HBV mono-infected patients, which translates into accelerated fibrosis rates and an increased risk of developing HCC[36]. Anti-iral therapy is considerably easier in HIV/HBV co-infected patients compared to HIV/HCV co-infected patients due to nucleos(t)ide reverse-transcriptase inhibitors (lamivudine, emtricitabine and tenofovir) that have both anti-HIV and anti-HBV activity.
Abnormal liver enzymes are common in HIV-positive patients occurring in up to two thirds of patients on cART[37]. Identifying the culprit drug can be difficult due to the use combination therapies. Patterns of liver injury include hypersensitivity, idiosyncratic hepatotoxicity, mitochondrial toxicity, immune reconstitution syndrome and hepatic steatosis[38-40]. Non-cirrhotic portal hypertension (NCPH) is an increasingly recognised clinical entity amongst HIV-positive patients especially those with previous didanosine exposure[41]. The pathogenesis of NCPH has been linked to a pro-thrombotic state. The histological spectrum includes nodular regenerative hyperplasia, hepatoportal sclerosis, peri-portal fibrosis and sclerosing portal venopathy[42-44].
Steatosis and steatohepatitis are common in diabetics, alcohol users and those with features of the metabolic syndrome[45,46]. Compared to the general population (14%-31%), NAFLD is more common in HIV-positive patients (30%-40%)[47,48]. The D:A:D study highlighted that the prevalence of the metabolic syndrome has increased over the last decade from 19% in 2000-2001 to 42% in 2006-2007[49]. This is likely to result in an increase in the prevalence of NAFLD, given that NAFLD is the hepatic manifestation of the metabolic syndrome. There is limited data available on the risk factors for NAFLD in HIV-positive patients but attention has focused on the role of cART because of its negative effects of insulin resistance, glucose metabolism and lipid metabolism. Central adiposity, male sex, low serum high-density lipoprotein levels, raised triglycerides levels and an increased ratio of alanine aminotransferase to aspartate aminotransferase have been suggested as risk factors for the development of NAFLD[46]. Raised gamma glutamyl transpeptidases and HOMA-IR > 2.5 are associated with NASH independent of cART and abdominal fat[50].
Hepatocellular carcinoma (HCC) cases in HIV-positive patients is expected to increase predominately due to the co-infection in chronic HBV and HCV infection. Data from the MORTAVIC study and the French Mortalite study has certainly demonstrated an increased number of deaths related to HCC[8,51]. HIV-positive patients with HCC tend to present at a younger age with symptomatic and multiple or invasive disease[36]. HIV-positive patients with cirrhosis should undergo 6-monthly surveillance with a liver ultrasound and serum alpha-feto protein. Standard treatment therapies (radio-frequencu ablation, transarterial chemo-embolisation, liver resection and liver transplantation) should be considered on an individual patient basis.
HIV-positive patients with liver disease should be managed in a multi-disciplinary environment with an experienced transplant hepatologist and HIV physician. Given their rapid disease kinetic, HIV-positive patients with end stage liver disease (ESLD) need to be identified early. The MELD score appears to be a sensitive predictor of patient outcome amongst HIV-positive patients pre-liver transplantation[52,53]. Even after adjusting for CD4+ counts and HIV RNA titres, the MELD score remains the only significant predictor of mortality[52].
Guidelines for liver transplantation in HIV-positive patients have evolved as our experience with this cohort has increased. The current United States National Institutes of Health multicenter trial guidelines for liver transplantation in HIV-positive patients with chronic liver disease are listed in Table 2.
| The criteria for liver transplantation are met |
| CD4+ cell count > 100 cells/μL (> 200 cells/μL with a previous history of opportunistic complications) |
| HIV viral load < 50 copies/mL (using ultrasensitive Amplicor Monitor PCR assay) |
| Absence of AIDS-defining illness1 |
| Absence progressive multi-focal leukoencephalopathy, chronic intestinal cryptosporidiosis (> 1 mo) or primary CNS lymphoma |
Optimal control of HIV disease is required for all HIV-positive patients undergoing consideration for liver transplantation. In patients with portal hypertension, splenic sequestration of T lymphocytes can lead to a fall in the CD4+ T cell count. In such cases a CD4+ cell count > 100 cells/μL is acceptable. Historically, a fall in the CD4+ cell count could also be precipitated by the use of PEG. In our opinion, CD4+ T cell percentages may represent a more sensitive indicator of immune reconstitution in HIV-positive patients with portal hypertension. HIV-positive patients undergoing evaluation for liver transplantation also require an undetectable HIV viral load (< 50 copies/mL) except for those that presently acutely. The inability to achieve an undetectable HIV RNA viral load before liver transplantation has been associated with an increased mortality rate (HR = 3.5, P < 0001)[53]. In addition to good therapeutic options available in the pre-transplant period, HIV-positive patients require future cART options based upon their previous regimens and genotype resistance testing.
Certain HIV-positive patients may not be able to tolerate cART medications pre-liver transplantation due to poor liver synthetic function. This group should not be automatically excluded from liver transplantation as long as control of their HIV is deemed possible post-liver transplantation. cART intolerance post-liver transplantation, however has been identified as an important predictor of survival[54]. A thorough knowledge of past opportunistic infections is also required. A distant history of an opportunistic infection in a patient that was not taking cART is not a contraindication to liver transplantation unless there is no effective treatment available for possible recurrence post-liver transplantation. Absolute contra-indications include multidrug resistant HIV infection, resistant fungal infections, chronic intestinal cryptosporidiosis, progressive multi-focal leukoencephalopathy and central nervous system lymphoma.
Standard surgical techniques with conventional arterial, venous and biliary anastomoses are recommended. Previous concerns regarding the possible transmission of HIV to the surgical team appear to be unfounded. The risk of HIV transmission is low and substantially lower than the risk of transmission of HBV and HCV[55]. In the event of HIV exposure, current regimens provide effective prophylaxis[55]. HIV infection is associated with a pro-thrombotic state and therefore concerns have been raised regarding an increased risk of vascular complications post transplantation[56]. Data appears to be conflicting regarding an increased incidence of hepatic artery thrombosis and at present no firm conclusions can be drawn[57,58]. In our institution, we introduce prophylactic subcutaneous heparin (5000 units every 8 h) once the international normalised ration is below 1.5 and the platelet count is greater than 50 × 109 cells/L.
Initial case series of HIV-positive patients undergoing liver transplantation reported poor outcomes[59,60]. It is important to note that this was before the introduction of cART regimens. Retrospective data since has demonstrated an increasing understanding of the complexities faced by this unique patient cohort. One of the largest studies performed analysed data provided by the US United Network for Organ Sharing (UNOS) liver transplant database (1997-2006) and identified 138 HIV-positive patients[61]. Overall survival rates were inferior in the HIV-positive cohort compared to a comparative HIV negative cohort (n = 30520) at 2- and 3-years post transplant (70% and 60% vs 81% and 77%, P < 0.047). Considerable data however, was missing from the HIV cohort raising the possibility that HIV infection may not have been optimally treated prior to liver transplantation.
Outcomes in HIV/HCV co-infected patients is clearly suboptimal when compared to other aetiologies; survival rates ranging between 64%-88% at 1 year and 33%-51% at 5 years[54,62-64]. To date, two prospective studies have been performed in HIV/HCV co-infected patients undergoing liver transplantation (Table 3), one conducted in the United States and the other in Spain[65,66]. The United States study reported outcomes in 89 HIV/HCV co-infected patients and 235 HCV mono-infected controls performed at 17 United States centers[66]. Compared to HCV controls, HIV/HCV co-infected patients were younger (49 years vs 54 years, P < 0.0001), had lower body mass index (BMI) at listing (25 kg/m2vs 28 kg/m2, P < 0.0001), more likely to have HBV co-infection (6% vs 1%, P = 0.02), were more likely to receive a non-heart beating graft (17% vs 4%, P = 0.0002), longer median warm ischaemia time (41 min vs 21 min, P = 0.001) and were less likely to be given tacrolimus-based (vs cyclosporine) initial immunosuppression (58% vs 80%, P < 0.0001). 1- and 3-year patient survival rates were 76% and 60% in HIV/HCV cohort compared to 92% and 79% in the HCV mono-infected cohort (P < 0.001). Graft loss was also significantly higher in the HIV/HCV co-infected cohort (P < 0.001). Multivariate analysis identified HIV infection as the only baseline factor associated with increased risk of death (HR = 2.3, P = 0.002) and graft loss (HR = 1.9, P = 0.01). Analysis of the HIV/HCV co-infected cohort only identified that receipt of a combined kidney-liver transplant (HR = 3.8, P = 0.003), BMI < 21 kg/m2 at enrolment (HR = 3.2, P = 0.01), receipt of an anti-HCV positive donor (HR = 2.5, P = 0.03), and older donor age (HR = 1.3 per decade, P = 0.04) were significant predictors of reduced graft survival. The cumulative incidence of acute cellular rejection (ACR) requiring treatment was significantly higher in HIV/HCV patients compared to HCV-mono-infected patients (39% vs 24% at year 3, HR = 2.1, P = 0.01). 50% of the cases of ACR occurred within the first 21 d following LT. Reasons for the increased incidence of ACR remain unclear but the immunosuppression protocol post transplantation was not standardized.
| n | Patient survival | Incidence of ACR | Risk factors for death amongst HIV/HCV recipients1 | |
| Miro et al[65], 2012 | HIV/HCV - 84 | 1, 3, 5 yr: 88%, 62%, 54% | 38% | HCV G1 |
| HCV - 252 | 1, 3, 5 yr: 90%, 76%, 71%1 | 20%1 | Donor risk index | |
| MELD score | ||||
| Center < 1 transplant/yr2 | ||||
| Terrault et al[66], 2012 | HIV/HCV - 89 | 1 and 3 yr: 92% and 79% | 39% | Combined liver-kidney transplant |
| HCV - 235 | 1 and 3 yr: 76% and 60%1 | 24%1 | BMI < 21 | |
| Anti-HCV positive donor | ||||
| Older donor |
The Spanish study enrolled 84 HIV/HCV co-infected patients and were matched with 252 HCV mono-infected patients[65]. This study reported a higher overall mortality amongst the HIV/HCV co-infected recipients (43%, n = 36 vs 30%, n = 75, P = 0.03) during a median of 3.6 years. Unsurprisingly, the leading cause of death was HCV recurrence in both patient cohorts but significantly increased in the HIV/HCV co-infected cohort (21% vs 12 %, P = 0.049). 1-year patient survival was similar between the two cohorts (88% vs 90%) but inferior patient survival outcomes were observed at 3 and 5 years in HIV/HCV co-infected recipients (62% vs 76% and 54% vs 71% respectively, P = 0.008). Independent risk factors for death amongst the HIV/HCV recipients included HCV genotype 1 (HR = 3.0, P = 0.008), donor risk index (HR = 9.5, P < 0.01) and a negative HCV RNA viral load before or after liver transplantation (HR= 0.14, P = 0.009). Important pre-transplant variables predictive of death included the MELD score (HR = 1.06, P = 0.023) and a transplant center with less than one liver transplant per year in a HIV-infected patient (HR = 2.3, P = 0.03). A higher incidence of ACR was once again reported amongst the HIV/HCV co-infected recipients (38% vs 20%, P < 0.001).
Recurrence of HCV post liver transplantation is universal in all patients with detectable HCV viraemia at the time of liver transplantation. The rate of HCV recurrence and therefore fibrosis is influenced by a variety of recipient, donor, viral, infectious and immunosuppression related factors[64,67,68]. An accelerated disease course is well recognised in HIV/HCV co-infected patient especially the aggressive severe fibrosing cholestatic variant of recurrent hepatitis C (FCH)[69]. FCH and sepsis appear to be the leading causes of death post liver transplantation amongst HIV/HCV co-infected patients[62,64,70]. No reliable markers are available to identify patients who will develop FCH but higher HCV viral loads immediately after liver transplantation at week 1 and week 2 may be an indicator for those at risk[71]. FCH usually occurs between 2 and 6 mo after liver transplantation and is associated with a high mortality (50% at 12 mo) and is invariably refractory to pegylated interferon (PEG-IFN) and ribavirin (RBV) antiviral therapy[72,73]. However, case reports are now emerging on the use of the new directly acting antivral (DAA) agents in patients with FCH resulting in a sustained viral response (SVR). Long-term fibrosis progression rates are also accelerated in HIV/HCV co-infected patients compared HCV mono-infected patients with an increased likelihood of progression to a fibrosis score ≥ 2[64].
Prior to the advent of DAAs, antiviral therapy post liver transplantation consisted of PEG-IFN and RBV for a minimum of 48 wk irrespective of HCV viral genotype[74]. Most institutions, as in our own, instigated treatment when histological disease was demonstrable (F ≥ 2). Reported SVR rates (38%) for the treatment of recurrent HCV post-liver transplantation in HCV mono-infected patients were inferior to patients pre-liver transplantation (60%)[75,76]. Data from a prospective study in HIV/HCV co-infected patients after liver transplantation demonstrated a SVR rate of only 21% compared to 36% in HCV mono-infected patients (P = 0.013)[77]. These poor SVR rates are explained by the combination of high discontinuation rates (40%), dose reductions (75%) and haematological toxicity commonly anaemia.
Data is now emerging on the use of the new DAAs in HIV/HCV co-infected patients following liver transplantation which have demonstrated improved SVR rates[78,79]. Certain caveats should be taken into account when considering anti-viral regimens: anti- HIV protease inhibitors should be avoided with boceprevir, a higher telaprevir dose is required in patients receiving efavirenz based cART. Sofosbuvir and ribavirin have been used on a compassionate-use basis patients with severe HCV recurrence and in patients with FCH post liver transplantation[80]. This study included both HCV mono-infected and HIV/HCV co-infected patients. Overall SVR rates were 59% with higher SVR rates (73%) reported in patients with early severe recurrence[80]. In addition, the use of sofosbuvir and ribavirin appeared safe and also resulted in an improvement in liver biochemistry, MELD and ascites. The SOLAR-1 study was a large, multicenter, randomized controlled trial that included liver transplant recipients with HCV recurrence with genotype 1 and 4 HCV[81]. Patients received ledipasvir, sofosbuvir and ribavirin for either 12 or 24 wk. The results demonstrated only 2% of patients discontinued treatment due to adverse events and encouraging SVR12 rates that varied with Child-Pugh class (A, 96%; B, 85%; C, 60%). Although HIV-HCV co-infected patients were not included in this study, these results are encouraging and are applicable to the HIV-HCV co-infected patients in the post-liver transplant period. Further studies are waited with DAAs for recurrent HCV in HIV/HCV co-infected patients post liver transplantation.
Patients co-infected with HBV and non-viral aetiologies including those that present with acute liver failure, have excellent short and long-term outcomes post-liver transplantation. Reported median survival at 1-year ranges between 75%-100% and 100% at 5 years[82-84]. The largest prospective study to date in HIV/HBV co-infected patients was conducted in 21 patients for a median of 42 mo with no patient suffering graft loss[85].
The key difference between HIV/HBV and HIV/HCV co-infected patients is the presence of highly potent anti-viral agents against HBV in the therapeutic armamentarium. Patients co-infected with HBV receiving cART will undoubtedly be receiving an oral nucleo(s)tide analogue that will have anti-viral actions against both HIV and HBV. Tenofovir in conjunction with emtricitabine (Truvada) is recommended[85,86]. The use of these highly efficacious, potent oral agents results in the majority of patients undergoing liver transplantation with an undetectable HBV viral load. Immuno-prophylaxis with Hepatitis B Immunoglobulin (HBIg) is recommended in the post-liver transplant period indefinitely. Reported data on the use of HBIg and oral anti-viral agents has demonstrated that this combination is highly effective at preventing HBV recurrence even in those who have a detectable HBV viral load at the time of liver transplantation[87]. Data on patients with HIV and non-viral liver disease undergoing liver transplantation is limited but evidence suggests that these patients have similar survival rates as HIV-negative patients[82,84].
Liver transplantation for HCC in HIV positive patients has been performed although the experience remains limited. A single center experience compared outcomes in 21 HIV-positive patients against 65 HIV-negative patients[88]. The authors reported a trend towards a higher dropout rate amongst the HIV positive patients but overall survival following liver transplantation at 1 and 3 years (81% and 74%, HIV positive group vs 93% and 85%, HIV negative group) and recurrence free survival at 1 and 3 years were similar between the two groups (69% and 69%, HIV positive group vs 89% and 84%, HIV negative group)[88].
Another study, this time across 3 centers, compared 30 HIV positive patients with 125 HIV negative patients[89]. Once again similar survival rates between the two groups were reported (77% and 65%, HIV positive group vs 85% and 70%, HIV negative group)[89]. The time to HCC recurrence was longer in the HIV positive group (27 mo) compared to the HIV negative group (10 mo, P < 0.01) but mortality remained similar suggesting the possible positive role of cART in attenuating hepato-carcinogenic progression.
Immunosuppression should be tailored to the individual taking into account aetiology of liver disease, renal function, risk factors for the metabolic syndrome and specifically to HIV patients the possible interactions with cART. Dual immunosuppression with calcineurin inhibitors and cortico-steroids is recommended post-liver transplantation. Target trough levels in the first 3 mo should be the same as HIV negative patients (cyclosporin, 100-250 ng/mL; tacrolimus, 8-10 ng/mL). Utilising data from HCV mono-infected patients post-LT, rapid withdrawal of cortico-steroids should be avoided due to the association of a more severe recurrence of HCV[90]. We therefore recommend that prednisolone, which is usually commenced at 20 mg/d, be withdrawn by a slow taper at 3 mo. Anti-fungal prophylaxis (fluconazole 50 mg/d) should be given for a minimum of 3 mo post-LT. Episodes of acute cellular rejection (ACR) should also be managed as one would in HIV negative patients namely moderate-severe episodes be treated with a 3-d course of intravenous methylprednisolone (1 g/d). Consideration of the introduction of mycophenalate mofetil after the 2nd episode of ACR is recommended.
Tacrolimus and cyclosporin are metabolised via the P450 cytochrome. In addition, non-nucleoside reverse-transcriptase inhibitors (NNRTIs) and protease inhibitors, which are commonly part of cART regimens, are also metabolised by the same pathway, therefore increasing the risk of drug - drug interactions (see Table 4). NNRTIs (e.g., efavirenz) decrease serum CNI concentrations by induction of the P450 cytochrome whilst PIs (e.g., ritonavir and lopinavir) are inducers resulting in increased CNI concentrations[91]. We have used tacrolimus doses as low as 1 mg per week in certain individuals. Raltegravir, a novel HIV-1 integrase inhibitor, is not metabolised via the P450 cytochrome and has been used successfully post transplantation in combination with nucleoside reverse-transcriptase inhibitors and standard CNI doses[92]. Meticulous monitoring and surveillance is required to reduce the possibility drug-drug interactions and toxicity.
| Drug | Effect on CNI level |
| Protease inhibitor | |
| Darunavir | ↑↑ |
| Fosamprenavir | ↑↑ |
| Lopinavir | ↑↑ |
| Ritonavir | ↑↑↑ |
| Saquinavir | ↑↑ |
| Non nucleoside reverse transverse inhibitors | |
| Efavirenz | ↓↓ |
| Nevirapine | ↓↓ |
| Integrase inhibitors | |
| Raltegravir | No effect |
At present no standardised cART regimen is utilised, but instead is tailored to the individual patient reflecting known resistance and mutations. The re-introduction of cART post-LT also varies between individual centers, with some continuing cART throughout the transplant period whilst others re-introduce the medication between 4-14 d. Our practice is to re-introduce cART medication once liver graft function has normalised thereby avoiding the possibility of confusion with the other causes of graft dysfunction immediately post-LT.
In a recent study of HIV/HCV patients, bacterial infections were identified as the principal aetiological agents of post-liver transplantation infections[93]. Risk factors associated with severe infections included a pre-liver transplantation MELD score > 15 (HR = 3.5, 95%CI: 1.7-7.1, P = 0.001), history of category C AIDS-defining events (HR = 4.0, 1.9-8.6, P < 0.001) and non-tacrolimus based immunosuppression (HR = 2.5, 1.3-4.8, P = 0.006). The same study also suggested that opportunistic infections namely CMV disease, disseminated HSV, invasive fungal infections and tuberculosis were increased in HIV positive patients, affecting 11% of their cohort. These “opportunistic” infections however can occur in HIV negative patients post-liver transplantation. This study also did not have a HIV negative comparative group therefore not allowing the authors to demonstrate that these deemed opportunistic infections were associated with HIV infection only. Reassuringly reported data from other studies have failed to demonstrate a higher incidence of opportunistic infections in comparison to HIV negative patients[94].
There is very little experience with re-transplantation in HIV positive patients and this appears to be limited to HIV/HCV co-infected patients[95]. In one study 14 HIV positive patients (13 with HIV/HCV co-infection) were compared to 157 HIV negative patients undergoing re-liver transplantation. The authors reported an inferior survival rate in the HIV positive group (42% vs 64%) at 3 years although this did not reach statistical significance (P = 0.2). HIV positive patients with a detectable HCV viral load at the time of re-transplantation do not appear to be appropriate re-transplant candidates due to poor 3 year survival rates (22% vs 65% in HIV negative patients, P = 0.008)[95]. A recent multinational study re-emphasised the poor outcomes In HIV positive patients with active HCV infection undergoing re-transplantation[96].
HIV infection is now regarded as a long-term illness with improving survival rates in patients maintained on cART. Subsequently, liver disease remains a common cause of morbidity and mortality in this cohort. HIV-positive patients with liver disease should be managed in a multi-disciplinary environment. Current data would suggest HIV-positive patients not co-infected with HCV have excellent outcomes following liver transplantation, similar to HIV negative patients. In stark contrast, published data has demonstrated inferior graft and patient survival rates in HIV/HCV co-infected patients predominately due to HCV recurrence. Emerging data with the use of DAAs in HIV/HCV co-infected patients would suggest that treatment of HCV in the pre- and post-transplant period will be more efficacious resulting in improved outcomes in this patient cohort. HIV/HCV co-infected patients would therefore be treated as HCV mono-infected patients with better outcomes post liver transplantation.
P- Reviewer: Charles B, Sobhonslidsuk A S- Editor: Yu J L- Editor: A E- Editor: Ma S
| 1. | Mocroft A, Brettle R, Kirk O, Blaxhult A, Parkin JM, Antunes F, Francioli P, D’Arminio Monforte A, Fox Z, Lundgren JD. Changes in the cause of death among HIV positive subjects across Europe: results from the EuroSIDA study. AIDS. 2002;16:1663-1671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 217] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 2. | Weber R, Sabin CA, Friis-Møller N, Reiss P, El-Sadr WM, Kirk O, Dabis F, Law MG, Pradier C, De Wit S. Liver-related deaths in persons infected with the human immunodeficiency virus: the D: A: D study. Arch Intern Med. 2006;166:1632-1641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 859] [Cited by in RCA: 834] [Article Influence: 43.9] [Reference Citation Analysis (0)] |
| 3. | Salmon-Ceron D, Lewden C, Morlat P, Bévilacqua S, Jougla E, Bonnet F, Héripret L, Costagliola D, May T, Chêne G. Liver disease as a major cause of death among HIV infected patients: role of hepatitis C and B viruses and alcohol. J Hepatol. 2005;42:799-805. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 248] [Cited by in RCA: 224] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 4. | Kuller LH, Tracy R, Belloso W, De Wit S, Drummond F, Lane HC, Ledergerber B, Lundgren J, Neuhaus J, Nixon D. Inflammatory and coagulation biomarkers and mortality in patients with HIV infection. PLoS Med. 2008;5:e203. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1319] [Cited by in RCA: 1323] [Article Influence: 77.8] [Reference Citation Analysis (0)] |
| 5. | Smith CJ, Ryom L, Weber R, Morlat P, Pradier C, Reiss P, Kowalska JD, de Wit S, Law M, el Sadr W. Trends in underlying causes of death in people with HIV from 1999 to 2011 (D: A: D): a multicohort collaboration. Lancet. 2014;384:241-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 667] [Cited by in RCA: 735] [Article Influence: 66.8] [Reference Citation Analysis (0)] |
| 6. | Joshi D, O’Grady J, Dieterich D, Gazzard B, Agarwal K. Increasing burden of liver disease in patients with HIV infection. Lancet. 2011;377:1198-1209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 230] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 7. | Pineda JA, Romero-Gómez M, Díaz-García F, Girón-González JA, Montero JL, Torre-Cisneros J, Andrade RJ, González-Serrano M, Aguilar J, Aguilar-Guisado M. HIV coinfection shortens the survival of patients with hepatitis C virus-related decompensated cirrhosis. Hepatology. 2005;41:779-789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 224] [Cited by in RCA: 211] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 8. | Rosenthal E, Pialoux G, Bernard N, Pradier C, Rey D, Bentata M, Michelet C, Pol S, Perronne C, Cacoub P. Liver-related mortality in human-immunodeficiency-virus-infected patients between 1995 and 2003 in the French GERMIVIC Joint Study Group Network (MORTAVIC 2003 Study). J Viral Hepat. 2007;14:183-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 107] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 9. | Bollepalli S, Mathieson K, Bay C, Hillier A, Post J, Van Thiel DH, Nadir A. Prevalence of risk factors for hepatitis C virus in HIV-infected and HIV/hepatitis C virus-coinfected patients. Sex Transm Dis. 2007;34:367-370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 10. | Fox J, Nastouli E, Thomson E, Muir D, McClure M, Weber J, Fidler S. Increasing incidence of acute hepatitis C in individuals diagnosed with primary HIV in the United Kingdom. AIDS. 2008;22:666-668. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 26] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 11. | Wang CC, Krantz E, Klarquist J, Krows M, McBride L, Scott EP, Shaw-Stiffel T, Weston SJ, Thiede H, Wald A. Acute hepatitis C in a contemporary US cohort: modes of acquisition and factors influencing viral clearance. J Infect Dis. 2007;196:1474-1482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 147] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 12. | Graham CS, Baden LR, Yu E, Mrus JM, Carnie J, Heeren T, Koziel MJ. Influence of human immunodeficiency virus infection on the course of hepatitis C virus infection: a meta-analysis. Clin Infect Dis. 2001;33:562-569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 705] [Cited by in RCA: 710] [Article Influence: 29.6] [Reference Citation Analysis (0)] |
| 13. | Bonnard P, Lescure FX, Amiel C, Guiard-Schmid JB, Callard P, Gharakhanian S, Pialoux G. Documented rapid course of hepatic fibrosis between two biopsies in patients coinfected by HIV and HCV despite high CD4 cell count. J Viral Hepat. 2007;14:806-811. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 47] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 14. | Soto B, Sánchez-Quijano A, Rodrigo L, del Olmo JA, García-Bengoechea M, Hernández-Quero J, Rey C, Abad MA, Rodríguez M, Sales Gilabert M. Human immunodeficiency virus infection modifies the natural history of chronic parenterally-acquired hepatitis C with an unusually rapid progression to cirrhosis. J Hepatol. 1997;26:1-5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 476] [Cited by in RCA: 466] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 15. | Poynard T, Mathurin P, Lai CL, Guyader D, Poupon R, Tainturier MH, Myers RP, Muntenau M, Ratziu V, Manns M. A comparison of fibrosis progression in chronic liver diseases. J Hepatol. 2003;38:257-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 317] [Cited by in RCA: 324] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 16. | Macías J, Berenguer J, Japón MA, Girón JA, Rivero A, López-Cortés LF, Moreno A, González-Serrano M, Iribarren JA, Ortega E. Fast fibrosis progression between repeated liver biopsies in patients coinfected with human immunodeficiency virus/hepatitis C virus. Hepatology. 2009;50:1056-1063. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 210] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 17. | Garba ML, Pilcher CD, Bingham AL, Eron J, Frelinger JA. HIV antigens can induce TGF-beta(1)-producing immunoregulatory CD8+ T cells. J Immunol. 2002;168:2247-2254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 106] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 18. | Safadi R, Ohta M, Alvarez CE, Fiel MI, Bansal M, Mehal WZ, Friedman SL. Immune stimulation of hepatic fibrogenesis by CD8 cells and attenuation by transgenic interleukin-10 from hepatocytes. Gastroenterology. 2004;127:870-882. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 186] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 19. | Graham CS, Curry M, He Q, Afdhal N, Nunes D, Fleming C, Horsburgh R, Craven D, Sherman KE, Koziel MJ. Comparison of HCV-specific intrahepatic CD4+ T cells in HIV/HCV versus HCV. Hepatology. 2004;40:125-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 48] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 20. | Balagopal A, Philp FH, Astemborski J, Block TM, Mehta A, Long R, Kirk GD, Mehta SH, Cox AL, Thomas DL. Human immunodeficiency virus-related microbial translocation and progression of hepatitis C. Gastroenterology. 2008;135:226-233. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 225] [Cited by in RCA: 217] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 21. | Benhamou Y, Bochet M, Di Martino V, Charlotte F, Azria F, Coutellier A, Vidaud M, Bricaire F, Opolon P, Katlama C. Liver fibrosis progression in human immunodeficiency virus and hepatitis C virus coinfected patients. The Multivirc Group. Hepatology. 1999;30:1054-1058. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 986] [Cited by in RCA: 907] [Article Influence: 34.9] [Reference Citation Analysis (0)] |
| 22. | Tuma P, Jarrin I, Del Amo J, Vispo E, Medrano J, Martin-Carbonero L, Labarga P, Barreiro P, Soriano V. Survival of HIV-infected patients with compensated liver cirrhosis. AIDS. 2010;24:745-753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 23. | Torriani FJ, Rodriguez-Torres M, Rockstroh JK, Lissen E, Gonzalez-García J, Lazzarin A, Carosi G, Sasadeusz J, Katlama C, Montaner J. Peginterferon Alfa-2a plus ribavirin for chronic hepatitis C virus infection in HIV-infected patients. N Engl J Med. 2004;351:438-450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 992] [Cited by in RCA: 955] [Article Influence: 45.5] [Reference Citation Analysis (0)] |
| 24. | Laguno M, Cifuentes C, Murillas J, Veloso S, Larrousse M, Payeras A, Bonet L, Vidal F, Milinkovic A, Bassa A. Randomized trial comparing pegylated interferon alpha-2b versus pegylated interferon alpha-2a, both plus ribavirin, to treat chronic hepatitis C in human immunodeficiency virus patients. Hepatology. 2009;49:22-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 77] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 25. | Chung RT, Andersen J, Volberding P, Robbins GK, Liu T, Sherman KE, Peters MG, Koziel MJ, Bhan AK, Alston B. Peginterferon Alfa-2a plus ribavirin versus interferon alfa-2a plus ribavirin for chronic hepatitis C in HIV-coinfected persons. N Engl J Med. 2004;351:451-459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 716] [Cited by in RCA: 686] [Article Influence: 32.7] [Reference Citation Analysis (0)] |
| 26. | Sulkowski M, Pol S, Mallolas J, Fainboim H, Cooper C, Slim J, Rivero A, Mak C, Thompson S, Howe AY. Boceprevir versus placebo with pegylated interferon alfa-2b and ribavirin for treatment of hepatitis C virus genotype 1 in patients with HIV: a randomised, double-blind, controlled phase 2 trial. Lancet Infect Dis. 2013;13:597-605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 154] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 27. | Sulkowski MS, Sherman KE, Dieterich DT, Bsharat M, Mahnke L, Rockstroh JK, Gharakhanian S, McCallister S, Henshaw J, Girard PM. Combination therapy with telaprevir for chronic hepatitis C virus genotype 1 infection in patients with HIV: a randomized trial. Ann Intern Med. 2013;159:86-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 80] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 28. | Dieterich D, Orkin C. Simeprevir (TMC435) plus pegylated interferon/ribavirin in patients co-infected with HCV genotype-1 and HIV-1: primary analysis of the C212 study. EACS. 2013;Abstract PS9/5. |
| 29. | Naggie S, Lalezari J. Sofosbuvir plus ribavirin for HCV genotype 1-3 infection in HIV co-infected patients (PHOTON-1). 21st Conference on Retroviruses and Opportunistic Infections;. 2014;Abstract 26. |
| 30. | Osinusi A, Townsend K, Kohli A, Nelson A, Seamon C, Meissner EG, Bon D, Silk R, Gross C, Price A. Virologic response following combined ledipasvir and sofosbuvir administration in patients with HCV genotype 1 and HIV co-infection. JAMA. 2015;313:1232-1239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 172] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 31. | Sulkowski MS, Eron JJ, Wyles D, Trinh R, Lalezari J, Wang C, Slim J, Bhatti L, Gathe J, Ruane PJ. Ombitasvir, paritaprevir co-dosed with ritonavir, dasabuvir, and ribavirin for hepatitis C in patients co-infected with HIV-1: a randomized trial. JAMA. 2015;313:1223-1231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 264] [Cited by in RCA: 263] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 32. | AASLD/IDSA/IAS-USA. Recommendations for testing, managing and treating hepatitis C. Accessed Jan. 2015; Available from: http://www.hcvguidelines.org. |
| 33. | Kellerman SE, Hanson DL, McNaghten AD, Fleming PL. Prevalence of chronic hepatitis B and incidence of acute hepatitis B infection in human immunodeficiency virus-infected subjects. J Infect Dis. 2003;188:571-577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 193] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 34. | Chapman LE, Sullivent EE, Grohskopf LA, Beltrami EM, Perz JF, Kretsinger K, Panlilio AL, Thompson ND, Ehrenberg RL, Gensheimer KF. Recommendations for postexposure interventions to prevent infection with hepatitis B virus, hepatitis C virus, or human immunodeficiency virus, and tetanus in persons wounded during bombings and other mass-casualty events--United States, 2008: recommendations of the Centers for Disease Control and Prevention (CDC). MMWR Recomm Rep. 2008;57:1-21; quiz CE1-CE4. [PubMed] |
| 35. | Colin JF, Cazals-Hatem D, Loriot MA, Martinot-Peignoux M, Pham BN, Auperin A, Degott C, Benhamou JP, Erlinger S, Valla D. Influence of human immunodeficiency virus infection on chronic hepatitis B in homosexual men. Hepatology. 1999;29:1306-1310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 334] [Cited by in RCA: 327] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 36. | Bräu N, Fox RK, Xiao P, Marks K, Naqvi Z, Taylor LE, Trikha A, Sherman M, Sulkowski MS, Dieterich DT. Presentation and outcome of hepatocellular carcinoma in HIV-infected patients: a U.S.-Canadian multicenter study. J Hepatol. 2007;47:527-537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 172] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 37. | Sulkowski MS, Mehta SH, Chaisson RE, Thomas DL, Moore RD. Hepatotoxicity associated with protease inhibitor-based antiretroviral regimens with or without concurrent ritonavir. AIDS. 2004;18:2277-2284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 121] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 38. | Bourezane Y, Salard D, Hoen B, Vandel S, Drobacheff C, Laurent R. DRESS (drug rash with eosinophilia and systemic symptoms) syndrome associated with nevirapine therapy. Clin Infect Dis. 1998;27:1321-1322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 39. | de Maat MM, ter Heine R, van Gorp EC, Mulder JW, Mairuhu AT, Beijnen JH. Case series of acute hepatitis in a non-selected group of HIV-infected patients on nevirapine-containing antiretroviral treatment. AIDS. 2003;17:2209-2214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 40. | Vrouenraets SM, Treskes M, Regez RM, Troost N, Smulders YM, Weigel HM, Frissen PH, Brinkman K. Hyperlactataemia in HIV-infected patients: the role of NRTI-treatment. Antivir Ther. 2002;7:239-244. [PubMed] |
| 41. | Maida I, Núñez M, Ríos MJ, Martín-Carbonero L, Sotgiu G, Toro C, Rivas P, Barreiro P, Mura MS, Babudieri S. Severe liver disease associated with prolonged exposure to antiretroviral drugs. J Acquir Immune Defic Syndr. 2006;42:177-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 119] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 42. | Sandrine PF, Sylvie A, André E, Abdoulaye D, Bernard L, André C. Nodular regenerative hyperplasia: a new serious antiretroviral drugs side effect? AIDS. 2007;21:1498-1499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 22] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 43. | Schiano TD, Kotler DP, Ferran E, Fiel MI. Hepatoportal sclerosis as a cause of noncirrhotic portal hypertension in patients with HIV. Am J Gastroenterol. 2007;102:2536-2540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 73] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 44. | Mallet V, Blanchard P, Verkarre V, Vallet-Pichard A, Fontaine H, Lascoux-Combe C, Pol S. Nodular regenerative hyperplasia is a new cause of chronic liver disease in HIV-infected patients. AIDS. 2007;21:187-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 91] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 45. | Crum-Cianflone N, Dilay A, Collins G, Asher D, Campin R, Medina S, Goodman Z, Parker R, Lifson A, Capozza T. Nonalcoholic fatty liver disease among HIV-infected persons. J Acquir Immune Defic Syndr. 2009;50:464-473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 155] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 46. | Guaraldi G, Squillace N, Stentarelli C, Orlando G, D’Amico R, Ligabue G, Fiocchi F, Zona S, Loria P, Esposito R. Nonalcoholic fatty liver disease in HIV-infected patients referred to a metabolic clinic: prevalence, characteristics, and predictors. Clin Infect Dis. 2008;47:250-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 181] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 47. | Angulo P. GI epidemiology: nonalcoholic fatty liver disease. Aliment Pharmacol Ther. 2007;25:883-889. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 258] [Cited by in RCA: 261] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 48. | Moyle G, Carr A. HIV-associated lipodystrophy, metabolic complications, and antiretroviral toxicities. HIV Clin Trials. 2002;3:89-98. [PubMed] |
| 49. | Worm SW, Friis-Møller N, Bruyand M, D’Arminio Monforte A, Rickenbach M, Reiss P, El-Sadr W, Phillips A, Lundgren J, Sabin C. High prevalence of the metabolic syndrome in HIV-infected patients: impact of different definitions of the metabolic syndrome. AIDS. 2010;24:427-435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 67] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 50. | Sterling RK, Smith PG, Brunt EM. Hepatic steatosis in human immunodeficiency virus: a prospective study in patients without viral hepatitis, diabetes, or alcohol abuse. J Clin Gastroenterol. 2013;47:182-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 69] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 51. | Salmon-Ceron D, Rosenthal E, Lewden C, Bouteloup V, May T, Burty C, Bonnet F, Costagliola D, Jougla E, Semaille C. Emerging role of hepatocellular carcinoma among liver-related causes of deaths in HIV-infected patients: The French national Mortalité 2005 study. J Hepatol. 2009;50:736-745. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 80] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 52. | Subramanian A, Sulkowski M, Barin B, Stablein D, Curry M, Nissen N, Dove L, Roland M, Florman S, Blumberg E. MELD score is an important predictor of pretransplantation mortality in HIV-infected liver transplant candidates. Gastroenterology. 2010;138:159-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 66] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 53. | Murillas J, Rimola A, Laguno M, de Lazzari E, Rascón J, Agüero F, Blanco JL, Moitinho E, Moreno A, Miró JM. The model for end-stage liver disease score is the best prognostic factor in human immunodeficiency virus 1-infected patients with end-stage liver disease: a prospective cohort study. Liver Transpl. 2009;15:1133-1141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 54. | de Vera ME, Dvorchik I, Tom K, Eghtesad B, Thai N, Shakil O, Marcos A, Demetris A, Jain A, Fung JJ. Survival of liver transplant patients coinfected with HIV and HCV is adversely impacted by recurrent hepatitis C. Am J Transplant. 2006;6:2983-2993. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 113] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 55. | Henderson DK. Postexposure chemoprophylaxis for occupational exposures to the human immunodeficiency virus. JAMA. 1999;281:931-936. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 41] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 56. | Ahonkhai AA, Gebo KA, Streiff MB, Moore RD, Segal JB. Venous thromboembolism in patients with HIV/AIDS: a case-control study. J Acquir Immune Defic Syndr. 2008;48:310-314. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 36] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 57. | Cherian PT, Alrabih W, Douiri A, Quaglia A, Heneghan MA, O’Grady J, Rela M, Heaton ND. Liver transplantation in human immunodeficiency virus-infected patients: procoagulant, but is antithrombotic prophylaxis required? Liver Transpl. 2012;18:82-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 58. | Gastaca M, Valdivieso A, Montejo M, Bustamante J, de Urbina JO. Is antithrombotic prophylaxis required after liver transplantation in HIV-infected recipients? Am J Transplant. 2012;12:2258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 59. | Tzakis AG, Cooper MH, Dummer JS, Ragni M, Ward JW, Starzl TE. Transplantation in HIV+ patients. Transplantation. 1990;49:354-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 127] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 60. | Dummer JS, Erb S, Breinig MK, Ho M, Rinaldo CR, Gupta P, Ragni MV, Tzakis A, Makowka L, Van Thiel D. Infection with human immunodeficiency virus in the Pittsburgh transplant population. A study of 583 donors and 1043 recipients, 1981-1986. Transplantation. 1989;47:134-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 83] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 61. | Mindikoglu AL, Regev A, Magder LS. Impact of human immunodeficiency virus on survival after liver transplantation: analysis of United Network for Organ Sharing database. Transplantation. 2008;85:359-368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 47] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 62. | Schreibman I, Gaynor JJ, Jayaweera D, Pyrsopoulos N, Weppler D, Tzakis A, Schiff ER, Regev A. Outcomes after orthotopic liver transplantation in 15 HIV-infected patients. Transplantation. 2007;84:697-705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 40] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 63. | Ragni MV, Belle SH, Im K, Neff G, Roland M, Stock P, Heaton N, Humar A, Fung JF. Survival of human immunodeficiency virus-infected liver transplant recipients. J Infect Dis. 2003;188:1412-1420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 161] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 64. | Duclos-Vallée JC, Féray C, Sebagh M, Teicher E, Roque-Afonso AM, Roche B, Azoulay D, Adam R, Bismuth H, Castaing D. Survival and recurrence of hepatitis C after liver transplantation in patients coinfected with human immunodeficiency virus and hepatitis C virus. Hepatology. 2008;47:407-417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 148] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 65. | Miro JM, Montejo M, Castells L, Rafecas A, Moreno S, Agüero F, Abradelo M, Miralles P, Torre-Cisneros J, Pedreira JD. Outcome of HCV/HIV-coinfected liver transplant recipients: a prospective and multicenter cohort study. Am J Transplant. 2012;12:1866-1876. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 119] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 66. | Terrault NA, Roland ME, Schiano T, Dove L, Wong MT, Poordad F, Ragni MV, Barin B, Simon D, Olthoff KM. Outcomes of liver transplant recipients with hepatitis C and human immunodeficiency virus coinfection. Liver Transpl. 2012;18:716-726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 170] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 67. | Joshi D, Pinzani M, Carey I, Agarwal K. Recurrent HCV after liver transplantation-mechanisms, assessment and therapy. Nat Rev Gastroenterol Hepatol. 2014;11:710-721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 23] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 68. | Gane EJ, Portmann BC, Naoumov NV, Smith HM, Underhill JA, Donaldson PT, Maertens G, Williams R. Long-term outcome of hepatitis C infection after liver transplantation. N Engl J Med. 1996;334:815-820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 787] [Cited by in RCA: 736] [Article Influence: 25.4] [Reference Citation Analysis (0)] |
| 69. | Roland ME, Barin B, Carlson L, Frassetto LA, Terrault NA, Hirose R, Freise CE, Benet LZ, Ascher NL, Roberts JP. HIV-infected liver and kidney transplant recipients: 1- and 3-year outcomes. Am J Transplant. 2008;8:355-365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 182] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 70. | Castells L, Escartín A, Bilbao I, Len O, Allende H, Vargas V, Ribera E, Lázaro JL, Bueno J, Balsells J. Liver transplantation in HIV-HCV coinfected patients: a case-control study. Transplantation. 2007;83:354-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 71. | Antonini TM, Sebagh M, Roque-Afonso AM, Teicher E, Roche B, Sobesky R, Coilly A, Vaghefi P, Adam R, Vittecoq D. Fibrosing cholestatic hepatitis in HIV/HCV co-infected transplant patients-usefulness of early markers after liver transplantation. Am J Transplant. 2011;11:1686-1695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 46] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 72. | McCaughan GW, Bowen DG. Pathogenesis of cholestatic hepatitis C. J Hepatol. 2011;54:392-394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 73. | Gopal DV, Rosen HR. Duration of antiviral therapy for cholestatic HCV recurrence may need to be indefinite. Liver Transpl. 2003;9:348-353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 48] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 74. | Gane EJ. The natural history of recurrent hepatitis C and what influences this. Liver Transpl. 2008;14 Suppl 2:S36-S44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 192] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 75. | Berenguer M. Systematic review of the treatment of established recurrent hepatitis C with pegylated interferon in combination with ribavirin. J Hepatol. 2008;49:274-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 292] [Cited by in RCA: 265] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 76. | Calmus Y, Duvoux C, Pageaux G, Wolf P, Rostaing L, Vanlemmens C, Botta-Fridlund D, Dharancy S, Gugenheim J, Durand F. Treatment of recurrent HCV infection following liver transplantation: results of a multicenter, randomized, versus placebo, trial of ribavirin alone as maintenance therapy after one year of PegIFNα-2a plus ribavirin. J Hepatol. 2012;57:564-571. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 77. | Castells L, Rimola A, Manzardo C, Valdivieso A, Montero JL, Barcena R, Abradelo M, Xiol X, Aguilera V, Salcedo M. Pegylated interferon plus ribavirin in HIV-infected patients with recurrent hepatitis C after liver transplantation: a prospective cohort study. J Hepatol. 2015;62:92-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 78. | Campos-Varela I, Straley S, Agudelo EZ, Carlson L, Terrault NA. Sofosbuvir, simeprevir, and ribavirin for the treatment of hepatitis C virus recurrence in human immunodeficiency virus/hepatitis C virus-coinfected liver transplant recipients. Liver Transpl. 2015;21:272-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 79. | Antonini TM, Furlan V, Teicher E, Haim-Boukobza S, Sebagh M, Coilly A, Bonhomme-Faivre L, Roque-Afonso AM, Vittecoq D, Samuel D. Therapy with boceprevir or telaprevir in HIV/hepatitis C virus co-infected patients to treat recurrence of hepatitis C virus infection after liver transplantation. AIDS. 2015;29:53-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 80. | Forns X, Charlton M, Denning J, McHutchison JG, Symonds WT, Brainard D, Brandt-Sarif T, Chang P, Kivett V, Castells L. Sofosbuvir compassionate use program for patients with severe recurrent hepatitis C after liver transplantation. Hepatology. 2015;61:1485-1494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 180] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 82. | Joshi D, Belgaumkar A. Liver Transplantation for HIV: analysis of outcomes suggest HIV/HCV coinfected patients have prohibitively poor survival at 5 years. Hepatology. 2008;48 Supp 1:311. |
| 83. | Tateo M, Roque-Afonso AM, Antonini TM, Medja F, Lombes A, Jardel C, Teicher E, Sebagh M, Roche B, Castaing D. Long-term follow-up of liver transplanted HIV/hepatitis B virus coinfected patients: perfect control of hepatitis B virus replication and absence of mitochondrial toxicity. AIDS. 2009;23:1069-1076. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 41] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 84. | Anadol E, Beckebaum S, Radecke K, Paul A, Zoufaly A, Bickel M, Hitzenbichler F, Ganten T, Kittner J, Stoll M. Orthotopic liver transplantation in human-immunodeficiency-virus-positive patients in Germany. AIDS Res Treat. 2012;2012:197501. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 85. | Terrault NA, Carter JT, Carlson L, Roland ME, Stock PG. Outcome of patients with hepatitis B virus and human immunodeficiency virus infections referred for liver transplantation. Liver Transpl. 2006;12:801-807. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 52] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 86. | Gazzard BG, Anderson J, Babiker A, Boffito M, Brook G, Brough G, Churchill D, Cromarty B, Das S, Fisher M. British HIV Association Guidelines for the treatment of HIV-1-infected adults with antiretroviral therapy 2008. HIV Med. 2008;9:563-608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 339] [Cited by in RCA: 341] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 87. | Coffin CS, Stock PG, Dove LM, Berg CL, Nissen NN, Curry MP, Ragni M, Regenstein FG, Sherman KE, Roland ME. Virologic and clinical outcomes of hepatitis B virus infection in HIV-HBV coinfected transplant recipients. Am J Transplant. 2010;10:1268-1275. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 75] [Cited by in RCA: 66] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 88. | Vibert E, Duclos-Vallée JC, Ghigna MR, Hoti E, Salloum C, Guettier C, Castaing D, Samuel D, Adam R. Liver transplantation for hepatocellular carcinoma: the impact of human immunodeficiency virus infection. Hepatology. 2011;53:475-482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 38] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 89. | Di Benedetto F, Tarantino G, Ercolani G, Baccarani U, Montalti R, De Ruvo N, Berretta M, Adani GL, Zanello M, Tavio M. Multicenter italian experience in liver transplantation for hepatocellular carcinoma in HIV-infected patients. Oncologist. 2013;18:592-599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 36] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 90. | Berenguer M, Royuela A, Zamora J. Immunosuppression with calcineurin inhibitors with respect to the outcome of HCV recurrence after liver transplantation: results of a meta-analysis. Liver Transpl. 2007;13:21-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 116] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 91. | Frassetto LA, Browne M, Cheng A, Wolfe AR, Roland ME, Stock PG, Carlson L, Benet LZ. Immunosuppressant pharmacokinetics and dosing modifications in HIV-1 infected liver and kidney transplant recipients. Am J Transplant. 2007;7:2816-2820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 131] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 92. | Tricot L, Teicher E, Peytavin G, Zucman D, Conti F, Calmus Y, Barrou B, Duvivier C, Fontaine C, Welker Y. Safety and efficacy of raltegravir in HIV-infected transplant patients cotreated with immunosuppressive drugs. Am J Transplant. 2009;9:1946-1952. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 124] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 93. | Moreno A, Cervera C, Fortún J, Blanes M, Montejo E, Abradelo M, Len O, Rafecas A, Martín-Davila P, Torre-Cisneros J. Epidemiology and outcome of infections in human immunodeficiency virus/hepatitis C virus-coinfected liver transplant recipients: a FIPSE/GESIDA prospective cohort study. Liver Transpl. 2012;18:70-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 40] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 94. | Neff GW, Bonham A, Tzakis AG, Ragni M, Jayaweera D, Schiff ER, Shakil O, Fung JJ. Orthotopic liver transplantation in patients with human immunodeficiency virus and end-stage liver disease. Liver Transpl. 2003;9:239-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 123] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 95. | Gastaca M, Aguero F, Rimola A, Montejo M, Miralles P, Lozano R, Castells L, Abradelo M, Mata Mde L, San Juan Rodríguez F. Liver retransplantation in HIV-infected patients: a prospective cohort study. Am J Transplant. 2012;12:2465-2476. [PubMed] |