Published online Nov 7, 2015. doi: 10.3748/wjg.v21.i41.11777
Peer-review started: May 7, 2015
First decision: June 2, 2015
Revised: June 18, 2015
Accepted: August 31, 2015
Article in press: August 31, 2015
Published online: November 7, 2015
Processing time: 189 Days and 4.5 Hours
Colon cancers develop adaptive mechanisms to survive under extreme conditions and display hallmarks of unlimited proliferation and resistance to cell death. The deregulation of cell death is a key factor that contributes to chemoresistance in tumors. In a physiological context, balance between cell proliferation and death, and protection against cell damage are fundamental processes for maintaining gut epithelial homeostasis. The mechanisms underlying anti-death cytoprotection and tumor resistance often bear common pathways, and although distinguishing them would be a challenge, it would also provide an opportunity to develop advanced anti-cancer therapeutics. This review will outline cell death pathways (i.e., apoptosis, necrosis, and necroptosis), and discuss cytoprotective strategies in normal intestinal epithelium and death resistance mechanisms of colon tumor. In colorectal cancers, the intracellular mechanisms of death resistance include the direct alteration of apoptotic and necroptotic machinery and the upstream events modulating death effectors such as tumor suppressor gene inactivation and pro-survival signaling pathways. The autocrine, paracrine and exogenous factors within a tumor microenvironment can also instigate resistance against apoptotic and necroptotic cell death in colon cancers through changes in receptor signaling or transporter uptake. The roles of cyclooxygenase-2/prostaglandin E2, growth factors, glucose, and bacterial lipopolysaccharides in colorectal cancer will be highlighted. Targeting anti-death pathways in the colon cancer tissue might be a promising approach outside of anti-proliferation and anti-angiogenesis strategies for developing novel drugs to treat refractory tumors.
Core tip: The mechanisms underlying anti-death cytoprotection and tumor resistance bear common pathways, and although distinguishing them would be a challenge, it would also provide an opportunity to develop advanced anti-cancer therapeutics. Autocrine, paracrine and exogenous factors within a tumor microenvironment may instigate apoptotic and necroptotic resistance in colon cancers. The roles of cyclooxygenase-2/prostaglandin E2, growth factors, glucose, and bacterial lipopolysaccharide will be highlighted. Targeting death resistance pathways in colon cancer tissue might be a promising approach outside of anti-proliferation and anti-angiogenesis strategies for developing novel drugs to treat refractory tumors.
- Citation: Huang CY, Yu LCH. Pathophysiological mechanisms of death resistance in colorectal carcinoma. World J Gastroenterol 2015; 21(41): 11777-11792
- URL: https://www.wjgnet.com/1007-9327/full/v21/i41/11777.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i41.11777
Colorectal carcinomas of epithelial origin are characterized by unlimited cell replication, death resistance, and metastasis[1]. In comparison to normal epithelial cells, cancer cells acquire the ability to avoid physiological cell turnover and exhibit an imbalance between renewal and demise, resulting in rapid expansion of tumor mass. It is believed that malignant cells develop adaptive mechanisms for surviving under the extreme conditions of tumor microenvironment, such as restricted oxygen supply and nutrient deprivation. The reprogramming of cancer cells not only contributes to their ability to hyperproliferate but also confers resistance to cell death against endogenous stress and exogenously applied cytotoxic drugs[2]. This review will outline pathways of cell death and discuss how cancer cells manipulate cytoprotective mechanisms to evade it.
Various types of cell death, i.e., apoptosis, necrosis, and necroptosis, was found in cancer tissues under metabolic stress or cytotoxic stimuli[3]. The signaling pathways of apoptosis and necroptosis will be discussed in the following sections. Although stress stimuli may also induce autophagy which is a catabolic process to remove protein aggregates and damaged organelles for recycling[4], this process is not described in the manuscript here since it may lead to either cell survival or apoptotic death.
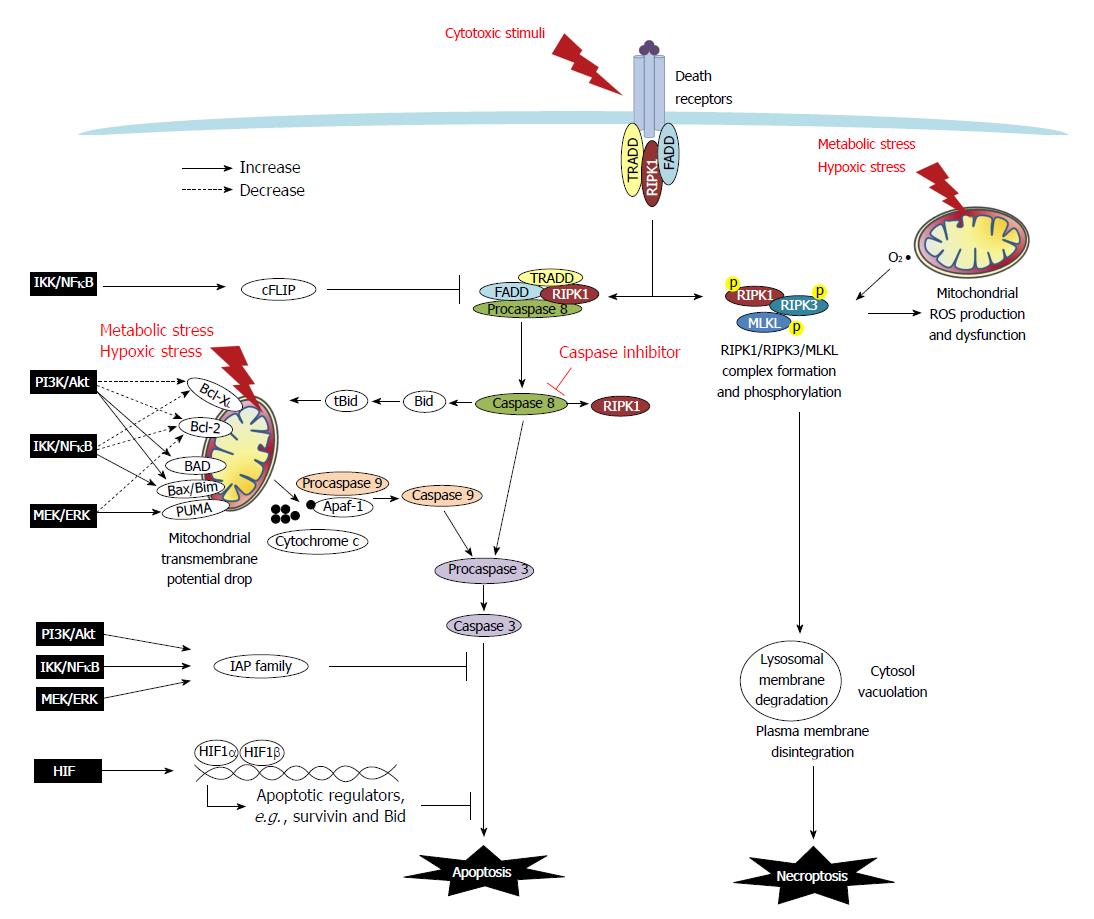
Apoptosis is a type of programmed cell death that is characterized by morphological and ultrastructural changes, including cell shrinkage, membrane blebbing, mitochondrial swelling, and chromatin condensation. Apoptosis may either be initiated extrinsically via death receptors such as tumor necrosis factor (TNF) receptor and Fas, or intrinsically via mitochondria-dependent pathways[5] (Figure 1). Moreover, anoikis, which is a form of detachment-induced apoptosis, has been demonstrated to occur in epithelial cells, as they normally require anchorage to basement membranes to establish a monolayer[6].
In the extrinsic apoptotic pathway, the recruitment of cytoplasmic molecules to receptors is initiated following the binding of TNFα or FasL. Docking molecules, including TNF receptor-associated death domain (TRADD), Fas-associated death domain (FADD), procaspase-8/FLICE/MACH, and receptor-interacting protein kinase (RIPK)-1, are recruited to receptor-associated lipid rafts to form a complex that facilitates the cleavage and activation of caspase-8[7,8]. The intrinsic apoptotic pathway occurs following endogenous stress and is associated with a drop in mitochondrial membrane potential. This pathway is regulated by the formation of a mitochondrial permeability transition pore (MPTP), which is composed of Bcl-2 family members and voltage-dependent anion channels on the outer mitochondrial membrane[5,9]. The ratio of Bcl-2 family proteins (i.e., anti-apoptotic Bcl-2 and Bcl-XL and pro-apoptotic Bax, Bad, Bak, Bid, Bim, and PUMA) is a key factor in determining the conformation of MPTP. Among the Bcl-2 members, Bid can be cleaved by caspase-8 and migrate to the mitochondria in its truncated form tBid to associate with Bax to increase membrane permeability. The drop of mitochondrial membrane potential leads to osmotic swelling of the matrix by water influx and release of cytochrome c from mitochondrial intramembranous space into the cytoplasm, followed by its complex formation with procaspase-9 and APAF-1. The activation of caspase-9 and/or -8 leads to caspase-3 cleavage, endonuclease activation, and ultimately nuclear DNA fragmentation, which is the hallmark of apoptosis[5,9] (Figure 1).
Regulatory proteins, such as FLICE-Like Inhibitory Proteins (FLIPs), inhibit the extrinsic apoptotic pathway by binding to FADD and causing dissociation of the FADD-caspase 8 complex. Additionally, families of inhibitor of apoptosis protein (IAP), including XIAP, cIAP, and survivin, bind to caspase-3 and -9 and thereby inhibit caspase activity. Moreover, XIAP-associated factor 1 (XAF1) negatively regulates the anti-apoptotic function of XIAP[5,9,10].
Necrosis is traditionally known as an uncontrolled form of cell death, characterized by morphological features of mitochondrial swelling, cytoplasmic vacuolation, cytosol density loss, and plasma membrane rupture. The resultant release of subcellular organelles and molecules is considered a potent trigger for tissue inflammation[11].
A novel form of programmed necrosis, termed necroptosis, has been recently identified. In this process, signaling pathways involving RIPK1/RIPK3-mediated phosphorylation activate the mixed lineage kinase domain-like protein (MLKL) to execute the final step of cell destruction[11] (Figure 1). The best defined necroptosis pathway was elucidated following the stimulation of cells with TNFα in the presence of ZVAD (a pan-caspase inhibitor)[11,12]. This observation has led to the development of a preliminary hypothesis that necroptosis may be a default mechanism for cells that are unable to die via apoptosis[12,13]. However, with increasing evidence of instances of necroptotic death occurring following various stimuli (e.g., oxygen and glucose deprivation, extensive DNA damage, hyperactivation of Poly(ADP-ribose)polymerase -1 (PARP), and free radical exposure), it is now clear that RIPK1/3-dependent necrosis is an independent mode of cell death that shares common pathways with apoptosis[14,15].
Early studies of necroptotic pathways by activating TNF receptor in the presence of caspase inhibition demonstrated that the adapter molecules FADD and TRADD recruited RIPK1, which subsequently undergo a series of ubiquitination and deubiquitination events before RIPK1 forming a complex with RIPK3 and MLKL in the cytosol for auto- or trans-phosphorylation[16]. The RIPK1/RIPK3/MLKL kinase complex has been proposed to mediate necrotic death via the induction of mitochondrial dysfunction[16]. Additionally, mitochondria-derived free radical production and lysosomal membrane disintegration have been reported to be facets of necroptotic machinery[17]. Free radical scavengers that suppress mitochondrial reactive oxygen species (ROS) were shown to inhibit the execution of necroptotic death induced by TNF and hypoxic stress, but had no effect to necroptosis induced by PARP[18,19]. Other reports revealed that hypoxia-induced mitochondrial ROS was upstream of RIPK1/RIPK3 activation in the necroptotic signaling pathway[19]. The order of intracellular events leading up to plasma membrane explosion and intracellular content spilling may vary depending on trigger type[17-19]. Overall, it is currently recognized that two modes of cell death are driven by RIPK1 through its kinase function, including apoptosis via its formation of a complex with caspase-8/FADD/TRADD and necroptosis via its formation of a complex with RIPK3/MLKL (Figure 1).
The intestinal epithelial monolayer is maintained in a state of dynamic equilibrium that is governed by the balance between crypt proliferation and surface/villus shedding and cell death. Newly proliferated cells that are derived from stem cells in the crypts migrate upward and differentiate into various cell types (e.g., absorptive and secretive epithelial cells, goblet cells, and endocrine cells); the cells then undergo detachment and apoptosis at an “extrusion zone” on the luminal surface with a turnover rate of 5-7 d[20,21]. Epithelial integrity and intestinal homeostasis are tightly controlled by the balancing of two physiological processes, namely cell proliferation and death.
Progressive inhibition of cell apoptosis has been associated with the transformation of normal colorectal epithelium into carcinoma[22]. Direct evidence of an inverse correlation between epithelial cell death and tumor susceptibility has been provided in recent studies. Mice that were deficient in pro-apoptotic molecules (e.g., Bak and Fas) displayed a higher incidence and higher numbers of aberrant crypt foci and colorectal tumors following induction with the carcinogen azoxymethane (AOM) or AOM/dextran sulfate sodium (DSS)[23,24]. Although a lack of Bak or Fas did not affect physiological apoptosis in colon cells, a decreased level of epithelial cell death was observed following exposure to pro-apoptotic triggers (e.g., gamma-radiation and genotoxic carcinogens)[23,24]. Moreover, PUMA-knockout mice exhibited reduced apoptosis in colonic crypts and increased colonic tumor susceptibility following an AOM/DSS challenge. A deficiency of PUMA enhanced the formation of spontaneous adenomas in the distal small intestines and colons of APC(Min/+) mice[25]. These studies indicate that cells that are unable to undergo apoptosis partly contribute to cancer progression.
Surface epithelial layers are constantly bombarded by orally acquired harmful substances and luminal bacteria, and are also exposed to potentially hypoxic conditions due to their location at the end of a capillary circuit that interfaces with an anaerobic lumen. When exposed to intrinsic stress or external stimuli, a normal epithelium exhibits excessive cell death (i.e., apoptosis, necrosis and necroptosis) and display barrier defects. However, cytoprotective strategies against cell death also exist to maintain gut homeostasis.
The cellular survival strategies include uptake of glucose and glutamine, free radical scavenging, transcriptional adaptation, and paracrine effects induced by cyclooxygenase (COX)-2/prostaglandin E2 (PGE2). In the following sections of this work, several facets of anti-death cytoprotective strategies that share common pathways to tumor resistance will be discussed. Understanding the similarity between epithelial cytoprotection and cancer resistance would help to identify distinct mechanisms undertaken by tumor cells for the search of advanced therapeutic targets.
One of the unique characteristic of the intestinal tract is that it possesses dual routes of nutrient supply, including hematologic and dietary sources. In small intestinal epithelium, apical glucose uptake is mediated by sodium-dependent glucose transporter 1 (SGLT1), while glucose transporter 2 (GLUT2) facilitates diffusive transport of intracellular glucose across the basolateral membrane and into the bloodstream[26]. Large intestinal epithelium normally expressed GLUT5 and GLUT6, but not the other glucose transporters[27]. We and others have previously shown that enhanced glucose uptake via SGLT1 can protect intestinal epithelial cells against various pro-apoptotic triggers, such as mesenteric ischemia/reperfusion, microbial challenges, and endotoxemia[28-31]. Energy production has been generally assumed as the main cytoprotective mechanism of glucose uptake; however, alternative pathways of SGLT1-mediated activation of phosphatidylinositide 3-kinase (PI3K)/Akt and nuclear factor kappa B (NFκB) pathways also partially contributes to cytoprotection[28,31]. Other than glucose, glutamine (a non-essential amino acid) is important for cell survival during conditions of stress. Glutamine can prevent epithelial cell apoptosis caused by hypoxia/reoxygenation, oxidants, endotoxins, heat stress, and TNFα[32-36]. The inhibition of gut epithelial cell apoptosis by glutamine is mediated through upregulation of autophagy and increased transcription of heat shock proteins[34,35,37].
Redox enzymes, including catalase (CAT), superoxide dismutase (SOD), glutathione reductase, and glutathione-S-transferase, suppress intracellular accumulation of free radicals. An intracellular redox system converts highly reactive free radicals, such as superoxide, hydrogen peroxide, and hydroxyl radical, into lower energy molecules. Intravenous injections of CAT and SOD have been shown to decrease intestinal inflammation and epithelial barrier dysfunction in animal models of mesenteric ischemia/reperfusion injury[38-40].
Hypoxia-inducible factor (HIF) is a transcription factor that is activated in epithelial cells under the low oxygen conditions of ischemic or inflamed gut[41,42]. The HIF family includes three proteins: HIF-1, HIF-2, and HIF-3. Activation of HIF is dependent on the stabilization of the oxygen-sensitive α subunit, which is subsequently translocated to the nucleus to form a functional complex with the β subunit and various other coactivators[43]. HIF-1α/2α forms a dimeric complex with HIF-1β, and triggers transcription by binding to the hypoxia response element of various gene promoter regions[44,45]. Under normoxic conditions, the hydroxyl hydroxylase (PHD)-mediated hydroxylation of proline residues on HIF-1α/2α leads to its ubiquitination and degradation. Low oxygen levels have been shown to result in a downregulation of PHD activity and to stabilize HIF-1α/2α levels[43]. HIF-1 activation has been implicated in maintaining epithelial barrier protection in models of intestinal ischemia/reperfusion, experimental colitis with inflammatory hypoxia, and in mouse ileal loops after exposure to bacterial toxins[41,42,46-48].
Cyclooxygenase (COX)-2, a catalyzing enzyme for PGE2 production, is involved in increased vascular permeability and blood flow during inflammation and wound healing. COX-2 also protects against colonic epithelial damage in animal models of chemical-induced colitis[49,50]. Oral supplementation of PGE2 increased proliferation and reduced apoptosis of intestinal epithelium in mice with colitis[49]. Moreover, PGE2 increased c-IAP2 expression in normal rat epithelial cells[51].
The detailed anti-death signaling pathways have primarily been delineated from observations made in adenocarcinoma cell line studies, and they will be discussed in the context of cancer cell death resistance in the next section.
Death and anti-death signaling in cancer cells has been extensively studied in the context of cell survival against exogenously applied cytotoxic drugs. Substantial efforts have also been made to uncover the pathways that lead to cancer cell death and survival under conditions of endogenous metabolic stress, with the goal of learning how to therapeutically manipulate tumor-specific machinery to produce anti-cancer effects. We will highlight the mechanisms of death resistance in cancer cells against exogenous or endogenous stress. These intracellular pathways include direct alteration of death machinery and modulation of upstream events such as tumor suppressor gene inactivation and pro-survival signaling. For additional discussion of drug-related resistance mechanisms, such as efflux pumps and enzymatic degradation, please see other review articles[52,53].
Defects in apoptotic signaling and increased use of anti-apoptotic pathways have been reported in colon cancer cells. Key regulatory proteins of apoptotic machinery, such as families of Bcl-2 and IAP, undergo changes in expression during the transition of an adenoma into a carcinoma and have therefore been utilized as prognostic biomarkers[54,55]. An overexpression of the anti-apoptotic Bcl-2 family member Bcl-XL is a known predictor of poor prognoses in patients with colonic adenocarcinomas[56]. Increased expression levels of anti-apoptotic regulators such as c-FLIP, XIAP, cIAP2, and survivin have also been correlated with disease progression and poor survival in colon cancer patients[57-61]. A recent report indicated that expression levels of the necroptotic adaptors RIPK1 and RIPK3 are significantly decreased in human colon cancer tissues compared to adjacent normal mucosa[62]. Overall, an increase of anti-apoptotic molecules and a decrease in pro-necroptotic kinases are both likely to contribute to death resistance in cancer cells (Table 1). The resistance mechanisms that occur upstream of the alteration of apoptotic regulators are further discussed below.
| Classification | Molecule | Expression in cancer tissues | Ref. |
| Bcl-2 family | Bcl-XL | Increased | [56] |
| IAP family | cFLIP | Increased | [57] |
| cIAP2 | Increased | [59,60] | |
| survivin | Increased | [55,61] | |
| XIAP | Increased | [58] | |
| RIP kinase family | RIPK1/RIPK3 | Decreased | [62] |
Mutations in oncogenes (RAS and β-catenin) and tumor suppressor genes (p53 and APC) have been identified to arise throughout the course of tumorigenesis[63]. The intriguing field of oncogene mutation and cell hyperproliferation has been reviewed comprehensively[64] and will not be discussed here. Instead, cancer cell death resistance that is imparted by mutation of the p53 tumor suppressor gene and the resultant functional consequences of this resistance will be the focus of this section.
Mutations in the p53 gene occur in half of all colorectal cancer cases and have been correlated with adenoma-to-carcinoma transitions and aggressive subsets of colorectal cancer[65,66]. Tumor cells harboring p53 mutations have long been known to be defective in the induction of apoptosis[67]. In addition to its pro-apoptotic role, it has become evident that p53 acts as a multifunctional transcription factor and is involved in physiological cellular responses to stressful stimuli (e.g., DNA damage and hypoxia), surveillance mechanisms that cause cell cycle arrest following cellular damage or oncogenic aberration, and regulation of metabolic pathways for a switch from glycolysis to oxidative phosphorylation[68,69]. The molecular mechanisms that are employed by p53 to induce cell death in the context of suppressing cancer progression include the transcriptional regulation of pro-apoptotic PUMA expression, the generation of oxidative free radicals within mitochondrial components, the reduction of COX-2/PGE2 synthesis, and the induction of death receptor 5[70-73].
A number of signaling molecules and transcription factors are involved in the dysregulation of death machinery in cancer cells (Figure 1). These include the PI3K/Akt, mitogen-activated protein kinase kinase (MEK)/extracellular signal-regulated kinase (ERK), IκB kinase (IKK)/inhibitor of NFκB (IκB)/NFκB, and HIF signaling pathways (Table 2).
| Pathway | Observation in experimental models | Ref. |
| PI3K/Akt | Increase of Bcl-2, Bcl-XL and survivin expression in colon cancer cell lines (SW480, SW620, HCT116, and HT29) | [74,75] |
| Inactivation of BAD by phosphorylation in colon cancer cell lines (HT29 and H508) | [76,77] | |
| Decreased expression of Bim and inactivation of Bax in colon cancer cell lines (HCT116 and DLD1), and increase of tumor growth in xenograft models | [78] | |
| MEK/ERK | Phosphorylation and stabilization of Bcl-2 in colon cancer cell lines (HCT116 and HT29) for increase of anoikis, and promotion of metastasis in xenograft models | [80] |
| Decreased expression of Bim and inactivation of Bax in colon cancer cell lines (HCT116 and DLD1), and increase of tumor growth in xenograft models | [78] | |
| Suppression of PUMA expression and activity in colon cancer cell lines (Lovo and SW1116) | [73] | |
| Dowregulation of XAF1 and upregulation of XIAP in colon cancer cell lines (HCT116, Lovo, DLD1, and SW1116) | [82] | |
| IKK/IκB/NFκB | Induction of cIAP-2 expression in colon cancer cell lines (Caco-2, HCT116, KM20, and KM12C) | [91] |
| Increase of Bcl-2, Bcl-XL, and cFLIP in colon cancer cell lines (COLO205 and HCT116) | [92] | |
| HIF | Binding to hypoxia-responsive element of the Bid promoter in colon cancer cells (SW480) for Bid downregulation | [94] |
| Binding to hypoxia-responsive element of the survivin promoter in breast cancer cells (MCF-7) for survivin upregulation | [93] |
Studies on colon cancer cell lines have shown that an upregulation of PI3K/Akt protein kinases has been associated with increased expression of anti-apoptotic Bcl-2 proteins (e.g., Bcl-2, Bcl-XL, and survivin)[74,75], phosphorylation and inactivation of pro-apoptotic Bad and Bax[76-78], and activation of XIAP[79]. In colon cancer and epithelial cells, the MEK/ERK signaling pathway mediates the phosphorylation and stabilization of Bcl-2[80], the inactivation of Bax and the degradation of Bim[78,81], the suppression of PUMA induction[73], the downregulation of XAF1 and the upregulation of XIAP expression[82].
The activation of the IKK/IκB/NFκB pathway, which serves as a putative proinflammatory signal, has been linked with anti-apoptotic effects in normal colonocytes and colon cancers alike. The activation of the IκB kinase complex (IKKα/β/γ) leads to the phosphorylation of NFκB-bound IκB and causes IκB to undergo ubiquitin-dependent degradation, enabling the liberated NFκB to translocate to the cell nucleus and act as a transcription factor[83]. Unlike other subunits in the complex, IKKα can shuttle between the nucleus and cytoplasm to facilitate NFκB-regulated gene expression[84]. An elegant study indicated that either epithelial-specific ablation of IKKγ (also called NEMO) or a deficiency of both IKKα and IKKβ can lead to increased levels of apoptosis in mouse colonocytes[85]. Using carcinogen-induced colon cancer models, epithelial-specific IKKβ-KO mice were shown to exhibit increased colonic cell apoptosis associated with a reduction of tumor growth compared to wild type mice[86]. Numerous studies of colonic, gastric and esophageal cancer cell lines have demonstrated that blocking NFκB-induced apoptosis under baseline conditions sensitizes cells to treatment with 5-fluorouricil (5-FU)[87-89]. The underlying mechanisms of this phenomenon included IKKα-mediated phosphorylation of CREB binding protein (a transcriptional coactivator), which induced a switch in binding preference from p53 to NFκB and led to a concurrent upregulation of NFκB-dependent anti-apoptotic genes, and the downregulation of p53-mediated pro-apoptotic genes[90]. Moreover, in colon cancer cells, the transcriptional targets of NFκB include the anti-apoptotic regulators Bcl-2, Bcl-XL, cFLIP and IAP[91,92].
HIF is upregulated in the hypoxic core of rapidly growing solid tumors and is therefore considered a biomarker of poor prognosis in colon cancer patients[45]. HIF-1 targets promoter sites of apoptotic regulators such as survivin and Bid, and directly modulates cell death pathways[93,94]. Other HIF-1-targeted genes included trefoil factor[47], COX-2[95], glucose metabolic enzymes [e.g., hexokinase (HK), glyceraldehyde-3-phosphate dehydrogenase (GAPDH), pyruvate kinase (PK), pyruvate dehydrogenase kinase (PDK)], and glucose transporters (e.g., GLUT-1 and -3)[45,96,97]. HIF-1α also directly activates promoter regions of various growth factors and receptors, including vascular endothelial growth factor (VEGF), c-Met [a receptor for hepatocyte growth factor (HGF)], HGF activator[98-100]. Moreover, several reports have shown that HIF-2α increases transcriptional and translational expression of amphiregulin [a member of the epidermal growth factor (EGF) family] and EGF receptor (EGFR), and favors autocrine growth signaling in various types of cancer[101-103].
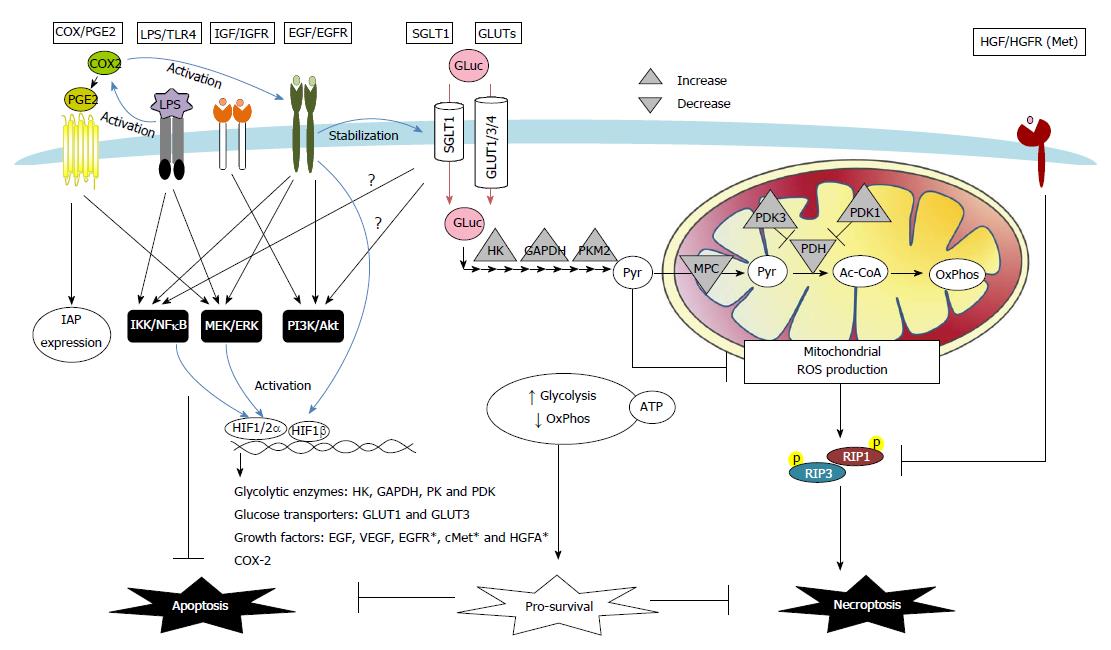
Autocrine, paracrine and exogenous factors in tumor microenvironments also instigate resistance against apoptotic and necroptotic cell death in colon cancers via changes in receptor signaling and transporter uptake (Figure 2). The roles of COX-2/PGE2, growth factors, glucose, and bacterial LPS are highlighted below.
Increased expression of COX-2 was observed in colon adenocarcinoma, and is considered one of the earliest events in tumor development[104,105]. Clinical studies have provided strong evidence that long term use of non-steroidal anti-inflammatory drugs reduce the incidence of colon cancers[106]. Various mechanisms have been proposed for the COX-2-mediated tumor-promoting activity, including increased blood flow, induction of growth factors, increase of cell proliferation, or modulation of apoptotic regulators[51,107,108]. COX-2/PGE2 induces synthesis of amphiregulin and activation of EGFR signaling in colon cancer cell lines[107,108]. Moreover, stimulation with PGE2 was found to suppress Fas-induced apoptosis in colon cancer cells via upregulation of IAP expression[51] (Figure 2).
A number of growth factors have been associated with death resistance in cancer, including epidermal, hepatocyte, and insulin-like growth factors (Figure 2). EGFR activation has long been known to induce epithelial cell proliferation, restitution, and tumorigenesis. In colorectal and gastric cancer cells, EGFR activation also exerts anti-apoptotic effects that are mediated by PI3K/Akt, ERK and IκBα/NFκB signaling, and protects cancer cells against anoikis and enhances epithelial-mesenchymal transition[109-112]. Moreover, both EGFR and its downstream Akt and ERK pathways are known to be involved in preventing apoptosis in stem-like cell populations in serum-deprived colorectal cancer cells[113]. A positive feedback loop between EGFR and HIF signaling pathways may also contribute to the increase of death resistance in cancer, as revealed by evidence that EGFR activation leads to increased expression and nuclear translocation of HIF-1α/1β in normoxic conditions[93,95,114]; furthermore, HIF-2α increases the transcript and protein expression of EGFR and amphiregulin[101-103]. Recent evidence has revealed a co-expression of EGFR and SGLT1 in human colorectal and oral squamous carcinoma[115,116]. Interactions between EGFR and SGLT1 have shown a positive correlation with cancer cell proliferation and survival through a mechanism that involves the stabilization of membranous SGLT1 proteins by EGFR in a tyrosine kinase-independent manner[117,118].
Elevated expression of the HGF receptor Met has been shown in human colorectal cancers compared to normal mucosa[119]. Recently, both the autocrine production of HGF and a positive feedback loop that was mediated by a constant activation of the HGF gene promoter due to microsatellite instability that was characterized by truncations of the promoter region were found in human colon carcinoma samples and cell lines[120]. Activation of HGF/Met signaling was shown to cause a downregulation in the expression of RIPK1 proteins, which correlated with a reduction in the number of necroptotic regions found in colon tumors[120]. Moreover, evidence of HIF-1α directly activating the promoter regions of Met and HGF activator (a serine protease that converts HGF to its active form) was found in pancreatic cancer and glioma cells[99,100]. Interestingly, increased activation of Met was found to be induced in human carcinoma Caco-2 cells following treatment with cetuximab (an EGFR inhibitor), which served as a chemoresistance mechanism[121].
Colon cancers express high levels of both insulin-like growth factor 1 (IGF1) and its receptor compared to normal mucosa. The overexpression of IGF1 receptors in human colon cancer cells was found to confer resistance to serum-deprivation induced apoptosis, which was associated with increased activation of Akt and an upregulation of Bcl-XL[122]. Another report demonstrated that inhibition of the IGF1 receptor sensitized human colon adenocarcinoma cells to cetuximab and restored cell death in drug-resistant cell clones[123].
Increased glucose dependency and altered glucose metabolism are associated with cancer cell transformation (Table 3). In normal cells, one glucose molecule is catalyzed into two ATPs and two pyruvate molecules in an anaerobic fashion by a cascade of glycolytic enzymes, including HK, GAPDH, and PK[124]. The final glycolytic product, pyruvate, is transported across the inner mitochondrial membrane (IMM) by mitochondrial pyruvate carrier (MPC) and is then converted to acetyl-CoA by pyruvate dehydrogenase (PDH) before entering into the tricarboxylic acid (TCA) cycle that occurs in the mitochondrial matrix[125,126]. A reduced substrate is generated by the TCA cycle and is then fed into the electron transport chain of the IMM, after which oxidative phosphorylation leads to the production of 36 ATPs. In contrast to normal cells, tumors exhibit high levels of glycolysis despite the presence of sufficient oxygen, which is a phenomenon known as the Warburg effect[127].
| Classification | Molecule | Expression and mechanism | Ref. |
| Glucose uptake | |||
| Transporters | GLUT1 | Abnormal expression of GLUT1 in colon cancer | [128,129] |
| Hypoxia-induced expression of GLUT1 by HIF-1 binding to the GLUT1 promoter | [96,131,132] | ||
| GLUT1-mediated glucose uptake promoted drug resistance in colon cancer cells | [136] | ||
| GLUT3,4 | Abnormal expression of GLUT3,4 in colon cancer | [19,27,130] | |
| SGLT1 | Abnormal expression of SGLT1 in colon cancer | [27,115] | |
| Stabilization of membrane SGLT1 expression is dependent on EGFR in a kinase-independent mechanism | [117,134] | ||
| Glucose metabolism | |||
| Enzymes | PK | Upregulation of PKM2 isoform in chemoresistant cancer cells | [132] |
| PDK-1 | PDK-1 as a novel Wnt target gene improved colon cancer cell survival via enhancement of glycolysis | [142] | |
| PDK-3 | HIF1-mediated upregulation of PDK-3 inhibited mitochondrial phosphorylation and promoted drug resistance | [143] | |
| HK, GAPDH | HIF1-dependent transcriptional upregulation | [45,96] | |
| PDH | Decreased expression in colon cancer cells | [141] | |
| Carriers | MPC | Reduction of MPC activity promoted glycolysis and maintenance of stemness properties | [144] |
| Products | ATP | Elevation of intracellular ATP promoted cancer cell survival and induced drug resistance | [145,146] |
| Pyruvate | Pyruvate prevented hypoxia-induced necroptosis through suppression of mitochondrial free radicals in an ATP-independent mechanism | [19] |
The abnormal expression of the GLUT isoforms 1, 3, and 4 and the SGLT1 protein have been widely documented in studies of human colon cancer samples[27,115,116,128-130]. A large body of evidence indicates that the upregulation of GLUT1 and several glycolytic enzymes is dependent on the transcriptional activity of HIF-1 in both human colon cancer tissues and drug-resistant cancer cell lines[96,131-133]. Recent studies have indicated that the stabilization of membrane SGLT1 expression in colon cancer cells is dependent on EGFR activation[117,134], suggesting that this pro-proliferative and anti-apoptotic growth factor is also involved in the mechanism that underlies enhanced glucose uptake (Figure 2).
Changes in glucose uptake and metabolism have been suggested to provide a survival advantage to tumor cells and also to contribute to anti-cancer drug resistance. A previous report has shown that high glucose levels can modulate the cytotoxicity and anti-proliferative effects of 5-FU in colon cancer cell lines[135]. The in vitro inhibition of GLUT1-mediated glucose uptake by phloretin was found to sensitize colon cancer cells to overcome apoptotic resistance to daunorubicin under conditions of hypoxia[136]. The modulation of glucose metabolic pathways by chemicals (e.g., 3-bromopyruvate, iodoacetate, dichloroacetate, or 2-deoxyglucose) was found to attenuate 5-FU resistance in colonic and gastric cancer cells[137-139]. Recent studies have indicated that high levels of intracellular glucose lead to an increased side population (SP) of stem-like cells within the overall cancer cell population; these cells exhibit high glycolytic activity and drug export ability, and are most resistant to cell death[140]. The underlying mechanism of SP cell expansion involves glucose-mediated suppression of AMP-activated protein kinase and an activation of Akt signaling[140].
PK is the final rate-limiting enzyme in the glycolytic pathway, and it catalyzes the conversion of phosphoenolpyruvate and ADP into pyruvate and ATP. PDH, which converts pyruvate into acetyl-CoA, can be phosphorylated and inactivated by PDK in physiological conditions. The PK isoform M2 (PKM2) is significantly upregulated in 5-FU-resistant colon cancer cell lines[132]. Moreover, decreased expression of PDH was reported in colorectal cancer cells[141]. A recent study has identified that PDK-1 is a novel Wnt target gene that can promote glycolysis and colon cancer cell survival[142]. Hypoxia-induced PDK-3 expression via the activation of HIF-1 has been shown to lead to an inhibition of mitochondrial phosphorylation and the promotion of drug resistance[143]. Additional research has indicated that reduced MPC activity promotes glycolysis and the maintenance of stemness in colon cancer cells[144].
ATP has generally been assumed to be the effector of glucose metabolism that promotes cancer growth, survival, and chemoresistance. A number of studies have shown that depleting ATP via glycolytic inhibition increases apoptosis in multidrug- resistant cancer cells under both normoxic and hypoxic conditions[145,146]. Moreover, direct delivery of liposome-encapsulated ATPs was found to be sufficient for promoting survival and inducing resistance to oxaliplatin in formerly drug-sensitive colon cancer cells[146]. Whether ATP-dependent chemoresistance is a result of cell hyperproliferation or the induction of anti-death mechanisms remains unknown.
It is notable that an energy-independent mechanism is also involved in glucose-mediated death resistance in cancer cells. Pyruvate not only serves as a link between glycolysis and mitochondrial respiration but also acts as a scavenger for oxidative free radicals through a non-enzymatic reaction[147]. Our recent studies have shown that pyruvate (uncoupled to ATP) plays a distinct role in promoting cancer cell survival[19]. Glycolytic pyruvate prevented RIPK1/3-dependent necroptosis caused by hypoxic stress in colon cancer cells through the suppression of mitochondrial free radicals[19]. Collectively, the manipulation of glucose uptake and metabolic pathways in conjunction with the application of cytotoxic drugs may promote anticancer effects and overcome chemoresistance (Figure 2).
With an increasing body of evidence suggesting that chemoresistance in colon cancers can be mediated by glucose, it is notable that dietary glutamine supplementation was shown to reduce tumor burden by increasing apoptosis and decreasing proliferation in the colons of mouse models that were submitted to colitis-associated cancer induction[148]. Although a glutamine-dependent chemopreventive effect on tumorigenesis was suggested to result from its anti-inflammatory activity, it remains unclear whether glutamine metabolism by first pass in epithelial cells (in place of glucose metabolism) may play a role in tumor suppression. The differential roles of glucose- and glutamine-dominant metabolism in the regulation of tumorigenesis warrant further investigation.
The innate immune receptor Toll-like receptor 4 (TLR4) forms a complex with CD14 and MD2 for sensing bacterial LPS. This receptor complex was originally identified on monocytes/macrophages and endothelial cells that are responsible for septic shock. Given the juxtaposition of commensal bacteria and intestinal mucosa, it had been assumed that normal gut epithelial cells were not equipped with LPS receptors. However, accumulating data showed that normal human colonocytes constitutively express CD14 in the absence of TLR4, and a strong immunoreactivity of CD14 and TLR4 was found in human colorectal carcinoma tissues, indicating a link between LPS/TLR4 signaling and tumor formation[149-151].
The activation of TLR4 signaling may contribute to tumorigenesis by promoting epithelial proliferation and/or by reducing cell death (Figure 2). In vitro studies in colon adenocarcinoma cell lines have demonstrated that LPS/TLR4 induced resistance to apoptosis via NFκB and ERK signaling, without modulating the rate of cell division[152,153]. Conversely, several studies have revealed enhancement of both cell viability and proliferation following the activation of LPS/TLR4 in cancer cell lines[154,155]. Using chemically induced colitis-associated mouse cancer models, numerous studies have shown that the genetic absence of TLR4 and its downstream signaling molecules (i.e., MyD88 and IKKβ) results in reduced tumor burdens[86,151,156]. A TLR4-dependent hyperproliferation of colonic epithelial cells was shown to be related to increased activation of COX/PGE2 and EGFR signaling in the mouse models of colon cancer[49,151,157].
Recently, we demonstrated that the abnormally upregulated TLR4 protein plays an anti-apoptotic role against its co-receptor CD14 during the development of colon cancer[158]. Stimulation with LPS induced CD14-mediated lipid signaling and led to colonic epithelial cell apoptosis, whereas TLR4 antagonistically promoted cell survival and cancer progression[29,158]. Our results showed that that dysfunction of this CD14/TLR4 antagonism in the context of the cellular death and survival response may contribute to the carcinogenic transformation of normal epithelial cells[158]. Finally, intracolonic administration of a TLR4 antagonist was shown to result in increased cancer cell apoptosis and reduced tumor burdern, suggesting the therapeutic potential of TLR4 blockade[158].
Greater understanding of the pathophysiological anti-death mechanisms that are present in cancer cells would enable advanced therapeutic interventions that could overcome chemoresistance. One of the key challenges of successful clinical translation is the ability to destroy tumors while sparing normal cells. By understanding the similarity between epithelial cytoprotection and cancer resistance, we may identify pathways that differ in normal and tumor cells for pharmacological manipulation. So far, abnormally expressed glucose transporters and TLR4, which are involved in both anti-death and pro-proliferative mechanisms, show great therapeutic potential. Drugs that target apoptotic machinery and signaling pathways, metabolic enzymes, and glucose transport are currently being investigated in clinical trials[159]. Agents to eliminate cancer stem cells, the cell type most resistant to death, are also in development[160]. In sum, targeting death resistance pathways may offer a promising therapeutic approach in addition to strategies such as inhibiting proliferation and angiogenesis and would offer hope to patients with refractory tumors.
P- Reviewer: Elpek GO, Perse M S- Editor: Ma YJ L- Editor: A E- Editor: Wang CH
| 2. | Cuezva JM, Krajewska M, de Heredia ML, Krajewski S, Santamaría G, Kim H, Zapata JM, Marusawa H, Chamorro M, Reed JC. The bioenergetic signature of cancer: a marker of tumor progression. Cancer Res. 2002;62:6674-6681. [PubMed] |
| 3. | Mohammad RM, Muqbil I, Lowe L, Yedjou C, Hsu HY, Lin LT, Siegelin MD, Fimognari C, Kumar NB, Dou QP. Broad targeting of resistance to apoptosis in cancer. Semin Cancer Biol. 2015;Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 605] [Cited by in RCA: 613] [Article Influence: 61.3] [Reference Citation Analysis (0)] |
| 4. | Viry E, Paggetti J, Baginska J, Mgrditchian T, Berchem G, Moussay E, Janji B. Autophagy: an adaptive metabolic response to stress shaping the antitumor immunity. Biochem Pharmacol. 2014;92:31-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 67] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 5. | Yang SY, Sales KM, Fuller B, Seifalian AM, Winslet MC. Apoptosis and colorectal cancer: implications for therapy. Trends Mol Med. 2009;15:225-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 71] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 6. | Loktionov A. Cell exfoliation in the human colon: myth, reality and implications for colorectal cancer screening. Int J Cancer. 2007;120:2281-2289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 72] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 7. | Humphries F, Yang S, Wang B, Moynagh PN. RIP kinases: key decision makers in cell death and innate immunity. Cell Death Differ. 2015;22:225-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 202] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 8. | Günther C, Neumann H, Neurath MF, Becker C. Apoptosis, necrosis and necroptosis: cell death regulation in the intestinal epithelium. Gut. 2013;62:1062-1071. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 271] [Cited by in RCA: 347] [Article Influence: 28.9] [Reference Citation Analysis (0)] |
| 9. | Shanmugathasan M, Jothy S. Apoptosis, anoikis and their relevance to the pathobiology of colon cancer. Pathol Int. 2000;50:273-279. [PubMed] |
| 10. | Zhang L, Yu J. Role of apoptosis in colon cancer biology, therapy, and prevention. Curr Colorectal Cancer Rep. 2013;9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 81] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 11. | Vandenabeele P, Galluzzi L, Vanden Berghe T, Kroemer G. Molecular mechanisms of necroptosis: an ordered cellular explosion. Nat Rev Mol Cell Biol. 2010;11:700-714. [PubMed] |
| 12. | Duprez L, Wirawan E, Vanden Berghe T, Vandenabeele P. Major cell death pathways at a glance. Microbes Infect. 2009;11:1050-1062. [PubMed] |
| 13. | Vandenabeele P, Declercq W, Van Herreweghe F, Vanden Berghe T. The role of the kinases RIP1 and RIP3 in TNF-induced necrosis. Sci Signal. 2010;3:re4. [PubMed] |
| 14. | Marshall KD, Baines CP. Necroptosis: is there a role for mitochondria? Front Physiol. 2014;5:323. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 107] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 15. | Declercq W, Vanden Berghe T, Vandenabeele P. RIP kinases at the crossroads of cell death and survival. Cell. 2009;138:229-232. [PubMed] |
| 16. | Linkermann A, Green DR. Necroptosis. N Engl J Med. 2014;370:455-465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 778] [Cited by in RCA: 895] [Article Influence: 81.4] [Reference Citation Analysis (0)] |
| 17. | Vanden Berghe T, Vanlangenakker N, Parthoens E, Deckers W, Devos M, Festjens N, Guerin CJ, Brunk UT, Declercq W, Vandenabeele P. Necroptosis, necrosis and secondary necrosis converge on similar cellular disintegration features. Cell Death Differ. 2010;17:922-930. [PubMed] |
| 18. | Sosna J, Voigt S, Mathieu S, Lange A, Thon L, Davarnia P, Herdegen T, Linkermann A, Rittger A, Chan FK. TNF-induced necroptosis and PARP-1-mediated necrosis represent distinct routes to programmed necrotic cell death. Cell Mol Life Sci. 2014;71:331-348. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 120] [Cited by in RCA: 131] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 19. | Huang CY, Kuo WT, Huang YC, Lee TC, Yu LC. Resistance to hypoxia-induced necroptosis is conferred by glycolytic pyruvate scavenging of mitochondrial superoxide in colorectal cancer cells. Cell Death Dis. 2013;4:e622. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 95] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 20. | Yen TH, Wright NA. The gastrointestinal tract stem cell niche. Stem Cell Rev. 2006;2:203-212. [PubMed] |
| 21. | Yu LC, Wang JT, Wei SC, Ni YH. Host-microbial interactions and regulation of intestinal epithelial barrier function: From physiology to pathology. World J Gastrointest Pathophysiol. 2012;3:27-43. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 157] [Cited by in RCA: 176] [Article Influence: 13.5] [Reference Citation Analysis (2)] |
| 22. | Bedi A, Pasricha PJ, Akhtar AJ, Barber JP, Bedi GC, Giardiello FM, Zehnbauer BA, Hamilton SR, Jones RJ. Inhibition of apoptosis during development of colorectal cancer. Cancer Res. 1995;55:1811-1816. [PubMed] |
| 23. | Duckworth CA, Pritchard DM. Suppression of apoptosis, crypt hyperplasia, and altered differentiation in the colonic epithelia of bak-null mice. Gastroenterology. 2009;136:943-952. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 24. | Liu F, Bardhan K, Yang D, Thangaraju M, Ganapathy V, Waller JL, Liles GB, Lee JR, Liu K. NF-κB directly regulates Fas transcription to modulate Fas-mediated apoptosis and tumor suppression. J Biol Chem. 2012;287:25530-25540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 115] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 25. | Qiu W, Carson-Walter EB, Kuan SF, Zhang L, Yu J. PUMA suppresses intestinal tumorigenesis in mice. Cancer Res. 2009;69:4999-5006. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 42] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 26. | Scheepers A, Joost HG, Schürmann A. The glucose transporter families SGLT and GLUT: molecular basis of normal and aberrant function. JPEN J Parenter Enteral Nutr. 2004;28:364-371. [PubMed] |
| 27. | Godoy A, Ulloa V, Rodríguez F, Reinicke K, Yañez AJ, García Mde L, Medina RA, Carrasco M, Barberis S, Castro T. Differential subcellular distribution of glucose transporters GLUT1-6 and GLUT9 in human cancer: ultrastructural localization of GLUT1 and GLUT5 in breast tumor tissues. J Cell Physiol. 2006;207:614-627. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 208] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 28. | Huang CY, Hsiao JK, Lu YZ, Lee TC, Yu LC. Anti-apoptotic PI3K/Akt signaling by sodium/glucose transporter 1 reduces epithelial barrier damage and bacterial translocation in intestinal ischemia. Lab Invest. 2011;91:294-309. [PubMed] |
| 29. | Yu LC, Flynn AN, Turner JR, Buret AG. SGLT-1-mediated glucose uptake protects intestinal epithelial cells against LPS-induced apoptosis and barrier defects: a novel cellular rescue mechanism? FASEB J. 2005;19:1822-1835. [PubMed] |
| 30. | Yu LC, Huang CY, Kuo WT, Sayer H, Turner JR, Buret AG. SGLT-1-mediated glucose uptake protects human intestinal epithelial cells against Giardia duodenalis-induced apoptosis. Int J Parasitol. 2008;38:923-934. [PubMed] |
| 31. | Palazzo M, Gariboldi S, Zanobbio L, Selleri S, Dusio GF, Mauro V, Rossini A, Balsari A, Rumio C. Sodium-dependent glucose transporter-1 as a novel immunological player in the intestinal mucosa. J Immunol. 2008;181:3126-3136. [PubMed] |
| 32. | Kallweit AR, Baird CH, Stutzman DK, Wischmeyer PE. Glutamine prevents apoptosis in intestinal epithelial cells and induces differential protective pathways in heat and oxidant injury models. JPEN J Parenter Enteral Nutr. 2012;36:551-555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 33. | Haynes TE, Li P, Li X, Shimotori K, Sato H, Flynn NE, Wang J, Knabe DA, Wu G. L-Glutamine or L-alanyl-L-glutamine prevents oxidant- or endotoxin-induced death of neonatal enterocytes. Amino Acids. 2009;37:131-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 142] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 34. | Ban K, Kozar RA. Glutamine protects against apoptosis via downregulation of Sp3 in intestinal epithelial cells. Am J Physiol Gastrointest Liver Physiol. 2010;299:G1344-G1353. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 22] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 35. | Sakiyama T, Musch MW, Ropeleski MJ, Tsubouchi H, Chang EB. Glutamine increases autophagy under Basal and stressed conditions in intestinal epithelial cells. Gastroenterology. 2009;136:924-932. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 112] [Cited by in RCA: 106] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 36. | Evans ME, Jones DP, Ziegler TR. Glutamine inhibits cytokine-induced apoptosis in human colonic epithelial cells via the pyrimidine pathway. Am J Physiol Gastrointest Liver Physiol. 2005;289:G388-G396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 40] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 37. | Ropeleski MJ, Riehm J, Baer KA, Musch MW, Chang EB. Anti-apoptotic effects of L-glutamine-mediated transcriptional modulation of the heat shock protein 72 during heat shock. Gastroenterology. 2005;129:170-184. [PubMed] |
| 38. | Riaz AA, Wan MX, Schäfer T, Dawson P, Menger MD, Jeppsson B, Thorlacius H. Allopurinol and superoxide dismutase protect against leucocyte-endothelium interactions in a novel model of colonic ischaemia-reperfusion. Br J Surg. 2002;89:1572-1580. [PubMed] |
| 39. | Simpson R, Alon R, Kobzik L, Valeri CR, Shepro D, Hechtman HB. Neutrophil and nonneutrophil-mediated injury in intestinal ischemia-reperfusion. Ann Surg. 1993;218:444-53; discussion 453-4. [PubMed] |
| 40. | Sun Z, Olanders K, Lasson A, Dib M, Annborn M, Andersson K, Wang X, Andersson R. Effective treatment of gut barrier dysfunction using an antioxidant, a PAF inhibitor, and monoclonal antibodies against the adhesion molecule PECAM-1. J Surg Res. 2002;105:220-233. [PubMed] |
| 41. | Keely S, Campbell EL, Baird AW, Hansbro PM, Shalwitz RA, Kotsakis A, McNamee EN, Eltzschig HK, Kominsky DJ, Colgan SP. Contribution of epithelial innate immunity to systemic protection afforded by prolyl hydroxylase inhibition in murine colitis. Mucosal Immunol. 2014;7:114-123. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 83] [Cited by in RCA: 98] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 42. | Hart ML, Grenz A, Gorzolla IC, Schittenhelm J, Dalton JH, Eltzschig HK. Hypoxia-inducible factor-1α-dependent protection from intestinal ischemia/reperfusion injury involves ecto-5’-nucleotidase (CD73) and the A2B adenosine receptor. J Immunol. 2011;186:4367-4374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 114] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 43. | Colgan SP, Taylor CT. Hypoxia: an alarm signal during intestinal inflammation. Nat Rev Gastroenterol Hepatol. 2010;7:281-287. [PubMed] |
| 44. | Zhao J, Du F, Luo Y, Shen G, Zheng F, Xu B. The emerging role of hypoxia-inducible factor-2 involved in chemo/radioresistance in solid tumors. Cancer Treat Rev. 2015;41:623-633. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 43] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 45. | Denko NC. Hypoxia, HIF1 and glucose metabolism in the solid tumour. Nat Rev Cancer. 2008;8:705-713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1203] [Cited by in RCA: 1311] [Article Influence: 77.1] [Reference Citation Analysis (0)] |
| 46. | Hirota SA, Fines K, Ng J, Traboulsi D, Lee J, Ihara E, Li Y, Willmore WG, Chung D, Scully MM. Hypoxia-inducible factor signaling provides protection in Clostridium difficile-induced intestinal injury. Gastroenterology. 2010;139:259-69.e3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 75] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 47. | Furuta GT, Turner JR, Taylor CT, Hershberg RM, Comerford K, Narravula S, Podolsky DK, Colgan SP. Hypoxia-inducible factor 1-dependent induction of intestinal trefoil factor protects barrier function during hypoxia. J Exp Med. 2001;193:1027-1034. [PubMed] |
| 48. | Karhausen J, Furuta GT, Tomaszewski JE, Johnson RS, Colgan SP, Haase VH. Epithelial hypoxia-inducible factor-1 is protective in murine experimental colitis. J Clin Invest. 2004;114:1098-1106. [PubMed] |
| 49. | Fukata M, Chen A, Klepper A, Krishnareddy S, Vamadevan AS, Thomas LS, Xu R, Inoue H, Arditi M, Dannenberg AJ. Cox-2 is regulated by Toll-like receptor-4 (TLR4) signaling: Role in proliferation and apoptosis in the intestine. Gastroenterology. 2006;131:862-877. [PubMed] |
| 50. | Okayama M, Hayashi S, Aoi Y, Nishio H, Kato S, Takeuchi K. Aggravation by selective COX-1 and COX-2 inhibitors of dextran sulfate sodium (DSS)-induced colon lesions in rats. Dig Dis Sci. 2007;52:2095-2103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 40] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 51. | Nishihara H, Kizaka-Kondoh S, Insel PA, Eckmann L. Inhibition of apoptosis in normal and transformed intestinal epithelial cells by cAMP through induction of inhibitor of apoptosis protein (IAP)-2. Proc Natl Acad Sci USA. 2003;100:8921-8926. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 107] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 52. | Modok S, Mellor HR, Callaghan R. Modulation of multidrug resistance efflux pump activity to overcome chemoresistance in cancer. Curr Opin Pharmacol. 2006;6:350-354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 181] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 53. | Sharom FJ. ABC multidrug transporters: structure, function and role in chemoresistance. Pharmacogenomics. 2008;9:105-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 653] [Cited by in RCA: 722] [Article Influence: 42.5] [Reference Citation Analysis (0)] |
| 54. | Miura K, Fujibuchi W, Ishida K, Naitoh T, Ogawa H, Ando T, Yazaki N, Watanabe K, Haneda S, Shibata C. Inhibitor of apoptosis protein family as diagnostic markers and therapeutic targets of colorectal cancer. Surg Today. 2011;41:175-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 67] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 55. | Kawasaki H, Toyoda M, Shinohara H, Okuda J, Watanabe I, Yamamoto T, Tanaka K, Tenjo T, Tanigawa N. Expression of survivin correlates with apoptosis, proliferation, and angiogenesis during human colorectal tumorigenesis. Cancer. 2001;91:2026-2032. [PubMed] |
| 56. | Biroccio A, Benassi B, D’Agnano I, D’Angelo C, Buglioni S, Mottolese M, Ricciotti A, Citro G, Cosimelli M, Ramsay RG. c-Myb and Bcl-x overexpression predicts poor prognosis in colorectal cancer: clinical and experimental findings. Am J Pathol. 2001;158:1289-1299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 108] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 57. | Heijink DM, Kleibeuker JH, Jalving M, Boersma-van Ek W, Koornstra JJ, Wesseling J, de Jong S. Independent induction of caspase-8 and cFLIP expression during colorectal carcinogenesis in sporadic and HNPCC adenomas and carcinomas. Cell Oncol. 2007;29:409-419. [PubMed] |
| 58. | Xiang G, Wen X, Wang H, Chen K, Liu H. Expression of X-linked inhibitor of apoptosis protein in human colorectal cancer and its correlation with prognosis. J Surg Oncol. 2009;100:708-712. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 55] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 59. | Krajewska M, Kim H, Kim C, Kang H, Welsh K, Matsuzawa S, Tsukamoto M, Thomas RG, Assa-Munt N, Piao Z. Analysis of apoptosis protein expression in early-stage colorectal cancer suggests opportunities for new prognostic biomarkers. Clin Cancer Res. 2005;11:5451-5461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 71] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 60. | Karasawa H, Miura K, Fujibuchi W, Ishida K, Kaneko N, Kinouchi M, Okabe M, Ando T, Murata Y, Sasaki H. Down-regulation of cIAP2 enhances 5-FU sensitivity through the apoptotic pathway in human colon cancer cells. Cancer Sci. 2009;100:903-913. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 47] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 61. | Fang YJ, Lu ZH, Wang GQ, Pan ZZ, Zhou ZW, Yun JP, Zhang MF, Wan DS. Elevated expressions of MMP7, TROP2, and survivin are associated with survival, disease recurrence, and liver metastasis of colon cancer. Int J Colorectal Dis. 2009;24:875-884. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 148] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 62. | Moriwaki K, Bertin J, Gough PJ, Orlowski GM, Chan FK. Differential roles of RIPK1 and RIPK3 in TNF-induced necroptosis and chemotherapeutic agent-induced cell death. Cell Death Dis. 2015;6:e1636. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 156] [Cited by in RCA: 203] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 63. | Ewing I, Hurley JJ, Josephides E, Millar A. The molecular genetics of colorectal cancer. Frontline Gastroenterol. 2014;5:26-30. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 73] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 64. | Hammoud SS, Cairns BR, Jones DA. Epigenetic regulation of colon cancer and intestinal stem cells. Curr Opin Cell Biol. 2013;25:177-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 71] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 65. | Laurent-Puig P, Blons H, Cugnenc PH. Sequence of molecular genetic events in colorectal tumorigenesis. Eur J Cancer Prev. 1999;8 Suppl 1:S39-S47. [PubMed] |
| 66. | Katkoori VR, Shanmugam C, Jia X, Vitta SP, Sthanam M, Callens T, Messiaen L, Chen D, Zhang B, Bumpers HL. Prognostic significance and gene expression profiles of p53 mutations in microsatellite-stable stage III colorectal adenocarcinomas. PLoS One. 2012;7:e30020. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 67. | Zamzami N, Kroemer G. p53 in apoptosis control: an introduction. Biochem Biophys Res Commun. 2005;331:685-687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 29] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 68. | Bedi A, Mookerjee B. Biological significance and molecular mechanisms of p53-induced apoptosis. Apoptosis. 1998;3:237-244. [PubMed] |
| 69. | Gottlieb E, Vousden KH. p53 regulation of metabolic pathways. Cold Spring Harb Perspect Biol. 2010;2:a001040. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 139] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 70. | Liu Y, Borchert GL, Surazynski A, Phang JM. Proline oxidase, a p53-induced gene, targets COX-2/PGE2 signaling to induce apoptosis and inhibit tumor growth in colorectal cancers. Oncogene. 2008;27:6729-6737. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 85] [Cited by in RCA: 84] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 71. | Taketani K, Kawauchi J, Tanaka-Okamoto M, Ishizaki H, Tanaka Y, Sakai T, Miyoshi J, Maehara Y, Kitajima S. Key role of ATF3 in p53-dependent DR5 induction upon DNA damage of human colon cancer cells. Oncogene. 2012;31:2210-2221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 68] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 72. | Edagawa M, Kawauchi J, Hirata M, Goshima H, Inoue M, Okamoto T, Murakami A, Maehara Y, Kitajima S. Role of activating transcription factor 3 (ATF3) in endoplasmic reticulum (ER) stress-induced sensitization of p53-deficient human colon cancer cells to tumor necrosis factor (TNF)-related apoptosis-inducing ligand (TRAIL)-mediated apoptosis through up-regulation of death receptor 5 (DR5) by zerumbone and celecoxib. J Biol Chem. 2014;289:21544-21561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 97] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 73. | Wang P, Qiu W, Dudgeon C, Liu H, Huang C, Zambetti GP, Yu J, Zhang L. PUMA is directly activated by NF-kappaB and contributes to TNF-alpha-induced apoptosis. Cell Death Differ. 2009;16:1192-1202. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 133] [Cited by in RCA: 138] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 74. | Singh AB, Sharma A, Dhawan P. Claudin-1 expression confers resistance to anoikis in colon cancer cells in a Src-dependent manner. Carcinogenesis. 2012;33:2538-2547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 67] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 75. | Bishnupuri KS, Luo Q, Murmu N, Houchen CW, Anant S, Dieckgraefe BK. Reg IV activates the epidermal growth factor receptor/Akt/AP-1 signaling pathway in colon adenocarcinomas. Gastroenterology. 2006;130:137-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 100] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 76. | Raufman JP, Shant J, Guo CY, Roy S, Cheng K. Deoxycholyltaurine rescues human colon cancer cells from apoptosis by activating EGFR-dependent PI3K/Akt signaling. J Cell Physiol. 2008;215:538-549. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 77. | Khor TO, Gul YA, Ithnin H, Seow HF. Positive correlation between overexpression of phospho-BAD with phosphorylated Akt at serine 473 but not threonine 308 in colorectal carcinoma. Cancer Lett. 2004;210:139-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 39] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 78. | Shao H, Jing K, Mahmoud E, Huang H, Fang X, Yu C. Apigenin sensitizes colon cancer cells to antitumor activity of ABT-263. Mol Cancer Ther. 2013;12:2640-2650. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 83] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 79. | Agarwal E, Chaudhuri A, Leiphrakpam PD, Haferbier KL, Brattain MG, Chowdhury S. Akt inhibitor MK-2206 promotes anti-tumor activity and cell death by modulation of AIF and Ezrin in colorectal cancer. BMC Cancer. 2014;14:145. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 58] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 80. | Huang CC, Wu DW, Lin PL, Lee H. Paxillin promotes colorectal tumor invasion and poor patient outcomes via ERK-mediated stabilization of Bcl-2 protein by phosphorylation at Serine 87. Oncotarget. 2015;6:8698-8708. [PubMed] |
| 81. | Ewings KE, Hadfield-Moorhouse K, Wiggins CM, Wickenden JA, Balmanno K, Gilley R, Degenhardt K, White E, Cook SJ. ERK1/2-dependent phosphorylation of BimEL promotes its rapid dissociation from Mcl-1 and Bcl-xL. EMBO J. 2007;26:2856-2867. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 146] [Cited by in RCA: 141] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 82. | Yu LF, Wang J, Zou B, Lin MC, Wu YL, Xia HH, Sun YW, Gu Q, He H, Lam SK. XAF1 mediates apoptosis through an extracellular signal-regulated kinase pathway in colon cancer. Cancer. 2007;109:1996-2003. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 34] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 83. | Kanarek N, London N, Schueler-Furman O, Ben-Neriah Y. Ubiquitination and degradation of the inhibitors of NF-kappaB. Cold Spring Harb Perspect Biol. 2010;2:a000166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 110] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 84. | Yamamoto Y, Verma UN, Prajapati S, Kwak YT, Gaynor RB. Histone H3 phosphorylation by IKK-alpha is critical for cytokine-induced gene expression. Nature. 2003;423:655-659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 482] [Cited by in RCA: 468] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 85. | Nenci A, Becker C, Wullaert A, Gareus R, van Loo G, Danese S, Huth M, Nikolaev A, Neufert C, Madison B. Epithelial NEMO links innate immunity to chronic intestinal inflammation. Nature. 2007;446:557-561. [PubMed] |
| 86. | Greten FR, Eckmann L, Greten TF, Park JM, Li ZW, Egan LJ, Kagnoff MF, Karin M. IKKbeta links inflammation and tumorigenesis in a mouse model of colitis-associated cancer. Cell. 2004;118:285-296. [PubMed] |
| 87. | Li Q, Yu YY, Zhu ZG, Ji YB, Zhang Y, Liu BY, Chen XH, Lin YZ. Effect of NF-kappaB constitutive activation on proliferation and apoptosis of gastric cancer cell lines. Eur Surg Res. 2005;37:105-110. [PubMed] |
| 88. | Liu T, Liu D, Liu J, Song JT, Gao SL, Li H, Hu LH, Liu BR. Effect of NF-κB inhibitors on the chemotherapy-induced apoptosis of the colon cancer cell line HT-29. Exp Ther Med. 2012;4:716-722. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 21] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 89. | Li J, Minnich DJ, Camp ER, Brank A, Mackay SL, Hochwald SN. Enhanced sensitivity to chemotherapy in esophageal cancer through inhibition of NF-kappaB. J Surg Res. 2006;132:112-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 90. | Huang WC, Ju TK, Hung MC, Chen CC. Phosphorylation of CBP by IKKalpha promotes cell growth by switching the binding preference of CBP from p53 to NF-kappaB. Mol Cell. 2007;26:75-87. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 200] [Cited by in RCA: 203] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 91. | Wang Q, Wang X, Evers BM. Induction of cIAP-2 in human colon cancer cells through PKC delta/NF-kappa B. J Biol Chem. 2003;278:51091-51099. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 84] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 92. | Kang HW, Kim JM, Cha MY, Jung HC, Song IS, Kim JS. Deguelin, an Akt inhibitor, down-regulates NF-κB signaling and induces apoptosis in colon cancer cells and inhibits tumor growth in mice. Dig Dis Sci. 2012;57:2873-2882. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 46] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 93. | Peng XH, Karna P, Cao Z, Jiang BH, Zhou M, Yang L. Cross-talk between epidermal growth factor receptor and hypoxia-inducible factor-1alpha signal pathways increases resistance to apoptosis by up-regulating survivin gene expression. J Biol Chem. 2006;281:25903-25914. [PubMed] |
| 94. | Erler JT, Cawthorne CJ, Williams KJ, Koritzinsky M, Wouters BG, Wilson C, Miller C, Demonacos C, Stratford IJ, Dive C. Hypoxia-mediated down-regulation of Bid and Bax in tumors occurs via hypoxia-inducible factor 1-dependent and -independent mechanisms and contributes to drug resistance. Mol Cell Biol. 2004;24:2875-2889. [PubMed] |
| 95. | Chang KY, Shen MR, Lee MY, Wang WL, Su WC, Chang WC, Chen BK. Epidermal growth factor-activated aryl hydrocarbon receptor nuclear translocator/HIF-1{beta} signal pathway up-regulates cyclooxygenase-2 gene expression associated with squamous cell carcinoma. J Biol Chem. 2009;284:9908-9916. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 96. | Marín-Hernández A, Gallardo-Pérez JC, Ralph SJ, Rodríguez-Enríquez S, Moreno-Sánchez R. HIF-1alpha modulates energy metabolism in cancer cells by inducing over-expression of specific glycolytic isoforms. Mini Rev Med Chem. 2009;9:1084-1101. [PubMed] |
| 97. | Airley RE, Mobasheri A. Hypoxic regulation of glucose transport, anaerobic metabolism and angiogenesis in cancer: novel pathways and targets for anticancer therapeutics. Chemotherapy. 2007;53:233-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 243] [Cited by in RCA: 255] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 98. | Jo JO, Kim SR, Bae MK, Kang YJ, Ock MS, Kleinman HK, Cha HJ. Thymosin β4 induces the expression of vascular endothelial growth factor (VEGF) in a hypoxia-inducible factor (HIF)-1α-dependent manner. Biochim Biophys Acta. 2010;1803:1244-1251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 40] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 99. | Kitajima Y, Ide T, Ohtsuka T, Miyazaki K. Induction of hepatocyte growth factor activator gene expression under hypoxia activates the hepatocyte growth factor/c-Met system via hypoxia inducible factor-1 in pancreatic cancer. Cancer Sci. 2008;99:1341-1347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 86] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 100. | Eckerich C, Zapf S, Fillbrandt R, Loges S, Westphal M, Lamszus K. Hypoxia can induce c-Met expression in glioma cells and enhance SF/HGF-induced cell migration. Int J Cancer. 2007;121:276-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 107] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 101. | Stiehl DP, Bordoli MR, Abreu-Rodríguez I, Wollenick K, Schraml P, Gradin K, Poellinger L, Kristiansen G, Wenger RH. Non-canonical HIF-2α function drives autonomous breast cancer cell growth via an AREG-EGFR/ErbB4 autocrine loop. Oncogene. 2012;31:2283-2297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 60] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 102. | Franovic A, Gunaratnam L, Smith K, Robert I, Patten D, Lee S. Translational up-regulation of the EGFR by tumor hypoxia provides a nonmutational explanation for its overexpression in human cancer. Proc Natl Acad Sci USA. 2007;104:13092-13097. [PubMed] |
| 103. | Chiavarina B, Martinez-Outschoorn UE, Whitaker-Menezes D, Howell A, Tanowitz HB, Pestell RG, Sotgia F, Lisanti MP. Metabolic reprogramming and two-compartment tumor metabolism: opposing role(s) of HIF1α and HIF2α in tumor-associated fibroblasts and human breast cancer cells. Cell Cycle. 2012;11:3280-3289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 75] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 104. | Eberhart CE, Coffey RJ, Radhika A, Giardiello FM, Ferrenbach S, DuBois RN. Up-regulation of cyclooxygenase 2 gene expression in human colorectal adenomas and adenocarcinomas. Gastroenterology. 1994;107:1183-1188. [PubMed] |
| 105. | Sano H, Kawahito Y, Wilder RL, Hashiramoto A, Mukai S, Asai K, Kimura S, Kato H, Kondo M, Hla T. Expression of cyclooxygenase-1 and -2 in human colorectal cancer. Cancer Res. 1995;55:3785-3789. [PubMed] |
| 106. | Nan H, Hutter CM, Lin Y, Jacobs EJ, Ulrich CM, White E, Baron JA, Berndt SI, Brenner H, Butterbach K. Association of aspirin and NSAID use with risk of colorectal cancer according to genetic variants. JAMA. 2015;313:1133-1142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 155] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 107. | Pai R, Soreghan B, Szabo IL, Pavelka M, Baatar D, Tarnawski AS. Prostaglandin E2 transactivates EGF receptor: a novel mechanism for promoting colon cancer growth and gastrointestinal hypertrophy. Nat Med. 2002;8:289-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 667] [Cited by in RCA: 648] [Article Influence: 28.2] [Reference Citation Analysis (0)] |
| 108. | Buchanan FG, Wang D, Bargiacchi F, DuBois RN. Prostaglandin E2 regulates cell migration via the intracellular activation of the epidermal growth factor receptor. J Biol Chem. 2003;278:35451-35457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 362] [Cited by in RCA: 365] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 109. | Yu Z, Cui B, Jin Y, Chen H, Wang X. Novel irreversible EGFR tyrosine kinase inhibitor 324674 sensitizes human colon carcinoma HT29 and SW480 cells to apoptosis by blocking the EGFR pathway. Biochem Biophys Res Commun. 2011;411:751-756. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 110. | Shi M, Shi H, Ji J, Cai Q, Chen X, Yu Y, Liu B, Zhu Z, Zhang J. Cetuximab inhibits gastric cancer growth in vivo, independent of KRAS status. Curr Cancer Drug Targets. 2014;14:217-224. [PubMed] |
| 111. | Sakuma K, Aoki M, Kannagi R. Transcription factors c-Myc and CDX2 mediate E-selectin ligand expression in colon cancer cells undergoing EGF/bFGF-induced epithelial-mesenchymal transition. Proc Natl Acad Sci USA. 2012;109:7776-7781. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 127] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 112. | Shant J, Cheng K, Marasa BS, Wang JY, Raufman JP. Akt-dependent NF-kappaB activation is required for bile acids to rescue colon cancer cells from stress-induced apoptosis. Exp Cell Res. 2009;315:432-450. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 43] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 113. | Feng Y, Dai X, Li X, Wang H, Liu J, Zhang J, Du Y, Xia L. EGF signalling pathway regulates colon cancer stem cell proliferation and apoptosis. Cell Prolif. 2012;45:413-419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 67] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 114. | Heeg S, Hirt N, Queisser A, Schmieg H, Thaler M, Kunert H, Quante M, Goessel G, von Werder A, Harder J. EGFR overexpression induces activation of telomerase via PI3K/AKT-mediated phosphorylation and transcriptional regulation through Hif1-alpha in a cellular model of oral-esophageal carcinogenesis. Cancer Sci. 2011;102:351-360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 40] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 115. | Guo GF, Cai YC, Zhang B, Xu RH, Qiu HJ, Xia LP, Jiang WQ, Hu PL, Chen XX, Zhou FF. Overexpression of SGLT1 and EGFR in colorectal cancer showing a correlation with the prognosis. Med Oncol. 2011;28 Suppl 1:S197-S203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 58] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 116. | Hanabata Y, Nakajima Y, Morita K, Kayamori K, Omura K. Coexpression of SGLT1 and EGFR is associated with tumor differentiation in oral squamous cell carcinoma. Odontology. 2012;100:156-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 34] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 117. | Weihua Z, Tsan R, Huang WC, Wu Q, Chiu CH, Fidler IJ, Hung MC. Survival of cancer cells is maintained by EGFR independent of its kinase activity. Cancer Cell. 2008;13:385-393. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 390] [Cited by in RCA: 395] [Article Influence: 23.2] [Reference Citation Analysis (0)] |
| 118. | Ren J, Bollu LR, Su F, Gao G, Xu L, Huang WC, Hung MC, Weihua Z. EGFR-SGLT1 interaction does not respond to EGFR modulators, but inhibition of SGLT1 sensitizes prostate cancer cells to EGFR tyrosine kinase inhibitors. Prostate. 2013;73:1453-1461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 34] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 119. | Seiden-Long IM, Brown KR, Shih W, Wigle DA, Radulovich N, Jurisica I, Tsao MS. Transcriptional targets of hepatocyte growth factor signaling and Ki-ras oncogene activation in colorectal cancer. Oncogene. 2006;25:91-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 62] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 120. | Seneviratne D, Ma J, Tan X, Kwon YK, Muhammad E, Melhem M, DeFrances MC, Zarnegar R. Genomic instability causes HGF gene activation in colon cancer cells, promoting their resistance to necroptosis. Gastroenterology. 2015;148:181-191.e17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 40] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 121. | Song N, Liu S, Zhang J, Liu J, Xu L, Liu Y, Qu X. Cetuximab-induced MET activation acts as a novel resistance mechanism in colon cancer cells. Int J Mol Sci. 2014;15:5838-5851. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 32] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 122. | Sekharam M, Zhao H, Sun M, Fang Q, Zhang Q, Yuan Z, Dan HC, Boulware D, Cheng JQ, Coppola D. Insulin-like growth factor 1 receptor enhances invasion and induces resistance to apoptosis of colon cancer cells through the Akt/Bcl-x(L) pathway. Cancer Res. 2003;63:7708-7716. [PubMed] |
| 123. | Alberobello AT, D’Esposito V, Marasco D, Doti N, Ruvo M, Bianco R, Tortora G, Esposito I, Fiory F, Miele C. Selective disruption of insulin-like growth factor-1 (IGF-1) signaling via phosphoinositide-dependent kinase-1 prevents the protective effect of IGF-1 on human cancer cell death. J Biol Chem. 2010;285:6563-6572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 124. | Fleming SE, Zambell KL, Fitch MD. Glucose and glutamine provide similar proportions of energy to mucosal cells of rat small intestine. Am J Physiol. 1997;273:G968-G978. [PubMed] |
| 125. | Hildyard JC, Ammälä C, Dukes ID, Thomson SA, Halestrap AP. Identification and characterisation of a new class of highly specific and potent inhibitors of the mitochondrial pyruvate carrier. Biochim Biophys Acta. 2005;1707:221-230. [PubMed] |
| 126. | Herzig S, Raemy E, Montessuit S, Veuthey JL, Zamboni N, Westermann B, Kunji ER, Martinou JC. Identification and functional expression of the mitochondrial pyruvate carrier. Science. 2012;337:93-96. [PubMed] |
| 127. | Hamanaka RB, Chandel NS. Targeting glucose metabolism for cancer therapy. J Exp Med. 2012;209:211-215. [PubMed] |
| 128. | de Wit M, Jimenez CR, Carvalho B, Belien JA, Delis-van Diemen PM, Mongera S, Piersma SR, Vikas M, Navani S, Pontén F. Cell surface proteomics identifies glucose transporter type 1 and prion protein as candidate biomarkers for colorectal adenoma-to-carcinoma progression. Gut. 2012;61:855-864. [PubMed] |
| 129. | Wincewicz A, Baltaziak M, Kanczuga-Koda L, Koda M, Sulkowska U, Sulkowski S. GLUT1 and Bcl-xL in relation to erythropoietin in human colorectal adenocarcinomas. Hepatogastroenterology. 2010;57:741-745. [PubMed] |
| 130. | Ha TK, Chi SG. CAV1/caveolin 1 enhances aerobic glycolysis in colon cancer cells via activation of SLC2A3/GLUT3 transcription. Autophagy. 2012;8:1684-1685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 46] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 131. | Chiacchiera F, Matrone A, Ferrari E, Ingravallo G, Lo Sasso G, Murzilli S, Petruzzelli M, Salvatore L, Moschetta A, Simone C. p38alpha blockade inhibits colorectal cancer growth in vivo by inducing a switch from HIF1alpha- to FoxO-dependent transcription. Cell Death Differ. 2009;16:1203-1214. [PubMed] |
| 132. | Shin YK, Yoo BC, Hong YS, Chang HJ, Jung KH, Jeong SY, Park JG. Upregulation of glycolytic enzymes in proteins secreted from human colon cancer cells with 5-fluorouracil resistance. Electrophoresis. 2009;30:2182-2192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 61] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 133. | Martinez-Balibrea E, Plasencia C, Ginés A, Martinez-Cardús A, Musulén E, Aguilera R, Manzano JL, Neamati N, Abad A. A proteomic approach links decreased pyruvate kinase M2 expression to oxaliplatin resistance in patients with colorectal cancer and in human cell lines. Mol Cancer Ther. 2009;8:771-778. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 51] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 134. | Chou CW, Wu MS, Huang WC, Chen CC. HDAC inhibition decreases the expression of EGFR in colorectal cancer cells. PLoS One. 2011;6:e18087. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 87] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 135. | Ma YS, Yang IP, Tsai HL, Huang CW, Juo SH, Wang JY. High glucose modulates antiproliferative effect and cytotoxicity of 5-fluorouracil in human colon cancer cells. DNA Cell Biol. 2014;33:64-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 46] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 136. | Cao X, Fang L, Gibbs S, Huang Y, Dai Z, Wen P, Zheng X, Sadee W, Sun D. Glucose uptake inhibitor sensitizes cancer cells to daunorubicin and overcomes drug resistance in hypoxia. Cancer Chemother Pharmacol. 2007;59:495-505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 173] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 137. | Xuan Y, Hur H, Ham IH, Yun J, Lee JY, Shim W, Kim YB, Lee G, Han SU, Cho YK. Dichloroacetate attenuates hypoxia-induced resistance to 5-fluorouracil in gastric cancer through the regulation of glucose metabolism. Exp Cell Res. 2014;321:219-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 51] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 138. | Tong J, Xie G, He J, Li J, Pan F, Liang H. Synergistic antitumor effect of dichloroacetate in combination with 5-fluorouracil in colorectal cancer. J Biomed Biotechnol. 2011;2011:740564. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 75] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 139. | Sánchez-Aragó M, Cuezva JM. The bioenergetic signature of isogenic colon cancer cells predicts the cell death response to treatment with 3-bromopyruvate, iodoacetate or 5-fluorouracil. J Transl Med. 2011;9:19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 45] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 140. | Liu PP, Liao J, Tang ZJ, Wu WJ, Yang J, Zeng ZL, Hu Y, Wang P, Ju HQ, Xu RH. Metabolic regulation of cancer cell side population by glucose through activation of the Akt pathway. Cell Death Differ. 2014;21:124-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 182] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 141. | Chen B, Liu Y, Jin X, Lu W, Liu J, Xia Z, Yuan Q, Zhao X, Xu N, Liang S. MicroRNA-26a regulates glucose metabolism by direct targeting PDHX in colorectal cancer cells. BMC Cancer. 2014;14:443. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 86] [Cited by in RCA: 97] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 142. | Pate KT, Stringari C, Sprowl-Tanio S, Wang K, TeSlaa T, Hoverter NP, McQuade MM, Garner C, Digman MA, Teitell MA. Wnt signaling directs a metabolic program of glycolysis and angiogenesis in colon cancer. EMBO J. 2014;33:1454-1473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 256] [Cited by in RCA: 351] [Article Influence: 31.9] [Reference Citation Analysis (0)] |
| 143. | Lu CW, Lin SC, Chen KF, Lai YY, Tsai SJ. Induction of pyruvate dehydrogenase kinase-3 by hypoxia-inducible factor-1 promotes metabolic switch and drug resistance. J Biol Chem. 2008;283:28106-28114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 215] [Cited by in RCA: 263] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 144. | Schell JC, Olson KA, Jiang L, Hawkins AJ, Van Vranken JG, Xie J, Egnatchik RA, Earl EG, DeBerardinis RJ, Rutter J. A role for the mitochondrial pyruvate carrier as a repressor of the Warburg effect and colon cancer cell growth. Mol Cell. 2014;56:400-413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 231] [Cited by in RCA: 290] [Article Influence: 26.4] [Reference Citation Analysis (0)] |
| 145. | Xu RH, Pelicano H, Zhou Y, Carew JS, Feng L, Bhalla KN, Keating MJ, Huang P. Inhibition of glycolysis in cancer cells: a novel strategy to overcome drug resistance associated with mitochondrial respiratory defect and hypoxia. Cancer Res. 2005;65:613-621. [PubMed] |
| 146. | Zhou Y, Tozzi F, Chen J, Fan F, Xia L, Wang J, Gao G, Zhang A, Xia X, Brasher H. Intracellular ATP levels are a pivotal determinant of chemoresistance in colon cancer cells. Cancer Res. 2012;72:304-314. [PubMed] |
| 147. | Kao KK, Fink MP. The biochemical basis for the anti-inflammatory and cytoprotective actions of ethyl pyruvate and related compounds. Biochem Pharmacol. 2010;80:151-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 136] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 148. | Tian Y, Wang K, Wang Z, Li N, Ji G. Chemopreventive effect of dietary glutamine on colitis-associated colon tumorigenesis in mice. Carcinogenesis. 2013;34:1593-1600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 29] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 149. | Martín-Villa JM, Ferre-López S, López-Suárez JC, Corell A, Pérez-Blas M, Arnaiz-Villena A. Cell surface phenotype and ultramicroscopic analysis of purified human enterocytes: a possible antigen-presenting cell in the intestine. Tissue Antigens. 1997;50:586-592. [PubMed] |
| 150. | Wang EL, Qian ZR, Nakasono M, Tanahashi T, Yoshimoto K, Bando Y, Kudo E, Shimada M, Sano T. High expression of Toll-like receptor 4/myeloid differentiation factor 88 signals correlates with poor prognosis in colorectal cancer. Br J Cancer. 2010;102:908-915. [PubMed] |
| 151. | Fukata M, Chen A, Vamadevan AS, Cohen J, Breglio K, Krishnareddy S, Hsu D, Xu R, Harpaz N, Dannenberg AJ. Toll-like receptor-4 promotes the development of colitis-associated colorectal tumors. Gastroenterology. 2007;133:1869-1881. [PubMed] |
| 152. | Tang X, Zhu Y. TLR4 signaling promotes immune escape of human colon cancer cells by inducing immunosuppressive cytokines and apoptosis resistance. Oncol Res. 2012;20:15-24. [PubMed] |
| 153. | Tang XY, Zhu YQ, Wei B, Wang H. Expression and functional research of TLR4 in human colon carcinoma. Am J Med Sci. 2010;339:319-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 43] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 154. | Ruemmele FM, Beaulieu JF, Dionne S, Levy E, Seidman EG, Cerf-Bensussan N, Lentze MJ. Lipopolysaccharide modulation of normal enterocyte turnover by toll-like receptors is mediated by endogenously produced tumour necrosis factor alpha. Gut. 2002;51:842-848. [PubMed] |
| 155. | Grondin V, Seksik P, Dumont S, Thomas G, Trugnan G, Fléjou JF, Masliah J, Wendum D, Bachelet M. Regulation of colon cancer cell proliferation and migration by MD-2 activity. Innate Immun. 2011;17:414-422. [PubMed] |
| 156. | Rakoff-Nahoum S, Medzhitov R. Regulation of spontaneous intestinal tumorigenesis through the adaptor protein MyD88. Science. 2007;317:124-127. [PubMed] |
| 157. | Santaolalla R, Sussman DA, Ruiz JR, Davies JM, Pastorini C, España CL, Sotolongo J, Burlingame O, Bejarano PA, Philip S. TLR4 activates the β-catenin pathway to cause intestinal neoplasia. PLoS One. 2013;8:e63298. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 86] [Cited by in RCA: 89] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 158. | Kuo WT, Lee TC, Yang HY, Chen CY, Au YC, Lu YZ, Wu LL, Wei SC, Ni YH, Lin BR. LPS receptor subunits have antagonistic roles in epithelial apoptosis and colonic carcinogenesis. Cell Death Differ. 2015;22:1590-1604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 52] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 159. | Vander Heiden MG. Exploiting tumor metabolism: challenges for clinical translation. J Clin Invest. 2013;123:3648-3651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 62] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 160. | Song L, Li Y, He B, Gong Y. Development of Small Molecules Targeting the Wnt Signaling Pathway in Cancer Stem Cells for the Treatment of Colorectal Cancer. Clin Colorectal Cancer. 2015;14:133-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 37] [Article Influence: 3.7] [Reference Citation Analysis (0)] |