Published online Oct 28, 2015. doi: 10.3748/wjg.v21.i40.11458
Peer-review started: April 30, 2015
First decision: June 2, 2015
Revised: June 25, 2015
Accepted: September 30, 2015
Article in press: September 30, 2015
Published online: October 28, 2015
Processing time: 179 Days and 11.2 Hours
AIM: To study all the aspects of drain management in pancreatic surgery.
METHODS: We conducted a systematic review according to the PRISMA guidelines. We searched the Cochrane Central Registry of Controlled Trials, EMBASE, Web of Science, and PubMed (MEDLINE) for relevant articles on drain management in pancreatic surgery. The reference lists of relevant studies were screened to retrieve any further studies. We included all articles that reported clinical studies on human subjects with elective pancreatic resection and that compared various strategies of intra-abdominal drain management, such as drain vs no drain, selective drain use, early vs late drain extraction, and the use of different types of drains.
RESULTS: A total of 19 studies concerned with drain management in pancreatic surgery involving 4194 patients were selected for this systematic review. We included studies analyzing the outcomes of pancreatic resection with and without intra-abdominal drains, studies comparing early vs late drain removal and studies analyzing different types of drains. The majority of the studies reporting equal or superior results for pancreatic resection without drains were retrospective and observational with significant selection bias. One recent randomized trial reported higher postoperative morbidity and mortality with routine omission of intra-abdominal drains. With respect to the timing of drain removal, all of the included studies reported superior results with early drain removal. Regarding the various types of drains, there is insufficient evidence to determine which type of drain is more suitable following pancreatic resection.
CONCLUSION: The prophylactic use of drains remains controversial. When drains are used, early removal is recommended. Further trials comparing types of drains are ongoing.
Core tip: This systematic review updates our current knowledge on the management of intra-abdominal drains in pancreatic surgery. Regarding the prophylactic use of intra-abdominal drains, current studies do not lead to definite conclusions whether routine drainage should or should not be advocated. When drains are used, early removal is recommended. There is not enough evidence regarding the type of drain. A new randomized controlled study is currently underway which aims to compare the closed suction drain vs the passive closed gravity drain.
- Citation: Čečka F, Loveček M, Jon B, Skalický P, Šubrt Z, Neoral Č, Ferko A. Intra-abdominal drainage following pancreatic resection: A systematic review. World J Gastroenterol 2015; 21(40): 11458-11468
- URL: https://www.wjgnet.com/1007-9327/full/v21/i40/11458.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i40.11458
High morbidity is a continuing concern in modern pancreatic surgery, with postoperative pancreatic fistula (POPF) being the most ominous complication[1-3]. POPF is not a life-threatening condition in most cases, but nevertheless, it prolongs the hospital stay, increases the cost of the treatment, and delays adjuvant therapy in malignant disease[4]. The rate of POPF is reported to be in the range of 10%-30% in the majority of papers[1,2,5]. As POPF has significant clinical and economic consequences, attention has focused on lowering the POPF rate.
Several methods have been studied in the past in order to lower the pancreatic fistula rate, including pharmacological prophylaxis with octreotide[6,7] and various technical modifications of pancreatic remnant management after pancreaticoduodenectomy (PD)[8] and after distal pancreatectomy (DP)[9,10]. However, the use of octreotide remains controversial, and none of the studied techniques proved to be superior.
In recent years, the issue of placement and management of intra-abdominal drains following pancreatic resection has attracted attention and is currently widely discussed[11-17]. The placement of prophylactic intra-abdominal drains has been common practice since the 19th century. The rationale for inserting intra-abdominal drains following resection was that the drains were thought to evacuate blood, bile, pancreatic juice and other fluids that may accumulate after surgery[18]. The drains were also thought to allow for early identification of postoperative complications, such as anastomotic dehiscence or early hemorrhage. Moreover, prophylactic intra-abdominal drainage was supposed to avoid the need for additional interventions for intra-abdominal collections by creating a controlled pancreatic fistula[18-24].
However, the controversy over drain placement in acute as well as elective surgeries has persisted since the beginning of modern surgery[25]. Many recent studies show that the use of drains might not be beneficial for patients after abdominal surgery (appendectomy, cholecystectomy, hepatectomy, colectomy, gastrectomy)[26-31]. In fact, the use of drains might be even harmful for the patient, as they can slow down recovery and the restoration of bowel movements, and further prolong the hospital stay; drains may even cause postoperative complications such as retrograde intra-abdominal infection, and hollow organ perforation[15,30]. This might be the result of an artificial access to the peritoneal cavity, the inflammatory response to the drain as a foreign body, increased pain due to the drain, or the loss of fluid and electrolytes[20]. The standard use of drains also interferes with attempts to accelerate recovery through ERAS (enhanced recovery after surgery) programs[32].
Pancreatic surgery is different from the surgery of hollow organs[20]. In contrast to enteric anastomosis dehiscence, which often presents with pneumoperitoneum and frequently causes peritonitis[33], a pancreatic leak is more frequent, but the clinical course is not usually as dramatic[2]. Pancreatic leak or pancreatic fistula can be easily diagnosed by analyzing the amylase concentration in the drain effluent[34]. However, the amylase concentration is increased in the majority of patients on the first postoperative day, even in those patients who will not develop a pancreatic fistula in their postoperative course; this implies that, in the majority of patients, the pancreatic anastomosis is not “water-tight”[35].
Management of intra-abdominal drains has become an important issue in modern pancreatic surgery, as previous studies found that the management of intra-abdominal drains can influence the rate of postoperative complications[18,20,36-38]. Recent systematic reviews and meta-analyses have focused on the routine usage of drains following elective pancreatic resection[20-22,24]. However, there are additional issues to address regarding the use of intra-abdominal drainage following pancreatic surgery, such as the timing of drain removal and the type of drain. For this reason we carried out a systematic review of studies dealing with all aspects of drain management in pancreatic surgery.
We searched the Cochrane Central Registry of Controlled Trials, EMBASE, Web of Science, and PubMed (MEDLINE) for relevant articles published from January 1990 to December 2014. The search was performed independently by two authors (FC and ML) using the terms: “Pancreatectomy”, “Drain”, “Pancreatic fistula”, “Pancreas”, and “Postoperative complication”. The full search strategy is shown in the Supplementary Appendix (Literature search).
The reference lists of relevant studies were screened to retrieve any further potential studies. No unpublished data or data from abstracts were encountered or used. No language restriction was applied to the search. Abstracts of all potentially relevant articles were read and assessed. All original papers studying the management of drains in pancreatic surgery were retrieved and included in the systematic review.
We included articles that reported clinical studies on human subjects with any type of elective pancreatic resection and that compared various strategies of intra-abdominal drain management: e.g., drain vs no-drain, selective drain use, early vs late drain removal, and the use of different types of drains. Studies reporting on drainage for acute pancreatitis were excluded. Studies were included irrespective of their design (prospective/retrospective, randomized controlled, non-randomized controlled, cohort studies/case-control studies) or the length of follow-up. Congress abstracts and personal communications were not considered.
All data from selected studies were analyzed independently by two reviewers (Čečka F and Loveček M). We extracted data on methodology, population, interventions including types of drains, outcome measures including POPF rate[39], postoperative morbidity and mortality. Missing data were obtained from the corresponding authors of the studies. Disagreements were resolved in group discussions. Our methodology followed the standard guidelines outlined in the Cochrane Handbook for Systematic Reviews of Interventions[40] and the PRISMA statement (Preferred Reporting Items for Systematic Reviews and Meta-Analyses)[41].
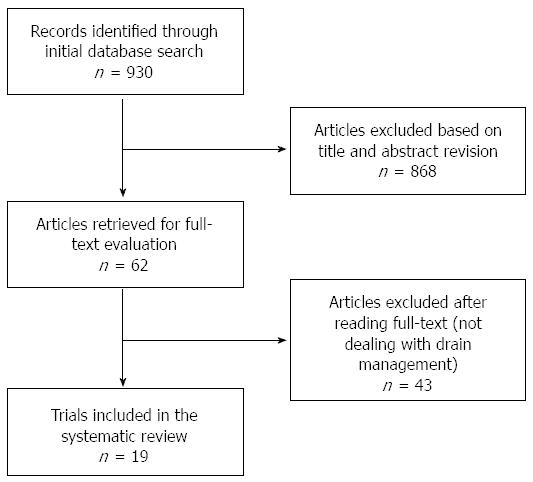
The initial search strategy retrieved 930 publications. Of these, 868 were excluded in the primary selection based on the title and abstract revision (not relevant, not dealing with drain management) and 43 were excluded in the secondary selection after reading the full-text of the potentially relevant studies. Subsequently, the reference lists of all reviewed articles were checked manually; however, this did not lead to identification of any additional studies. Nineteen studies were identified and included in the systematic review, representing a total of 4194 patients (samples ranging from 22 to 1122)[11-17,36-38,42-50]. The reviewers reached agreement on the application of the eligibility criteria for study selection. A flowchart of the literature search strategy according to the PRISMA statement is shown in Figure 1. Only three studies were randomized: published in 2001[13] (n = 179), in 2010[37] (n = 114), and in 2014[17] (n = 137 patients); the last study was the only multi-center study, and the first two were single-center studies. Except for the three randomized studies, all other studies were either retrospective observational, time-cohort or pilot studies.
Table 1 lists the studies analyzing the outcomes of pancreatic resections without drains. A retrospective study comparing two cohorts was published by the group from Memorial Sloan-Kettering Cancer Center (MSKCC) in New York[43]. The authors reported comparable results in both groups (comparable postoperative complications, POPF rate, CT-guided drainage, and length of hospital stay). The only difference was shorter operating time in the group of patients without drains (P = 0.0001). The same group from MSKCC conducted a RCT of drain vs no-drain following pancreatic resection[13]. In this trial, the authors described an equal rate of postoperative complications in both groups. However, patients with drain had a higher rate of intra-abdominal collections and fistulas (22% vs 9%, P < 0.02) and a higher rate of POPF itself.
| Ref. | Year | Type of study | Type of resection, n | Type of drain | Groups, n | Age (yr), range | POPF | Morbidity | Reoperation | Operation time (min) | Blood loss (mL) | Hospital stay (d) |
| Jeekel et al[44] | 1992 | Pilot study | PD 22 | NA | No drain n = 22 | 43-79 | NA | 5 (23) | 1 (5) | NA | NA | NA |
| Heslin et al[43] | 1998 | Retrospective | PD 89 | Closed-suction | Drain n = 51 | 65 ± 21 | 3 (6) | 23 (45) | 2 (4) | 386 ± 201 | 1100 ± 101 | 12 ± 11 |
| No drain n = 38 | 65 ± 21 | 1 (3) | 15 (39) | 3 (8) | 292 ± 131 | 1100 ± 101 | 12 ± 11 | |||||
| Conlon et al[13] | 2001 | RCT | PD 139/DP 40 | Closed-suction (Jackson-Pratt) | Drain n = 88 | 66 (23-81)2 | 11 (13) | 55 (63) | 8 (9) | 330/190 | 800/600 | 9 + |
| No drain n = 91 | 69 (33-87)2 | 0 (0) | 52 (57) | 4 (4) | 329/180 | 800/500 | 9 + | |||||
| Fischer et al[15] | 2011 | Time cohort | PD 153, DP 73 | Closed-suction | Drain n = 179 | 63 (53-72)3 | 79 (44) | 117 (65) | 8 (4) | 401 (310-490)3 | 400 (200-700)3 | 7 (7-10)3 |
| No drain n = 47 | 59 (51-70)3 | 5 (11) | 22 (47) | 0 (0) | 400 (314-458)3 | 250 (150-500)3 | 7 (6-8)3 | |||||
| Paulus et al[16] | 2012 | Retrospective | DP 69 | Closed-suction (Jackson-Pratt or Blake) | Drain n = 39 | 52 (44-66)2 | 6 (15) | 15 (50) | 11 (28) | 249 (196-290)2 | 450 (300-750)2 | 9 (7-17)2 |
| No drain n = 30 | 58 (52-68)2 | 0 (0) | 20 (51) | 8 (27) | 195 (176-260)2 | 200 (100-300)2 | 6.5 (5-8)2 | |||||
| Lim et al[46] | 2013 | Selective | PD 54 | Multichannel open silicone drain | Drain n = 27 | 62 (40-76)2 | 6 (22) | 19 (70) | 2 (7) | 300 (180-540)2 | 400 (50-2000)2 | 15 (11-56)2 |
| No drain n = 27 | 62 (38-78)2 | 0 (0) | 15 (56) | 1 (4) | 270 (170-420)2 | 300 (100-2000)2 | 10 (8-26)2 | |||||
| Mehta et al[47] | 2013 | Retrospective | 709 PD | Closed-suction (Jackson-Pratt or Blake) | Drain n = 251 | 604 | 61 (24) | 171 (68) | 14 (6) | 2944 | 5724 | 13.84 |
| No drain n = 458 | 62.54 | 48 (10) | 248 (54) | 26 (6) | 2014 | 2824 | 11.34 | |||||
| Adham et al[11] | 2013 | Retrospective | 148 PD, 66 DP, 20 CPR, 8 E | Closed-suction (shirley) | Drain n = 130 | 61.5 (20-85)2 | 21 (16) | 83 (64) | 16 (12) | 235 ± 711 | 471 ± 5681 | 16 (2-98)2 |
| No drain n = 112 | 66.5 (19-85)2 | 14 (13) | 45 (67) | 17 (15) | 265 ± 841 | 379 ± 3871 | 18 (7-131)2 | |||||
| Correa-Gallego et al[14] | 2013 | Retrospective | 739 PD, 350 DP, 31 CPR, 2 CSP | Closed-suction (Jackson-Pratt) | Drain PD n = 386 | 65 ± 131 | 149 (27) | 301 (54) | 3 (< 1) | 295 (250-339)3 | 525 (350-800)3 | 8 (7-11)3 |
| Drain DP n = 154 | 191 (154-229)3 | 400 (200-800)3 | 7 (6-9)3 | |||||||||
| No drain PD n = 353 | 102 (18) | 272 (48) | 2 (< 1) | 206 (180-247)3 | 400 (250-700)3 | 7 (6-10)3 | ||||||
| No drain DP n = 196 | 152 (118-188)3 | 200 (100-400)3 | 5 (5-7)3 | |||||||||
| Behrman et al[12] | 2014 | Propensity-score match cohort | 232 DP | NA | Drain n = 116 | 57 + | 25 (22) | 50 (43) | 1 (1) | 222 + | NA | 6 + |
| no draín n = 116 | 59 + | 8 (7) | 35 (30) | 3 (3) | 228 + | NA | 6 + | |||||
| Kunstman et al[45] | 2014 | Routine non-drainer | 265 PD | NA | Drain n = 6 | 64.24 | 21 (8) | 220 (83) | NA | NA | NA | 6 (3-8)3 |
| No drain n = 259 | ||||||||||||
| Van Buren et al[17] | 2014 | RCT | 137 PD | Closed-suction | Drain n = 68 | 62 ± 121 | 21 (31) | 50 (74) | 2 (3) | 425 ± 1511 | 460 ± 3521 | 7 (6-9)3 |
| No drain n = 69 | 64 ± 131 | 14 (20) | 52 (75) | 6 (9) | 407 ± 1571 | 443 ± 3441 | 8 (7-14)3 |
Another trial from this department was published in 2013[14]. Six high-volume surgeons were paired according to their operative drainage practices into routine drainers, selective drainers and routine non-drainers. The group of patients with intra-abdominal drainage had a higher POPF rate (P < 0.001) and higher overall morbidity (P = 0.03). However, the patients with drains had significantly higher blood loss in pancreaticoduodenectomy (P < 0.001) as well as in distal pancreatectomy (P < 0.001). Furthermore, the patients in the drained group had longer operating times for both pancreaticoduodenectomy (P < 0.001) and distal pancreatectomy (P < 0.001). The most important fact is that mortality was significantly higher in the no-drain group (3% vs 1%, P = 0.02)
The study by Paulus et al[16] analyzed abdominal drainage following distal pancreatectomy. There were no differences between the groups regarding overall complications (P = 0.91) or intra-abdominal complications (P = 0.58). Estimated blood loss was higher in the drain group (P = 0.0003).
Lim et al[46] avoided abdominal drainage after uncomplicated pancreaticoduodenectomy in 27 patients at low risk of POPF; these patients were matched to 27 patients undergoing PD with intra-abdominal drainage. Overall morbidity (P = 0.4) and mortality (P = 1) were similar in both groups. The POPF rate (P = 0.009) and hospital stay (P = 0.004) were significantly reduced in the no drainage group.
Mehta et al[47] analyzed 709 patients undergoing PD. Compared with the no drain group, patients with a primary drain had a higher overall morbidity (P < 0.001) and POPF rate (P < 0.0001), as well as a longer hospital stay (P = 0.001). Operation time (P = 0.021) and blood loss (P < 0.0001) were significantly higher in the drain-group. It is worth noting that intra-abdominal drainage did not prevent the need for secondary drainage in this study (P = 0.358).
The study by Adham et al[11] was retrospective and surgeon-dependent. One surgeon always used an intra-abdominal drain, whereas the second surgeon shifted from using a systematic drain to a no drain policy over the duration of the study. There was no difference in overall complications (P = 0.11), post-pancreatectomy hemorrhage (P = 0.33) or POPF rate (P = 0.34). The requirement for an interventional procedure was equivalent in both groups (P = 0.15).
Behrman et al[12] used data from the American College of Surgeons-National Surgical Quality Improvement Program. In this study, 116 patients without drains following distal pancreatectomy were matched using propensity scores with 116 patients with drains. The overall POPF rate (P < 0.01) and overall morbidity (P < 0.05) were more common in patients who received a drain. The placement of a drain did not reduce the need for postoperative interventional procedures (0.29).
Kunstman et al[45] calculated FRS (fistula risk score) for 265 patients, 259 of whom were managed without operative drains. The authors reported an unusually high rate of postoperative morbidity (83%) with an acceptable clinically relevant postoperative pancreatic fistula (CR-POPF) rate (8%). The authors concluded that the FRS reliably predicts the absence of CR-POPF in low risk patients and provides an objective way to characterize POPF risk[45].
A time cohort study was published by Fisher[15]. Morbidity (P = 0.02) and POPF (P < 0.0001) were higher in patients with drains. However, postoperative percutaneous drainage (P = 0.001) and readmission rates (P = 0.007) were higher in the no drain group. Based on this preliminary experience, the authors conducted a multicenter randomized controlled trial[17]. A total of 752 patients were planned to be included in the study in order to detect any significant difference between the groups. However, the trial was stopped early by the Data Safety Monitoring Board because of excess mortality in the patients undergoing PD without routine intraperitoneal drainage[17]. After 90 d of follow-up, there were 8 deaths (12%) in the no-drain group and only 2 deaths (3%) in the drain group (P = 0.097). There were more intra-abdominal abscesses (P = 0.033) and abdominal fluid collections (P = 0.033) in the no-drain group. The POPF rate in both groups was not significantly different (P = 0.155); however, 14 out of 21 patients in the drain group had asymptomatic POPF grade A. All 20 patients in the drain group had clinically relevant POPF.
Table 2 lists the studies analyzing the timing of drain removal. Balzano et al[42] reported a series of 123 patients with DP. The authors preferred cautious drain management, i.e., maintaining the drain until the daily output diminished to 5 mL in 24 h. Thirty-nine out of 42 patients with POPF were discharged home with the drain and maintained it for a mean duration of 36 d. The authors did not compare this approach to early drain removal.
| Ref. | Year | Type of study | Type of resection, n | Type of drain | Groups, n | Age (yr) | Drain duration (d) | POPF | Morbidity | Reoperation | Op time (min) | Blood loss (mL) | Hospital stay (d) |
| Balzano et al[42] | 2005 | Retrospective | 123 DP | Open silicone 28 CH drain | 123 | 59 (19-85)2 | 36 ± 17 ++ | 42 (34) | 60 (49) | 5 (4) | 246 ± 871 | 635 ± 5231 | 11.8 ± 6.11 |
| Kawai et al[38] | 2006 | Time cohort | 104 PD | 10-mm Penrose (silicon multitubular flat drain) | 52 early | 66 ± 101 | POD 4 | 2 (4) | 19 (37) | 0 | 407 ± 761 | 1270 ± 12201 | 42 ± 131 |
| 52 late | 67 ± 101 | POD 8 | 12 (23) | 35 (67) | 0 | 383 ± 591 | 1287 ± 13741 | 35 ± 251 | |||||
| Bassi et al[37] | 2010 | RCT | 75 PD, 39 DP | Flat penrose drain 12 mm | 57 early | 56 ± 141 | POD 3 | 1 (2) | 22 (39) | 0 | 285 ± 971 | NA | 8.7 ± 41 |
| 57 late | 57 ± 131 | POD after 5 | 15 (26) | 35 (61) | 1 (2) | 291 ± 861 | NA | 10.8 ± 6.91 |
A time cohort study was published by Kawai et al[38]. In the first period, the drain was removed on postoperative day (POD) 8, whereas in the second period the drain was removed on POD 4. The POPF rate (P = 0.0038) as well as intra-abdominal infections (P = 0.0003) and infected intra-abdominal collections (P = 0.0079) were significantly lower in the second period. According to the authors, increasing infections occurred around POD 7, with positive cultures of drainage fluid increasing to 31% on POD 7. This suggests that prolonged placement of a drain might be a major cause of postoperative infectious intra-abdominal complications[38].
Bassi et al[37] published a RCT comparing early drain removal (POD 3) vs late drain removal (after POD 5) in patients at low risk for POPF. Patients with a high risk of POPF development (amylase value ≥ 5000 U/L on POD 1) were excluded. Early drain removal was associated with a decreased POPF rate (P = 0.007), abdominal complications (P = 0.002), and pulmonary complications (P = 0.007). The median hospital stay was also shorter in patients with early drain removal (P = 0.018).
Only four studies compared various types of drains following pancreatic resection; these results are described in Table 3.
| Ref. | Year | Type of study | Type of resection, n | Type of drain | Morbidity | POPF |
| Aimoto et al[36] | 2008 | Time cohort | 33 PD | Duple drain (n = 14) | 10 (71) | 14 (100) |
| Blake drain (n = 19) | 2 (11) | 19 (100) | ||||
| Schmidt et al[48] | 2009 | Retrospective | 510 PD | Penrose drain (n = 241) | 241 (47) | 8 (3) |
| Closed-suction (n = 269) | 269 (53) | 38 (14) | ||||
| Yoshikawa et al[49] | 2011 | Time cohort | 97 DP | Penrose drain (n = 56) | 56 (58) | 40 (71) |
| Closed-suction (n = 41) | 41 (42) | 26 (63) | ||||
| Yui et al[50] | 2014 | Time cohort | 109 DP | Penrose drain (n = 52) | 28 (54) | 22 (42) |
| Closed-suction (n = 57) | 25 (44) | 15 (26) |
Aimoto et al[36] compared closed-suction drains (Blake) vs closed passive drains (Duple) for efficacy in a retrospective study of 33 patients following PD. Only patients with a soft pancreas who developed CR-POPF were included. Overall morbidity was significantly lower in the patients with a Blake drain compared to those with a Duple drain (P < 0.01). The authors concluded that the Blake drains controlled POPF grade B more successfully than did the Duple drains in this study[36].
Schmidt et al[48] analyzed the clinical predictors and patient outcomes of pancreatic fistula following PD in 510 patients over a period of 23 years. The authors compared patients with closed-suction drains vs open Penrose drains. There was a significantly higher POPF rate in patients with closed-suction drains compared to passive Penrose drains (P < 0.001). However, the comparison of drain types was not the primary end-point of this study.
Yoshikawa et al[49] studied 97 patients undergoing distal pancreatectomy. In the first period, the authors used Penrose drains, and closed suction drains were used in the second period. The authors stated that closed-suction drains tended to reduce the persistent drainage period and significantly shorten the postoperative stay; however, no exact data were reported.
Yui et al[50] described a retrospective comparison of two cohorts of patients undergoing distal pancreatectomy after introducing a new policy for peri-and post-operative management. This new policy included the use of ultrasonically activated scissors, early drain removal and a different type of drain (two open Penrose drains vs one closed suction drain). Because several factors changed at the same time, the contribution of each factor remains unclear.
This systematic review aimed to evaluate the current knowledge about drain management following pancreatic resection. This topic has been divided into three issues: (1) whether to use routine intra-abdominal drains at all; (2) when to remove the drains; and (3) what type of drain is preferred.
Our review is based on a comprehensive literature search and systematic data aggregation. Nineteen studies met the inclusion criteria. Drain management following pancreatic resection has attracted much attention, especially in the past five years; most of the studies in this review have been published within this period. The first systematic review and meta-analysis assessing drain management was published in 2011; this analyzed the results of the first 4 studies[13,37,38,43]. Diener et al[18] included two studies reporting the result of drain omission and two studies analyzing the timing of drain removal. The authors concluded that the evidence is still unclear and that a treatment recommendation could not be made. Other studies have been published since that time, and progress has been made to date[18-24].
Although surgical drains had previously been considered as mandatory following pancreatic resection, a new approach to pancreatic resection emerged without the necessity for intra-abdominal drain insertion with the pilot study by Jeekel et al[44], who described 22 cases of pancreaticoduodenectomy without drains. The authors concluded that intra-abdominal drainage did not improve the results of pancreatic resection, and thus, it should not be considered mandatory. A number of studies have been published since the first pilot study; the majority of the studies were retrospective[11,14,16,43,47] or time cohort[15] in design. These studies showed data suggesting that pancreatic resection can be safely performed without routine drainage; they described either comparable results regarding postoperative morbidity and POPF rate in both groups[11,16,43] or even superior results without a drain[12,14,15,47]. These retrospective observational trials are inevitably subject to selection bias or bias due to the uneven distribution of the involved surgeons’ expertise among treatment groups. These studies described higher estimated blood loss or longer operating times in the drain group[14,16,43,47], which suggest that these cases were more difficult or demanding, with a higher risk of postoperative complications regardless of the use of intra-abdominal drains[51]. Another source of bias is the surgeon’s preference. In the study by Paulus, only one of three surgeons was responsible for those patients who did not receive a drain[16]. In the study by Adham, one surgeon always used an abdominal drain, while the second surgeon shifted from systematically using a drain to a no-drain policy over the duration of the study[11]. In the study by Correa-Gallego, the six high-volume surgeons involved in the trial were paired according to their operative drainage practices into routine drainers (operative drains placed in over 95% of cases), selective drainers (drains placed in 50% of cases) and routine non-drainers (drains placed in less than 15% of cases)[14].
Such selection bias is excluded in the randomization process in RCTs[13,17]. The first RCT by Conlon showed an equal rate of morbidity in both groups but a higher rate of POPF in the drain group[13]. The last RCT by Van Buren et al[17] seems to be in direct contrast to the previous RCT as well as the observational retrospective cohorts.
The Van Buren group had planned to test the hypothesis that “abandoning routine drainage following pancreatic resection would not increase the incidence or severity of postoperative morbidity or mortality”. This study was conducted in 9 high-volume academic pancreatic surgery centers and planned to involve 752 patients. However, the Data Safety Monitoring Board stopped the study because of excess mortality in the patients without drainage (12% vs 3%, P = 0.097)[17]. PD without drain was associated with increased morbidity and clinically relevant POPF in this study.
The differences between the results can be explained in several ways. The multicenter approach seems to provide more validity and generalizability of results[51]. Furthermore, the authors from Memorial Sloan Kettering Cancer Center (MSKCC) did not use what are the currently generally accepted definitions of postoperative morbidity and POPF.
Further analysis of the POPF rate in the multicenter study by Van Buren shows that the overall POPF rate was higher in the drain group, although not significantly so (31% vs 20%, P = 0.155). However, clinically relevant POPF was higher in the no-drain group (10% vs 20%, P = 0.104). It is apparent from the results that all of the patients in the group without drains had symptomatic fistula. One hypothesis states that some of the patients would have had asymptomatic fistula if they had had an intra-abdominal drain. In high-risk patients who have a leak from the pancreaticojejunal anastomosis, any excess pancreatic juice is removed via the intra-abdominal drain if it is present. After a minor leak has healed, the intra-abdominal drain is removed, and an asymptomatic POPF grade A is classified in the patient. However, in patients with no drain, the pancreatic juice would congest in the retroperitoneum and peripancreatic area with subsequent complications. Subsequent digestion and destruction of the surrounding tissue may be followed by the development of peripancreatic fluid collections, intra-abdominal or retroperitoneal abscesses, delayed gastric emptying, and postoperative hemorrhage.
The controversy regarding the routine abandonment of drains following pancreatic resection is also evident in the study by Correa-Gallego et al[14]. Even at the MSKCC, where a RCT[13] showed that routine intra-abdominal drains could be abandoned, twelve years later two out of 6 high-volume pancreatic surgeons still routinely use intra-abdominal drains and two other surgeons drain selectively.
Even though the retrospective observational trials carry a significant risk of selection bias, all of them uniformly suggest that routine abandonment of drains can be safely performed at least in a subset of patients[14,16,43,47]. Therefore, a selective approach to drain placement according to the individual risk-benefit assessment was recommended by some authors[19]. Drains should be placed in high risk patients, whereas they can be omitted in low risk patients[19]. This approach was also adopted in the study by Lim et al[46]. They established a predictive model of POPF based on body mass index (BMI), pathologic grading of fatty infiltration, and fibrosis in the pancreatic transection margin. Intra-abdominal drainage was avoided in patients at low risk of POPF after uncomplicated PD. The POPF rate (P = 0.009) and hospital stay (P = 0.004) were significantly reduced in the no-drainage group. It is not clear whether better results in the no-drain group were due to the avoidance of drainage or due to a lower risk of POPF in the patients[46].
A useful model for determining the risk of POPF is the fistula risk score (FRS)[52]. A simple 10-point FRS based on pancreatic gland texture, certain pathology, pancreatic duct diameter and intraoperative blood loss accurately predicts subsequent CR-POPF.
The fistula risk score was later calculated by McMillan et al[53] for the patients in the multicenter RCT published by Van Buren. This work found no differences between the treatment cohorts in terms of the fistula risk score. The authors concluded that patients with moderate and high risk of CR-POPF should undergo routine drain placement to ensure optimal treatment of the fistula and its consequences, and in patients with low risk, drain placement should be left to the discretion of the surgeon[53].
It is impossible to determine whether the complications seen with the use of drains are because of the drains themselves or because of other factors related to the patient or to the disease that increase the rate of complications.
Traditionally, intra-abdominal drains were inserted following pancreatic resection and maintained until the risk of POPF diminished. This meant in most cases maintaining the drains until the daily output had decreased to below 5 mL per 24 h[42]. Keeping the drains for a longer period of time could reduce the patient’s comfort; however, some authors believed that this approach could lower the rate of delayed complications[42]. Surgically placed drains are normally removed “at the surgeon’s discretion” with no clear specification as to when the drains should be removed. However, with the introduction of fast-track protocols, the need for reducing hospital-stay, and ultimately providing high-quality cost-effective care, more attention has been paid to drain management. Drain management and especially the timing of drain removal are key factors[37,38].
Both studies comparing early vs later drain removal showed superior results for early drain removal, even though there were certain flaws in the study designs. The study published by Kawai et al[38] was a time-cohort study, which carries significant bias; the study published by Bassi was criticized for analyzing both procedures (pancreaticoduodenectomy and distal pancreatectomy) together, even though the two procedures are different, with different POPF rates[5] and a different course of POPF development[54]. Furthermore, the authors in both studies used flat Penrose drains, which are now considered obsolete[49,55].
Not much attention has been paid to the various types of drains that are used following intra-abdominal surgery[18,51]. Two types of surgical drains exist: open drains and closed drains. Open drains evacuate collected fluid through an artificial catheter inserted into the postoperative wound. Open drains are considered obsolete because of frequent retrograde infection[55]. Closed drainage is believed to reduce the risk of retrograde microbial contamination compared with open drainage[23].
Closed drains include two types: passive gravity drains and closed-suction drains. The majority of authors prefer various modifications of closed suction drains (Jackson-Pratt, Blake, Shirley)[11,13-17,43,47]. However, some surgeons believe that negative pressure might pose potential hazards to the patients[56], increase the risk of pancreatic fistula or lead to delayed hemorrhage[23]. Therefore, passive gravity drains are preferred by some authors[42,46]. Various types of drains have been studied retrospectively in neck dissection[57] and in liver resection[58]; RCTs were conducted to study the types of drains in cholecystectomy[55] and cardiac surgery[59]. The situation in pancreatic surgery is different, as the pancreatico-enteric anastomosis is not water-tight in most cases, as indicated by an increased amylase level on the 1st POD[34,60,61]; more attention must be paid to the choice of drain type in order to decrease the clinically significant POPF rate. Only four studies have compared the various types of drains in pancreatic surgery[36,48-50]; however, two of them[48,49] were retrospective observational, and most importantly, the comparison of the types of drains was not the primary outcome of the studies, and the studies took place over a very long time period. Furthermore, their results are contradictory.
Diener et al[18] stated that the role of different types of drains remains unclear. Strobel also noted that the type of drainage is unknown[51]. Some surgeons believe that negative pressure might increase the risk of pancreatic fistula or lead to delayed hemorrhage[16,23]. Furthermore, there are case reports suggesting that closed-suction drains may have caused small bowel perforations[62-64].
Grobmyer et al[65] conducted an ex-vivo study comparing various types of closed-suction drains. The authors demonstrated that commonly used closed-suction drains generate vacuum pressure from-75 to-175 mm Hg and that the practice of “stripping” the drain tubing can generate a maximal pressure of-225 mm Hg and significantly higher sustained pressures than the suction bulb alone. This negative pressure may hinder wound healing or even promote the formation of POPF[65].
On the other hand, a study in favor of closed-suction drains was that by Aimoto et al[36], which reported that Blake drains controlled grade B POPF more successfully than closed passive Duple drains. The main conservative management of POPF is sufficient control of the fistula by adequate drainage of the enzyme-rich pancreatic fluid.
Furthermore, most of the recent studies analyzing the role of drains in pancreatic surgery used closed-suction drains[11,13-17,43,47].
A new randomized controlled study is currently underway to compare closed suction drainage vs passive closed gravity drains in patients undergoing pancreaticoduodenectomy or distal pancreatectomy (DRAPA: DRAins in PAncreatic surgery)[66]. This study is registered at clinicaltrials.gov under the number NCT01988519 and plans to enroll 223 patients. The primary end-point of this study is the rate of POPF occurrence, and the secondary end-point is postoperative morbidity[66].
In conclusion, the postoperative pancreatic fistula remains a significant problem after pancreatic resection. The pancreatico-enteric anastomosis as well as the suture of the pancreatic resection line is not absolutely water-tight, which is proven by increased amylase in the drain fluid from the first postoperative day. The majority of the fistulas are asymptomatic. The goal of postoperative management including the management of intra-abdominal drains is to decrease the rate of symptomatic pancreatic fistula. The study by Van Buren et al[17] proved that routine omission of intra-abdominal drains leads to worse results in terms of increased postoperative mortality. Although many retrospective studies have reported superior results from pancreatic resection without drains, these studies were influenced by selection bias due to their retrospective nature. Current studies do not lead to definitive conclusions, and further studies are needed to clarify this issue. When drains are used, early removal is recommended. The final issue that has not yet been clarified is the preferred type of drain. Only a few retrospective studies have compared the various types of drains. However, the analysis of drain types was not the primary goal in two of them, and their results were contradictory. A prospective randomized trial is ongoing; it aims to compare closed-suction drainage with closed passive gravity drains. This review also emphasizes the importance of well-designed randomized controlled studies, which are least likely to be influenced by bias and thus provide the highest level of evidence.
The authors are grateful to Ian McColl, MD, PhD for assistance with the manuscript.
Postoperative pancreatic fistula is the most ominous complication following pancreatic surgery. New methods are being studied in order to reduce the rate and clinical significance of the pancreatic fistula. Manipulation with intra-abdominal drains is considered to be one of the important measures.
This review discusses three important issues regarding drain management: (1) whether to use routine intra-abdominal drains at all; (2) when to remove the drains; and (3) what type of drain is preferred.
Most other reviews and meta-analyses have focused only on the question of routine usage or elimination of intra-abdominal drains following pancreatic surgery. However, there are additional issues to address regarding the drains, such as timing of drain removal or the type of drain. This systematic review addresses all aspects of drain management in pancreatic surgery.
Although many retrospective studies have reported superior results from pancreatic resection without drains, these studies were influenced by selection bias due to their retrospective nature. Current studies do not lead to definitive conclusions, and therefore, further studies are needed to clarify this issue. When drains are used, early removal is recommended in patients at low risk of pancreatic fistula. The final issue that has not yet been clarified is the preferred type of drain. A prospective randomized trial is ongoing that aims to compare closed-suction drainage with closed passive gravity drains.
Pancreatic resections are highly invasive surgical procedures that carry significant morbidity. Prophylactic intra-abdominal drains were traditionally considered to help to reduce postoperative complications.
This is a good systematic review about intra-abdominal drainage following pancreatic resection according to the PRISMA guidelines. This study analyzed the outcomes of pancreatic resection with and without intra-abdominal drains, comparing early vs late drain removal and analyzing different types of drains.
P-Reviewer: Bradley EL, Fu DL S-Editor: Yu J L-Editor: A E-Editor: Zhang DN
| 1. | Büchler MW, Wagner M, Schmied BM, Uhl W, Friess H, Z’graggen K. Changes in morbidity after pancreatic resection: toward the end of completion pancreatectomy. Arch Surg. 2003;138:1310-134; discussion 1315. [PubMed] |
| 2. | Butturini G, Daskalaki D, Molinari E, Scopelliti F, Casarotto A, Bassi C. Pancreatic fistula: definition and current problems. J Hepatobiliary Pancreat Surg. 2008;15:247-251. [PubMed] |
| 3. | DeOliveira ML, Winter JM, Schafer M, Cunningham SC, Cameron JL, Yeo CJ, Clavien PA. Assessment of complications after pancreatic surgery: A novel grading system applied to 633 patients undergoing pancreaticoduodenectomy. Ann Surg. 2006;244:931-97; discussion 931-97;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 578] [Cited by in RCA: 623] [Article Influence: 34.6] [Reference Citation Analysis (0)] |
| 4. | Čečka F, Jon B, Šubrt Z, Ferko A. Clinical and economic consequences of pancreatic fistula after elective pancreatic resection. Hepatobiliary Pancreat Dis Int. 2013;12:533-539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 5. | Pratt W, Maithel SK, Vanounou T, Callery MP, Vollmer CM. Postoperative pancreatic fistulas are not equivalent after proximal, distal, and central pancreatectomy. J Gastrointest Surg. 2006;10:1264-178; discussion 1264-178;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 99] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 6. | Čečka F, Jon B, Šubrt Z, Ferko A. The effect of somatostatin and its analogs in the prevention of pancreatic fistula after elective pancreatic surgery. Eur Surg. 2012;44:99-108. [RCA] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 7. | Klempa I, Schwedes U, Usadel KH. [Prevention of postoperative pancreatic complications following duodenopancreatectomy using somatostatin]. Chirurg. 1979;50:427-431. [PubMed] |
| 8. | Shrikhande SV, Qureshi SS, Rajneesh N, Shukla PJ. Pancreatic anastomoses after pancreaticoduodenectomy: do we need further studies? World J Surg. 2005;29:1642-1649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 83] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 9. | Diener MK, Seiler CM, Rossion I, Kleeff J, Glanemann M, Butturini G, Tomazic A, Bruns CJ, Busch OR, Farkas S. Efficacy of stapler versus hand-sewn closure after distal pancreatectomy (DISPACT): a randomised, controlled multicentre trial. Lancet. 2011;377:1514-1522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 468] [Cited by in RCA: 404] [Article Influence: 28.9] [Reference Citation Analysis (0)] |
| 10. | Cečka F, Jon B, Subrt Z, Ferko A. Surgical technique in distal pancreatectomy: a systematic review of randomized trials. Biomed Res Int. 2014;2014:482906. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 11. | Adham M, Chopin-Laly X, Lepilliez V, Gincul R, Valette PJ, Ponchon T. Pancreatic resection: drain or no drain? Surgery. 2013;154:1069-1077. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 53] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 12. | Behrman SW, Zarzaur BL, Parmar A, Riall TS, Hall BL, Pitt HA. Routine drainage of the operative bed following elective distal pancreatectomy does not reduce the occurrence of complications. J Gastrointest Surg. 2015;19:72-9; discussion 79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 46] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 13. | Conlon KC, Labow D, Leung D, Smith A, Jarnagin W, Coit DG, Merchant N, Brennan MF. Prospective randomized clinical trial of the value of intraperitoneal drainage after pancreatic resection. Ann Surg. 2001;234:487-93; discussion 493-4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 407] [Cited by in RCA: 383] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 14. | Correa-Gallego C, Brennan MF, D’angelica M, Fong Y, Dematteo RP, Kingham TP, Jarnagin WR, Allen PJ. Operative drainage following pancreatic resection: analysis of 1122 patients resected over 5 years at a single institution. Ann Surg. 2013;258:1051-1058. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 125] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 15. | Fisher WE, Hodges SE, Silberfein EJ, Artinyan A, Ahern CH, Jo E, Brunicardi FC. Pancreatic resection without routine intraperitoneal drainage. HPB (Oxford). 2011;13:503-510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 81] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 16. | Paulus EM, Zarzaur BL, Behrman SW. Routine peritoneal drainage of the surgical bed after elective distal pancreatectomy: is it necessary? Am J Surg. 2012;204:422-427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 36] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 17. | Van Buren G, Bloomston M, Hughes SJ, Winter J, Behrman SW, Zyromski NJ, Vollmer C, Velanovich V, Riall T, Muscarella P. A randomized prospective multicenter trial of pancreaticoduodenectomy with and without routine intraperitoneal drainage. Ann Surg. 2014;259:605-612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 276] [Cited by in RCA: 273] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 18. | Diener MK, Tadjalli-Mehr K, Wente MN, Kieser M, Büchler MW, Seiler CM. Risk-benefit assessment of closed intra-abdominal drains after pancreatic surgery: a systematic review and meta-analysis assessing the current state of evidence. Langenbecks Arch Surg. 2011;396:41-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 61] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 19. | Kaminsky PM, Mezhir JJ. Intraperitoneal drainage after pancreatic resection: a review of the evidence. J Surg Res. 2013;184:925-930. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 20. | Nitsche U, Müller TC, Späth C, Cresswell L, Wilhelm D, Friess H, Michalski CW, Kleeff J. The evidence based dilemma of intraperitoneal drainage for pancreatic resection - a systematic review and meta-analysis. BMC Surg. 2014;14:76. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 21. | Rondelli F, Desio M, Vedovati MC, Balzarotti Canger RC, Sanguinetti A, Avenia N, Bugiantella W. Intra-abdominal drainage after pancreatic resection: is it really necessary? A meta-analysis of short-term outcomes. Int J Surg. 2014;12 Suppl 1:S40-S47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 22. | van der Wilt AA, Coolsen MM, de Hingh IH, van der Wilt GJ, Groenewoud H, Dejong CH, van Dam RM. To drain or not to drain: a cumulative meta-analysis of the use of routine abdominal drains after pancreatic resection. HPB (Oxford). 2013;15:337-344. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 51] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 23. | Wang Q, Jiang YJ, Li J, Yang F, Di Y, Yao L, Jin C, Fu DL. Is routine drainage necessary after pancreaticoduodenectomy? World J Gastroenterol. 2014;20:8110-8118. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 27] [Cited by in RCA: 26] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 24. | Zhou Y, Zhang X, Wu L, Ye F, Su X, Li B. Evidence-based value of prophylactic intraperitoneal drainage following pancreatic resection: a meta-analysis. Pancreatology. 2014;14:302-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 25. | Robinson JO. Surgical drainage: an historical perspective. Br J Surg. 1986;73:422-426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 70] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 26. | Belghiti J, Kabbej M, Sauvanet A, Vilgrain V, Panis Y, Fekete F. Drainage after elective hepatic resection. A randomized trial. Ann Surg. 1993;218:748-753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 150] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 27. | Kim J, Lee J, Hyung WJ, Cheong JH, Chen J, Choi SH, Noh SH. Gastric cancer surgery without drains: a prospective randomized trial. J Gastrointest Surg. 2004;8:727-732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 75] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 28. | Lewis RT, Goodall RG, Marien B, Park M, Lloyd-Smith W, Wiegand FM. Simple elective cholecystectomy: to drain or not. Am J Surg. 1990;159:241-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 50] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 29. | Merad F, Hay JM, Fingerhut A, Yahchouchi E, Laborde Y, Pélissier E, Msika S, Flamant Y. Is prophylactic pelvic drainage useful after elective rectal or anal anastomosis? A multicenter controlled randomized trial. French Association for Surgical Research. Surgery. 1999;125:529-535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 127] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 30. | Petrowsky H, Demartines N, Rousson V, Clavien PA. Evidence-based value of prophylactic drainage in gastrointestinal surgery: a systematic review and meta-analyses. Ann Surg. 2004;240:1074-1084; discussion 1074-1084. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 325] [Cited by in RCA: 309] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 31. | Stone HH, Hooper CA, Millikan WJ. Abdominal drainage following appendectomy and cholecystectomy. Ann Surg. 1978;187:606-612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 49] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 32. | Berberat PO, Ingold H, Gulbinas A, Kleeff J, Müller MW, Gutt C, Weigand M, Friess H, Büchler MW. Fast track--different implications in pancreatic surgery. J Gastrointest Surg. 2007;11:880-887. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 88] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 33. | Čečka F, Šubrt Z, Sotona O. How to distinguish between surgical and non-surgical pneumoperitoneum? Signa Vitae. 2014;9:9-15. |
| 34. | Molinari E, Bassi C, Salvia R, Butturini G, Crippa S, Talamini G, Falconi M, Pederzoli P. Amylase value in drains after pancreatic resection as predictive factor of postoperative pancreatic fistula: results of a prospective study in 137 patients. Ann Surg. 2007;246:281-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 251] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 35. | Lee CW, Pitt HA, Riall TS, Ronnekleiv-Kelly SS, Israel JS, Leverson GE, Parmar AD, Kilbane EM, Hall BL, Weber SM. Low drain fluid amylase predicts absence of pancreatic fistula following pancreatectomy. J Gastrointest Surg. 2014;18:1902-1910. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 52] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 36. | Aimoto T, Uchida E, Nakamura Y, Matsushita A, Katsuno A, Chou K, Kawamoto M, Taniai N, Yoshida H, Tajiri T. Efficacy of a Blake drainR on pancreatic fistula after pancreaticoduodenectomy. Hepatogastroenterology. 2008;55:1796-1800. [PubMed] |
| 37. | Bassi C, Molinari E, Malleo G, Crippa S, Butturini G, Salvia R, Talamini G, Pederzoli P. Early versus late drain removal after standard pancreatic resections: results of a prospective randomized trial. Ann Surg. 2010;252:207-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 355] [Cited by in RCA: 385] [Article Influence: 25.7] [Reference Citation Analysis (0)] |
| 38. | Kawai M, Tani M, Terasawa H, Ina S, Hirono S, Nishioka R, Miyazawa M, Uchiyama K, Yamaue H. Early removal of prophylactic drains reduces the risk of intra-abdominal infections in patients with pancreatic head resection: prospective study for 104 consecutive patients. Ann Surg. 2006;244:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 343] [Cited by in RCA: 363] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 39. | Bassi C, Dervenis C, Butturini G, Fingerhut A, Yeo C, Izbicki J, Neoptolemos J, Sarr M, Traverso W, Buchler M. Postoperative pancreatic fistula: an international study group (ISGPF) definition. Surgery. 2005;138:8-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3282] [Cited by in RCA: 3506] [Article Influence: 175.3] [Reference Citation Analysis (34)] |
| 40. | Clarke M, Horton R. Bringing it all together: Lancet-Cochrane collaborate on systematic reviews. Lancet. 2001;357:1728. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 205] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 41. | Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6:e1000100. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11206] [Cited by in RCA: 11000] [Article Influence: 687.5] [Reference Citation Analysis (0)] |
| 42. | Balzano G, Zerbi A, Cristallo M, Di Carlo V. The unsolved problem of fistula after left pancreatectomy: the benefit of cautious drain management. J Gastrointest Surg. 2005;9:837-842. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 81] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 43. | Heslin MJ, Harrison LE, Brooks AD, Hochwald SN, Coit DG, Brennan MF. Is intra-abdominal drainage necessary after pancreaticoduodenectomy? J Gastrointest Surg. 1998;2:373-378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 93] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 44. | Jeekel J. No abdominal drainage after Whipple’s procedure. Br J Surg. 1992;79:182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 43] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 45. | Kunstman JW, Kuo E, Fonseca AL, Salem RR. Evaluation of a recently described risk classification scheme for pancreatic fistulae development after pancreaticoduodenectomy without routine post-operative drainage. HPB (Oxford). 2014;16:987-993. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 46. | Lim C, Dokmak S, Cauchy F, Aussilhou B, Belghiti J, Sauvanet A. Selective policy of no drain after pancreaticoduodenectomy is a valid option in patients at low risk of pancreatic fistula: a case-control analysis. World J Surg. 2013;37:1021-1027. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 47. | Mehta VV, Fisher SB, Maithel SK, Sarmiento JM, Staley CA, Kooby DA. Is it time to abandon routine operative drain use? A single institution assessment of 709 consecutive pancreaticoduodenectomies. J Am Coll Surg. 2013;216:635-42; discussion 642-4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 48. | Schmidt CM, Choi J, Powell ES, Yiannoutsos CT, Zyromski NJ, Nakeeb A, Pitt HA, Wiebke EA, Madura JA, Lillemoe KD. Pancreatic fistula following pancreaticoduodenectomy: clinical predictors and patient outcomes. HPB Surg. 2009;2009:404520. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 78] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 49. | Yoshikawa K, Konishi M, Takahashi S, Gotohda N, Kato Y, Kinoshita T. Surgical management for the reduction of postoperative hospital stay following distal pancreatectomy. Hepatogastroenterology. 2011;58:1389-1393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 50. | Yui R, Satoi S, Toyokawa H, Yanagimoto H, Yamamoto T, Hirooka S, Yamaki S, Ryota H, Michiura T, Inoue K. Less morbidity after introduction of a new departmental policy for patients who undergo open distal pancreatectomy. J Hepatobiliary Pancreat Sci. 2014;21:72-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 51. | Strobel O, Büchler MW. Drainage after pancreaticoduodenectomy: controversy revitalized. Ann Surg. 2014;259:613-615. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 52. | Callery MP, Pratt WB, Kent TS, Chaikof EL, Vollmer CM. A prospectively validated clinical risk score accurately predicts pancreatic fistula after pancreatoduodenectomy. J Am Coll Surg. 2013;216:1-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 653] [Cited by in RCA: 911] [Article Influence: 70.1] [Reference Citation Analysis (2)] |
| 53. | McMillan MT, Fisher WE, Van Buren G, McElhany A, Bloomston M, Hughes SJ, Winter J, Behrman SW, Zyromski NJ, Velanovich V. The value of drains as a fistula mitigation strategy for pancreatoduodenectomy: something for everyone? Results of a randomized prospective multi-institutional study. J Gastrointest Surg. 2015;19:21-30; discussion 30-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 74] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 54. | Sauvanet A, Partensky C, Sastre B, Gigot JF, Fagniez PL, Tuech JJ, Millat B, Berdah S, Dousset B, Jaeck D. Medial pancreatectomy: a multi-institutional retrospective study of 53 patients by the French Pancreas Club. Surgery. 2002;132:836-843. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 114] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 55. | Sarr MG, Parikh KJ, Minken SL, Zuidema GD, Cameron JL. Closed-suction versus Penrose drainage after cholecystectomy. A prospective, randomized evaluation. Am J Surg. 1987;153:394-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 56. | Barie PS. Are we draining the life from our patients? Surg Infect (Larchmt). 2002;3:159-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 57. | Batstone MD, Lowe D, Shaw RJ, Brown JS, Vaughan ED, Rogers SN. Passive versus active drainage following neck dissection: a non-randomised prospective study. Eur Arch Otorhinolaryngol. 2009;266:121-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 58. | Tanaka K, Kumamoto T, Nojiri K, Takeda K, Endo I. The effectiveness and appropriate management of abdominal drains in patients undergoing elective liver resection: a retrospective analysis and prospective case series. Surg Today. 2013;43:372-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 27] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 59. | Roberts N, Boehm M, Bates M, Braidley PC, Cooper GJ, Spyt TJ. Two-center prospective randomized controlled trial of Blake versus Portex drains after cardiac surgery. J Thorac Cardiovasc Surg. 2006;132:1042-1046. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 60. | Cloyd JM, Kastenberg ZJ, Visser BC, Poultsides GA, Norton JA. Postoperative serum amylase predicts pancreatic fistula formation following pancreaticoduodenectomy. J Gastrointest Surg. 2014;18:348-353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 44] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 61. | Sutcliffe RP, Battula N, Haque A, Ali A, Srinivasan P, Atkinson SW, Rela M, Heaton ND, Prachalias AA. Utility of drain fluid amylase measurement on the first postoperative day after pancreaticoduodenectomy. World J Surg. 2012;36:879-883. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 50] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 62. | Benjamin PJ. Faeculent peritonitis: a complication of vacuum drainage. Br J Surg. 1980;67:453-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 63. | Gray AJ, Copeland GP. Small bowel perforation following vacuum suction drainage. J R Coll Surg Edinb. 1985;30:324-325. [PubMed] |
| 64. | Reed MW, Wyman A, Thomas WE, Zeiderman MR. Perforation of the bowel by suction drains. Br J Surg. 1992;79:679. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 65. | Grobmyer SR, Graham D, Brennan MF, Coit D. High-pressure gradients generated by closed-suction surgical drainage systems. Surg Infect (Larchmt). 2002;3:245-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 53] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 66. | Čečka F, Loveček M, Jon B, Skalický P, Šubrt Z, Ferko A. DRAPA trial--closed-suction drains versus closed gravity drains in pancreatic surgery: study protocol for a randomized controlled trial. Trials. 2015;16:207. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |