Published online Oct 28, 2015. doi: 10.3748/wjg.v21.i40.11282
Peer-review started: June 2, 2015
First decision: June 23, 2015
Revised: July 12, 2015
Accepted: September 2, 2015
Article in press: September 2, 2015
Published online: October 28, 2015
Processing time: 144 Days and 12.6 Hours
In recent years, the incidence of inflammatory bowel disease (IBD) has been on the rise, extending to countries where it was infrequent in the past. As a result, the gap between high and low incidence countries is decreasing. The disease, therefore, has an important economic impact on the healthcare system. Advances in recent years in pharmacogenetics and clinical pharmacology have allowed for the development of treatment strategies adjusted to the patient profile. Concurrently, new drugs aimed at inflammatory targets have been developed that may expand future treatment options. This review examines advances in the optimization of existing drug treatments and the development of novel treatment options for IBD.
Core tip: The incidence and prevalence of inflammatory bowel disease (IBD) has been increasing worldwide. In recent years, the treatment objectives, the monitoring of IBD, and the drug treatments for controlling the disorder have been evolving. This review summarizes recent developments in pharmacogenetics, clinical pharmacology, and the use of new drug molecules that may expand IBD treatment options in the future.
- Citation: Gómez-Gómez GJ, Masedo &, Yela C, Martínez-Montiel MDP, Casís B. Current stage in inflammatory bowel disease: What is next? World J Gastroenterol 2015; 21(40): 11282-11303
- URL: https://www.wjgnet.com/1007-9327/full/v21/i40/11282.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i40.11282
Inflammatory bowel disease (IBD), Crohn’s disease (CD), and ulcerative colitis (UC) are important public health problems. According to recent studies, the annual incidence of UC varies between 19.2-24.3 cases per 100000 inhabitants in Europe and 6.3 cases per 100000 inhabitants in Asia and the Middle East[1]. For CD, the estimated incidence is 12.7-20.2 cases per 100000 inhabitants in Europe and the United States vs five cases per 100000 inhabitants in Asia and the Middle East. The incidence of IBD is currently growing in areas where the disease was previously infrequent. As a result, the gap between high- and low-incidence countries is closing[2]. This rise runs parallel to technological development, improvements in living standards, and a greater interest in this disease among physicians[3]. The underlying pathogenesis remains uncertain, although the most widely accepted theory revolves around changes in the host immune response in genetically susceptible individuals to the intestinal microbiota that is triggered by environmental stimuli. None of these alterations alone can cause the disease, and the interactions among these four factors in the pathogenesis are very complex. In recent decades there have been important advances regarding each of these factors. Progress in the field of genetics has resulted from the performance of genome-wide association studies (GWAS), although they only account for 20%-25% of the cases of IBD[4]. Knowledge of epigenetic mechanisms could explain the influence of environmental factors and the microbiota upon IBD and the low correlation to concrete genes[5,6]. These developments have opened the door to personalized medicine[7].
Knowledge of the immunological mechanisms involved in the manifestation of IBD has led to the development of new biological drugs. The first major advance is represented by the anti-tumor necrosis factor (TNF)-α drugs, which have revolutionized the treatment of IBD, since they are able to induce and maintain mucosal healing of the disease[8], a key factor for modifying the natural course of the disorder[9,10]. Nevertheless, despite these advances, one-third of all patients with CD fail to respond to anti-TNF-α therapy (primary non-responders), and 10% do not tolerate or do not respond to any of the drugs used to treat CD[11,12]. In the case of UC, the reported colectomy rate reaches up to 21% after an initial response to anti-TNF-α drugs[13]. This has led to the search for new therapeutic targets and further optimization of existing treatment options. Clinical pharmacology allows us to determine therapeutic drug concentrations (thiopurine agents and anti-TNF-α drugs) and, if needed, to explain their loss of responsiveness and their adverse effects. In the coming years, personalized medicine, where treatments will be prescribed according to the risk factors in each individual patient and the probability of achieving response to a given drug substance, will be initiated. There have been developments in the way IBD is monitored, with the adoption of reliable and scantly aggressive techniques, such as noninvasive imaging tests, stool markers, breath tests, etc.[14], which fall beyond the scope of this review. We provide below a description of the current advances in pharmacogenetics and possible new drug substances.
Personalized medicine seeks to find the ideal drug for each individual patient at the appropriate dose and administered via the best possible route. This approach allows for increased effectiveness, with the least risk of side effects, and at the lowest possible cost. Physicians try to identify patients with more serious disease, with a view to introducing early and more effective treatment in order to prevent long-term complications, distinguishing them from those individuals with less severe disease and a more favorable prognosis in which aggressive treatment poses a higher risk of undesired effects. Patient response to drug treatment is dependent upon many factors, including the severity of the disease and genetic and environmental factors.
Pharmacogenetics is the study of the association between the different polymorphisms of a gene and the variability of response to treatment or its toxicity with a given drug. It has been estimated that polymorphisms can account for 20%-95% of the variability of a response to a drug[15].
A number of drugs are currently available for the treatment of IBD: 5-aminosalicylates, corticosteroids, immunosuppressors (thiopurine drugs, calcineurinic agents, methotrexate), and biological agents (anti-TNF-α drugs).
The aminosalicylates are among the main agents used to treat patients with UC, and their colon cancer chemoprophylactic effect allows them to be used in UC with pancolonic disease involvement. The metabolization of both sulfasalazine and mesalazine is mediated by the enzyme N-acetyltransferase (NAT). For almost six decades, the population has been divided into fast and slow acetylators. There are two NAT isoenzymes (NAT1 and NAT2), and different polymorphisms have been described in different ethnic groups[16]. NAT1 metabolizes mesalazine, and it has no demonstrable associations with clinical effects. NAT2 metabolizes salazopyrin derived from sulfasalazine breakdown. In 1983, a link between NAT2 slow acetylators, who accumulate higher drug levels in blood, and an increased number of side effects was shown. Twenty-five years later, and thanks to our knowledge of single nucleotide polymorphisms (SNPs), it has been possible to confirm the association between NAT2 with a slow acetylator phenotype and dose-dependent side effects[17]. There are fewer studies on 5-acetylsalicylic acid (5-ASA) than with immunosuppressors and biological drugs, since 5-ASA is only used to reduce side effects that are usually not serious. However, since more prolonged treatment with 5-ASA was proposed due to its chemoprotective effect against colon cancer, the pharmacogenetic studies have become more important.
Glucocorticoids (GLCs) are used in moderate and severe flare-ups of IBD, and although they are very effective, 16%-20% of all patients are refractory to GLCs in the Caucasian population, and 28%-36% are corticodependent[18-20]. The GLCs exert their anti-inflammatory effect by inhibiting T cell activation and cytokine secretion, following binding of the drug to the intracellular glucocorticoid receptors (GR-alpha), which modify their structural conformation as a result. Three potential mechanisms can cause GLCS treatment to be ineffective: inadequate receptor function; an excess of proinflammatory cytokines, which would reduce affinity between the drug and its intracellular receptor; and a decrease in intracellular corticosteroids secondary to expulsion from the cell[21]. This latter mechanism is dependent upon glycoprotein P-170 (P-gp), which is found in lymphocytes and in the apical membrane of the enterocytes, among other locations. An increase in P-gp at cell surface level causes drug release into the bloodstream. This protein is encoded by the ABCB1/MDR1 gene of chromosome 7. The expression of this gene is reportedly increased in IBD presenting a greater need for surgery because of a poor response to drug treatment[22]. Different allelic variants (the most widely studied being C3435T and G2677T) are associated with an increased risk of developing extensive UC, although no association to CD has been observed[23]. Studies with larger patient series and stable corticosteroid doses are needed to determine the precise relationship between P-gp and the lack of response to such drugs.
The studies that have explored the different cytokines implicated in corticosteroid response offer contradictory results, and the underlying polymorphisms have not been established[24].
Genetic studies related to the gene encoding for the intracellular glucocorticoid receptor (hGR) have also been performed. Polymorphism N363S is associated with a good response[25], while polymorphism ER22/23EK is associated with corticosteroid resistance[26]. Knowing the genetic susceptibility of corticosteroid resistant patients is an important step forward, since it would help avoid important morbidity among patients who stand to derive no benefit from such treatment. None of these pharmacogenetic markers are of use in routine clinical practice.
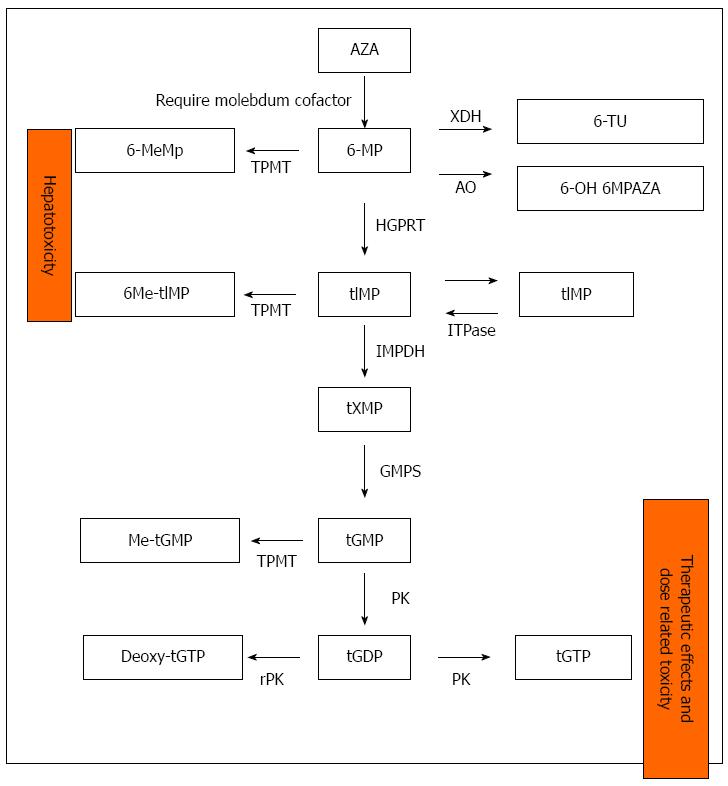
Thiopurine drugs are used to maintain remission in patients with moderate to severe IBD. The effects are only observed after 3 mo of treatment. Purine metabolization is complex and involves different enzymes; this results in important genetic variability in the efficacy and toxicity of these drug substances (Figure 1).
The thiopurine drugs (TPs) are able to control the disease in 66% of patients, although an important proportion (10%-25%) must suspend the medication because of serious (leukopenia, pancreatitis, infections, or malignancies) or mild side effects (rash, nausea, vomiting, flu syndrome, or joint pain). A clear association has been demonstrated between thiopurine methyltransferase (TMPT) deficiency and bone marrow suppression, although this explains only one-third of adverse effects. No clear explanation has been found for the remaining effects. Knowing in advance whether these drugs will be tolerated and the risk of side effects in a given patient may prove useful in daily practice.
TPMT: TPMT is the most widely studied enzyme in IBD. The identification of genetic mutations before onset of treatment with TPs is currently the only pharmacogenetic test performed in IBD.
In 1980, Weinshilboum and Sladeck were the first to describe the trimodal distribution of TMPT in Caucasian patients: 90% of the subjects have normal TMPT activity, almost 10% have intermediate activity, and 0.3% have almost zero activity. Posteriorly, over 30 allelic variants have been described, with different distributions depending on the ethnic group considered. The correlation between genotype and phenotype (expressed enzyme activity) is very good in 77%-99% of the cases. The differences can be explained by genetic and epigenetic factors (such as the use of concomitant drugs that inhibit TMPT), the age of the patient, and the existence of recent transfusions - since two different enzyme populations (donor and recipient) may be measured[27,28]. The genetic study of this enzyme allows us to distinguish among homozygous individuals (without enzyme activity) at a high risk of suffering bone marrow suppression; ultra-fast methylators (high enzyme activity) with high liver toxicity and a low response to treatment; and patients with normal and intermediate enzyme activity, which are the individuals that stand to benefit most from this medication and are at lesser risk of adverse effects. Furthermore, it can help determine which dose a treatment should be started (Table 1).
| TMPT genotype | TPMT fenotype (pmol/mgHb) | Treatment dosis(mg/kg) |
| Homozygous | < 10 | Avoid or consider 0.1-0.2 |
| Heterozygous | 10-24 | 1-1.5 |
| Wild type (normal) | 25-35 | 2-2.5 |
| Wild type (high) | > 35 | 0.5 + 100 mg allopurinol |
Allopurinol reduces the levels of 6-methylmercaptopurin (6-MeMp) and increases those of 6-thioguanine (6TG). Although its mechanism of action is not clear, it has been suggested that the drug inhibits the enzyme xanthine oxidase through competitive inhibition or reduces the availability of its substrate[29,30]. In daily practice, the TP dose should be reduced by 25% in those patients who require allopurinol and present with a normal TPMT genotype (Table 1). Taking into account that only about one-third of all cases of bone marrow suppression in patients receiving TPs are explained by genetic disposition and that the origin is, therefore, multifactorial and will require constant laboratory test monitoring, many authors have questioned whether this strategy is cost-effective. Nevertheless, most current clinical guides do not recommend genetic study before starting treatment with TPs. On the other hand, such studies are not available in many hospitals; laboratory test monitoring is, therefore, the safest alternative.
Less scientific evidence is available regarding the other enzymes.
The enzyme xanthine oxidase (XO) is the second most important enzyme in the metabolism of TPs. It is found in many tissues, although its main activity is located in the small bowel and liver. Until a few years ago, it was only known that XO activity varied from one person to another. Discordant variations depending on patient gender or ethnicity had been described[31,32], together with changes induced by environmental factors, such as smoking and the diet. In 2008, a Japanese group described three polymorphisms of the gene encoding for XO (G514A, A3326C and A3662G) that are linked with enzyme activity, with the population being divided into low, normal, or high activity[33]. These polymorphisms have not been studied in Caucasians or in patients with IBD. At present, we are only able to extrapolate that individuals with low enzyme activity would be at greater risk of suffering adverse effects, while those with high activity would experience treatment failure.
The enzyme inosine triphosphate pyrophosphatase (ITPase) controls the intracellular levels of inosine triphosphate (ITP), transforming it into inosine monophosphate, which acts as a substrate for other enzymes. When the enzyme is deficient, ITP accumulates in enterocytes. Deficiencies of this enzyme have been known for almost half a century and have been evaluated in different ethnic groups (with incidences of 5%-7% among Caucasians and African populations and up to 15% in Asian populations). Five polymorphisms have been described, of which only two are associated with enzyme inactivity: C94A, with an activity between 0% and less than 25% of normal and IVS2 + 21AC, with an activity of 60% of normal[24].
The results from IBD studies are conflicting regarding side effects (pseudoinfluenza syndrome, rash, and pancreatitis) and bone marrow toxicity of azathioprine (Table 2). These studies, in general, have few patients and an even smaller number of patients with the relevant polymorphisms and have been carried out in different ethnic groups. Reliable conclusions, therefore, cannot be drawn. Pseudoinfluenza syndrome causes a large number of patients to abandon the medication. Currently, he importance of the genotype of ITPase in relation to side effects and early treatment suspension is not known.
| Study (year) | No. of patients | Conclusion |
| Marinaki et al[34] (2004) | 130 | Significant association with flu-illness, rash, and pancreatitis |
| No association with mielotoxicity | ||
| Allorge et al[35] (2005) | 72 | No association with flu-illness, rash, pancreatitis, or mielotoxicity |
| Gearry et al[36] (2004) | 147 | No association with flu-illness, rash, and pancreatitis |
| De Ridder et al[37] (2006) | 72 | No association with side effects |
| Hindorf et al[38] (2006) | 60 | No association with side effects |
| von Ahsen et al[39] (2005) | 71 | Early withdrawal of therapy but no association with specific adverse events |
| Ansari et al[40] (2008) | 202 | Association with flu-like symptoms but not withdrawal of therapy |
| van Dieren et al[41] (2005) | 109 | Not associated with an increased risk for the development of leucopenia and other side effects |
| Zelinkova et al[42] (2006) | 262 | Increased risk of leucopenia |
| Uchiyama et al[43] (2009) | 16 | Increase risk of mielotoxicity (leucopenia) |
| Shipkova et al[44] (2011) | 160 | Increase risk of mielotoxicity |
| Kim et al[45] (2010) | 248 | No association with leucopenia |
| Zabala-Fernández et al[46] (2011) | 232 | Significant association with artralgia |
Prior to the year 2006, it was believed that the conversion of azathioprine to 6-mercaptopurine (6-MP) was not mediated by enzyme action. That year, Eklund demonstrated that it was catalyzed by the glutathione-S-transferases (GSTs)[47]. This author analyzed 14 variants, where GST-A1, GST-A2, and GST-M1 were the three with the highest enzyme activity. All of them are polymorphic. The studies in patients with IBD only allow us to affirm that individuals with low enzyme activity will have low 6-MP levels and, therefore, will not respond to the medication, while patients with ultra-fast activity are at an increased risk for adverse effects due to high 6-MP levels. If this is confirmed, the clinical application would be evident, since the problem could be overcome by directly prescribing 6-MP at the correct dose.
Other enzymes, such as inosine monophosphate dehydrogenase, hypoxanthine phosphoribosyltransferase, etc., have been studied, although their application at the present time is unclear.
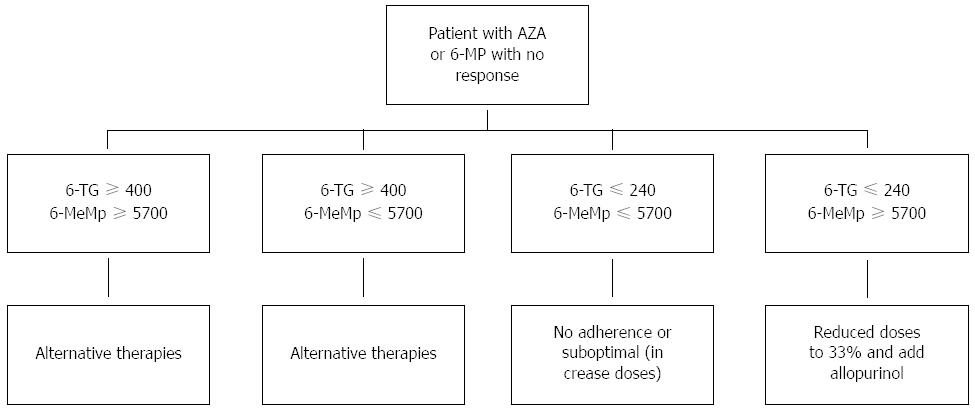
Likewise, studies have attempted to determine the relationship between the levels of metabolites (6-TG and 6MeMP) and the response to treatment or development of adverse effects. Once again, the results have been contradictory, with some studies describing a relationship between activity and the metabolite levels, while others indicating the opposite. A meta-analysis suggested that 6-TG levels above 260 pmol/8 × 108 imply that the patient has a greater probability of disease remission[48]. Blood 6-TG levels above 400 pmol/8 × 108 red blood cells increase the risk of bone marrow toxicity[49], and 6-MeMP levels above 5700 pmol/8 × 108 red blood cells increase the risk of liver toxicity[50,51]. At present, metabolite determination is not available on a generalized basis in clinical practice, and its use is controversial. However, in patients lacking a clinical response, it may help in deciding medication changes (Figure 2).
Methotrexate (MTX) is usually used as an alternative to treatment with TPs in CD both for flare-ups and as a maintenance therapy. Its usefulness in UC is more controversial.
The mechanism of action of MTX in IBD has not been clearly established. The drug is a folic acid antagonist and blocks purine and pyrimidine synthesis. Those tissues characterized by greater cellular regeneration (turnover) show more toxic effects. Consequently, the main adverse effects of MTX are bone marrow suppression, mucositis, and gastrointestinal and hepatic alterations. Folic acid supplementation reduces these side effects, although up to 30% of all patients have to suspend the medication.
Most pharmacogenetic studies of MTX have focused on patients with hematological tumors and rheumatoid arthritis (RA). In both of these disease conditions, the dosage and administration route are very different from those used in IBD; the extrapolation of results is therefore not possible.
Herrlinger has carried out the only study to date in patients with IBD. Patients with the 1298C allele of the enzyme MTHFR are more susceptible to adverse effects, although these data are in contrast with those recorded for other diseases[52].
Infliximab (IFX) is a chimeric IgG1 monoclonal antibody (Ab) targeted to TNF-α. It is used for induction and maintenance in patients with moderate to severe flare-ups of IBD. IFX is very useful and has been shown to produce mucosal healing and to reduce the number of flare-ups, hospital admissions, and surgeries. A number of clinical factors indicative of a good response have been described, such as the start of patient treatment at a young age, colonic location of the disease, and associated immunosuppressor therapy. It seems that a shorter duration of the disease, non-smoking, and elevated C-reactive protein levels at the start of therapy also favor a good response[53,54]. Even so, 25% of all patients are primary non-responders, and 20%-30% lose responsiveness over time (secondary non-responders). Most of these latter cases are a consequence of the production of antibodies against IFX[11-13]. Furthermore, the drug is expensive and has potentially serious side effects.
Table 3 summarizes the genetic studies on treatment of IBD with IFX. Studies have been carried out on genes that encode TNF-α, the TNF-α receptor, genes regulating the expression of TNF-α (NOD2/CARD2), apoptotic mechanisms, and other proinflammatory cytokines. To date, it has not been possible to demonstrate an association between drug response and a specific gene. There are two main issues: on the one hand, many studies failed to reach statistically significant results because of the small number of patients involved, and on the other hand, the studies are not always comparable, since different response criteria were used (C-reactive protein, CDAI score, Harvey-Bradshaw index), patients with different degrees of disease activity were included, and different doses were administered.
| Study (year) | Patients recruited | Response criteria | Genes investigated | Conclusion |
| Taylor et al[55] (2001) | 75 | CDAI | Polymorphims TNF/LTA region | Homozygosity for the LTA1-1-1-1 haplotype may identify subgroups with poorer response |
| Louis et al[56] (2002) | 226 | CDAI | TNFA | No association with treatment outcome |
| Mascheretti et al[57] (2002) | 90 | CDAI | TNF and TNFR polymorphism | 196Arg homozygotes had poorer clinical response than 196Met |
| 444 | heterozygotes and homozygotes (P = 0.036) | |||
| No predictive treatment outcome | ||||
| Mascheretti et al[58] (2002) | 534 | CDAI | CARD15/NOD2 | A strong relation to susceptibility to CD but not association with treatment outcome |
| Vermeire et al[59] (2002) | 245 | CDAI | CARD15/NOD2 | Not predictive of treatment outcome |
| Pierik et al[60] (2004) | 166 | CDAI | TNF/TNFR | Biological response to infliximab was lower in patients carrying TNFR1-36G |
| Matsukura et al[61] (2008) | TNFRSF1A | 28% of G allele heterozygotes and homozygotes responded | ||
| 80 | HBI | TNFRSF1B | compared to 73% of A allele homozygotes (P = 0.04) | |
| 5% of patients with AT haplotype responded compared to 20%-40% of patients with other haplotypes (P = 0.01) | ||||
| Louis et al[62] (2004) | 200 | CDAI | FcγRIIIa | Positive (V/V genotype) association with good treatment outcome |
| Urcelay et al[63] (2005) | 40 | CDAI | IBD5(5q31) | Polymorphims TT is associated with negative response |
| Hlavaty et al[64] (2005) | 287 | CDAI | FASL/CASP9 | Positive association |
| Hlavaty et al[65] (2007) | 287 | CDAI | FASL | Negative association (stadistical model) |
| Willot et al[66] (2006) | 189 | CRP | CRP | Polymorphims evaluated are not associated with treatment outcome |
| Dideberg et al[67] (2006) | 214 | CDAI | TNF/LTA region | No association |
| Dideberg et al[68] (2006) | 186 | CDAI and CRP | ADAM17 | Minor allele homozygotes for each SNP associated with clinicalresponse (P < 0.002) |
| Jürgens et al[69] (2010) | 90 | CAI | IL-23RIL-2/IL-21 | Homozygous carriers of IBDrisk-increasing IL-23R variants more likely to respond to infliximab than homozygous carriers of IBD risk-decreasing IL-23R variants (P = 0.001) |
| Dubinsky et al[70] (2010) | 94 | HBI and Partial Mayo score | rs2241880 2q37/ATG16L1rs21889625q31rs6908425 6p22/CDKAL1rs762421 21q22/ICOSLGrs2395185 6p21/HLA-DAQ1rs2836878 21q22/BRWD1 | Six known susceptibility lociassociated with primary nonresponse(P < 0.05). Only the 21q22.2/BRWDIloci remained significant in thepredictive model |
Perhaps, in the near future, information regarding the genotype of TNF-α and its receptor may help us to identify non-responders to anti-TNF-α therapy.
Understanding the pharmacokinetics of a drug is very important when adjusting the dose required to guarantee therapeutic concentrations, since many factors influence drug concentration in blood. In clinical practice, the determination of blood drug levels has been used to monitor treatments with drug substances that have a narrow therapeutic margin or window. In IBD, such monitoring has been applied to cyclosporine A and tacrolimus; and in recent years, it has become particularly important in the management of anti-TNF-α drugs.
Differences in the administration route, degradation, and clearance of anti-TNF-α determine its concentration in blood and, in turn, its treatment response. In this regard, achieving adequate blood drug levels is correlated with clinical and endoscopic remission of the disease[71,72]. When the anti-TNF-α drug is administered intravenously (e.g., IFX), the maximum blood drug concentration is reached immediately after infusion, with little variability among patients. However, when the anti-TNF-α drug is administered subcutaneously (e.g., antidrug antibodies (ADAs), golimumab, and certolizumab), the maximum concentration is reached after approximately 10 days, and the bioavailability ranges between 50%-100%[73,74].
The clearance of anti-TNF-α drugs from blood is complex and multifactorial. Variables that increase drug clearance include those that depend upon or reflect the severity of the disease (hypoalbuminemia, decreased hemoglobin levels, C-reactive protein elevation, TNF-α, leukocytosis, and increased IFX intestinal losses), demographic parameters (increased body mass, male gender, and age under 40 years) and immunogenicity (the development of antibodies against the drug)[75]. The concomitant use of immunosuppressors (TP drugs and MTX) is associated with decreased immunogenicity and increased anti-TNF-α drug levels.
Up to 40% of all patients that respond to anti-TNF-α drug treatment will require one or more dose adjustments in order to maintain treatment efficacy. After 1 year of treatment, efficacy is maintained in only one-third of all responders[76].
The need for dose adjustment of these drugs may occur at two time points during treatment: at the start (primary failure) or during the maintenance phase (secondary failure).
Primary failure, or a primary lack of response, refers to the absence of improvement in signs and symptoms of the disease that leads to treatment suspension during the induction phase[77]. The time point at which this primary lack of response is assessed varies among different studies. In patients treated with IFX, as in the ACCENT I trial[78], assessment was made 2 wk after the first IFX infusion. In contrast, assessment in the ACCENT II trial was made 10-14 wk after induction[79]. In studies including patients treated with ADA, the response was assessed in week 4 in the CLASSIC I trial[80] and in week 6 in the CLASIC II trial[81]. Among the different published studies, the lack of primary response to treatment rate with anti-TNF-α drugs varied from 10%-40% and was found to be higher in UC than in CD. Lack of response was noted particularly in severe presentations of UC. This observation could be explained by increased drug clearance as a result of a greater inflammatory intestinal surface[77].
Secondary failure, or secondary lack of response, is defined as failure occurring in the course of treatment in those patients that have responded to induction therapy and is observed in approximately 13% of the patients treated with IFX[82] and in 10%-24% of those treated with ADA[83]. Failure is more frequent in the first year of therapy.
Table 4 and Table 5 describe the risk factors associated with primary and secondary failure[11,84].
| Crohn's disease | Ulcerative colitis |
| Duration of the disease > 2 yr | Old age |
| Smoking | Anti-neutrophil cytoplasmic (ANC) antibodies |
| Extensive small bowel involvement | Negative antibodies against Saccharomyces cerevisiae |
| Low C-reactive protein levels | Previous exposure to anti-TNF-α drugs |
| Genetic mutations or polymorphisms of the apoptosis and caspase 6 genes and locus IBD 5 |
| Individual differences in bioavailability and pharmacokinetics |
| Symptoms not due to inflammatory bowel disease |
| Lack of adherence to therapy |
| Drug loss in stools |
| Intermittent treatments |
| Non-inflammatory symptoms |
| Structuring disease pattern |
| Smoking |
| Development of antibodies |
In secondary failure, the most widely investigated factor is the development of ADAs. When a loss of treatment response occurs and once the possible existence of intercurrent processes and lack of adherence to therapy have been discarded, the patient requires dose adjustment or a switch to some other molecule. In this context, it is very useful to know the blood drug levels and whether or not ADAs have developed.
The development of ADAs is conditioned by the patient immune condition and is much more common in intermittent treatments (over 60% of the cases) than in maintenance therapy (10%-20%). Furthermore, those patients who have developed ADAs and remain without treatment for extended periods exhibit slow clearance of these antibodies[85].
Many studies have been carried out to determine when and how antibody titers should be measured in order to perform the necessary adjustments and to define the interval during which therapeutic blood drug levels are present. In routine clinical practice, if this is not possible, dose adjustment is made empirically, shortening the interval between doses or switching to another molecule when loss of response occurs or an immune-mediated reaction is observed. Three different methods can be used to measure drug levels.
The most widely used option is the enzyme linked immunosorbent assay (ELISA), which can also be used to determine ADA titers. This technique requires the anti-TNF-α drug to be undetectable in blood, since it binds to the antibodies and forms immune complexes that are not detected.
Radioimmunoassay (RIA) is similar to ELISA, although its use is limited since it involves the use of a radioactive reagent. Furthermore, as for ELISAs, it cannot detect the presence of ADAs if there are detectable drug levels present in blood.
Another method for determining the drug and antibody is variable mobility testing, which allows for the detection of ADAs (against IFX and ADA) in the presence of drug in blood.
The determination of anti-TNF-α drug levels is performed immediately before administration of the next dose. ADAs are only detected when the drug levels are undetectable, since the most widely used technique in clinical practice is the ELISA.
Different studies have correlated the development of ADAs with an increased risk of infusion reactions and increased drug clearance from blood[86]. However, not all studies have established correlations to loss of response. In this regard, a systematic review published by Chaparro et al[87] found no differences between maintenance or loss of response according to whether ADAs develop or not, while the meta-analysis conducted by Nanda et al[88] found that patients who develop ADAs have a 3-fold greater risk of loss of treatment response than patients who do not develop such antibodies.
Although there is great variability among studies in defining the minimum effective concentration of anti-TNF-α drugs, the determination of drug levels has been correlated with improved disease control and to clinical and endoscopic remission. This is important in designing treatment algorithms[71]. To date, the management approach in clinical practice depends on the drug values and on the presence or absence of ADAs (Table 6).
| Anti-TNF-αdrug levels | Antibodies | Action |
| Low | Negative | Increase dose |
| Low | Positive | Switch drug |
| High | Not determined | Switch to a drug with a different mechanism of action |
Current studies are evaluating the role of routine anti-TNF-α drug level measurements in blood during therapy, as is done with other drug substances, in order to facilitate better dose adjustment[86]. However, the different studies propose different cutoff values when defining the therapeutic range. Therefore, studies are needed to determine the adequate therapeutic interval or window.
The biological drugs currently authorized for the treatment of IBD are monoclonal antibodies targeted to TNF-α (IFX, ADA, certolizumab, and golimumab) and monoclonal antibodies targeted to the leukocyte integrins (natalizumab, approved by the United States Food and Drug Administration (FDA) for refractory CD; and vedolizumab, approved by the European Medicines Agency (EMA) and the FDA).
A number of lines of research have been developed with the aim of blocking the inflammatory process at different levels. In the coming years, new drugs will be introduced that will contribute to the expansion of therapeutic options.
The drug options that are presently in the most advanced stages of development are described below.
Interleukins (ILs) are soluble inflammatory response messenger (signaling) molecules. Their role in IBD has been clearly established, and research in this field has been particularly wide-ranging and advanced[89].
Anti-IL-12/23 drugs: Ustekinumab is the drug with the most advanced results available to date. Ustekinumab is a fully humanized IgG1κ anti-IL-12/23 monoclonal antibody. It specifically binds to the p40 protein subunit shared by both of the mentioned ILs. Binding prevents the mentioned subunit from interacting with the IL-12Rβ1 receptor protein, which is expressed on the surface of immune cells - thereby inhibiting innate and adaptive immune response stimulation. In an inflammatory environment, naïve CD4+ T cells are induced to interferon (IFN)-γ producing Th1 cells by the action of IL-12 and to Th17 cells by the action of IL-23. The Th17 cells, in turn, are responsible for the production of proinflammatory cytokines, such as IL-17, IL-17F, IL-6, and TNF-α[90,91]. Blocking this pathway has been successfully employed in animal models[92,93], and both ILs play a key role in the inflammatory processes of CD[91,94,95].
The results of a first double-blind and placebo (PB)-controlled phase II clinical trial were published in 2008[96]. This study had a complicated design that included two patient populations. In population 1, the results at the primary endpoint were discouraging, with a clinical response rate in week 8 of 49% in the group of patients treated with ustekinumab vs 40% in the PB group (P = 0.34). However, in a subgroup of 49 patients previously treated with IFX, statistical significance vs PB was reached, with a response rate of 59% and 26%, respectively (P = 0.05), in week 8. In population 2, the clinical response rate with ustekinumab in week 8 was 43% in the subcutaneous treatment group and 54% in the intravenous treatment group. Failure of the study to confirm the primary endpoint was attributed to the high percentage response observed in the PB group. No serious adverse events were detected in week 8, and the recorded problems were similar to those seen in the PB group. Overall, the final conclusion was favorable regarding the capacity of the active drug to elicit a clinical response in the induction phase.
The CERTIFI trial was published in 2012[97]. This was a randomized, double-blind, PB-controlled phase IIa study on the efficacy of ustekinumab in patients with moderate to severe CD refractory to IFX. A total of 526 patients resistant to treatment (50% of the subjects having received at least two anti-TNF-α drugs) were randomized to three intravenous induction treatment arms (1, 3, or 6 mg/kg of ustekinumab) and a PB arm. The 145 patients responding to ustekinumab in week 6 were randomized to subcutaneous maintenance therapy in weeks 8 and 16 with PB vs ustekinumab 90 mg. The primary endpoint was the clinical response rate in week 6, where the recorded percentages were 36.6%, 34.1%, and 39.7% (1, 3, or 6 mg/kg of ustekinumab) vs 23.5% in the PB arm. Only the 6 mg dose reached statistical significance vs PB. In the maintenance phase, 41.7% of the patients treated with ustekinumab showed clinical remission vs 27.4% of the patients in the PB arm (P = 0.03), and the clinical response rate was 69.5% vs 42.5%, respectively (P < 0.001). The safety profile was found to be similar to that of other biological drugs.
The publication of the results of three phase III clinical trials is currently pending. The first of these studies (UNITI-1)[98] is a randomized, double-blind trial designed to evaluate the efficacy and safety of induction with ustekinumab in patients with moderate to severe CD who have failed or are intolerant to anti-TNF-α therapy. The primary endpoint is clinical response in week 6, while the secondary endpoints are remission and clinical response in week 8. A total of 769 patients have been randomized to three arms: (1) intravenous PB; (2) intravenous ustekinumab 130; and (3) intravenous ustekinumab 6 mg/kg as a single dose. The study ended in July 2013. The second study (UNITI-2)[99] has the same design as the first, although the included patients are naïve to biological drugs and show failure or intolerance to immunosuppressors or corticosteroids. This study ended in October 2014 and includes a total of 642 patients. Lastly, the IM-UNITI[100] trial is also a randomized, double-blind, PB-controlled, parallel group multicenter study. This trial was designed to determine efficacy in the maintenance phase of CD and is currently in the recruitment stage. The study plans to include 1310 patients from the two previously mentioned trials, with conclusion in November 2018.
There are two other anti-IL-12/23 molecules: briakinumab (ABT-874), which targets the p40 subunit, and apilimod mesylate, which is a small molecule administered via the oral route that inhibits the transcription of IL-12 and IL-23. The initial results with both molecules have not been significant[101,102].
Anti-IL-6 drugs: Interleukin-6 is a proinflammatory cytokine produced by different types of cells. It participates in a series of processes including T lymphocyte activation and immunoglobulin secretion through the differentiation of B cells into plasma cells[103,104]. Interleukin-6 exerts its action via membrane or soluble receptors[105]. In healthy individuals, the IL-6 levels are low and increase in the context of immune processes[103]. This cytokine is increased in CD in the same way as its soluble receptor, and its levels are correlated with the C-reactive protein concentrations[106,107].
Tocilizumab is an IgG1 monoclonal antibody indicated in RA. It binds specifically to the soluble and membrane receptors of IL-6. In one study, 36 patients with active CD were randomly assigned to two treatment arms (intravenous 8 mg/kg every 2 wk or every 4 wk) or PB[108]. The clinical response rate in the group administered tocilizumab every 2 wk was 80% vs 31% in the PB arm, although only 20% achieved clinical remission. The drug was well tolerated, although studies in RA have shown neutropenia, altered liver biochemical parameters, and hyperlipidemia. In this regard, dyslipidemia might prove to be a safety problem of the drug long term[109].
Two phase II studies involving two monoclonal antibodies (BMS-945429, formerly ALD518, and PF-04236921) targeted to IL-6 are currently ongoing. No results are yet available, since patient recruitment has ended only recently[110,111].
Other anti-interleukin drugs: Interleukin-2 is another molecule that plays a key role in T cell activation and proliferation.
Basiliximab and daclizumab are monoclonal antibodies targeted to CD25, which is the alpha-chain of the IL-2 receptor. Both drugs are used for the prevention of renal graft rejection. Initial studies with basiliximab documented clinical remission in eight out of 10 patients with UC refractory to corticosteroid therapy[112], and a later study recorded a remission rate of up to 65%, without a control group though[113]. In the case of daclizumab, a comparative study vs PB failed to demonstrate positive efficacy results[114].
IL-13 is produced by naive T cells and activates natural killer (NK) cells, which in turn synthesize IL-13. IL-13 has been shown to play a key role in the pathogenesis of UC[115,116].
Two phase II trials (IMA-648 [arunkinzumab] and CAT-345 [tralokinumab]) published in 2014 randomized the patients to the antibody[117,118] at different doses (in the case of the first study) or PB. Neither study recorded differences in terms of treatment response or clinical remission. Both studies documented a tendency towards lower activity index scores, and the safety profile was favorable. A third phase II study in patients with perianal CD has been designed to evaluate the efficacy and safety of another antibody targeted to IL-13 (QAX576) vs IFX for comparison[119]. Ten patients have been included, and results from the trial are pending after the end of the recruitment phase.
Vidofludimus is an immunosuppressor that inhibits the release of IL-17 and IFN-γ [by interfering with the janus kinase/signal transducer and activator of transcription (JAK/STAT) pathway and nuclear factor kappa B (NF-κB)], which blocks the enzyme dihydroorotate dehydrogenase (DHODH). This is a small molecule administered via the oral route. The results of the ENTRANCE study, a non-controlled multicenter trial, were published in 2013[120]. This study evaluated 34 patients with corticosteroid-dependent IBD (CD and UC) treated with vidofludimus 35 mg/d orally administered (per os, po) over 12 wk. The primary endpoint was clinical remission without corticosteroids in week 12, and this was reached by 56% of the patients with CD and 50% of those with UC. The drug was well tolerated, and no serious adverse events were reported - although additional studies with larger patient series are needed to confirm this finding.
Other targets currently under investigation are antibodies against IL-17 (AMG 827), although the study that has been closed because of worsening of patient symptoms[121], IL-18 (GSK1070806)[122], and IL-21(PF-05230900)[123]. These interleukins had been implicated previously in the pathogenesis of IBD[124-126].
Anti-inflammatory interleukins: Lastly, studies have been performed to evaluate the efficacy of the anti-inflammatory interleukins IL-10, IL-11, and IFN-β. The results to date have not been encouraging[127-131].
Chemokines are small cytokines that induce chemotaxis by interacting with the chemokine transmembrane receptors bound to protein G[132,133]. Their ligand is CCL25, which in the intestine is fundamentally expressed by the luminal epithelial cells[134]. In IBD, and especially in CD, high levels of CCL25 and of T lymphocytes expressing CCR9 have been detected[135].
Vercirnon: CCX-282B (vercirnon) is a molecule administered via the oral route that acts as a CCR9 receptor antagonist and has been suggested to reduce lymphocyte trafficking towards the intestine[136]. The data derived from the PROTECT-1 multicenter trial were published in 2013[137]. This was a double-blind multicenter study randomizing 436 patients with CD to three vercirnon treatment groups (250 mg/d po, 250 mg twice daily po, 500 mg/d po) vs PB over an induction phase lasting 12 wk. Subsequently, in the case of clinical response, the patients were again randomized to vercirnon (250 mg twice daily po) vs PB until week 36. The results were not statistically significant, although in week 8 a tendency towards greater efficacy, in terms of clinical response, was observed in the group administered 500 mg/d vs PB (49% vs 60% with odds ratio (OR) = 1.53, P = 0.111). In week 52, significance was reached in terms of the percentage of patients in remission (47% vs 31% with OR = 2.01, P = 0.12). The safety profile was found to be favorable, with similar serious and non-serious adverse events rates in both groups.
BMS-936557: Regarding UC, a parallel line of research is based on IFN-γ-induced protein 10 (IP-10, also known as CXCL10). This protein is secreted by monocytes, endothelial cells, and fibroblasts in response to stimulation by IFN-γ[138]. This protein is implicated in chemotaxis and interaction phenomena with T cells through the CXCR3 receptor[139]. Inhibition of this pathway could incline the Th1 response towards a Th2 response[140]. In patients with UC, CXCL10 is over-expressed in plasma and colon tissue[141], and in animal studies it has shown a reasonable efficacy profile warranting the start of evaluations in humans[142]. BMS-936557 (MDX-1100) is a monoclonal antibody targeted to protein IP-10. Its safety profile has been evaluated in phase I studies[143], and based on the evidence obtained in patients with RA[144], a specific double-blind multicenter trial on active UC has been started[145]. This phase II trial randomized 109 patients to PB or four doses of intravenous 10 mg/kg BMS-936557 every 2 wk. The primary endpoint (clinical response on day 57) was better among the patients that had received the antibody, although statistical significance was not reached (52.7% vs 35.2%, P = 0.083). Nevertheless, the results were statistically significant in the group of subjects with the highest titers in blood (87.5% vs 37%, P < 0.001). The number of infections was greater in the active treatment group (12.7%) compared with PB (5.8%), and drug suspension because of adverse events was necessary in 3.6% of cases. At present, we are awaiting the publication of the results of two other phase II trials randomizing patients to different doses of the molecule vs PB in application to moderate or severe CD[146] or UC[147].
Other chemokine antagonists: There are other lines of research involving chemokines in autoimmune diseases, such as the binding of CXCL10 to its CXC3 receptor[148], which has been shown to be reduced in patients with CD, in parallel to reduction in the C-reactive protein levels[149]. On the other hand, it has been reported that patients with UC show serum and tissues elevations of exotaxin-1, a chemokine that acts by recruiting eosinophils at the intestinal level.
Bertilizumab is a humanized IgG4 monoclonal antibody that blocks exotaxin-1 activity[150]. A phase II study involving 42 patients with moderate or severe UC is planned, with the aim of assessing the efficacy and safety of the drug[151].
Another line of investigation involves the generation of anti-TNF-α polyclonal antibodies through active immunotherapy. This strategy is based on the use of a TNF-α derivative as a vaccine. The compound is known as TNK-kinoid (TNF-K), and while biologically inactive, it can interrupt B cell tolerance of their own cytokines, resulting in the production of high titers of antibodies[152]. A first phase I/II study was presented at Digestive Disease Week in 2011[153], involving 21 patients with moderate to severe CD assigned to different doses on an open-label basis. The safety profile was found to be favorable, with no serious adverse events, and antibodies were generated in 81% of the cases. Clinical remission in week 12 was achieved by 50% of the patients. The results of a second randomized phase II study were published in 2012, involving patients with moderate to severe CD and loss of response or with intolerance to conventional anti-TNF-α drug therapy. The trial included 68 patients randomized vs PB into two cross-over treatment arms with intramuscular doses of 180 μg on days 0, 7, 28, 84, 91, and 112, with switching of the active drug to PB in the fifth dose and of PB to the active drug in the third dose in the control group. The safety data were again favorable in this case, with only one serious adverse event related to worsening of CD, although the efficacy results were not reported[154].
The Janus kinases (JAKs) are a group of proteins corresponding to enzymes associated to cytokine receptors. They form part of a complex system of signal transmission from outside the cell towards the nucleus, activating transcription of the genes that intervene in important cell processes, such as growth, differentiation, proliferation, or migration. The process begins when the membrane receptor is stimulated by a chemical messenger, e.g., a cytokine. This receptor activates JAK, which undergoes auto-phosphorylation and, in turn, phosphorylates the STAT protein. The latter protein then binds to another phosphorylated STAT protein (i.e., it undergoes dimerization) and is translocated to the cell nucleus where DNA transcription factors are activated. This system, known as the JAK/STAT system[155], has been implicated in the pathogenesis of different diseases[156,157], including specifically IBD[158].
Tofacitinib: This is a small molecule administered via the oral route that selectively inhibits JAK1 and JAK3, affecting the signaling pathways of cytokines such as IL-2, 4, 7, 9, 15, and 21[159,160]. Blocking these pathways could suppress the activation and proliferation of lymphocytes while maintaining T cell regulatory function[161,162]. In 2002, the United States FDA approved the drug for the treatment of RA. In the case of IBD, the initial data are not encouraging in reference to CD regarding disease response or remission - although the drug is associated to a significant decrease in biomarkers (C-reactive protein and calprotectin)[162]. In reference to UC, the results of a randomized, double-blind phase II trial involving four treatment doses (0.5, 3, 10, or 15 mg/d po) or PB in 194 patients with moderate to severe UC were published[163]. The primary endpoint was clinical response in week 8. In the high dose groups (10 and 15 mg, respectively), the clinical response was greater than in the PB series - statistical significance being reached with the 15 mg dose (61% and 78% vs 42%, P = 0.10 and P < 0.001). Clinical remission reached statistical significance vs PB with both doses (48% and 41% vs 10%, P < 0.001). A subsequent sub-analysis demonstrated improvement in patient quality of life[164]. Further studies involving larger patient samples are needed to confirm the efficacy and safety of the drug.
Inhibition of IL-13: In this line of research, studies have been performed on the inhibition of the mentioned pathway with monoclonal antibodies targeted to IL-13, which is responsible for activating the JAK/STAT pathway[165]. The studies are cited in the section on interleukin antagonist drugs.
Adhesion molecules constitute one of the most advanced lines of research in IBD. Adhesion molecules are transmembrane receptors with three domains (intracellular, transmembrane, and extracellular) that induce cellular changes following stimulation by external molecules. These molecules include the integrins and lymphocyte homing receptors.
Natalizumab: The first anti-adhesion drug investigated was natalizumab, and it consists of an IgG4 monoclonal antibody targeted to integrin subunit α4. Natalizumab initially showed favorable results in CD[166,167], although the risk of progressive multifocal leukoencephalopathy (PML) secondary to reactivation of the JC virus has limited its use[168,169]. Natalizumab acts by blocking the interaction of α4β7 with mucosal vascular addressin cell adhesion molecule-1 (MadCAM-1) and the interaction of α4β1 with vascular cell adhesion molecule-1 (VCAM-1), which is critical to lymphocyte trafficking towards the central nervous system - thereby giving rise to the risk of JC virus reactivation[170].
Vedolizumab: Vedolizumab is another IgG1 monoclonal antibody that binds to integrin α4β7, preventing it from binding to its specific intestinal ligand, MadCAM-1. As a result, T lymphocyte migration towards the inflamed intestinal areas is inhibited. In contrast to natalizumab, it does not bind to integrins α4β1 and αEβ7 and does not antagonize the interaction of integrin α4 with VCAM-1. At present, and based on the results of clinical trials[171,172], vedolizumab has been approved by both the FDA and the EMA for the treatment of patients with moderate to severe CD or UC who fail to respond to conventional treatment or therapy with anti-TNF-α drugs.
AMG 181: AMG81 is another humanized IgG2 monoclonal antibody likewise targeted to integrin α4β7[173]. Early-stage studies warrant the safety, pharmacological, and tolerability profile of the drug. AMG 181 is currently being investigated in the context of two randomized, PB-controlled trials in application to both CD[174] and UC[175]. More evidence will be obtained in the coming years.
AJM300: AJM300 is a small molecule administered via the oral route that inhibits the α4 receptor. It is known to inhibit the binding of integrin α4β1/α4β7 - expressing cells to VCAM-1/MAdCAM-1 and has efficacy in the prevention of colitis in animal studies[176]. The results of a double-blind multicenter phase IIa trial were published in 2012[177]. The study involved the randomization of 102 patients with active-moderate UC to receive AJM 300 at a dose of 960 mg or PB three times daily for 8 wk. The primary endpoint was the clinical response rate in week 8, which was found to be 62.7% vs 25.5% in the AJM300 group and PB group, respectively (OR = 5.35; P = 0.0002). The secondary endpoints (clinical remission and mucosal healing in week 8) were also favorable to the study molecule (23.5% vs 3.9% with OR = 7.81; P = 0.0099 and 58.8% vs 29.4% with OR = 4.65; P = 0.0014). No serious adverse events (progressive multifocal leukoencephalopathy) were documented over the short term.
Etrolizumab: Etrolizumab is a humanized monoclonal antibody targeted to the B7 subunit present in integrins α4β7 and αEβ7. The results of a double-blind multicenter phase II trial were published in 2013[178]. The study involved the randomization of 124 patients with refractory moderate to severe UC to etrolizumab in two treatment arms (subcutaneous 100 mg monthly or subcutaneous 300 mg monthly plus a loading dose of subcutaneous 420 mg between weeks 0 and 2) or PB. The primary endpoint was the clinical remission rate in week 10, with significant results in both active treatment arms vs PB (20.5% and 10.3% vs 0%, P = 0.004 and P = 0.049). In the subgroup of patients naïve to anti-TNF-α drug treatment, the differences obtained with the 100 mg dose were even greater (43.8% vs 0%, P = 0.007). The adverse event rates were similar in all three groups.
MECA-367 and PF-00547,659: The pharmacological properties of two monoclonal antibodies (MECA-367 and PF-00547,659) targeted to MAdCAM, with inhibition of binding of the latter to integrin α4β7, were presented in 2009[179], but subsequent data are only available for PF-00547,659. In 2011, a double-blind study randomized 80 patients with active UC to different single or multiple subcutaneous or intravenous doses of PF-00547,659 vs PB. Good efficacy vs PB was recorded, with endoscopic improvement of the lesions, a good safety and tolerability profile, and no immunogenicity. Results of the TURANDOT trial were published this year[180]. This is a double-blind, PB-controlled multicenter efficacy and safety study in 357 patients with moderate to severe UC randomized to PB or to doses of the antibody (7.5, 22.5, 75, or 225 mg every 4 wk for three doses). The primary endpoint was the remission rate in week 12, and the secondary endpoints were the response rate and mucosal healing rate in week 12. Remission and mucosal healing were significantly greater in the 22.5 mg and 75 mg dose groups vs PB, while response was significantly greater for the 22.5 mg and 225 mg groups vs PB. The safety profile remained favorable.
Laquinimod: Laquinimod is a molecule administered via the oral route, with great bioavailability and with purported regulatory activity upon antigen-presenting cells (APCs) and T lymphocytes[181,182]. A phase II trial randomizing 180 patients to different doses of active treatment vs PB reported that increasing dose was inversely proportional to the percentage response or remission[183]. Specifically, the lowest dose (0.5 mg) elicited a clinical response in 55.2% of the patients treated with the active drug vs in 31.7% of the patients administered PB, with respective remission rates of 48.3% vs 15.9% in week 8.
Masitinib: Gastrointestinal mast cells are usually found beneath the epithelial surfaces and are able to release cytokines, chemokines, prostaglandins, histamine, and heparin. The proliferation of these cells increases at intestinal mucosal and submucosal levels in CD[184,185]. Masitinib is a selective tyrosine kinase inhibitor that targets the c-kit receptor (expressed by mast cells), platelet-derived growth factor receptor-α/β, lymphocyte-specific kinase, Lck/Yes-related protein, fibroblast growth factor receptor 3, and the focal adhesion kinase activation pathway[186]. A phase IIb/III phase trial with 450 CD patients is currently underway with this molecule[187].
Visiluzimab, rituximab, and abatacept: Attempts have been made to invert the natural inflammation process in which T cell proliferation vs apoptosis is observed. Visiluzimab (a humanized monoclonal antibody against T cell receptor CD3) and rituximab (a chimeric monoclonal antibody targeted to B cell receptor CD20) were evaluated in IBD, where they were shown to have an unfavorable safety profile[188-193]. Abatacept is a recombinant protein that blocks T cell co-stimulation by the antigen-presenting cells (APCs). Its use has been approved for RA, although the results for IBD have been discouraging[194,195].
Morgensen: Immunosuppressive cytokine transforming growth factor (TGF)-β1 is a secreted cytokine with known functions in growth, proliferation, differentiation, and apoptosis. It has been linked to immune regulating functions, depending on the cell upon which it acts and the environment in which it is found. Diminished TGF-β1 activity has been reported in CD. This is due to the binding of an intracellular protein called SMAD 7 to the TGF-β1 receptor[196]. A molecule known as Morgensen (GED301) was first described in 2001. This is an antisense oligonucleotide that hybridizes to the human SMAD7 messenger RNA (mRNA) and facilitates RNase H-mediated RNA degradation through a classic antisense mechanism. Its release is pH-dependent; accordingly, it is released in the ileum and right colon[196]. Favorable safety results from a phase I study in 15 patients with CD were published in 2012[197]. Recently, a phase II trial has evaluated 166 patients with active CD assigned to three active drug treatment groups vs PB[198]. The clinical remission rates associated with the two highest drug doses were 55% and 65% vs 10% for PB (P = 0.001), while the clinical response rates were 58% and 72% vs 18% (P = 0.04). The total and serious adverse event rates were similar, with no reported neoplasms.
RPC1063 and GLPG0974: Lastly, the results of two randomized, double-blind, PB controlled trials were presented at the European Crohn’s and Colitis Organisation (ECCO) meeting in 2015 on two new molecules: RPC1063 and GLPG0974.
RPC1063 is a molecule administered via the oral route with selectivity for sphingosine 1-phosphate (S1P) 1 and 5 receptor modulator. The study included 197 patients with moderate or severe UC that were administered 0.5 mg or 1 mg of the active drug vs PB, once daily[199]. The primary endpoint (the proportion of subjects in remission in week 8) reached statistical significance in the highest dose group (16.4% vs 6.2%, P = 0.048). The adverse events profiles were comparable between groups, with approximately 31% of patients experiencing a treatment emergent adverse event.
GLPG0974 is a selective free fatty acid receptor antagonist. Binding of the fatty acids to their receptor induces neutrophil activation and migration. The results were assessed in 45 patients with mild to moderate UC treated with GLPG0974 during 4 wk (200 mg/12 h po vs PB)[200]. A decrease in calprotectin levels and myeloperoxidase-positive cells was recorded, although there was no difference in terms of response, clinical remission, or mucosal healing. The safety and tolerability profile was favorable.
Stem cells: Different stem cell therapies have been used in CD and UC. Stem cell therapy involves the use of autologous hematopoietic stem cell transplantation in CD, mesenchymal stem cells administered systemically or locally in perianal fistulas, and other cell treatments where experience is more limited, such as regulatory T cells and dendritic cells. We refer readers to two recent reviews carried out by our group in this field[201,202].
Herbal remedies: Studies have been made that specifically compare treatment with herbal remedies vs PB or even conventional therapy, although there are discrepancies in the results due to the lack of homogeneity among the different studies. The findings in terms of safety are favorable, and the predictable costs are lower than in the case of conventional treatment. We recommend a recent systematic review, which affords a more detailed analysis of this subject[203] (Table 7). Among the different herbal remedies employed, special mention must be made of Andorgraphis paniculata extract, known as HMPL-004, which has been found to reduce TNF, IL-1β, IFN-γ, and IL-22 in the development of experimental colitis[204].
| Author | n | Producto | Comparador | Indicación | Remisión/response herbal vs PB or drug (%) |
| Langmead | 44 | Aloe vera | Placebo | Induction remission CU | 30 vs 7 |
| Ben-Arye | 23 | Triticum aestivum | Placebo | Induction remission CU | 91 vs 42 |
| Khan | 14 | Bovine colostrum enema | Placebo | Induction remission CU | |
| Sandborn | 224 | HMPL-004 | Placebo | Induction remission CU | 38/60 vs 25/40 |
| Fukunaga | 30 | Xilei-san suppository | Placebo | Induction remission CU | 46 vs 0 |
| Zhang | 35 | XIlei-san enema | Enema dexametasona | Induction remission CU | |
| Tang | 120 | HMPL-004 | Mesalazina | Induction remission CU | 21 vs 16 |
| Gupta | 30 | Boswellia serrata | Sulfasalazina | Induction remission CU | 70 vs 40 |
| Cheng | 153 | Jian Pi Ling tablet | Sulfasalazina | Induction remission CU | 53 vs 28/19 |
| Placebo | |||||
| Wang | 106 | Kui Jie Quing enemas | Sulfasalazina | Induction remission CU | 72 vs 9 |
| Prednisolona | |||||
| Cheng | 118 | Yukui tang ablets | Prednisolonoa | Induction remission CU | 33 vs 17 |
| Neomicina | |||||
| Vitamina B | |||||
| Fernández Bañares | 105 | Plantago ovata sedes | Mesalazine | Maintenance remission CU | 60 vs 65 |
| Hanai | 89 | Curcumin | Placebo | Maintenance remission CU | 95 vs 79 |
| Greenfield | 43 | Oenothera biennis | Evening primrose oil and olive oil | Maintenance remission CU | |
| Omer | 40 | Artemisia absinthium | Placebo | Treatment and prevention recurrence EC | 65 vs 0 |
| Krebs | 20 | Artemisia absinthium | Placebo | Treatment and prevention recurrence EC | 80 vs 20 |
| Gerhardt | 102 | Boswellia serrata extract | Mesalazine | Treatment and prevention recurrence EC | 36 vs 31 |
| Ren | 20 | Tripterygium wilfordii | Placebo | Treatment and prevention recurrence EC | |
| Holtmeier | 108 | Boswellia serrata extract | Placebo | Treatment and prevention recurrence EC | 60 vs 55 |
| Tao | 45 | Tripterygium wilfordii | Mesalazine | Treatment and prevention recurrence EC | 68 vs 61 |
| Liao | 39 | Tripterygium wilfordii | Sulphasalazine | Treatment and prevention recurrence EC | 94 vs 75 |
Fecal transplant: Fecal transplant is a therapeutic alternative in gastrointestinal processes, such as Clostridium difficile-infection, metabolic syndrome, constipation, pouchitis, irritable bowel syndrome, and IBD[205]. In IBD, effectiveness appears to be related to the stability of the colonization of donated bacteria[206]. The experience in CD is limited to six patients in total[207,208]. In UC, there are data available for up to 106 patients[206,208-212]. The study with more patients included 62 cases with UC, finding clinical improvement in 92% and clinical remission in 68%. In the remaining studies, the results have not been as favorable as the aforementioned studies, with clinical remission ranging from 0% to 30% and clinical response from 0% to 70%. The relatively small number of patients evaluated so far does not allow for the establishment of firm conclusions, but it stresses the importance of the microbiota in the pathogenesis of IBD.
At present there are a large number of ongoing studies in various stages of research on new molecules for the treatment of IBD. An analysis of mucosalhealing is needed in order to evaluate fully the impact of these therapies. In this way, it is expected to change the course of treating IBD. Among the different alternatives, anti-adhesion molecules and interleukin drugs are promising anti-TNF-α treatments.
With developments in the near future in pharmacogenetics, clinical pharmacology, the use of indices that try to classify patients by defining profiles of severity, and new drug molecules, personalized tailoring of treatment strategies will be possible for IBD.
P- Reviewer: Lakatos PL, Marie JC S- Editor: Ma YJ L- Editor: Filipodia E- Editor: Liu XM
| 1. | Molodecky NA, Soon IS, Rabi DM, Ghali WA, Ferris M, Chernoff G, Benchimol EI, Panaccione R, Ghosh S, Barkema HW. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology. 2012;142:46-54.e42; quiz e30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3789] [Cited by in RCA: 3527] [Article Influence: 271.3] [Reference Citation Analysis (5)] |
| 2. | Burisch J, Pedersen N, Čuković-Čavka S, Brinar M, Kaimakliotis I, Duricova D, Shonová O, Vind I, Avnstrøm S, Thorsgaard N, Andersen V, Krabbe S, Dahlerup JF, Salupere R, Nielsen KR, Olsen J, Manninen P, Collin P, Tsianos EV, Katsanos KH, Ladefoged K, Lakatos L, Björnsson E, Ragnarsson G, Bailey Y, Odes S, Schwartz D, Martinato M, Lupinacci G, Milla M, De Padova A, D’Incà R, Beltrami M, Kupcinskas L, Kiudelis G, Turcan S, Tighineanu O, Mihu I, Magro F, Barros LF, Goldis A, Lazar D, Belousova E, Nikulina I, Hernandez V, Martinez-Ares D, Almer S, Zhulina Y, Halfvarson J, Arebi N, Sebastian S, Lakatos PL, Langholz E, Munkholm P; EpiCom-group. East-West gradient in the incidence of inflammatory bowel disease in Europe: the ECCO-EpiCom inception cohort. Gut. 2014;63:588-597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 311] [Cited by in RCA: 289] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 3. | Lakatos PL. Environmental factors affecting inflammatory bowel disease: have we made progress? Dig Dis. 2009;27:215-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 79] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 4. | Knights D, Lassen KG, Xavier RJ. Advances in inflammatory bowel disease pathogenesis: linking host genetics and the microbiome. Gut. 2013;62:1505-1510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 323] [Cited by in RCA: 344] [Article Influence: 28.7] [Reference Citation Analysis (0)] |
| 5. | Kalla R, Ventham NT, Kennedy NA, Quintana JF, Nimmo ER, Buck AH, Satsangi J. MicroRNAs: new players in IBD. Gut. 2015;64:504-517. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 188] [Cited by in RCA: 207] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 6. | Zuk O, Hechter E, Sunyaev SR, Lander ES. The mystery of missing heritability: Genetic interactions create phantom heritability. Proc Natl Acad Sci USA. 2012;109:1193-1198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1086] [Cited by in RCA: 1062] [Article Influence: 81.7] [Reference Citation Analysis (0)] |
| 7. | Patel KK, Babyatsky MW. Medical education: a key partner in realizing personalized medicine in gastroenterology. Gastroenterology. 2008;134:656-661. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 8. | Cohen LB, Nanau RM, Delzor F, Neuman MG. Biologic therapies in inflammatory bowel disease. Transl Res. 2014;163:533-556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 38] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 9. | Laharie D, Filippi J, Roblin X, Nancey S, Chevaux JB, Hébuterne X, Flourié B, Capdepont M, Peyrin-Biroulet L. Impact of mucosal healing on long-term outcomes in ulcerative colitis treated with infliximab: a multicenter experience. Aliment Pharmacol Ther. 2013;37:998-1004. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 91] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 10. | Papi C, Aratari A. Mucosal healing as a treatment for IBD? Expert Rev Gastroenterol Hepatol. 2014;8:457-459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 11. | Kopylov U, Ben-Horin S, Seidman E. Therapeutic drug monitoring in inflammatory bowel disease. Ann Gastroenterol. 2014;27:304-312. [PubMed] |
| 12. | Ben-Horin S, Kopylov U, Chowers Y. Optimizing anti-TNF treatments in inflammatory bowel disease. Autoimmun Rev. 2014;13:24-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 238] [Cited by in RCA: 307] [Article Influence: 25.6] [Reference Citation Analysis (0)] |
| 13. | Ferrante M, Vermeire S, Fidder H, Schnitzler F, Noman M, Van Assche G, De Hertogh G, Hoffman I, D’Hoore A, Van Steen K. Long-term outcome after infliximab for refractory ulcerative colitis. J Crohns Colitis. 2008;2:219-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 173] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 14. | Nancey S, Roblin X. [Non-invasive follow up of patients with inflammatory bowel diseases]. Rev Prat. 2014;64:1256-1261. [PubMed] |
| 15. | Evans WE, McLeod HL. Pharmacogenomics--drug disposition, drug targets, and side effects. N Engl J Med. 2003;348:538-549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1228] [Cited by in RCA: 1093] [Article Influence: 49.7] [Reference Citation Analysis (0)] |
| 16. | Sim E, Lack N, Wang CJ, Long H, Westwood I, Fullam E, Kawamura A. Arylamine N-acetyltransferases: structural and functional implications of polymorphisms. Toxicology. 2008;254:170-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 85] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 17. | Chen M, Xia B, Chen B, Guo Q, Li J, Ye M, Hu Z. N-acetyltransferase 2 slow acetylator genotype associated with adverse effects of sulphasalazine in the treatment of inflammatory bowel disease. Can J Gastroenterol. 2007;21:155-158. [PubMed] |
| 18. | Faubion WA, Loftus EV, Harmsen WS, Zinsmeister AR, Sandborn WJ. The natural history of corticosteroid therapy for inflammatory bowel disease: a population-based study. Gastroenterology. 2001;121:255-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 833] [Cited by in RCA: 792] [Article Influence: 33.0] [Reference Citation Analysis (0)] |
| 19. | Munkholm P, Langholz E, Davidsen M, Binder V. Frequency of glucocorticoid resistance and dependency in Crohn’s disease. Gut. 1994;35:360-362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 487] [Cited by in RCA: 431] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 20. | Reinisch W, Gasché C, Wyatt J, Moser G, Lochs H, Vogelsang H, Gangl A. Steroid dependency in Crohn’s disease. Lancet. 1995;345:859. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 21. | Farrell RJ, Kelleher D. Glucocorticoid resistance in inflammatory bowel disease. J Endocrinol. 2003;178:339-346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 171] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 22. | Farrell RJ, Murphy A, Long A, Donnelly S, Cherikuri A, O’Toole D, Mahmud N, Keeling PW, Weir DG, Kelleher D. High multidrug resistance (P-glycoprotein 170) expression in inflammatory bowel disease patients who fail medical therapy. Gastroenterology. 2000;118:279-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 196] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 23. | Onnie CM, Fisher SA, Pattni R, Sanderson J, Forbes A, Lewis CM, Mathew CG. Associations of allelic variants of the multidrug resistance gene (ABCB1 or MDR1) and inflammatory bowel disease and their effects on disease behavior: a case-control and meta-analysis study. Inflamm Bowel Dis. 2006;12:263-271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 63] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 24. | Smith MA, Marinaki AM, Sanderson JD. Pharmacogenomics in the treatment of inflammatory bowel disease. Pharmacogenomics. 2010;11:421-437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 25. | De Iudicibus S, Stocco G, Martelossi S, Drigo I, Norbedo S, Lionetti P, Pozzi E, Barabino A, Decorti G, Bartoli F. Association of BclI polymorphism of the glucocorticoid receptor gene locus with response to glucocorticoids in inflammatory bowel disease. Gut. 2007;56:1319-1320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 49] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 26. | van Rossum EF, Koper JW, Huizenga NA, Uitterlinden AG, Janssen JA, Brinkmann AO, Grobbee DE, de Jong FH, van Duyn CM, Pols HA. A polymorphism in the glucocorticoid receptor gene, which decreases sensitivity to glucocorticoids in vivo, is associated with low insulin and cholesterol levels. Diabetes. 2002;51:3128-3134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 226] [Cited by in RCA: 228] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 27. | Derijks LJ, Wong DR. Pharmacogenetics of thiopurines in inflammatory bowel disease. Curr Pharm Des. 2010;16:145-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 51] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 28. | Van Asseldonk DP, de Boer NK, Peters GJ, Veldkamp AI, Mulder CJ, Van Bodegraven AA. On therapeutic drug monitoring of thiopurines in inflammatory bowel disease; pharmacology, pharmacogenomics, drug intolerance and clinical relevance. Curr Drug Metab. 2009;10:981-997. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 36] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 29. | Priest VL, Begg EJ, Gardiner SJ, Frampton CM, Gearry RB, Barclay ML, Clark DW, Hansen P. Pharmacoeconomic analyses of azathioprine, methotrexate and prospective pharmacogenetic testing for the management of inflammatory bowel disease. Pharmacoeconomics. 2006;24:767-781. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 68] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 30. | Winter J, Walker A, Shapiro D, Gaffney D, Spooner RJ, Mills PR. Cost-effectiveness of thiopurine methyltransferase genotype screening in patients about to commence azathioprine therapy for treatment of inflammatory bowel disease. Aliment Pharmacol Ther. 2004;20:593-599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 116] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 31. | Guerciolini R, Szumlanski C, Weinshilboum RM. Human liver xanthine oxidase: nature and extent of individual variation. Clin Pharmacol Ther. 1991;50:663-672. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 75] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 32. | Relling MV, Lin JS, Ayers GD, Evans WE. Racial and gender differences in N-acetyltransferase, xanthine oxidase, and CYP1A2 activities. Clin Pharmacol Ther. 1992;52:643-658. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 199] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 33. | Kudo M, Moteki T, Sasaki T, Konno Y, Ujiie S, Onose A, Mizugaki M, Ishikawa M, Hiratsuka M. Functional characterization of human xanthine oxidase allelic variants. Pharmacogenet Genomics. 2008;18:243-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 64] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 34. | Marinaki AM, Ansari A, Duley JA, Arenas M, Sumi S, Lewis CM, Shobowale-Bakre el-M, Escuredo E, Fairbanks LD, Sanderson JD. Adverse drug reactions to azathioprine therapy are associated with polymorphism in the gene encoding inosine triphosphate pyrophosphatase (ITPase). Pharmacogenetics. 2004;14:181-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 241] [Cited by in RCA: 235] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 35. | Allorge D, Hamdan R, Broly F, Libersa C, Colombel JF. ITPA genotyping test does not improve detection of Crohn’s disease patients at risk of azathioprine/6-mercaptopurine induced myelosuppression. Gut. 2005;54:565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 59] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 36. | Gearry RB, Roberts RL, Barclay ML, Kennedy MA. Lack of association between the ITPA 94C>A polymorphism and adverse effects from azathioprine. Pharmacogenetics. 2004;14:779-781. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 88] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 37. | De Ridder L, Van Dieren JM, Van Deventer HJ, Stokkers PC, Van der Woude JC, Van Vuuren AJ, Benninga MA, Escher JC, Hommes DW. Pharmacogenetics of thiopurine therapy in paediatric IBD patients. Aliment Pharmacol Ther. 2006;23:1137-1141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 38. | Hindorf U, Lindqvist M, Peterson C, Söderkvist P, Ström M, Hjortswang H, Pousette A, Almer S. Pharmacogenetics during standardised initiation of thiopurine treatment in inflammatory bowel disease. Gut. 2006;55:1423-1431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 131] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 39. | von Ahsen N, Armstrong VW, Behrens C, von Tirpitz C, Stallmach A, Herfarth H, Stein J, Bias P, Adler G, Shipkova M. Association of inosine triphosphatase 94C>A and thiopurine S-methyltransferase deficiency with adverse events and study drop-outs under azathioprine therapy in a prospective Crohn disease study. Clin Chem. 2005;51:2282-2288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 72] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 40. | Ansari A, Arenas M, Greenfield SM, Morris D, Lindsay J, Gilshenan K, Smith M, Lewis C, Marinaki A, Duley J. Prospective evaluation of the pharmacogenetics of azathioprine in the treatment of inflammatory bowel disease. Aliment Pharmacol Ther. 2008;28:973-983. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 131] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 41. | van Dieren JM, van Vuuren AJ, Kusters JG, Nieuwenhuis EE, Kuipers EJ, van der Woude CJ. ITPA genotyping is not predictive for the development of side effects in AZA treated inflammatory bowel disease patients. Gut. 2005;54:1664. [PubMed] |
| 42. | Zelinkova Z, Derijks LJ, Stokkers PC, Vogels EW, van Kampen AH, Curvers WL, Cohn D, van Deventer SJ, Hommes DW. Inosine triphosphate pyrophosphatase and thiopurine s-methyltransferase genotypes relationship to azathioprine-induced myelosuppression. Clin Gastroenterol Hepatol. 2006;4:44-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 124] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 43. | Uchiyama K, Nakamura M, Kubota T, Yamane T, Fujise K, Tajiri H. Thiopurine S-methyltransferase and inosine triphosphate pyrophosphohydrolase genes in Japanese patients with inflammatory bowel disease in whom adverse drug reactions were induced by azathioprine/6-mercaptopurine treatment. J Gastroenterol. 2009;44:197-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 56] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 44. | Shipkova M, Franz J, Abe M, Klett C, Wieland E, Andus T. Association between adverse effects under azathioprine therapy and inosine triphosphate pyrophosphatase activity in patients with chronic inflammatory bowel disease. Ther Drug Monit. 2011;33:321-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 36] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 45. | Kim JH, Cheon JH, Hong SS, Eun CS, Byeon JS, Hong SY, Kim BY, Kwon SH, Kim SW, Han DS. Influences of thiopurine methyltransferase genotype and activity on thiopurine-induced leukopenia in Korean patients with inflammatory bowel disease: a retrospective cohort study. J Clin Gastroenterol. 2010;44:e242-e248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 71] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 46. | Zabala-Fernández W, Barreiro-de Acosta M, Echarri A, Carpio D, Lorenzo A, Castro J, Martínez-Ares D, Pereira S, Martin-Granizo I, Corton M. A pharmacogenetics study of TPMT and ITPA genes detects a relationship with side effects and clinical response in patients with inflammatory bowel disease receiving Azathioprine. J Gastrointestin Liver Dis. 2011;20:247-253. [PubMed] |
| 47. | Eklund BI, Moberg M, Bergquist J, Mannervik B. Divergent activities of human glutathione transferases in the bioactivation of azathioprine. Mol Pharmacol. 2006;70:747-754. [PubMed] |
| 48. | Osterman MT, Kundu R, Lichtenstein GR, Lewis JD. Association of 6-thioguanine nucleotide levels and inflammatory bowel disease activity: a meta-analysis. Gastroenterology. 2006;130:1047-1053. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 307] [Cited by in RCA: 287] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 49. | Hindorf U, Lindqvist M, Hildebrand H, Fagerberg U, Almer S. Adverse events leading to modification of therapy in a large cohort of patients with inflammatory bowel disease. Aliment Pharmacol Ther. 2006;24:331-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 132] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 50. | Cuffari C, Théorêt Y, Latour S, Seidman G. 6-Mercaptopurine metabolism in Crohn’s disease: correlation with efficacy and toxicity. Gut. 1996;39:401-406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 260] [Cited by in RCA: 247] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 51. | Dubinsky MC, Lamothe S, Yang HY, Targan SR, Sinnett D, Théorêt Y, Seidman EG. Pharmacogenomics and metabolite measurement for 6-mercaptopurine therapy in inflammatory bowel disease. Gastroenterology. 2000;118:705-713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 771] [Cited by in RCA: 722] [Article Influence: 28.9] [Reference Citation Analysis (0)] |
| 52. | Herrlinger KR, Cummings JR, Barnardo MC, Schwab M, Ahmad T, Jewell DP. The pharmacogenetics of methotrexate in inflammatory bowel disease. Pharmacogenet Genomics. 2005;15:705-711. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 51] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 53. | Vermeire S, Louis E, Carbonez A, Van Assche G, Noman M, Belaiche J, De Vos M, Van Gossum A, Pescatore P, Fiasse R. Demographic and clinical parameters influencing the short-term outcome of anti-tumor necrosis factor (infliximab) treatment in Crohn’s disease. Am J Gastroenterol. 2002;97:2357-2363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 169] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 54. | Parsi MA, Achkar JP, Richardson S, Katz J, Hammel JP, Lashner BA, Brzezinski A. Predictors of response to infliximab in patients with Crohn’s disease. Gastroenterology. 2002;123:707-713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 183] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 55. | Taylor KD, Plevy SE, Yang H, Landers CJ, Barry MJ, Rotter JI, Targan SR. ANCA pattern and LTA haplotype relationship to clinical responses to anti-TNF antibody treatment in Crohn’s disease. Gastroenterology. 2001;120:1347-1355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 158] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 56. | Louis E, Vermeire S, Rutgeerts P, De Vos M, Van Gossum A, Pescatore P, Fiasse R, Pelckmans P, Reynaert H, D’Haens G. A positive response to infliximab in Crohn disease: association with a higher systemic inflammation before treatment but not with -308 TNF gene polymorphism. Scand J Gastroenterol. 2002;37:818-824. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 57. | Mascheretti S, Hampe J, Kühbacher T, Herfarth H, Krawczak M, Fölsch UR, Schreiber S. Pharmacogenetic investigation of the TNF/TNF-receptor system in patients with chronic active Crohn’s disease treated with infliximab. Pharmacogenomics J. 2002;2:127-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 108] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 58. | Mascheretti S, Hampe J, Croucher PJ, Nikolaus S, Andus T, Schubert S, Olson A, Bao W, Fölsch UR, Schreiber S. Response to infliximab treatment in Crohn’s disease is not associated with mutations in the CARD15 (NOD2) gene: an analysis in 534 patients from two multicenter, prospective GCP-level trials. Pharmacogenetics. 2002;12:509-515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 107] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 59. | Vermeire S, Louis E, Rutgeerts P, De Vos M, Van Gossum A, Belaiche J, Pescatore P, Fiasse R, Pelckmans P, Vlietinck R. NOD2/CARD15 does not influence response to infliximab in Crohn’s disease. Gastroenterology. 2002;123:106-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 154] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 60. | Pierik M, Vermeire S, Steen KV, Joossens S, Claessens G, Vlietinck R, Rutgeerts P. Tumour necrosis factor-alpha receptor 1 and 2 polymorphisms in inflammatory bowel disease and their association with response to infliximab. Aliment Pharmacol Ther. 2004;20:303-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 120] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 61. | Matsukura H, Ikeda S, Yoshimura N, Takazoe M, Muramatsu M. Genetic polymorphisms of tumour necrosis factor receptor superfamily 1A and 1B affect responses to infliximab in Japanese patients with Crohn’s disease. Aliment Pharmacol Ther. 2008;27:765-770. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 62] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 62. | Louis E, El Ghoul Z, Vermeire S, Dall’Ozzo S, Rutgeerts P, Paintaud G, Belaiche J, De Vos M, Van Gossum A, Colombel JF. Association between polymorphism in IgG Fc receptor IIIa coding gene and biological response to infliximab in Crohn’s disease. Aliment Pharmacol Ther. 2004;19:511-519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 160] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 63. | Urcelay E, Mendoza JL, Martinez A, Fernandez L, Taxonera C, Diaz-Rubio M, de la Concha EG. IBD5 polymorphisms in inflammatory bowel disease: association with response to infliximab. World J Gastroenterol. 2005;11:1187-1192. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 57] [Cited by in RCA: 57] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 64. | Hlavaty T, Pierik M, Henckaerts L, Ferrante M, Joossens S, van Schuerbeek N, Noman M, Rutgeerts P, Vermeire S. Polymorphisms in apoptosis genes predict response to infliximab therapy in luminal and fistulizing Crohn’s disease. Aliment Pharmacol Ther. 2005;22:613-626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 144] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 65. | Hlavaty T, Ferrante M, Henckaerts L, Pierik M, Rutgeerts P, Vermeire S. Predictive model for the outcome of infliximab therapy in Crohn’s disease based on apoptotic pharmacogenetic index and clinical predictors. Inflamm Bowel Dis. 2007;13:372-379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 54] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 66. | Willot S, Vermeire S, Ohresser M, Rutgeerts P, Paintaud G, Belaiche J, De Vos M, Van Gossum A, Franchimont D, Colombel JF. No association between C-reactive protein gene polymorphisms and decrease of C-reactive protein serum concentration after infliximab treatment in Crohn’s disease. Pharmacogenet Genomics. 2006;16:37-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 34] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 67. | Dideberg V, Louis E, Farnir F, Bertoli S, Vermeire S, Rutgeerts P, De Vos M, Van Gossum A, Belaiche J, Bours V. Lymphotoxin alpha gene in Crohn’s disease patients: absence of implication in the response to infliximab in a large cohort study. Pharmacogenet Genomics. 2006;16:369-373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 68. | Dideberg V, Théâtre E, Farnir F, Vermeire S, Rutgeerts P, De Vos M, Belaiche J, Franchimont D, Van Gossum A, Louis E. The TNF/ADAM 17 system: implication of an ADAM 17 haplotype in the clinical response to infliximab in Crohn’s disease. Pharmacogenet Genomics. 2006;16:727-734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 69. | Jürgens M, Laubender RP, Hartl F, Weidinger M, Seiderer J, Wagner J, Wetzke M, Beigel F, Pfennig S, Stallhofer J. Disease activity, ANCA, and IL23R genotype status determine early response to infliximab in patients with ulcerative colitis. Am J Gastroenterol. 2010;105:1811-1819. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 134] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 70. | Dubinsky MC, Mei L, Friedman M, Dhere T, Haritunians T, Hakonarson H, Kim C, Glessner J, Targan SR, McGovern DP. Genome wide association (GWA) predictors of anti-TNFalpha therapeutic responsiveness in pediatric inflammatory bowel disease. Inflamm Bowel Dis. 2010;16:1357-1366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 112] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 71. | Colombel JF, Sandborn WJ, Reinisch W, Mantzaris GJ, Kornbluth A, Rachmilewitz D, Lichtiger S, D’Haens G, Diamond RH, Broussard DL. Infliximab, azathioprine, or combination therapy for Crohn’s disease. N Engl J Med. 2010;362:1383-1395. [PubMed] |
| 72. | Mazor Y, Almog R, Kopylov U, Ben Hur D, Blatt A, Dahan A, Waterman M, Ben-Horin S, Chowers Y. Adalimumab drug and antibody levels as predictors of clinical and laboratory response in patients with Crohn’s disease. Aliment Pharmacol Ther. 2014;40:620-628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 155] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 73. | Vaughn BP, Martinez-Vazquez M, Patwardhan VR, Moss AC, Sandborn WJ, Cheifetz AS. Proactive therapeutic concentration monitoring of infliximab may improve outcomes for patients with inflammatory bowel disease: results from a pilot observational study. Inflamm Bowel Dis. 2014;20:1996-2003. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 74. | Lobo ED, Hansen RJ, Balthasar JP. Antibody pharmacokinetics and pharmacodynamics. J Pharm Sci. 2004;93:2645-2668. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 689] [Cited by in RCA: 671] [Article Influence: 33.6] [Reference Citation Analysis (0)] |
| 75. | Colombel JF, Feagan BG, Sandborn WJ, Van Assche G, Robinson AM. Therapeutic drug monitoring of biologics for inflammatory bowel disease. Inflamm Bowel Dis. 2012;18:349-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 101] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 76. | Ben-Horin S, Chowers Y. Review article: loss of response to anti-TNF treatments in Crohn’s disease. Aliment Pharmacol Ther. 2011;33:987-995. [PubMed] |
| 77. | Papamichael K, Gils A, Rutgeerts P, Levesque BG, Vermeire S, Sandborn WJ, Vande Casteele N. Role for therapeutic drug monitoring during induction therapy with TNF antagonists in IBD: evolution in the definition and management of primary nonresponse. Inflamm Bowel Dis. 2015;21:182-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 191] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 78. | Hanauer SB, Feagan BG, Lichtenstein GR, Mayer LF, Schreiber S, Colombel JF, Rachmilewitz D, Wolf DC, Olson A, Bao W. Maintenance infliximab for Crohn’s disease: the ACCENT I randomised trial. Lancet. 2002;359:1541-1549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2987] [Cited by in RCA: 3055] [Article Influence: 132.8] [Reference Citation Analysis (0)] |
| 79. | Sands BE, Anderson FH, Bernstein CN, Chey WY, Feagan BG, Fedorak RN, Kamm MA, Korzenik JR, Lashner BA, Onken JE. Infliximab maintenance therapy for fistulizing Crohn’s disease. N Engl J Med. 2004;350:876-885. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1581] [Cited by in RCA: 1553] [Article Influence: 74.0] [Reference Citation Analysis (0)] |
| 80. | Hanauer SB, Sandborn WJ, Rutgeerts P, Fedorak RN, Lukas M, MacIntosh D, Panaccione R, Wolf D, Pollack P. Human anti-tumor necrosis factor monoclonal antibody (adalimumab) in Crohn’s disease: the CLASSIC-I trial. Gastroenterology. 2006;130:323-333; quiz 591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1153] [Cited by in RCA: 1194] [Article Influence: 62.8] [Reference Citation Analysis (0)] |
| 81. | Sandborn WJ, Hanauer SB, Rutgeerts P, Fedorak RN, Lukas M, MacIntosh DG, Panaccione R, Wolf D, Kent JD, Bittle B. Adalimumab for maintenance treatment of Crohn’s disease: results of the CLASSIC II trial. Gut. 2007;56:1232-1239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 708] [Cited by in RCA: 771] [Article Influence: 42.8] [Reference Citation Analysis (0)] |
| 82. | Gisbert JP, Panés J. Loss of response and requirement of infliximab dose intensification in Crohn’s disease: a review. Am J Gastroenterol. 2009;104:760-767. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 360] [Cited by in RCA: 399] [Article Influence: 24.9] [Reference Citation Analysis (0)] |
| 83. | Peters CP, Eshuis EJ, Toxopeüs FM, Hellemons ME, Jansen JM, D’Haens GR, Fockens P, Stokkers PC, Tuynman HA, van Bodegraven AA. Adalimumab for Crohn’s disease: long-term sustained benefit in a population-based cohort of 438 patients. J Crohns Colitis. 2014;8:866-875. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 68] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 84. | Yanai H, Hanauer SB. Assessing response and loss of response to biological therapies in IBD. Am J Gastroenterol. 2011;106:685-698. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 233] [Cited by in RCA: 265] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 85. | Moss AC. Optimizing the use of biological therapy in patients with inflammatory bowel disease. Gastroenterol Rep (Oxf). 2015;3:63-68. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 58] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 86. | Warman A, Straathof JW, Derijks LJ. Therapeutic drug monitoring of infliximab in inflammatory bowel disease patients in a teaching hospital setting: results of a prospective cohort study. Eur J Gastroenterol Hepatol. 2015;27:242-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 41] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 87. | Chaparro M, Guerra I, Muñoz-Linares P, Gisbert JP. Systematic review: antibodies and anti-TNF-α levels in inflammatory bowel disease. Aliment Pharmacol Ther. 2012;35:971-986. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 44] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 88. | Nanda KS, Cheifetz AS, Moss AC. Impact of antibodies to infliximab on clinical outcomes and serum infliximab levels in patients with inflammatory bowel disease (IBD): a meta-analysis. Am J Gastroenterol. 2013;108:40-47; quiz 48. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 288] [Cited by in RCA: 281] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 89. | McLean MH, Neurath MF, Durum SK. Targeting interleukins for the treatment of inflammatory bowel disease-what lies beyond anti-TNF therapy? Inflamm Bowel Dis. 2014;20:389-397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 42] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 90. | Parrello T, Monteleone G, Cucchiara S, Monteleone I, Sebkova L, Doldo P, Luzza F, Pallone F. Up-regulation of the IL-12 receptor beta 2 chain in Crohn’s disease. J Immunol. 2000;165:7234-7239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 89] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 91. | Iwakura Y, Ishigame H. The IL-23/IL-17 axis in inflammation. J Clin Invest. 2006;116:1218-1222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 708] [Cited by in RCA: 790] [Article Influence: 41.6] [Reference Citation Analysis (1)] |
| 92. | Neurath MF, Fuss I, Kelsall BL, Stüber E, Strober W. Antibodies to interleukin 12 abrogate established experimental colitis in mice. J Exp Med. 1995;182:1281-1290. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 981] [Cited by in RCA: 1043] [Article Influence: 34.8] [Reference Citation Analysis (0)] |
| 93. | Elson CO, Cong Y, Weaver CT, Schoeb TR, McClanahan TK, Fick RB, Kastelein RA. Monoclonal anti-interleukin 23 reverses active colitis in a T cell-mediated model in mice. Gastroenterology. 2007;132:2359-2370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 346] [Cited by in RCA: 352] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 94. | Duerr RH, Taylor KD, Brant SR, Rioux JD, Silverberg MS, Daly MJ, Steinhart AH, Abraham C, Regueiro M, Griffiths A. A genome-wide association study identifies IL23R as an inflammatory bowel disease gene. Science. 2006;314:1461-1463. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2428] [Cited by in RCA: 2310] [Article Influence: 121.6] [Reference Citation Analysis (1)] |
| 95. | Kastelein RA, Hunter CA, Cua DJ. Discovery and biology of IL-23 and IL-27: related but functionally distinct regulators of inflammation. Annu Rev Immunol. 2007;25:221-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 566] [Cited by in RCA: 593] [Article Influence: 32.9] [Reference Citation Analysis (0)] |
| 96. | Sandborn WJ, Feagan BG, Fedorak RN, Scherl E, Fleisher MR, Katz S, Johanns J, Blank M, Rutgeerts P; Ustekinumab Crohn's Disease Study Group. A randomized trial of Ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with moderate-to-severe Crohn’s disease. Gastroenterology. 2008;135:1130-1141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 577] [Cited by in RCA: 585] [Article Influence: 34.4] [Reference Citation Analysis (0)] |
| 97. | Sandborn WJ, Gasink C, Gao LL, Blank MA, Johanns J, Guzzo C, Sands BE, Hanauer SB, Targan S, Rutgeerts P. Ustekinumab induction and maintenance therapy in refractory Crohn’s disease. N Engl J Med. 2012;367:1519-1528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 810] [Cited by in RCA: 873] [Article Influence: 67.2] [Reference Citation Analysis (0)] |
| 98. | A Study to Evaluate the Safety and Efficacy of Ustekinumab in Patients With Moderately to Severely Active Crohn’s Disease Who Have Failed or Are Intolerant to Tumor Necrosis Factor (TNF) Antagonist Therapy (UNITI-1). 2015. Available from: http://clinicaltrials.gov/ct2/show/results/NCT01369329. |
| 101. | Panaccione R, Sandborn WJ, Gordon GL, Lee SD, Safdi A, Sedghi S, Feagan BG, Hanauer S, Reinisch W, Valentine JF. Briakinumab for treatment of Crohn’s disease: results of a randomized trial. Inflamm Bowel Dis. 2015;21:1329-1340. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 37] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 102. | Sands BE, Jacobson EW, Sylwestrowicz T, Younes Z, Dryden G, Fedorak R, Greenbloom S. Randomized, double-blind, placebo-controlled trial of the oral interleukin-12/23 inhibitor apilimod mesylate for treatment of active Crohn’s disease. Inflamm Bowel Dis. 2010;16:1209-1218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 81] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 103. | Melton L, Coombs A. Actemra poised to launch IL-6 inhibitors. Nat Biotechnol. 2008;26:957-959. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 105. | Jones SA, Horiuchi S, Topley N, Yamamoto N, Fuller GM. The soluble interleukin 6 receptor: mechanisms of production and implications in disease. FASEB J. 2001;15:43-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 439] [Cited by in RCA: 484] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 106. | Mitsuyama K, Toyonaga A, Sasaki E, Ishida O, Ikeda H, Tsuruta O, Harada K, Tateishi H, Nishiyama T, Tanikawa K. Soluble interleukin-6 receptors in inflammatory bowel disease: relation to circulating interleukin-6. Gut. 1995;36:45-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 166] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 107. | Hosokawa T, Kusugami K, Ina K, Ando T, Shinoda M, Imada A, Ohsuga M, Sakai T, Matsuura T, Ito K. Interleukin-6 and soluble interleukin-6 receptor in the colonic mucosa of inflammatory bowel disease. J Gastroenterol Hepatol. 1999;14:987-996. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 87] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 108. | Ito H, Takazoe M, Fukuda Y, Hibi T, Kusugami K, Andoh A, Matsumoto T, Yamamura T, Azuma J, Nishimoto N. A pilot randomized trial of a human anti-interleukin-6 receptor monoclonal antibody in active Crohn’s disease. Gastroenterology. 2004;126:989-996; discussion 947. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 479] [Cited by in RCA: 471] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 109. | Nishimoto N, Ito K, Takagi N. Safety and efficacy profiles of tocilizumab monotherapy in Japanese patients with rheumatoid arthritis: meta-analysis of six initial trials and five long-term extensions. Mod Rheumatol. 2010;20:222-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 73] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 110. | Available from: http://www.clinicaltrials.gov/ct2/show/study/NCT01545050?show_locs=Y. |
| 112. | Creed TJ, Norman MR, Probert CS, Harvey RF, Shaw IS, Smithson J, Anderson J, Moorghen M, Gupta J, Shepherd NA. Basiliximab (anti-CD25) in combination with steroids may be an effective new treatment for steroid-resistant ulcerative colitis. Aliment Pharmacol Ther. 2003;18:65-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 79] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 113. | Creed TJ, Probert CS, Norman MN, Moorghen M, Shepherd NA, Hearing SD, Dayan CM; BASBUC INVESTIGATORS. Basiliximab for the treatment of steroid-resistant ulcerative colitis: further experience in moderate and severe disease. Aliment Pharmacol Ther. 2006;23:1435-1442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 63] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 114. | Van Assche G, Sandborn WJ, Feagan BG, Salzberg BA, Silvers D, Monroe PS, Pandak WM, Anderson FH, Valentine JF, Wild GE. Daclizumab, a humanised monoclonal antibody to the interleukin 2 receptor (CD25), for the treatment of moderately to severely active ulcerative colitis: a randomised, double blind, placebo controlled, dose ranging trial. Gut. 2006;55:1568-1574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 86] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 115. | Heller F, Florian P, Bojarski C, Richter J, Christ M, Hillenbrand B, Mankertz J, Gitter AH, Bürgel N, Fromm M. Interleukin-13 is the key effector Th2 cytokine in ulcerative colitis that affects epithelial tight junctions, apoptosis, and cell restitution. Gastroenterology. 2005;129:550-564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 35] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 116. | Danese S. IBD: of mice and men-shedding new light on IL-13 activity in IBD. Nat Rev Gastroenterol Hepatol. 2011;8:128-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 117. | Reinisch W, Panés J, Khurana S, Toth G, Hua F, Comer GM, Hinz M, Page K, O’Toole M, Moorehead TM. Anrukinzumab, an anti-interleukin 13 monoclonal antibody, in active UC: efficacy and safety from a phase IIa randomised multicentre study. Gut. 2015;64:894-900. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 129] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 118. | Danese S, Rudziński J, Brandt W, Dupas JL, Peyrin-Biroulet L, Bouhnik Y, Kleczkowski D, Uebel P, Lukas M, Knutsson M. Tralokinumab for moderate-to-severe UC: a randomised, double-blind, placebo-controlled, phase IIa study. Gut. 2015;64:243-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 131] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 120. | Herrlinger KR, Diculescu M, Fellermann K, Hartmann H, Howaldt S, Nikolov R, Petrov A, Reindl W, Otte JM, Stoynov S. Efficacy, safety and tolerability of vidofludimus in patients with inflammatory bowel disease: the ENTRANCE study. J Crohns Colitis. 2013;7:636-643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 55] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 121. | Targan S, Feagan B, Vermeire S, Panaccione R, Melmed G, Blosch C, Newmark R, Zhang N. A Randomized, Double-Blind, Placebo-Controlled Study to Evaluate the Safety, Tolerability, and Efficacy of AMG 827 in Subjects With Moderate to Severe Crohn’s Disease. Gastroenterology. 2012;143:e26. [DOI] [Full Text] |
| 124. | Pallone F, Fina D, Caruso R, Monteleone G. Role of IL-21 in inflammatory bowel disease. Expert Rev Clin Immunol. 2010;6:537-541. [PubMed] |
| 125. | Rovedatti L, Kudo T, Biancheri P, Sarra M, Knowles CH, Rampton DS, Corazza GR, Monteleone G, Di Sabatino A, Macdonald TT. Differential regulation of interleukin 17 and interferon gamma production in inflammatory bowel disease. Gut. 2009;58:1629-1636. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 252] [Cited by in RCA: 283] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 126. | Ludwiczek O, Kaser A, Novick D, Dinarello CA, Rubinstein M, Tilg H. Elevated systemic levels of free interleukin-18 (IL-18) in patients with Crohn’s disease. Eur Cytokine Netw. 2005;16:27-33. [PubMed] |
| 127. | Fedorak RN, Gangl A, Elson CO, Rutgeerts P, Schreiber S, Wild G, Hanauer SB, Kilian A, Cohard M, LeBeaut A. Recombinant human interleukin 10 in the treatment of patients with mild to moderately active Crohn’s disease. The Interleukin 10 Inflammatory Bowel Disease Cooperative Study Group. Gastroenterology. 2000;119:1473-1482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 389] [Cited by in RCA: 348] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 128. | Schreiber S, Fedorak RN, Nielsen OH, Wild G, Williams CN, Nikolaus S, Jacyna M, Lashner BA, Gangl A, Rutgeerts P. Safety and efficacy of recombinant human interleukin 10 in chronic active Crohn’s disease. Crohn’s Disease IL-10 Cooperative Study Group. Gastroenterology. 2000;119:1461-1472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 351] [Cited by in RCA: 317] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 129. | Colombel JF, Rutgeerts P, Malchow H, Jacyna M, Nielsen OH, Rask-Madsen J, Van Deventer S, Ferguson A, Desreumaux P, Forbes A. Interleukin 10 (Tenovil) in the prevention of postoperative recurrence of Crohn’s disease. Gut. 2001;49:42-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 163] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 130. | Herrlinger KR, Witthoeft T, Raedler A, Bokemeyer B, Krummenerl T, Schulzke JD, Boerner N, Kueppers B, Emmrich J, Mescheder A. Randomized, double blind controlled trial of subcutaneous recombinant human interleukin-11 versus prednisolone in active Crohn’s disease. Am J Gastroenterol. 2006;101:793-797. [PubMed] |
| 131. | Pena Rossi C, Hanauer SB, Tomasevic R, Hunter JO, Shafran I, Graffner H. Interferon beta-1a for the maintenance of remission in patients with Crohn’s disease: results of a phase II dose-finding study. BMC Gastroenterol. 2009;9:22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 132. | Charo IF, Ransohoff RM. The many roles of chemokines and chemokine receptors in inflammation. N Engl J Med. 2006;354:610-621. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1834] [Cited by in RCA: 1943] [Article Influence: 102.3] [Reference Citation Analysis (0)] |
| 133. | Zabel BA, Agace WW, Campbell JJ, Heath HM, Parent D, Roberts AI, Ebert EC, Kassam N, Qin S, Zovko M. Human G protein-coupled receptor GPR-9-6/CC chemokine receptor 9 is selectively expressed on intestinal homing T lymphocytes, mucosal lymphocytes, and thymocytes and is required for thymus-expressed chemokine-mediated chemotaxis. J Exp Med. 1999;190:1241-1256. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 396] [Cited by in RCA: 376] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 134. | Kunkel EJ, Campbell JJ, Haraldsen G, Pan J, Boisvert J, Roberts AI, Ebert EC, Vierra MA, Goodman SB, Genovese MC. Lymphocyte CC chemokine receptor 9 and epithelial thymus-expressed chemokine (TECK) expression distinguish the small intestinal immune compartment: Epithelial expression of tissue-specific chemokines as an organizing principle in regional immunity. J Exp Med. 2000;192:761-768. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 522] [Cited by in RCA: 518] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 135. | Papadakis KA, Prehn J, Moreno ST, Cheng L, Kouroumalis EA, Deem R, Breaverman T, Ponath PD, Andrew DP, Green PH. CCR9-positive lymphocytes and thymus-expressed chemokine distinguish small bowel from colonic Crohn’s disease. Gastroenterology. 2001;121:246-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 167] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 136. | Walters MJ, Wang Y, Lai N, Baumgart T, Zhao BN, Dairaghi DJ, Bekker P, Ertl LS, Penfold ME, Jaen JC. Characterization of CCX282-B, an orally bioavailable antagonist of the CCR9 chemokine receptor, for treatment of inflammatory bowel disease. J Pharmacol Exp Ther. 2010;335:61-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 128] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 137. | Keshav S, Vaňásek T, Niv Y, Petryka R, Howaldt S, Bafutto M, Rácz I, Hetzel D, Nielsen OH, Vermeire S. A randomized controlled trial of the efficacy and safety of CCX282-B, an orally-administered blocker of chemokine receptor CCR9, for patients with Crohn’s disease. PLoS One. 2013;8:e60094. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 107] [Cited by in RCA: 113] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 138. | Luster AD, Unkeless JC, Ravetch JV. Gamma-interferon transcriptionally regulates an early-response gene containing homology to platelet proteins. Nature. 1985;315:672-676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 691] [Cited by in RCA: 785] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 139. | Dufour JH, Dziejman M, Liu MT, Leung JH, Lane TE, Luster AD. IFN-gamma-inducible protein 10 (IP-10; CXCL10)-deficient mice reveal a role for IP-10 in effector T cell generation and trafficking. J Immunol. 2002;168:3195-3204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 816] [Cited by in RCA: 887] [Article Influence: 38.6] [Reference Citation Analysis (0)] |
| 140. | Romagnani P, Maggi L, Mazzinghi B, Cosmi L, Lasagni L, Liotta F, Lazzeri E, Angeli R, Rotondi M, Filì L. CXCR3-mediated opposite effects of CXCL10 and CXCL4 on TH1 or TH2 cytokine production. J Allergy Clin Immunol. 2005;116:1372-1379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 88] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 141. | Torres J, Danese S, Colombel JF. New therapeutic avenues in ulcerative colitis: thinking out of the box. Gut. 2013;62:1642-1652. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 58] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 142. | Sasaki S, Yoneyama H, Suzuki K, Suriki H, Aiba T, Watanabe S, Kawauchi Y, Kawachi H, Shimizu F, Matsushima K. Blockade of CXCL10 protects mice from acute colitis and enhances crypt cell survival. Eur J Immunol. 2002;32:3197-3205. [PubMed] |
| 143. | Hardi R, Mayer L, Targan SR, Yellin M, Cardarelli P, Das K. A phase 1 open-label, single-dose, dose-escalation study of MDX-1100, a high-affinity, neutralizing, fully human Igg1(kappa) anti-CXCL10 (Ip10) monoclonal antibody, in ulcerative colitis. Gastroenterology. 2008;134:A99-A100. [RCA] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 144. | Yellin M, Paliienko I, Balanescu A, Ter-Vartanian S, Tseluyko V, Xu LA, Tao X, Cardarelli PM, Leblanc H, Nichol G. A phase II, randomized, double-blind, placebo-controlled study evaluating the efficacy and safety of MDX-1100, a fully human anti-CXCL10 monoclonal antibody, in combination with methotrexate in patients with rheumatoid arthritis. Arthritis Rheum. 2012;64:1730-1739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 151] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 145. | Mayer L, Sandborn WJ, Stepanov Y, Geboes K, Hardi R, Yellin M, Tao X, Xu LA, Salter-Cid L, Gujrathi S. Anti-IP-10 antibody (BMS-936557) for ulcerative colitis: a phase II randomised study. Gut. 2014;63:442-450. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 114] [Cited by in RCA: 118] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 147. | Available from: http://clinicaltrials.gov/ct2/show/record/NCT01294410?term=BMS-936557&rank=2&show_locs=Y. |
| 148. | Antonelli A, Ferrari SM, Giuggioli D, Ferrannini E, Ferri C, Fallahi P. Chemokine (C-X-C motif) ligand (CXCL)10 in autoimmune diseases. Autoimmun Rev. 2014;13:272-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 324] [Cited by in RCA: 445] [Article Influence: 37.1] [Reference Citation Analysis (0)] |
| 149. | Grip O, Janciauskiene S. Atorvastatin reduces plasma levels of chemokine (CXCL10) in patients with Crohn’s disease. PLoS One. 2009;4:e5263. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 56] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 150. | Coburn LA, Horst SN, Chaturvedi R, Brown CT, Allaman MM, Scull BP, Singh K, Piazuelo MB, Chitnavis MV, Hodges ME. High-throughput multi-analyte Luminex profiling implicates eotaxin-1 in ulcerative colitis. PLoS One. 2013;8:e82300. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 43] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 151. | Available from: http://www.clinicaltrials.gov/ct2/show/record/NCT01671956?term=bertilimumab&rank=1. |
| 152. | Zagury D, Le Buanec H, Bizzini B, Burny A, Lewis G, Gallo RC. Active versus passive anti-cytokine antibody therapy against cytokine-associated chronic diseases. Cytokine Growth Factor Rev. 2003;14:123-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 38] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 153. | Vandepapeliere P, Malan F, Rogler G, van der Bijl A, Kruger F. Safety, Immunogenicity and Clinical Phase I-II Results of TNFa-Kinoid Immunotherapeutic in Crohn’s Disease Patients. Gastroenterology. 2011;140 Suppl 1:S123. [DOI] [Full Text] |
| 154. | Dewit O, Hebuterne X, Dupas J, Howaldt S, Bures J, Schreiber S, Pace A, Klaus J, Bouhnik Y, Reinshagen M. Results of a phase II, randomized, double blind, controlled trial of the efficacy of active therapeutic immunization with TNFKinoid in patients with moderate to severe Crohn’s disease with secondary resistance to TNFα antagonist. Gastroenterology. 2012;142:S567-S568. [DOI] [Full Text] |
| 155. | Aaronson DS, Horvath CM. A road map for those who don’t know JAK-STAT. Science. 2002;296:1653-1655. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 967] [Cited by in RCA: 993] [Article Influence: 43.2] [Reference Citation Analysis (0)] |
| 156. | Seavey MM, Dobrzanski P. The many faces of Janus kinase. Biochem Pharmacol. 2012;83:1136-1145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 94] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 157. | O’Shea JJ, Plenge R. JAK and STAT signaling molecules in immunoregulation and immune-mediated disease. Immunity. 2012;36:542-550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 692] [Cited by in RCA: 876] [Article Influence: 67.4] [Reference Citation Analysis (0)] |
| 158. | Ghoreschi K, Jesson MI, Li X, Lee JL, Ghosh S, Alsup JW, Warner JD, Tanaka M, Steward-Tharp SM, Gadina M. Modulation of innate and adaptive immune responses by tofacitinib (CP-690,550). J Immunol. 2011;186:4234-4243. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 532] [Cited by in RCA: 499] [Article Influence: 35.6] [Reference Citation Analysis (0)] |
| 159. | Flanagan ME, Blumenkopf TA, Brissette WH, Brown MF, Casavant JM, Shang-Poa C, Doty JL, Elliott EA, Fisher MB, Hines M. Discovery of CP-690,550: a potent and selective Janus kinase (JAK) inhibitor for the treatment of autoimmune diseases and organ transplant rejection. J Med Chem. 2010;53:8468-8484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 242] [Cited by in RCA: 266] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 160. | Changelian PS, Moshinsky D, Kuhn CF, Flanagan ME, Munchhof MJ, Harris TM, Whipple DA, Doty JL, Sun J, Kent CR. The specificity of JAK3 kinase inhibitors. Blood. 2008;111:2155-2157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 79] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 161. | Sewgobind VD, Quaedackers ME, van der Laan LJ, Kraaijeveld R, Korevaar SS, Chan G, Weimar W, Baan CC. The Jak inhibitor CP-690,550 preserves the function of CD4CD25FoxP3 regulatory T cells and inhibits effector T cells. Am J Transplant. 2010;10:1785-1795. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 55] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 162. | Sandborn WJ, Ghosh S, Panes J, Vranic I, Spanton Ch, Niezychowski W. Phase 2 randomized study of CP-690,550, an oral janus kinase inhibitor, in active Crohn’s disease. Gastroenterology. 2011;140:S110. [DOI] [Full Text] |
| 163. | Sandborn WJ, Ghosh S, Panes J, Vranic I, Su C, Rousell S, Niezychowski W; Study A3921063 Investigators. Tofacitinib, an oral Janus kinase inhibitor, in active ulcerative colitis. N Engl J Med. 2012;367:616-624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 585] [Cited by in RCA: 631] [Article Influence: 48.5] [Reference Citation Analysis (0)] |
| 164. | Panés J, Su C, Bushmakin AG, Cappelleri JC, Mamolo C, Healey P. Randomized trial of tofacitinib in active ulcerative colitis: analysis of efficacy based on patient-reported outcomes. BMC Gastroenterol. 2015;15:14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 72] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 165. | Mannon P, Reinisch W. Interleukin 13 and its role in gut defence and inflammation. Gut. 2012;61:1765-1773. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 90] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 166. | Natalizumab induction and maintenance therapy for Crohn’s disease. N Engl J Med. 2015;372:2074. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 167. | Targan SR, Feagan BG, Fedorak RN, Lashner BA, Panaccione R, Present DH, Spehlmann ME, Rutgeerts PJ, Tulassay Z, Volfova M. Natalizumab for the treatment of active Crohn’s disease: results of the ENCORE Trial. Gastroenterology. 2007;132:1672-1683. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 508] [Cited by in RCA: 456] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 168. | Kleinschmidt-DeMasters BK, Tyler KL. Progressive multifocal leukoencephalopathy complicating treatment with natalizumab and interferon beta-1a for multiple sclerosis. N Engl J Med. 2005;353:369-374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 810] [Cited by in RCA: 766] [Article Influence: 38.3] [Reference Citation Analysis (0)] |
| 169. | Van Assche G, Van Ranst M, Sciot R, Dubois B, Vermeire S, Noman M, Verbeeck J, Geboes K, Robberecht W, Rutgeerts P. Progressive multifocal leukoencephalopathy after natalizumab therapy for Crohn’s disease. N Engl J Med. 2005;353:362-368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 813] [Cited by in RCA: 763] [Article Influence: 38.2] [Reference Citation Analysis (0)] |
| 170. | Lobatón T, Vermeire S, Van Assche G, Rutgeerts P. Review article: anti-adhesion therapies for inflammatory bowel disease. Aliment Pharmacol Ther. 2014;39:579-594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 140] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 171. | Feagan BG, Rutgeerts P, Sands BE, Hanauer S, Colombel JF, Sandborn WJ, Van Assche G, Axler J, Kim HJ, Danese S. Vedolizumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2013;369:699-710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1576] [Cited by in RCA: 1865] [Article Influence: 155.4] [Reference Citation Analysis (1)] |
| 172. | Sandborn WJ, Feagan BG, Rutgeerts P, Hanauer S, Colombel JF, Sands BE, Lukas M, Fedorak RN, Lee S, Bressler B. Vedolizumab as induction and maintenance therapy for Crohn’s disease. N Engl J Med. 2013;369:711-721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1416] [Cited by in RCA: 1568] [Article Influence: 130.7] [Reference Citation Analysis (1)] |
| 173. | Pan WJ, Köck K, Rees WA, Sullivan BA, Evangelista CM, Yen M, Andrews JM, Radford-Smith GL, Prince PJ, Reynhardt KO. Clinical pharmacology of AMG 181, a gut-specific human anti-α4β7 monoclonal antibody, for treating inflammatory bowel diseases. Br J Clin Pharmacol. 2014;78:1315-1333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 174. | Available from: http://clinicaltrials.gov/ct2/show/NCT01696396?term=amg181&rank=3. |
| 175. | Available from: http://clinicaltrials.gov/ct2/show/NCT01694485?term=amg181&rank=4. |
| 176. | Sugiura T, Kageyama S, Andou A, Miyazawa T, Ejima C, Nakayama A, Dohi T, Eda H. Oral treatment with a novel small molecule alpha 4 integrin antagonist, AJM300, prevents the development of experimental colitis in mice. J Crohns Colitis. 2013;7:e533-e542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 50] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 177. | Watanabe M, Yoshimura N, Motoya S, Tominaga K, Iwakiri R, Watanebe K, Hibi T. AJM300, an Oral α4 Integrin Antagonist, for Active Ulcerative Colitis: A Multicenter, Randomized, Double-Blind, Placebo-Controlled Phase 2A Study. Gastroenterology. 2013;146; S82. |
| 178. | Vermeire S, O’Byrne S, Williams M, Mansfield J, Feagan B, Panés J, Baumgart D, Schreiber S, Dotan I, Sandbron W. Differentiation between etrolizumab (rhuMAb beta7) and placebo in the Eucalyptus phase II randomized double-blind placebo-controlled induction study to evaluate efficacy and safety in patients with refractory moderate-to-severely active ulcerative colitis. Gastroenterology. 2013;144:S-36. [DOI] [Full Text] |
| 179. | Pullen N, Molloy E, Carter D, Syntin P, Clemo F, Finco-Kent D, Reagan W, Zhao S, Kawabata T, Sreckovic S. Pharmacological characterization of PF-00547659, an anti-human MAdCAM monoclonal antibody. Br J Pharmacol. 2009;157:281-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 54] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 180. | Vermeire S, Sandborn W, Danese S, Hebuterne X, Salzberg B, Klopocka M, Tarabar D, Vanasek T, Gregus M, Hellstern P. Sparrow M, Gorelick KJ, Ahmad A, Hassan-Zahraee M, Pradhan V, Cataldi F, Reinisch W. TURANDOT: a randomized, multicenter double-blind, placebo-controlled study of the safety and efficacy of Anti-MAdCAM Antibody PF-00547659 (PF) in patients with moderate to severe Ulcerative Colitis (UC). J Crohns Colitis. 2015;Suppl 1:S13. |
| 181. | Brück W, Wegner C. Insight into the mechanism of laquinimod action. J Neurol Sci. 2011;306:173-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 97] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 182. | Yang JS, Xu LY, Xiao BG, Hedlund G, Link H. Laquinimod (ABR-215062) suppresses the development of experimental autoimmune encephalomyelitis, modulates the Th1/Th2 balance and induces the Th3 cytokine TGF-beta in Lewis rats. J Neuroimmunol. 2004;156:3-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 133] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 183. | D’haens G, Colombel J, Sandborn W, Rutgeerts P, Feagan B. Safety and efficacy of laquinimod in inducing clinical and biochemical improvement in active Crohn’s disease: results of an exploratory trial. Gastroenterology. 2013;144:S21. [DOI] [Full Text] |
| 184. | He SH. Key role of mast cells and their major secretory products in inflammatory bowel disease. World J Gastroenterol. 2004;10:309-318. [PubMed] |
| 185. | Beunk L, Verwoerd A, van Overveld FJ, Rijkers GT. Role of mast cells in mucosal diseases: current concepts and strategies for treatment. Expert Rev Clin Immunol. 2013;9:53-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 186. | D’allard D, Gay J, Descarpentries C, Frisan E, Adam K, Verdier F, Floquet C, Dubreuil P, Lacombe C, Fontenay M. Tyrosine kinase inhibitors induce down-regulation of c-Kit by targeting the ATP pocket. PLoS One. 2013;8:e60961. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 187. | Available from: http://www.clinicaltrialsregister.eu/ctr-search/search?query=masitinib and crohn. |
| 188. | Baumgart DC, Targan SR, Dignass AU, Mayer L, van Assche G, Hommes DW, Hanauer SB, Mahadevan U, Reinisch W, Plevy SE. Prospective randomized open-label multicenter phase I/II dose escalation trial of visilizumab (HuM291) in severe steroid-refractory ulcerative colitis. Inflamm Bowel Dis. 2010;16:620-629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 33] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 189. | Baumgart DC, Lowder JN, Targan SR, Sandborn WJ, Frankel MB. Transient cytokine-induced liver injury following administration of the humanized anti-CD3 antibody visilizumab (HuM291) in Crohn’s disease. Am J Gastroenterol. 2009;104:868-876. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 190. | Blombery P, Prince HM, Levinson M, Pianko S, Maxwell E, Bhathal P. Rituximab-induced immunodysregulatory ileocolitis in a patient with follicular lymphoma. J Clin Oncol. 2011;29:e110-e112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 19] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 191. | El Fassi D, Nielsen CH, Kjeldsen J, Clemmensen O, Hegedüs L. Ulcerative colitis following B lymphocyte depletion with rituximab in a patient with Graves’ disease. Gut. 2008;57:714-715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 105] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 192. | Ardelean DS, Gonska T, Wires S, Cutz E, Griffiths A, Harvey E, Tse SM, Benseler SM. Severe ulcerative colitis after rituximab therapy. Pediatrics. 2010;126:e243-e246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 99] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 193. | Goetz M, Atreya R, Ghalibafian M, Galle PR, Neurath MF. Exacerbation of ulcerative colitis after rituximab salvage therapy. Inflamm Bowel Dis. 2007;13:1365-1368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 199] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 194. | Hanauer SB, Sandborn WJ, Sands BE, Rutgeers P, Panaccione R, Bressler B, Whiteside M, Swanink R, Aranda R, Luo A. A randomized placebo-controlled trial of abatacept for moderately-to-severely active Crohn’s disease (CD). Gastroenterology. 2010;138:S-86. [DOI] [Full Text] |
| 195. | Sandborn WJ, Colombel JF, Sands BE, Rutgeerts P, Targan SR, Panaccione R, Bressler B, Geboes K, Schreiber S, Aranda R. Abatacept for Crohn’s disease and ulcerative colitis. Gastroenterology. 2012;143:62-69.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 107] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 196. | Monteleone G, Kumberova A, Croft NM, McKenzie C, Steer HW, MacDonald TT. Blocking Smad7 restores TGF-beta1 signaling in chronic inflammatory bowel disease. J Clin Invest. 2001;108:601-609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 435] [Cited by in RCA: 458] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 197. | Monteleone G, Fantini MC, Onali S, Zorzi F, Sancesario G, Bernardini S, Calabrese E, Viti F, Monteleone I, Biancone L. Phase I clinical trial of Smad7 knockdown using antisense oligonucleotide in patients with active Crohn’s disease. Mol Ther. 2012;20:870-876. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 112] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 198. | Monteleone G, Neurath MF, Ardizzone S, Di Sabatino A, Fantini MC, Castiglione F, Scribano ML, Armuzzi A, Caprioli F, Sturniolo GC. Mongersen, an oral SMAD7 antisense oligonucleotide, and Crohn’s disease. N Engl J Med. 2015;372:1104-1113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 317] [Cited by in RCA: 314] [Article Influence: 31.4] [Reference Citation Analysis (0)] |
| 199. | Sandborn W, Feagan B, Wolf D, D’Haens G, Vermeire S, Hanauer S, Ghosh S, Smith H, Cravets M, Frohna P. OP024. A randomized, double-blind, placebo-controlled induction trial of an oral S1P receptor modulator (RPC1063) in moderate to severe Ulcerative Colitis: Results of the TOUCHSTONE study. J Crohns Colitis. 2015;Suppl 1:S15. |
| 200. | Vermeire S, Kojecky V, Knoflicek V, Reinisch W, Van Kaem T, Namour F, Beetens J, Vanhoutte F. DOP030. GLPG0974, an FFA2 antagonist, in ulcerative colitis: efficacy and safety in a multicenter proof-of-concept study. J Crohns Colitis. 2015;Suppl 1:S39. |
| 201. | Martínez-Montiel Mdel P, Gómez-Gómez GJ, Flores AI. Therapy with stem cells in inflammatory bowel disease. World J Gastroenterol. 2014;20:1211-1227. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 51] [Cited by in RCA: 51] [Article Influence: 4.6] [Reference Citation Analysis (2)] |
| 202. | Flores AI, Gómez-Gómez GJ, Masedo-González Á, Martínez-Montiel MP. Stem cell therapy in inflammatory bowel disease: A promising therapeutic strategy? World J Stem Cells. 2015;7:343-351. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 203. | Ng SC, Lam YT, Tsoi KK, Chan FK, Sung JJ, Wu JC. Systematic review: the efficacy of herbal therapy in inflammatory bowel disease. Aliment Pharmacol Ther. 2013;38:854-863. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 93] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 204. | Michelsen KS, Wong MH, Ko B, Thomas LS, Dhall D, Targan SR. HMPL-004 (Andrographis paniculata extract) prevents development of murine colitis by inhibiting T-cell proliferation and TH1/TH17 responses. Inflamm Bowel Dis. 2013;19:151-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 205. | Rossen NG, MacDonald JK, de Vries EM, D’Haens GR, de Vos WM, Zoetendal EG, Ponsioen CY. Fecal microbiota transplantation as novel therapy in gastroenterology: A systematic review. World J Gastroenterol. 2015;21:5359-5371. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 160] [Cited by in RCA: 160] [Article Influence: 16.0] [Reference Citation Analysis (1)] |
| 206. | Angelberger S, Reinisch W, Makristathis A, Lichtenberger C, Dejaco C, Papay P, Novacek G, Trauner M, Loy A, Berry D. Temporal bacterial community dynamics vary among ulcerative colitis patients after fecal microbiota transplantation. Am J Gastroenterol. 2013;108:1620-1630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 252] [Cited by in RCA: 259] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 207. | Vermeire S, Joossens M, Verbeke K, Hildebrand F, Machiels K, Van den Broeck K, Van Assche G, Paul J. Rutgeerts, Jeroen Raes23. Pilot Study on the Safety and Efficacy of Faecal Microbiota Transplantation in Refractory Crohn. Gastroenterology. 2012;142 Suppl 5:S360. [DOI] [Full Text] |
| 208. | Greenberg A, Aroniadis O, Shelton C, Brandt LJ. Long-term followup study of fecal microbiota transplantation (FMT) for Inflammatory Bowel Disease (IBD). ACG Annu Sci Meet Abstr. San Diego: EEUU 2013; P1629. |
| 209. | Borody TJ, Warren EF, Leis S, Surace R, Ashman O. Treatment of ulcerative colitis using fecal bacteriotherapy. J Clin Gastroenterol. 2003;37:42-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 210. | Kunde S, Pham A, Bonczyk S, Crumb T, Duba M, Conrad H, Cloney D, Kugathasan S. Safety, tolerability, and clinical response after fecal transplantation in children and young adults with ulcerative colitis. J Pediatr Gastroenterol Nutr. 2013;56:597-601. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 227] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 211. | Kump PK, Gröchenig HP, Lackner S, Trajanoski S, Reicht G, Hoffmann KM, Deutschmann A, Wenzl HH, Petritsch W, Krejs GJ. Alteration of intestinal dysbiosis by fecal microbiota transplantation does not induce remission in patients with chronic active ulcerative colitis. Inflamm Bowel Dis. 2013;19:2155-2165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 188] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 212. | Kump PK, Gröchenig HP, Spindelböck W, Gorkiewicz G, Wenzl H, Petritsch W, Reicht G. Preliminary clinical results of repeatedly fecal microbiota transplantation (FMT) in chronic active ulcerative colitis. United European Gastroenterol J. 2013;1 Suppl 1:A57. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |