Published online Oct 28, 2015. doi: 10.3748/wjg.v21.i40.11236
Peer-review started: April 9, 2015
First decision: May 18, 2015
Revised: June 2, 2015
Accepted: August 28, 2015
Article in press: August 31, 2015
Published online: October 28, 2015
Processing time: 197 Days and 10.1 Hours
Since their discovery two decades ago, CD4+CD25+Foxp3+ regulatory T cells (Tregs) have become the subject of intense investigation by immunologists. Unlike other T cells, which promote an immune response, Tregs actively inhibit inflammation when activated by their cognate antigen, thus raising hope that these cells could be engineered into a highly targeted, antigen-specific, immunosuppressant therapy. Although Tregs represent less than 10% of circulating CD4+T cells, they have been shown to play an essential role in preventing or limiting inflammation in a variety of animal models and human diseases. In particular, spontaneous intestinal inflammation has been shown to occur in the absence of Tregs, suggesting that there may be a Treg defect central to the pathogenesis of human inflammatory bowel disease (IBD). However, over the past decade, multiple groups have reported no qualitative or quantitative deficits in Tregs from the intestines and blood of IBD patients to explain why these cells fail to regulate inflammation in Crohn’s disease and ulcerative colitis. In this review, we will discuss the history of Tregs, what is known about them in IBD, and what progress and obstacles have been seen with efforts to employ them for therapeutic benefit.
Core tip: Regulatory T cells (Tregs) have received much interest in animal models of inflammatory bowel disease (IBD), but have yet to demonstrate a clear defect in human Crohn’s disease or ulcerative colitis. This review will detail our current knowledge about this important regulatory arm of the immune system in human IBD, and discuss the potential role for Tregs as immunotherapy.
- Citation: Lord JD. Promises and paradoxes of regulatory T cells in inflammatory bowel disease. World J Gastroenterol 2015; 21(40): 11236-11245
- URL: https://www.wjgnet.com/1007-9327/full/v21/i40/11236.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i40.11236
In the summer of 1995, Shimon Sakaguchi published the first report of what later came to be recognized as regulatory T cells (Tregs) by demonstrating that mice depleted of CD4+CD25+ T cells spontaneously developed multiorgan autoimmunity, including gastrointestinal (GI) inflammation[1]. More importantly, such autoimmunity could be prevented by administration of these CD4+CD25+ Tregs, suggesting that they might someday represent a potent cellular therapy for autoimmune and chronic inflammatory conditions. In the twenty years since this initial report, well over 10000 original manuscripts have been published concerning Tregs, making them one of the most intensely studied T cell populations of the 21st century.
Interest in Tregs took a quantum leap forward shortly after the turn of the millennium, when it was discovered that the gene FOXP3 was central to Treg development and function, and could serve as an excellent marker for these relatively rare cells. A genetic defect in the FOXP3 gene which precluded Treg development was found to be the cause of a mouse multiorgan inflammatory condition called scurfy[2]. At roughly the same time, a similar human condition called immune polyendocrinopathy enteropathy X-linked (IPEX) was reported to result from mutations in the human FOXP3 gene resulting in humans with no Tregs[3,4]. As the name implies, an inflammatory enteropathy, resembling severe pan-intestinal Crohn’s disease, is a central feature of IPEX, and generally causes fatal malnutrition in the absence of a hematopoietic cell transplant (HCT).This condition made it clear that the Tregs which had been receiving increasing attention in murine models were also critical for intestinal immune homeostasis in humans.
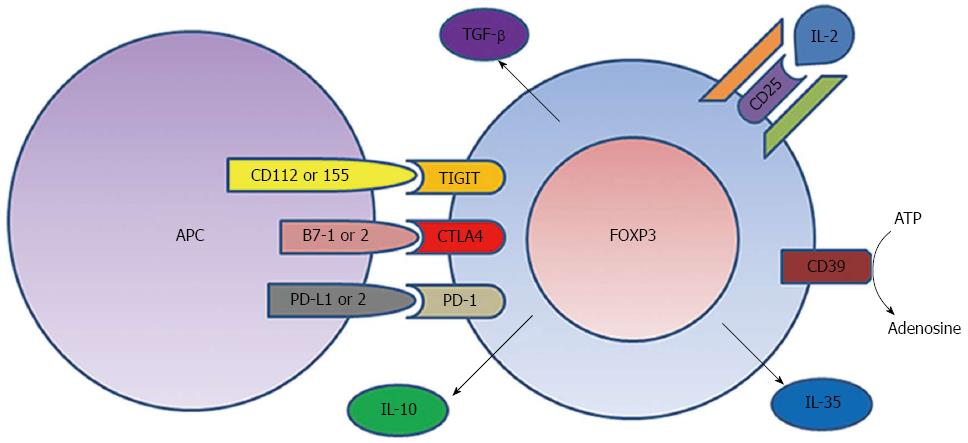
We now know that FOXP3+ Tregs reside within the intestinal lamina propria and represent up to 10% of circulating CD4+ T cells in humans[5-8]. Tregs recognize specific MHC-II-bound peptide antigens though a clonally unique T cell receptor (TCR), just like any other CD4+ T cells[7,9]. However, while other T cells will deliver pro-inflammatory signals upon TCR ligation, Tregs do the opposite. They inhibit the activation of bystander T cells in a contact-dependent manner[10]. While no single molecular mechanism for this inhibition has been elucidated, several regulatory signals appear to be important (Figure 1), augmentation of which would represent an attractive opportunity for IBD therapy.
By definition, Tregs express more CD25 than any other T cells[1], and because CD25 is an essential component of the high-affinity IL-2 receptor, Tregs may absorb local IL-2, depriving nearby T cells of this T cell growth and survival factor when its concentration is limiting. However, IL-2 is evidently not essential for pro-inflammatory T cell growth and survival because mice genetically engineered to lack CD25[11] or the beta chain of the IL-2 receptor (CD122)[12] do not develop immunodeficiency, but rather a lymphoproliferative disorder including spontaneous autoimmunity and IBD. This was evidently due to a lack of Tregs[13], as the latter are uniquely dependent upon IL-2. Thus, depriving other T cells of IL-2 is certainly not central to the inhibitory effect of Tregs in vivo.
Tregs also constitutively express more of the immunoregulatory CTLA4 molecule (CD152) than other T cells[8,14,15], and this molecule appears to be necessary for Treg inhibitory function[15,16]. CTLA4 can bind up B7-1 (CD80) and B7-2 (CD86) costimulatory molecules on the surface of antigen presenting cells (APC), preventing them from costimulating CD28 receptors on other T cells[17]. Mice lacking the CTLA4 gene develop multiorgan autoimmunity[18] not unlike mice lacking Tregs. Similarly, patients who receive the CTLA4-blocking antibody ipilimumab as a cancer immunotherapy can develop spontaneous autoimmunity, including enterocolitis in over 20% of recipients[19,20], thus demonstrating the importance of this molecule in maintaining intestinal immune homeostasis. However, whether CTLA4’s role is primarily mediated through Tregs is unclear, as ipilimumab also limits CTLA4 engagement on activated T cells.
TIGIT, a molecule analogous to CTLA4, is also enriched on a subset of Tregs[21,22], and likewise binds costimulatory molecules (CD112, CD155) on APC, preventing them from ligating a costimulatory receptor (CD226) on effector T cells, and thereby inhibiting the latter[23]. TIGIT+ Tregs have been reported to selectively inhibit Th1 and Th17 cells, the CD4+ T cell populations commonly associated with autoimmune and inflammatory conditions like IBD[24]. Tregs also express PD-1 (CD279)[25], an inhibitory receptor that interacts with PD-L1 (CD274) and PD-L2 (B7-DC, CD273) on APCs and has, like CTLA4, recently become a target for cancer immunotherapy[26-29]. Like CTLA4 blockade, PD-1 blockade has caused spontaneous intestinal inflammation in clinical trials, albeit at a lower rate, affecting < 10% of recipients[30,31].
In addition to their contact-dependent immunomodulatory mechanisms, Tregs may control inflammation through soluble factors. CD39 is an ectonucleotidase preferentially expressed by Tregs, which hydrolizes ATP and ADP to AMP, and ultimately adenosine[32,33]. ATP has been reported to enhance pro-inflammatory Th17 cells[34,35], while adenosine may inhibit effector T cells through the A2A receptor[36-39], so this surface receptor may change the local environment of the Tregs to regulate inflammation. Reduced Treg expression of CD39 has been described in lupus[40] and multiple sclerosis[32,32,41], but has not yet been described in IBD.
Tregs have also been reported to control inflammation through cytokines. TGF-β is expressed by Tregs, and has immunomodulatory properties, although it may function as a cell-surface protein on Tregs[42], and may not be necessary for Treg inhibitory function[43]. IL-10 is likewise an immunomodulatory cytokine made by Tregs[42], and is essential for preventing spontaneous bowel inflammation in mice[44] and humans[45]. However, the immunoregulatory roles of IL-10 and TGF-β may be more appropriately ascribed to other “regulatory T cell” populations that do not express FOXP3, namely Tr1[46,47] and Th3 cells[48], which are beyond the scope of this review. More recently, FOXP3+ Tregs have been shown to mediate their inhibitory function through the cytokine IL-35[49,50].
A number of clinical observations and experiments in animal models[51,52] have suggested that Tregs or their inhibitory mechanisms are critical for preventing spontaneous intestinal inflammation, and thus suggested that a defect in Tregs may be central to the pathogenesis of UC and/or Crohn’s disease. Out of 38 distinct animal models of IBD reviewed in 2003, nine involved Tregs or their inhibitory mechanisms[51,53]. As an iatrogenic inflammatory bowel disease, human gastrointestinal graft vs host disease (GVHD) following HCT has been associated with evidence of decreased Tregs in the blood[54] and intestinal mucosa[55].
Despite this wealth of data implicating Tregs in intestinal immune homeostasis, direct evaluation of Tregs in the intestines of IBD patients has not identified obvious defects. The first report of CD4+CD25+ Tregs isolated from the intestinal lamina propria (LP) of IBD patients, published more than a decade ago, demonstrated that these cells are present, express CTLA4, and show in vitro suppressive activity against other T cells which is no different from those of controls[56]. This and subsequent reports found that these Tregs paradoxically represent a greater fraction of LP CD4+ T cells in the intestines of IBD patients than healthy control subjects[5] and are no less common in bowel affected by IBD than in bowel inflamed for other reasons, such as infection[51]. Paradoxically, Tregs are even more common in actively inflamed than uninflamed IBD mucosa[5,57-59], with a reciprocal drop in circulating Treg frequency in the peripheral blood of symptomatic IBD patients likely reflecting sequestration of these cells to the site of inflammation. Thus, the mucosal inflammation of IBD appears to be different from that of IPEX in that it does not result from any local dearth of FOXP3+ cells.
Confounding these analyses was the discovery that FOXP3 expression could be induced de novo in human T cells that were originally FOXP3 negative by TCR activation in the presence of TGF-β[60,61]. Thus the seemingly paradoxical excess of FOXP3+ cells in the inflamed mucosa of an IBD patient could simply be locally activated T cells. Complicating matters, by some accounts, T cells induced to express FOXP3 by activation are nonetheless effective regulators of other immune cells in vitro[62,63]. Whether these “induced Tregs” (iTregs) have all the same suppressive function in vivo as constitutively FOXP3+“natural” Tregs (nTregs) has been debated[64], and is difficult to establish experimentally in humans. One significant difference between iTregs and nTregs concerns their ability to make cytokines. Classical nTregs do not make pro-inflammatory cytokines, such as IL-2 or IFN-γ, and additionally show demethylation of CpG sites in the FOXP3 promoter[6]. In contrast, iTregs generated from effector T cells retain their ability to produce these cytokines[64], and do not demethylate their FOXP3 promoter[65], although they do up-regulate CD25 and CTLA4 to resemble nTregs[64], making it difficult to discern the two Treg populations by surface markers. Adding to the complexity, it has become clear that the “nTregs” that constitutively express FOXP3 in vivo are actually a mix of Tregs that either acquired FOXP3 expression in the thymus (tTregs) or periphery (pTregs), thus reflecting their antigen specificity and perhaps phenotype[66].
The nuclear protein Helios has been shown to be constitutively expressed by thymically-derived tTregs, but not in vitro-generated iTregs[67], making this a potentially unique marker with which to distinguish at least these two populations. The fraction of FOXP3+ LP T cells that express Helios is no lower in IBD patients than controls[68], suggesting that the paradoxically increased FOXP3+ T cells in IBD are not exclusively iTregs. However, there is evidence that activation-induced FOXP3+ T cells may acquire Helios expression[69], thus compromising the reliability of Helios as a marker for distinguishing iTregs from nTregs.
The TCR gene is uniquely rearranged in each nascent T cell, making it a stable genetic marker with which to identify T cells from a common clonal origin. By comparing the TCR Vβ hypervariable domain repertoires of FOXP3+ and FOXP3- T cell populations from the colon LP, it has been shown that these are predominantly distinct populations, even in IBD[68]. Indeed, LP Helios£ Tregs show no more similarity in their TCR repertoire to effector T cells than they do to Helios+ Tregs[68]. Thus, the paradoxically increased mucosal FOXP3+ cells in IBD cannot be explained solely by activation-induced FOXP3 expression among effector T cells.
Several groups have noted that an unusually high fraction of mucosal Tregs from IBD patients are able to produce IL-17A[70-72]. IL-17A is a potent pro-inflammatory cytokine associated with neutrophil recruitment[73], and hence thought to play a central role in anti-bacterial immune responses. It is made by a subset of effector T cells, called Th17 cells, which can be identified by CCR6[74] and CD161 expression[75], and have been implicated in multiple autoimmune conditions[76]. Thus, by sharing characteristics with a potentially pathogenic class of T cells, the copious intestinal FOXP3+ Tregs present in IBD could paradoxically promote rather than suppress intestinal inflammation.
Like iTregs, Th17 cells require TGF-β for their development, but additionally require IL-6, which in turn suppresses the formation of FOXP3+ Tregs[77,78]. The differentiation of Th17 cells is governed by the transcription factor RORγt[74,79] instead of FOXP3. In cells that express both transcription factors, FOXP3 physically interacts with RORγt in the nucleus to prevent the latter from promoting IL-17A expression[80]. This interaction requires a region of the FOXP3 protein encoded by exon 2 of the FOXP3 mRNA[80], which is deleted in a splice variant (Δexon 2) that represents approximately half the FOXP3 transcripts expressed by humans[81]. This would suggest that IL-17-producing FOXP3+ T cells, as seen in IBD, could be exclusively expressing the Δexon 2 variant of FOXP3. However, no predominance of Δexon 2 relative to full-length FOXP3 expression is seen in IBD, nor are there cells which exclusively express Δexon 2, even among IL-17-expressing FOXP3+ T cells[57]. Thus, how Th17-like FOXP3+ T cells arise in IBD remains a mystery, but could be due to an increased responsiveness to IL-6, as has been seen in T cells from multiple sclerosis patients[82].
With the recent advent of inexpensive, high-throughput nucleic acid sequencing techniques, the bacterial flora, or “microbiome”, of the GI tract has recently come under intense scrutiny. Differences between the intestinal microbiomes of people with and without IBD have been described by many independent researchers[83-86], although it is difficult to determine whether such differences are a cause or effect of IBD once sufficient inflammation has occurred in the GI tract to diagnose an individual with IBD. Nonetheless, a leading hypothesis about the pathogenesis of IBD dictates that the immune system is losing tolerance to intestinal commensal flora, suggesting a dominant role for the microbiome.
Studies in germ-free mice have demonstrated that the gut microbiome is important for development of the normal intestinal immune system, as reviewed elsewhere[87]. This includes IL-10-producing, peripherally-induced FOXP3+ Tregs, whose development can be driven by specific intestinal microbiota in animal models[88,89]. While some intestinal Treg development may simply be due to exposure to luminal peptide antigens, non-peptide bacterial products, such as short-chain fatty acids[90] or specific polysaccharides[88], are important for Treg induction in the gut. Likewise, ingested micronutrients, such as retinoic acid, have been shown to contribute to the peripheral generation of FOXP3+ Tregs in the gut[91]. Thus, exposure of the intestinal mucosa to the fecal stream may be an important means by which the mucosal immune system develops tolerance, or perhaps fails to do so in IBD.
Contemporaneous with the growth of research on Tregs in the early 21st century was the use of biopharmaceutical therapy for IBD and other inflammatory conditions involving TNF-α blockade. Perhaps as a consequence, a number of groups analyzed the effect of anti-TNF agents, particularly infliximab, on circulating FOXP3+ Tregs, and found that the latter were enriched in the peripheral blood of patients demonstrating a good clinical response to therapy[92-95]. This suggests that the blockade of TNF-αin vivo may enhance Treg development, expansion, or viability if this cytokine normally inhibits Tregs in the setting of inflammation. Alternatively, because anti-TNF drugs can cause apoptosis of TNF-producing cells, and Tregs do not make TNF-α, it is possible this effect reflects a selective “pruning” of the FOXP3- effector T cell population rather than expansion of FOXP3+ Tregs. However, caution should be taken in drawing conclusions about IBD from peripheral blood analyses, as the intestinal lamina propria houses more lymphocytes than the circulation. Thus selective sequestration or release of cell populations to or from the gut can actually cause the blood to reflect the opposite of what is actually happening at the site of inflammation in IBD. Indeed, the effect of anti-TNF agents on intramucosal Tregs has been less clear, with some researchers reporting a drop in FOXP3+ cells on therapy[94], and others reporting an increase[95]. Further confounding these analyses is the observation that histological IBD activity correlates inversely with Treg frequency in tissue sections[5,57-59], such that a drop in tissue Tregs in the setting of effective therapy could obscure any local enrichment, and if mediated by a release of Tregs into circulation, produce the observed increase in blood Tregs.
The effect of other immunosuppressive therapies on Tregs has been less intensely studied in IBD, but data exists from other conditions for which these drugs are used. In liver transplant recipients, use of the immunosuppressive drug azathioprine has been paradoxically associated with decreased colonic FOXP3+ cells, although only as cotherapy with prednisone and calcineurin inhibitors[96]. Likewise, in autoimmune hepatitis, azathioprine use, again in conjunction with prednisone, resulted in decreased intrahepatic Tregs, although a higher ratio of these Tregs to other lymphocytes correlated with biochemical remission[97]. Although these effects could be attributed to cotherapy with prednisone, studies in asthmatics have shown no effect of oral glucocorticoids on circulating Treg frequency[97]. Furthermore, as with anti-TNF agents, it is difficult to demonstrate that changes in Tregs associated with a given therapy represent a cause or effect of changes in inflammatory activity. Whether the newer anti-integrin biopharmaceutical vedolizumab will have an effect on intramucosal Tregs has yet to be seen, but a similar agent, natalizumab, did not alter the ratio of Tregs to other T cells in the intestinal mucosa of Crohn’s patients receiving it[98].
Shortly after their discovery, Tregs were proposed as a potential therapy for autoimmune or inflammatory disease in more reviews and editorials than can be listed here. Indeed, in many animal models, adoptive transfer of Tregs proved effective for the prevention or treatment of inflammatory conditions, including IBD[99]. However, more than a decade later, the application of Tregs to human disease has been surprisingly limited. Given their rarity in peripheral blood, a major obstacle to therapeutic application of Tregs has been simply having enough Tregs to administer, so much work went into expanding or generating Tregs in vitro into a large, stable population with stable suppressive function. The earliest and most extensive efforts applying Tregs as anti-inflammatory therapy have been directed at GVHD complicating HCT[100-102], a condition which, like IBD, commonly involves deregulated intestinal inflammation. As an alternative to adoptive transfer of in vitro expanded Tregs, in vivo expansion of Tregs post HCT through the use of low-dose IL-2 has demonstrated efficacy against GVHD[103-105]. Low dose IL-2 also expanded Tregs in type-I diabetes[106,107], but it paradoxically accelerated autoimmunity, even when given with the immunosuppressant rapamycin, perhaps because it also expanded eosinophils and NK cells[107]. However, some efficacy has been seen with adoptive transfer of Tregs in type-I diabetes[108,109].
The first trial of adoptive transfer of Tregs as a therapy for IBD was recently published as an 8-wk, open-label, dose-ranging study involving 20 Crohn’s patients[110]. In contrast to the aforementioned trials in GVHD and diabetes, the transferred Tregs were selected and cloned to be specific for a dietary antigen (chicken egg ovalbumin) so that antigen-specific activation of the transferred cells could be stimulated in the gastrointestinal tract through an egg-intensive diet (meringue cake). 40% of recipients demonstrated clinical improvement, although the most improvement was paradoxically seen in recipients of the smallest number of Tregs (106), and only minimal improvement was observed by objective measures of inflammation, such as C reactive protein and fecal calprotectin. Thus, the efficacy of Tregs as IBD therapy was neither straightforward nor overwhelming, suggesting that other factors, such as Treg antigen specificity or inhibitory function, may be more important than Treg numbers. Curiously, the number of circulating FOXP3+ T cells decreased in responders, while rising in non-responders. However, the frequency of Tregs in the intestines was not evaluated, so this dichotomy could reflect mucosal Treg sequestration if such a phenomenon was associated with therapeutic response.
Despite extensive interest in Tregs as central mediators of intestinal immune homeostasis, there is surprisingly little evidence that a defect in Tregs is associated with either form of human IBD. The fact that inflammation persists in Crohn’s and UC despite an excess of Tregs in the mucosa relative to healthy bowel indicates that the inflammation of IBD is resistant to their presence. Whether the mucosal Tregs of IBD patients are intrinsically defective in their ability to regulate mucosal inflammation in vivo is unknown, but in vitro assays have shown no such functional defect[56,58,59]. Alternatively, Treg-extrinsic factors could undermine the immunoregulatory function of Tregs. Other immune cells, such as FOXP3-negative effector T cells, could be resistant to the inhibitory function of Tregs in IBD, as has been described in multiple sclerosis and diabetes[82,111]. Mucosal dendritic and other antigen presenting cells with which Tregs and other T cells interact could deliver signals which undermine Treg-mediated inhibition. Finally, the mucosal microenvironment in general, including soluble factors and components of the extracellular matrix, such as hyaluronic acid[112], could be actively detrimental to, or passively unsupportive of, the inhibitory function of Tregs in IBD. A better understanding of the factors that undermine Treg function in IBD will be necessary before the promise of Tregs as an IBD therapy can ultimately be realized.
P- Reviewer: Miyoshi E S- Editor: Yu J L- Editor: A E- Editor: Zhang DN
| 1. | Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Pillars article: immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor α-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J. Immunol. 1995. J Immunol. 2011;186:3808-3821. [PubMed] |
| 2. | Brunkow ME, Jeffery EW, Hjerrild KA, Paeper B, Clark LB, Yasayko SA, Wilkinson JE, Galas D, Ziegler SF, Ramsdell F. Disruption of a new forkhead/winged-helix protein, scurfin, results in the fatal lymphoproliferative disorder of the scurfy mouse. Nat Genet. 2001;27:68-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1827] [Cited by in RCA: 1926] [Article Influence: 80.3] [Reference Citation Analysis (0)] |
| 3. | Bennett CL, Christie J, Ramsdell F, Brunkow ME, Ferguson PJ, Whitesell L, Kelly TE, Saulsbury FT, Chance PF, Ochs HD. The immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) is caused by mutations of FOXP3. Nat Genet. 2001;27:20-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2432] [Cited by in RCA: 2489] [Article Influence: 103.7] [Reference Citation Analysis (0)] |
| 4. | Wildin RS, Ramsdell F, Peake J, Faravelli F, Casanova JL, Buist N, Levy-Lahad E, Mazzella M, Goulet O, Perroni L. X-linked neonatal diabetes mellitus, enteropathy and endocrinopathy syndrome is the human equivalent of mouse scurfy. Nat Genet. 2001;27:18-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1371] [Cited by in RCA: 1380] [Article Influence: 57.5] [Reference Citation Analysis (0)] |
| 5. | Maul J, Loddenkemper C, Mundt P, Berg E, Giese T, Stallmach A, Zeitz M, Duchmann R. Peripheral and intestinal regulatory CD4+ CD25(high) T cells in inflammatory bowel disease. Gastroenterology. 2005;128:1868-1878. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 470] [Cited by in RCA: 497] [Article Influence: 24.9] [Reference Citation Analysis (0)] |
| 6. | Miyara M, Yoshioka Y, Kitoh A, Shima T, Wing K, Niwa A, Parizot C, Taflin C, Heike T, Valeyre D. Functional delineation and differentiation dynamics of human CD4+ T cells expressing the FoxP3 transcription factor. Immunity. 2009;30:899-911. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1588] [Cited by in RCA: 1811] [Article Influence: 113.2] [Reference Citation Analysis (0)] |
| 7. | Baecher-Allan C, Brown JA, Freeman GJ, Hafler DA. CD4+CD25high regulatory cells in human peripheral blood. J Immunol. 2001;167:1245-1253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1386] [Cited by in RCA: 1408] [Article Influence: 58.7] [Reference Citation Analysis (0)] |
| 8. | Dieckmann D, Plottner H, Berchtold S, Berger T, Schuler G. Ex vivo isolation and characterization of CD4(+)CD25(+) T cells with regulatory properties from human blood. J Exp Med. 2001;193:1303-1310. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 839] [Cited by in RCA: 849] [Article Influence: 35.4] [Reference Citation Analysis (0)] |
| 9. | Suri-Payer E, Amar AZ, Thornton AM, Shevach EM. CD4+CD25+ T cells inhibit both the induction and effector function of autoreactive T cells and represent a unique lineage of immunoregulatory cells. J Immunol. 1998;160:1212-1218. [PubMed] |
| 10. | Thornton AM, Shevach EM. CD4+CD25+ immunoregulatory T cells suppress polyclonal T cell activation in vitro by inhibiting interleukin 2 production. J Exp Med. 1998;188:287-296. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1907] [Cited by in RCA: 1955] [Article Influence: 72.4] [Reference Citation Analysis (0)] |
| 11. | Willerford DM, Chen J, Ferry JA, Davidson L, Ma A, Alt FW. Interleukin-2 receptor alpha chain regulates the size and content of the peripheral lymphoid compartment. Immunity. 1995;3:521-530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 837] [Cited by in RCA: 851] [Article Influence: 28.4] [Reference Citation Analysis (0)] |
| 12. | Suzuki H, Kündig TM, Furlonger C, Wakeham A, Timms E, Matsuyama T, Schmits R, Simard JJ, Ohashi PS, Griesser H. Deregulated T cell activation and autoimmunity in mice lacking interleukin-2 receptor beta. Science. 1995;268:1472-1476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 676] [Cited by in RCA: 681] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 13. | Malek TR, Yu A, Vincek V, Scibelli P, Kong L. CD4 regulatory T cells prevent lethal autoimmunity in IL-2Rbeta-deficient mice. Implications for the nonredundant function of IL-2. Immunity. 2002;17:167-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 595] [Cited by in RCA: 616] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 14. | Jonuleit H, Schmitt E, Stassen M, Tuettenberg A, Knop J, Enk AH. Identification and functional characterization of human CD4(+)CD25(+) T cells with regulatory properties isolated from peripheral blood. J Exp Med. 2001;193:1285-1294. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 918] [Cited by in RCA: 933] [Article Influence: 38.9] [Reference Citation Analysis (0)] |
| 15. | Takahashi T, Tagami T, Yamazaki S, Uede T, Shimizu J, Sakaguchi N, Mak TW, Sakaguchi S. Immunologic self-tolerance maintained by CD25(+)CD4(+) regulatory T cells constitutively expressing cytotoxic T lymphocyte-associated antigen 4. J Exp Med. 2000;192:303-310. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1642] [Cited by in RCA: 1694] [Article Influence: 67.8] [Reference Citation Analysis (0)] |
| 16. | Read S, Malmström V, Powrie F. Cytotoxic T lymphocyte-associated antigen 4 plays an essential role in the function of CD25(+)CD4(+) regulatory cells that control intestinal inflammation. J Exp Med. 2000;192:295-302. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1694] [Cited by in RCA: 1665] [Article Influence: 66.6] [Reference Citation Analysis (0)] |
| 17. | Teft WA, Kirchhof MG, Madrenas J. A molecular perspective of CTLA-4 function. Annu Rev Immunol. 2006;24:65-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 380] [Cited by in RCA: 406] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 18. | Waterhouse P, Penninger JM, Timms E, Wakeham A, Shahinian A, Lee KP, Thompson CB, Griesser H, Mak TW. Lymphoproliferative disorders with early lethality in mice deficient in Ctla-4. Science. 1995;270:985-988. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2111] [Cited by in RCA: 2136] [Article Influence: 71.2] [Reference Citation Analysis (0)] |
| 19. | Beck KE, Blansfield JA, Tran KQ, Feldman AL, Hughes MS, Royal RE, Kammula US, Topalian SL, Sherry RM, Kleiner D, Quezado M, Lowy I, Yellin M, Rosenberg SA, Yang JC. Enterocolitis in patients with cancer after antibody blockade of cytotoxic T-lymphocyte-associated antigen 4. J Clin Oncol. 2006;24:2283-2289. [PubMed] |
| 20. | Lord JD, Hackman RC, Moklebust A, Thompson JA, Higano CS, Chielens D, Steinbach G, McDonald GB. Refractory colitis following anti-CTLA4 antibody therapy: analysis of mucosal FOXP3+ T cells. Dig Dis Sci. 2010;55:1396-1405. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 82] [Cited by in RCA: 81] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 21. | Zhang Y, Maksimovic J, Naselli G, Qian J, Chopin M, Blewitt ME, Oshlack A, Harrison LC. Genome-wide DNA methylation analysis identifies hypomethylated genes regulated by FOXP3 in human regulatory T cells. Blood. 2013;122:2823-2836. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 105] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 22. | Bin Dhuban K, d’Hennezel E, Nashi E, Bar-Or A, Rieder S, Shevach EM, Nagata S, Piccirillo CA. Coexpression of TIGIT and FCRL3 identifies Helios+ human memory regulatory T cells. J Immunol. 2015;194:3687-3696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 95] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 23. | Levin SD, Taft DW, Brandt CS, Bucher C, Howard ED, Chadwick EM, Johnston J, Hammond A, Bontadelli K, Ardourel D. Vstm3 is a member of the CD28 family and an important modulator of T-cell function. Eur J Immunol. 2011;41:902-915. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 275] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 24. | Joller N, Lozano E, Burkett PR, Patel B, Xiao S, Zhu C, Xia J, Tan TG, Sefik E, Yajnik V. Treg cells expressing the coinhibitory molecule TIGIT selectively inhibit proinflammatory Th1 and Th17 cell responses. Immunity. 2014;40:569-581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 669] [Cited by in RCA: 732] [Article Influence: 66.5] [Reference Citation Analysis (0)] |
| 25. | Raimondi G, Shufesky WJ, Tokita D, Morelli AE, Thomson AW. Regulated compartmentalization of programmed cell death-1 discriminates CD4+CD25+ resting regulatory T cells from activated T cells. J Immunol. 2006;176:2808-2816. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 139] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 26. | Weber JS, D’Angelo SP, Minor D, Hodi FS, Gutzmer R, Neyns B, Hoeller C, Khushalani NI, Miller WH, Lao CD. Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): a randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2015;16:375-384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1853] [Cited by in RCA: 2105] [Article Influence: 210.5] [Reference Citation Analysis (0)] |
| 27. | Robert C, Long GV, Brady B, Dutriaux C, Maio M, Mortier L, Hassel JC, Rutkowski P, McNeil C, Kalinka-Warzocha E. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015;372:320-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3964] [Cited by in RCA: 4346] [Article Influence: 434.6] [Reference Citation Analysis (0)] |
| 28. | Armand P, Nagler A, Weller EA, Devine SM, Avigan DE, Chen YB, Kaminski MS, Holland HK, Winter JN, Mason JR. Disabling immune tolerance by programmed death-1 blockade with pidilizumab after autologous hematopoietic stem-cell transplantation for diffuse large B-cell lymphoma: results of an international phase II trial. J Clin Oncol. 2013;31:4199-4206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 352] [Cited by in RCA: 370] [Article Influence: 30.8] [Reference Citation Analysis (0)] |
| 29. | Hamid O, Robert C, Daud A, Hodi FS, Hwu WJ, Kefford R, Wolchok JD, Hersey P, Joseph RW, Weber JS. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N Engl J Med. 2013;369:134-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2712] [Cited by in RCA: 2723] [Article Influence: 226.9] [Reference Citation Analysis (0)] |
| 30. | Gibney GT, Kudchadkar RR, DeConti RC, Thebeau MS, Czupryn MP, Tetteh L, Eysmans C, Richards A, Schell MJ, Fisher KJ. Safety, correlative markers, and clinical results of adjuvant nivolumab in combination with vaccine in resected high-risk metastatic melanoma. Clin Cancer Res. 2015;21:712-720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 206] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 31. | Brahmer JR, Drake CG, Wollner I, Powderly JD, Picus J, Sharfman WH, Stankevich E, Pons A, Salay TM, McMiller TL. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. J Clin Oncol. 2010;28:3167-3175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2330] [Cited by in RCA: 2414] [Article Influence: 160.9] [Reference Citation Analysis (0)] |
| 32. | Borsellino G, Kleinewietfeld M, Di Mitri D, Sternjak A, Diamantini A, Giometto R, Höpner S, Centonze D, Bernardi G, Dell’Acqua ML. Expression of ectonucleotidase CD39 by Foxp3+ Treg cells: hydrolysis of extracellular ATP and immune suppression. Blood. 2007;110:1225-1232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 866] [Cited by in RCA: 957] [Article Influence: 53.2] [Reference Citation Analysis (0)] |
| 33. | Deaglio S, Dwyer KM, Gao W, Friedman D, Usheva A, Erat A, Chen JF, Enjyoji K, Linden J, Oukka M. Adenosine generation catalyzed by CD39 and CD73 expressed on regulatory T cells mediates immune suppression. J Exp Med. 2007;204:1257-1265. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1917] [Cited by in RCA: 1843] [Article Influence: 102.4] [Reference Citation Analysis (0)] |
| 34. | Killeen ME, Ferris L, Kupetsky EA, Falo L, Mathers AR. Signaling through purinergic receptors for ATP induces human cutaneous innate and adaptive Th17 responses: implications in the pathogenesis of psoriasis. J Immunol. 2013;190:4324-4336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 91] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 35. | Paustian C, Taylor P, Johnson T, Xu M, Ramirez N, Rosenthal KS, Shu S, Cohen PA, Czerniecki BJ, Koski GK. Extracellular ATP and Toll-like receptor 2 agonists trigger in human monocytes an activation program that favors T helper 17. PLoS One. 2013;8:e54804. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 36. | Ohta A, Sitkovsky M. Role of G-protein-coupled adenosine receptors in downregulation of inflammation and protection from tissue damage. Nature. 2001;414:916-920. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 982] [Cited by in RCA: 1064] [Article Influence: 44.3] [Reference Citation Analysis (0)] |
| 37. | Lappas CM, Rieger JM, Linden J. A2A adenosine receptor induction inhibits IFN-gamma production in murine CD4+ T cells. J Immunol. 2005;174:1073-1080. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 289] [Cited by in RCA: 312] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 38. | Ohta A, Ohta A, Madasu M, Kini R, Subramanian M, Goel N, Sitkovsky M. A2A adenosine receptor may allow expansion of T cells lacking effector functions in extracellular adenosine-rich microenvironments. J Immunol. 2009;183:5487-5493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 162] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 39. | Linden J, Cekic C. Regulation of lymphocyte function by adenosine. Arterioscler Thromb Vasc Biol. 2012;32:2097-2103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 119] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 40. | Loza MJ, Anderson AS, O’Rourke KS, Wood J, Khan IU. T-cell specific defect in expression of the NTPDase CD39 as a biomarker for lupus. Cell Immunol. 2011;271:110-117. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 30] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 41. | Fletcher JM, Lonergan R, Costelloe L, Kinsella K, Moran B, O’Farrelly C, Tubridy N, Mills KH. CD39+Foxp3+ regulatory T Cells suppress pathogenic Th17 cells and are impaired in multiple sclerosis. J Immunol. 2009;183:7602-7610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 356] [Cited by in RCA: 390] [Article Influence: 24.4] [Reference Citation Analysis (0)] |
| 42. | Nakamura K, Kitani A, Strober W. Cell contact-dependent immunosuppression by CD4(+)CD25(+) regulatory T cells is mediated by cell surface-bound transforming growth factor beta. J Exp Med. 2001;194:629-644. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1190] [Cited by in RCA: 1198] [Article Influence: 49.9] [Reference Citation Analysis (0)] |
| 43. | Piccirillo CA, Letterio JJ, Thornton AM, McHugh RS, Mamura M, Mizuhara H, Shevach EM. CD4(+)CD25(+) regulatory T cells can mediate suppressor function in the absence of transforming growth factor beta1 production and responsiveness. J Exp Med. 2002;196:237-246. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 456] [Cited by in RCA: 435] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 44. | Kühn R, Löhler J, Rennick D, Rajewsky K, Müller W. Interleukin-10-deficient mice develop chronic enterocolitis. Cell. 1993;75:263-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3189] [Cited by in RCA: 3226] [Article Influence: 100.8] [Reference Citation Analysis (0)] |
| 45. | Glocker EO, Kotlarz D, Boztug K, Gertz EM, Schäffer AA, Noyan F, Perro M, Diestelhorst J, Allroth A, Murugan D. Inflammatory bowel disease and mutations affecting the interleukin-10 receptor. N Engl J Med. 2009;361:2033-2045. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1149] [Cited by in RCA: 1092] [Article Influence: 68.3] [Reference Citation Analysis (0)] |
| 46. | Levings MK, Roncarolo MG. T-regulatory 1 cells: a novel subset of CD4 T cells with immunoregulatory properties. J Allergy Clin Immunol. 2000;106:S109-S112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 116] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 47. | Asseman C, Powrie F. Interleukin 10 is a growth factor for a population of regulatory T cells. Gut. 1998;42:157-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 75] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 48. | Fukaura H, Kent SC, Pietrusewicz MJ, Khoury SJ, Weiner HL, Hafler DA. Induction of circulating myelin basic protein and proteolipid protein-specific transforming growth factor-beta1-secreting Th3 T cells by oral administration of myelin in multiple sclerosis patients. J Clin Invest. 1996;98:70-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 361] [Cited by in RCA: 338] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 49. | Collison LW, Workman CJ, Kuo TT, Boyd K, Wang Y, Vignali KM, Cross R, Sehy D, Blumberg RS, Vignali DA. The inhibitory cytokine IL-35 contributes to regulatory T-cell function. Nature. 2007;450:566-569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1324] [Cited by in RCA: 1515] [Article Influence: 84.2] [Reference Citation Analysis (0)] |
| 50. | Collison LW, Pillai MR, Chaturvedi V, Vignali DA. Regulatory T cell suppression is potentiated by target T cells in a cell contact, IL-35- and IL-10-dependent manner. J Immunol. 2009;182:6121-6128. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 195] [Cited by in RCA: 204] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 51. | Uhlig HH, Coombes J, Mottet C, Izcue A, Thompson C, Fanger A, Tannapfel A, Fontenot JD, Ramsdell F, Powrie F. Characterization of Foxp3+CD4+CD25+ and IL-10-secreting CD4+CD25+ T cells during cure of colitis. J Immunol. 2006;177:5852-5860. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 360] [Cited by in RCA: 365] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 52. | Gad M. Regulatory T cells in experimental colitis. Curr Top Microbiol Immunol. 2005;293:179-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 53. | Mizoguchi A, Mizoguchi E, Bhan AK. Immune networks in animal models of inflammatory bowel disease. Inflamm Bowel Dis. 2003;9:246-259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 48] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 54. | Miura Y, Thoburn CJ, Bright EC, Phelps ML, Shin T, Matsui EC, Matsui WH, Arai S, Fuchs EJ, Vogelsang GB. Association of Foxp3 regulatory gene expression with graft-versus-host disease. Blood. 2004;104:2187-2193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 238] [Cited by in RCA: 243] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 55. | Rieger K, Loddenkemper C, Maul J, Fietz T, Wolff D, Terpe H, Steiner B, Berg E, Miehlke S, Bornhäuser M. Mucosal FOXP3+ regulatory T cells are numerically deficient in acute and chronic GvHD. Blood. 2006;107:1717-1723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 189] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 56. | Makita S, Kanai T, Oshima S, Uraushihara K, Totsuka T, Sawada T, Nakamura T, Koganei K, Fukushima T, Watanabe M. CD4+CD25bright T cells in human intestinal lamina propria as regulatory cells. J Immunol. 2004;173:3119-3130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 201] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 57. | Lord JD, Valliant-Saunders K, Hahn H, Thirlby RC, Ziegler SF. Paradoxically increased FOXP3+ T cells in IBD do not preferentially express the isoform of FOXP3 lacking exon 2. Dig Dis Sci. 2012;57:2846-2855. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 37] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 58. | Saruta M, Yu QT, Fleshner PR, Mantel PY, Schmidt-Weber CB, Banham AH, Papadakis KA. Characterization of FOXP3+CD4+ regulatory T cells in Crohn’s disease. Clin Immunol. 2007;125:281-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 157] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 59. | Yu QT, Saruta M, Avanesyan A, Fleshner PR, Banham AH, Papadakis KA. Expression and functional characterization of FOXP3+ CD4+ regulatory T cells in ulcerative colitis. Inflamm Bowel Dis. 2007;13:191-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 149] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 60. | Chen W, Jin W, Hardegen N, Lei KJ, Li L, Marinos N, McGrady G, Wahl SM. Conversion of peripheral CD4+CD25- naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J Exp Med. 2003;198:1875-1886. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3466] [Cited by in RCA: 3765] [Article Influence: 179.3] [Reference Citation Analysis (0)] |
| 61. | Fantini MC, Becker C, Monteleone G, Pallone F, Galle PR, Neurath MF. Cutting edge: TGF-beta induces a regulatory phenotype in CD4+CD25- T cells through Foxp3 induction and down-regulation of Smad7. J Immunol. 2004;172:5149-5153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 858] [Cited by in RCA: 910] [Article Influence: 43.3] [Reference Citation Analysis (0)] |
| 62. | Walker MR, Kasprowicz DJ, Gersuk VH, Benard A, Van Landeghen M, Buckner JH, Ziegler SF. Induction of FoxP3 and acquisition of T regulatory activity by stimulated human CD4+CD25- T cells. J Clin Invest. 2003;112:1437-1443. [PubMed] |
| 63. | Walker MR, Carson BD, Nepom GT, Ziegler SF, Buckner JH. De novo generation of antigen-specific CD4+CD25+ regulatory T cells from human CD4+CD25- cells. Proc Natl Acad Sci USA. 2005;102:4103-4108. [PubMed] |
| 64. | Allan SE, Crome SQ, Crellin NK, Passerini L, Steiner TS, Bacchetta R, Roncarolo MG, Levings MK. Activation-induced FOXP3 in human T effector cells does not suppress proliferation or cytokine production. Int Immunol. 2007;19:345-354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 691] [Cited by in RCA: 686] [Article Influence: 38.1] [Reference Citation Analysis (0)] |
| 65. | Janson PC, Winerdal ME, Marits P, Thörn M, Ohlsson R, Winqvist O. FOXP3 promoter demethylation reveals the committed Treg population in humans. PLoS One. 2008;3:e1612. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 174] [Cited by in RCA: 175] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 66. | Shevach EM, Thornton AM. tTregs, pTregs, and iTregs: similarities and differences. Immunol Rev. 2014;259:88-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 449] [Cited by in RCA: 469] [Article Influence: 42.6] [Reference Citation Analysis (0)] |
| 67. | Thornton AM, Korty PE, Tran DQ, Wohlfert EA, Murray PE, Belkaid Y, Shevach EM. Expression of Helios, an Ikaros transcription factor family member, differentiates thymic-derived from peripherally induced Foxp3+ T regulatory cells. J Immunol. 2010;184:3433-3441. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1089] [Cited by in RCA: 1080] [Article Influence: 72.0] [Reference Citation Analysis (0)] |
| 68. | Lord J, Chen J, Thirlby RC, Sherwood AM, Carlson CS. T-cell receptor sequencing reveals the clonal diversity and overlap of colonic effector and FOXP3+ T cells in ulcerative colitis. Inflamm Bowel Dis. 2015;21:19-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 69. | Akimova T, Beier UH, Wang L, Levine MH, Hancock WW. Helios expression is a marker of T cell activation and proliferation. PLoS One. 2011;6:e24226. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 255] [Cited by in RCA: 308] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 70. | Hovhannisyan Z, Treatman J, Littman DR, Mayer L. Characterization of interleukin-17-producing regulatory T cells in inflamed intestinal mucosa from patients with inflammatory bowel diseases. Gastroenterology. 2011;140:957-965. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 283] [Cited by in RCA: 285] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 71. | Ueno A, Jijon H, Chan R, Ford K, Hirota C, Kaplan GG, Beck PL, Iacucci M, Fort Gasia M, Barkema HW. Increased prevalence of circulating novel IL-17 secreting Foxp3 expressing CD4+ T cells and defective suppressive function of circulating Foxp3+ regulatory cells support plasticity between Th17 and regulatory T cells in inflammatory bowel disease patients. Inflamm Bowel Dis. 2013;19:2522-2534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 173] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 72. | Kryczek I, Wu K, Zhao E, Wei S, Vatan L, Szeliga W, Huang E, Greenson J, Chang A, Roliński J. IL-17+ regulatory T cells in the microenvironments of chronic inflammation and cancer. J Immunol. 2011;186:4388-4395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 217] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 73. | Fossiez F, Banchereau J, Murray R, Van Kooten C, Garrone P, Lebecque S. Interleukin-17. Int Rev Immunol. 1998;16:541-551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 182] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 74. | Annunziato F, Cosmi L, Santarlasci V, Maggi L, Liotta F, Mazzinghi B, Parente E, Filì L, Ferri S, Frosali F. Phenotypic and functional features of human Th17 cells. J Exp Med. 2007;204:1849-1861. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1399] [Cited by in RCA: 1478] [Article Influence: 82.1] [Reference Citation Analysis (0)] |
| 75. | Cosmi L, De Palma R, Santarlasci V, Maggi L, Capone M, Frosali F, Rodolico G, Querci V, Abbate G, Angeli R. Human interleukin 17-producing cells originate from a CD161+CD4+ T cell precursor. J Exp Med. 2008;205:1903-1916. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 546] [Cited by in RCA: 582] [Article Influence: 34.2] [Reference Citation Analysis (0)] |
| 76. | Harrington LE, Mangan PR, Weaver CT. Expanding the effector CD4 T-cell repertoire: the Th17 lineage. Curr Opin Immunol. 2006;18:349-356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 431] [Cited by in RCA: 435] [Article Influence: 22.9] [Reference Citation Analysis (0)] |
| 77. | Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5130] [Cited by in RCA: 5473] [Article Influence: 288.1] [Reference Citation Analysis (0)] |
| 78. | Kimura A, Naka T, Kishimoto T. IL-6-dependent and -independent pathways in the development of interleukin 17-producing T helper cells. Proc Natl Acad Sci USA. 2007;104:12099-12104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 267] [Cited by in RCA: 270] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 79. | Ivanov II, McKenzie BS, Zhou L, Tadokoro CE, Lepelley A, Lafaille JJ, Cua DJ, Littman DR. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126:1121-1133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3769] [Cited by in RCA: 4132] [Article Influence: 217.5] [Reference Citation Analysis (0)] |
| 80. | Zhou L, Lopes JE, Chong MM, Ivanov II, Min R, Victora GD, Shen Y, Du J, Rubtsov YP, Rudensky AY. TGF-beta-induced Foxp3 inhibits T(H)17 cell differentiation by antagonizing RORgammat function. Nature. 2008;453:236-240. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1569] [Cited by in RCA: 1541] [Article Influence: 90.6] [Reference Citation Analysis (0)] |
| 81. | Allan SE, Passerini L, Bacchetta R, Crellin N, Dai M, Orban PC, Ziegler SF, Roncarolo MG, Levings MK. The role of 2 FOXP3 isoforms in the generation of human CD4+ Tregs. J Clin Invest. 2005;115:3276-3284. [PubMed] |
| 82. | Schneider A, Long SA, Cerosaletti K, Ni CT, Samuels P, Kita M, Buckner JH. In active relapsing-remitting multiple sclerosis, effector T cell resistance to adaptive T(regs) involves IL-6-mediated signaling. Sci Transl Med. 2013;5:170ra15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 123] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 83. | Frank DN, St Amand AL, Feldman RA, Boedeker EC, Harpaz N, Pace NR. Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc Natl Acad Sci USA. 2007;104:13780-13785. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3731] [Cited by in RCA: 3430] [Article Influence: 190.6] [Reference Citation Analysis (1)] |
| 84. | Manichanh C, Rigottier-Gois L, Bonnaud E, Gloux K, Pelletier E, Frangeul L, Nalin R, Jarrin C, Chardon P, Marteau P. Reduced diversity of faecal microbiota in Crohn’s disease revealed by a metagenomic approach. Gut. 2006;55:205-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1633] [Cited by in RCA: 1676] [Article Influence: 88.2] [Reference Citation Analysis (0)] |
| 85. | Sokol H, Seksik P, Rigottier-Gois L, Lay C, Lepage P, Podglajen I, Marteau P, Doré J. Specificities of the fecal microbiota in inflammatory bowel disease. Inflamm Bowel Dis. 2006;12:106-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 334] [Cited by in RCA: 323] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 86. | Conte MP, Schippa S, Zamboni I, Penta M, Chiarini F, Seganti L, Osborn J, Falconieri P, Borrelli O, Cucchiara S. Gut-associated bacterial microbiota in paediatric patients with inflammatory bowel disease. Gut. 2006;55:1760-1767. [PubMed] |
| 87. | Izcue A, Coombes JL, Powrie F. Regulatory lymphocytes and intestinal inflammation. Annu Rev Immunol. 2009;27:313-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 388] [Cited by in RCA: 388] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 88. | Round JL, Mazmanian SK. Inducible Foxp3+ regulatory T-cell development by a commensal bacterium of the intestinal microbiota. Proc Natl Acad Sci USA. 2010;107:12204-12209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1484] [Cited by in RCA: 1674] [Article Influence: 111.6] [Reference Citation Analysis (0)] |
| 89. | Atarashi K, Tanoue T, Oshima K, Suda W, Nagano Y, Nishikawa H, Fukuda S, Saito T, Narushima S, Hase K. Treg induction by a rationally selected mixture of Clostridia strains from the human microbiota. Nature. 2013;500:232-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1875] [Cited by in RCA: 2138] [Article Influence: 178.2] [Reference Citation Analysis (2)] |
| 90. | Smith PM, Howitt MR, Panikov N, Michaud M, Gallini CA, Bohlooly-Y M, Glickman JN, Garrett WS. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science. 2013;341:569-573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2937] [Cited by in RCA: 3948] [Article Influence: 329.0] [Reference Citation Analysis (1)] |
| 91. | Mucida D, Park Y, Kim G, Turovskaya O, Scott I, Kronenberg M, Cheroutre H. Reciprocal TH17 and regulatory T cell differentiation mediated by retinoic acid. Science. 2007;317:256-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1505] [Cited by in RCA: 1590] [Article Influence: 88.3] [Reference Citation Analysis (0)] |
| 92. | Boschetti G, Nancey S, Sardi F, Roblin X, Flourié B, Kaiserlian D. Therapy with anti-TNFα antibody enhances number and function of Foxp3(+) regulatory T cells in inflammatory bowel diseases. Inflamm Bowel Dis. 2011;17:160-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 93] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 93. | Di Sabatino A, Biancheri P, Piconese S, Rosado MM, Ardizzone S, Rovedatti L, Ubezio C, Massari A, Sampietro GM, Foschi D. Peripheral regulatory T cells and serum transforming growth factor-β: relationship with clinical response to infliximab in Crohn’s disease. Inflamm Bowel Dis. 2010;16:1891-1897. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 39] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 94. | Li Z, Arijs I, De Hertogh G, Vermeire S, Noman M, Bullens D, Coorevits L, Sagaert X, Schuit F, Rutgeerts P. Reciprocal changes of Foxp3 expression in blood and intestinal mucosa in IBD patients responding to infliximab. Inflamm Bowel Dis. 2010;16:1299-1310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 90] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 95. | Ricciardelli I, Lindley KJ, Londei M, Quaratino S. Anti tumour necrosis-alpha therapy increases the number of FOXP3 regulatory T cells in children affected by Crohn’s disease. Immunology. 2008;125:178-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 86] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 96. | Verdonk RC, Haagsma EB, Jonker MR, Bok LI, Zandvoort JH, Kleibeuker JH, Faber KN, Dijkstra G. Effects of different immunosuppressive regimens on regulatory T-cells in noninflamed colon of liver transplant recipients. Inflamm Bowel Dis. 2007;13:703-709. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 97. | Taubert R, Hardtke-Wolenski M, Noyan F, Wilms A, Baumann AK, Schlue J, Olek S, Falk CS, Manns MP, Jaeckel E. Intrahepatic regulatory T cells in autoimmune hepatitis are associated with treatment response and depleted with current therapies. J Hepatol. 2014;61:1106-1114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 113] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 98. | Kurmaeva E, Lord JD, Zhang S, Bao JR, Kevil CG, Grisham MB, Ostanin DV. T cell-associated α4β7 but not α4β1 integrin is required for the induction and perpetuation of chronic colitis. Mucosal Immunol. 2014;7:1354-1365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 32] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 99. | Asseman C, Fowler S, Powrie F. Control of experimental inflammatory bowel disease by regulatory T cells. Am J Respir Crit Care Med. 2000;162:S185-S189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 51] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 100. | Brunstein CG, Miller JS, Cao Q, McKenna DH, Hippen KL, Curtsinger J, Defor T, Levine BL, June CH, Rubinstein P. Infusion of ex vivo expanded T regulatory cells in adults transplanted with umbilical cord blood: safety profile and detection kinetics. Blood. 2011;117:1061-1070. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 816] [Cited by in RCA: 829] [Article Influence: 59.2] [Reference Citation Analysis (0)] |
| 101. | Di Ianni M, Falzetti F, Carotti A, Terenzi A, Del Papa B, Perruccio K, Ruggeri L, Sportoletti P, Rosati E, Marconi P. Immunoselection and clinical use of T regulatory cells in HLA-haploidentical stem cell transplantation. Best Pract Res Clin Haematol. 2011;24:459-466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 41] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 102. | Martelli MF, Di Ianni M, Ruggeri L, Falzetti F, Carotti A, Terenzi A, Pierini A, Massei MS, Amico L, Urbani E. HLA-haploidentical transplantation with regulatory and conventional T-cell adoptive immunotherapy prevents acute leukemia relapse. Blood. 2014;124:638-644. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 290] [Cited by in RCA: 304] [Article Influence: 27.6] [Reference Citation Analysis (0)] |
| 103. | Kennedy-Nasser AA, Ku S, Castillo-Caro P, Hazrat Y, Wu MF, Liu H, Melenhorst J, Barrett AJ, Ito S, Foster A. Ultra low-dose IL-2 for GVHD prophylaxis after allogeneic hematopoietic stem cell transplantation mediates expansion of regulatory T cells without diminishing antiviral and antileukemic activity. Clin Cancer Res. 2014;20:2215-2225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 164] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 104. | Koreth J, Matsuoka K, Kim HT, McDonough SM, Bindra B, Alyea EP, Armand P, Cutler C, Ho VT, Treister NS. Interleukin-2 and regulatory T cells in graft-versus-host disease. N Engl J Med. 2011;365:2055-2066. [PubMed] |
| 105. | Matsuoka K, Koreth J, Kim HT, Bascug G, McDonough S, Kawano Y, Murase K, Cutler C, Ho VT, Alyea EP. Low-dose interleukin-2 therapy restores regulatory T cell homeostasis in patients with chronic graft-versus-host disease. Sci Transl Med. 2013;5:179ra43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 314] [Cited by in RCA: 380] [Article Influence: 31.7] [Reference Citation Analysis (0)] |
| 106. | Hartemann A, Bensimon G, Payan CA, Jacqueminet S, Bourron O, Nicolas N, Fonfrede M, Rosenzwajg M, Bernard C, Klatzmann D. Low-dose interleukin 2 in patients with type 1 diabetes: a phase 1/2 randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol. 2013;1:295-305. [PubMed] [DOI] [Full Text] |
| 107. | Long SA, Rieck M, Sanda S, Bollyky JB, Samuels PL, Goland R, Ahmann A, Rabinovitch A, Aggarwal S, Phippard D. Rapamycin/IL-2 combination therapy in patients with type 1 diabetes augments Tregs yet transiently impairs β-cell function. Diabetes. 2012;61:2340-2348. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 234] [Cited by in RCA: 258] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 108. | Marek-Trzonkowska N, Mysliwiec M, Dobyszuk A, Grabowska M, Techmanska I, Juscinska J, Wujtewicz MA, Witkowski P, Mlynarski W, Balcerska A. Administration of CD4+CD25highCD127- regulatory T cells preserves β-cell function in type 1 diabetes in children. Diabetes Care. 2012;35:1817-1820. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 311] [Cited by in RCA: 352] [Article Influence: 27.1] [Reference Citation Analysis (0)] |
| 109. | Marek-Trzonkowska N, Myśliwiec M, Dobyszuk A, Grabowska M, Derkowska I, Juścińska J, Owczuk R, Szadkowska A, Witkowski P, Młynarski W. Therapy of type 1 diabetes with CD4(+)CD25(high)CD127-regulatory T cells prolongs survival of pancreatic islets - results of one year follow-up. Clin Immunol. 2014;153:23-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 239] [Cited by in RCA: 297] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 110. | Desreumaux P, Foussat A, Allez M, Beaugerie L, Hébuterne X, Bouhnik Y, Nachury M, Brun V, Bastian H, Belmonte N. Safety and efficacy of antigen-specific regulatory T-cell therapy for patients with refractory Crohn’s disease. Gastroenterology. 2012;143:1207-17.e1-2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 249] [Cited by in RCA: 296] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 111. | Schneider A, Rieck M, Sanda S, Pihoker C, Greenbaum C, Buckner JH. The effector T cells of diabetic subjects are resistant to regulation via CD4+ FOXP3+ regulatory T cells. J Immunol. 2008;181:7350-7355. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 253] [Cited by in RCA: 245] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 112. | Bollyky PL, Lord JD, Masewicz SA, Evanko SP, Buckner JH, Wight TN, Nepom GT. Cutting edge: high molecular weight hyaluronan promotes the suppressive effects of CD4+CD25+ regulatory T cells. J Immunol. 2007;179:744-747. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 144] [Article Influence: 8.0] [Reference Citation Analysis (0)] |