Published online Sep 21, 2015. doi: 10.3748/wjg.v21.i35.10215
Peer-review started: April 1, 2015
First decision: May 18, 2015
Revised: June 3, 2015
Accepted: July 18, 2015
Article in press: July 18, 2015
Published online: September 21, 2015
Processing time: 170 Days and 20.9 Hours
AIM: To evaluate the changes of shear-wave velocity (Vs) by acoustic radiation force impulse after treatment in chronic hepatitis C.
METHODS: Eighty-seven patients with chronic hepatitis C were consecutively treated with combinations of interferon (IFN) plus ribavirin (RBV). Vs value (m/s) was measured with acoustic radiation force impulse before treatment, at end of treatment (EOT), 1 year after EOT, and 2 years after EOT.
RESULTS: In patients with a sustained virological response (SVR) (n = 41), Vs significantly decreased at EOT [1.19 (1.07-1.37), P = 0.0004], 1 year after EOT [1.10 (1.00-1.22), P = 0.0001], and 2 years after EOT [1.05 (0.95-1.16), P < 0.0001] compared with baseline [1.27 (1.11-1.49)]. In patients with a relapse (n = 26), Vs did not significantly decrease at EOT [1.23 (1.12-1.55)], 1 year after EOT [1.20 (1.12-1.80)], and 2 years after EOT [1.41 (1.08-2.01)] compared with baseline [1.39 (1.15-1.57)]. In patients with a nonvirological response (n = 20), Vs did not significantly decrease at EOT [1.64 (1.43-2.06)], 1 year after EOT [1.66 (1.30-1.95)], and 2 years after EOT [1.61 (1.36-2.37)] compared with baseline [1.80 (1.54-2.01)]. Among genotype 1 patients, baseline Vs was significantly lower in SVR patients [1.28 (1.04-1.40)] than in non-SVR patients [1.56 (1.20-1.83)] (P = 0.0142).
CONCLUSION: Reduction of Vs values was shown in SVR patients after IFN-plus-RBV therapy by acoustic radiation force impulse.
Core tip: The estimation of the stage of liver fibrosis is important for the prediction of hepatitis outcome, response to treatment, and evaluation of treatment outcomes in patients with chronic liver disease. Methods for noninvasive assessment of liver fibrosis have been developed. Shear-wave velocity (Vs) measured by acoustic radiation force impulse (ARFI) correlate with liver fibrosis stages in various liver diseases. To evaluate Vs value changes measured by ARFI after interferon (IFN) plus ribavirin (RBV) in chronic hepatitis C patients. Reduction of Vs values was shown in sustained virological response patients after IFN-plus-RBV therapy by ARFI.
- Citation: Osakabe K, Ichino N, Nishikawa T, Sugiyama H, Kato M, Shibata A, Asada W, Kawabe N, Hashimoto S, Murao M, Nakano T, Shimazaki H, Kan T, Nakaoka K, Takagawa Y, Ohki M, Kurashita T, Takamura T, Yoshioka K. Changes of shear-wave velocity by interferon-based therapy in chronic hepatitis C. World J Gastroenterol 2015; 21(35): 10215-10223
- URL: https://www.wjgnet.com/1007-9327/full/v21/i35/10215.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i35.10215
Chronic hepatitis C virus (HCV) infection affects approximately 170 million people worldwide[1]. HCV usually causes chronic infection, which can result in chronic hepatitis, liver cirrhosis, and hepatocellular carcinoma[2,3]. Therefore, an estimation of the stage of liver fibrosis is important for the prediction of hepatitis outcome, response to treatment, and evaluation of treatment outcomes in patients with chronic liver disease[4].
The aim of the treatment for chronic hepatitis C is the elimination of the virus. Several studies have shown that sustained virological response (SVR) after antiviral therapy can reduce fibrosis[5-8]. In addition, partial regression of liver fibrosis has also been reported in patients with nonresponse and relapse to antiviral therapy with pegylated interferon (peg-IFN) and ribavirin (RBV)[5,6].
Methods of treatment using IFN have continued to advance[9]. Combination therapy of peg-IFN and RBV with telaprevir, boceprevir, or simeprevir has recently become available[10-12]. Furthermore, an IFN-free therapy with daclatasvir, asunaprevir, sofosbuvir, ledipasvir, and paritaprevir/ritonavir/ombitasvir plus dasabuvir has also become available[13-20]. Thus, it is likely that IFN-based therapy will be replaced by IFN-free therapies. The reduction of fibrosis after IFN-free therapies should be evaluated in the future.
To estimate the effect of antiviral therapy, the evaluations of viral load and ALT levels are useful. However, the evaluation of liver fibrosis is also important. Liver biopsy is currently considered the gold standard for assessing the stage of fibrosis in chronic liver disease. However, it is an invasive procedure, with rare but potentially life-threatening complications. In addition, the accuracy of liver biopsy in assessing fibrosis has limitations because of sampling errors and interobserver variability[21-23].
Methods for noninvasive assessment of liver fibrosis have been developed. Liver stiffness (LS)[24-32] by transient elastography (TE) with Fibroscan and shear-wave velocity (Vs) measured by acoustic radiation force impulse (ARFI)[33-37] correlate with liver fibrosis stages in various liver diseases.
The aim of the present study was to evaluate the usefulness of Vs values measured by ARFI for the assessment of liver fibrosis regression after IFN-based therapy.
This study was performed in strict accordance with the ethical guidelines of the Declaration of Helsinki and was approved by the ethics committee of Fujita Health University. All study participants provided written informed consent.
Eighty-seven patients with chronic hepatitis C were consecutively treated with combinations of IFNs and RBV in Fujita Health University Hospital from October 2009 to February 2014 (Table 1).
| All | SVRs | Relapsers | NVRs | P value | |
| Number of patients | 87 | 41 | 26 | 20 | |
| Gender (female/male) | 47/40 | 17/24 | 20/6 | 10/10 | N.S. |
| Age (yr) | 59 (50-66) | 52 (44-63)2 | 63 (54-66)2 | 62 (51-67) | 0.01252 |
| Fibrosis stage (F0-2/F3,4)1 | 38/24 | 23/43 | 12/84 | 3/1234 | < 0.00013 0.01544 |
| Inflammatory grade (A0,1/A2,3)1 | 25/37 | 14/133 | 8/12 | 3/123 | 0.03843 |
| Genotype (1/2)2 | 57/28 | 17/24234 | 21/424 | 19/034 | 0.00042 < 0.00013 0.02814 |
| Platelet count (× 104/μL) | 14.2 (11.7-18.8) | 15.7 (12.7-19.5)3 | 14.4 (11.4-17.9) | 11.1 (9.1-15.7)3 | 0.01163 |
| Prothrombin time (%) | 97 (91-110) | 99 (91-111) | 96 (91-110) | 97 (88-103) | NS |
| Albumin (g/dL) | 4.2 (4.0-4.5) | 4.3 (4.2-4.6)3 | 4.2 (3.9-4.5) | 4.2 (3.9-4.2)3 | 0.01413 |
| Total bilirubin (mg/dL) | 0.8 (0.6-1.0) | 0.7 (0.5-1.1) | 0.7 (0.6-1.0) | 0.9 (0.7-1.0) | NS |
| AST (IU/L) | 45 (32-69) | 40 (27-59)3 | 43 (32-59) | 57 (46-88)3 | 0.01873 |
| ALT (IU/L) | 51 (35-86) | 51 (30-75) | 47 (33-94) | 58 (42-95) | NS |
| γ-GTP (IU/L) | 37 (23-67) | 47 (23-83) | 28 (21-43)4 | 44 (27-110)4 | 0.02054 |
| ALP (U/L) | 261 (212-345) | 244 (208-267)3 | 287 (220-389) | 358 (223-422)3 | 0.00343 |
| Hyaluronic acid (ng/mL) | 80 (42-169) | 47 (33-94)3 | 86 (54-168)4 | 163 (97-301)34 | 0.00033 0.02764 |
| HCV RNA (logIU/mL) | 6.3 (5.2-6.8) | 5.7 (4.8-6.8)3 | 6.4 (5.8-6.7) | 6.7 (6.3-7.1)3 | 0.01093 |
| APRI | 0.91 (0.54-1.68) | 0.82 (0.40-1.16)3 | 0.95 (0.57-1.58) | 1.59 (0.85-2.84)3 | 0.00833 |
| FIB-4 | 2.57 (1.66-4.00) | 2.04 (1.49-2.70)23 | 2.95 (2.14-4.49)2 | 4.52 (2.82-5.96)3 | 0.01082 0.00013 |
| Vs (m/s) | |||||
| Pretreatment | 1.36 (1.16-1.72) | 1.27 (1.11-1.49)3 | 1.39 (1.15-1.57)4 | 1.80 (1.54-2.01)34 | 0.00073 0.00364 |
| End of treatment | 1.27 (1.12-1.56) | 1.19 (1.07-1.37)3 | 1.23 (1.12-1.55)4 | 1.64 (1.43-2.06)34 | < 0.00013 0.01244 |
| 1 yr after treatment | 1.20 (1.06-1.50) | 1.10 (1.00-1.22)23 | 1.20 (1.12-1.80)2 | 1.66 (1.30-1.95)3 | 0.01922 0.00133 |
| 2 yr after treatment | 1.17 (1.03-1.48) | 1.05 (0.95-1.16)23 | 1.41 (1.08-2.01)2 | 1.61 (1.36-2.37)3 | 0.00962 0.00043 |
Seventy patients were treated with peg-IFN-α2b (1.5 mg/kg per week) and RBV (600-1000 mg/d), 9 patients with peg-IFN-α2a (180 mg/wk) and RBV (600-1000 mg/d), and 8 patients with IFN-β (1-6 MU/d) and RBV (600-1000 mg/d).
The planned treatment duration was 24, 48, or 72 wk according to HCV genotype, viral load, and response to treatment in the first 12 wk. The responses to IFN therapy were categorized into three types: SVR, where negativity of HCV RNA persisted 6 mo after the end of treatment (EOT); relapse, where HCV RNA became negative during treatment but relapsed to positive after EOT; and nonvirological response (NVR) where HCV RNA remained positive throughout treatment.
The biochemical, serological, and virological examinations were performed within 2 d of the Vs measurements: serum alanine aminotransferase (ALT), aspartate aminotransferase (AST), albumin, total bilirubin, γ-glutamyl transpeptidase (γ-GTP), alkaline phosphatase (ALP), hyaluronic acid, prothrombin time, platelet count, aminotransferase-to-platelet ratio index (APRI), and fibrosis-4 (FIB-4). APRI values were calculated using the following formula: AST (/ULN)/platelets (109/L) × 100[38]. FIB-4 values were calculated using the following formula: age (years) × AST (IU/L)/platelets (109/L) × [ALT (IU/L)]1/2[39].
Vs measurement by ARFI was performed using a Siemens ACUSON S2000 ultrasound system (Siemens Japan Co., Ltd., Tokyo, Japan) before treatment, at EOT (within 6 mo after EOT), 1 year after EOT (7-18 mo after EOT) and 2 years after EOT (19-30 mo after EOT).
The region in the liver to be examined for elastic properties was targeted with a region-of-interest (ROI) cursor while performing B-mode imaging. Tissue at ROI was mechanically excited using acoustic push pulses to generate localized tissue displacements. The displacements resulted in the propagation of the shear wave away from the region of excitation, which was tracked using ultrasonic correlation-based methods. The maximal displacement was estimated for multiple ultrasound tracking beams laterally adjacent to the single push-beam. By measuring the time to peak displacement at each lateral location, the shear wave propagation velocity was reconstructed. The examination was performed on the right lobe of the liver from the right intercostal space. A measurement depth of 2-3 cm below the liver capsule was chosen. Ten successful acquisitions at different locations were performed on each patient. The results are expressed in meters/second (m/s), and the median value was calculated. The shear wave propagation velocity was considered to be proportional to the square root of tissue elasticity.
The procedures were performed by two investigators (NT and HS) who were blind to clinical, serological and histological data. The correlation in Vs measurement between two operators was satisfactory (r = 0.934).
Patients were classified according to the responses to IFN plus RBV therapy. Patients with genotype 1 were also examined. The groups were compared by chi-squared test and Mann-Whitney U-test. Data were expressed as median and interquartile range.
In the follow-up phase of the study, the significance of changes in Vs values between the pairs of 4 points was evaluated by the Wilcoxon signed-rank test.
The statistical analysis was performed using JMP® software (SAS Institute, Cary, NC, United States).
Forty one of 87 patients (47.1%) achieved SVR, 26 (29.9%) relapsed, and 20 (23.0%) had NVR (Table 1). Age, genotype, and FIB-4 significantly differed between patients with SVR and those who relapsed (P = 0.0125, P = 0.0004, and P < 0.0108, respectively).
Fibrosis stage, inflammatory grade, genotype, platelet count, albumin, AST, ALP, hyaluronic acid, HCV RNA, APRI, and FIB-4 significantly differed between patients with SVR and those with NVR (P < 0.0001, P = 0.0384, P < 0.0001, P = 0.0116, P = 0.0141, P = 0.0187, P = 0.0034, P = 0.0003, P = 0.0109, P = 0.0083 and P = 0.0001, respectively).
Fibrosis stage, genotype, γ-GTP, and hyaluronic acid significantly differed between patients who relapsed and those with NVR (P = 0.0154, P = 0.0281, P = 0.0205, and P = 0.0276, respectively).
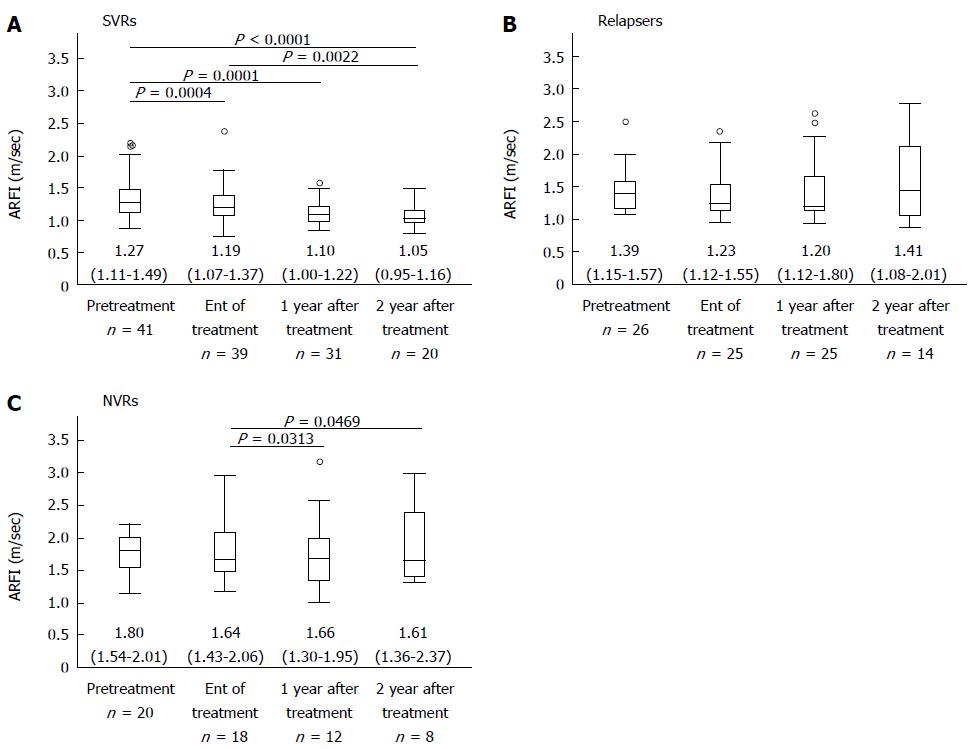
Vs values at pretreatment were significantly higher in patients with NVR than in those achieving SVR and those who relapsed (P = 0.0007, and P = 0.0036, respectively). Vs values at EOT were significantly higher in patients with NVR than those who achieved SVR and those who relapsed (P < 0.0001, and P = 0.0124, respectively). Vs values at 1 year after EOT were significantly lower in SVR patients than in patients who relapsed and in those with NVR (P = 0.0192, and P = 0.0013, respectively). Vs values at 2 years after EOT were significantly lower in patients achieving SVR than in patients who relapsed or had NVR (P = 0.0096, and P = 0.0004, respectively).
In 39 patients achieving SVR whose Vs values were measured at both points, Vs values were significantly lower at EOT [1.19 (1.07-1.36)] than at pretreatment [1.28 (1.11-1.51)] (P = 0.0004). Vs values were significantly lower at 1 year after treatment [1.10 (1.00-1.23)] than at pretreatment [1.27 (1.11-1.46)] (P = 0.0001) in 33 patients in whom Vs values were measured at both points. Vs values were significantly lower at 2 years after EOT [1.05 (0.96-1.16)] than at pretreatment [1.25 (0.97-1.47)] (P < 0.0001) in 22 patients for whom Vs values were measured at both points. Vs values were significantly lower at 2 years after treatment [1.07 (0.96-1.17)] than at EOT [1.20 (1.05-1.33)] (P = 0.0022) in 20 patients for whom Vs values were measured at both points (Figure 1A).
In patients who relapsed, Vs values did not differ significantly among pretreatment, EOT, 1 year, and 2 years after EOT (Figure 1B).
In 11 patients with NVR whose Vs values were measured at both time points, Vs values were significantly higher at 1 year after EOT [1.72 (1.43-2.00)] than at EOT [1.53 (1.28-1.75)] (P = 0.0313). Vs values were significantly higher at 2 years after EOT [1.58 (1.33-1.68)] than at EOT [1.37 (1.20-1.64)] (P = 0.0469) in 7 patients in whom Vs was measured at both points (Figure 1C).
Fibrosis stages were deduced from Vs values according to cut-off values for fibrosis stages[40]. The cut-off value was 1.28 m/s for F2, 1.44 m/s for F3, and 1.73 m/s for F4. The deduced fibrosis stages of the first and the last measurements were compared among patients with the deduced fibrosis stage F3 or F4 at the first measurement (Table 2). Thirteen of 41 (31.7%) SVR patients had deduced stage F3 or F4 at pretreatment. Nine of 13 patients (69.2%) had two point reduction of deduced fibrosis stage at the last measurement during the median observation period of 2 years. Two patients (15.4%) had 1-point reduction. In patients who relapsed, 12 of 26 patients (46.2%) had deduced stage F3 or F4 at pretreatment. Two patients (16.7%) had 1-point reduction of deduced stage during the median observation period of 2.8 years. One patient (8.3%) had 1-point reduction. Three patients (25.0%) had 1-point progression of deduced stage. In patients with NVRs, 16 of 20 (80.0%) patients had deduced stage F3 or F4 at pretreatment. Five of 16 patients (31.3%) had 1-point reduction of deduced stage during the median observation period of 1.6 years. Two patients (12.5%) had 1-point progression of deduced stage.
| SVRs | Relapsers | NVRs | |
| (n = 13) | (n = 12) | (n = 16) | |
| 2-point reduction | 9 (69.2) | 2 (16.7) | 0 |
| 1-point reduction | 2 (15.4) | 1 (8.3) | 5 (31.3) |
| No changes | 2 (15.4) | 6 (50.0) | 9 (56.2) |
| 1-point progression | 0 | 3 (25.0) | 2 (12.5) |
| Interval (yr) | 2.0 (1.0-2.5) | 2.8 (1.7-3.1) | 1.6 (0.7-2.4) |
Fifty seven patients (23 men, 34 women, median age 59 years; age range 50-66 years) had HCV genotype 1 (Table 3). Seventeen of 57 patients (29.8%) obtained SVR, 40 (70.2%) had non-SVR (21 patients relapsed and 19 patients had NVRs).
| All | SVRs | Relapsers and NVRs | P value | |
| Number of patients | 57 | 17 | 40 | |
| Gender (female/male) | 34/23 | 7/10 | 25/15 | NS |
| Age (yr) | 59 (50-66) | 51 (41-58) | 62 (54-67) | 0.0016 |
| Fibrosis stage (F0-F2 /F3,4)1 | 25/17 | 12/0 | 13/17 | < 0.0001 |
| Inflammatory grade (A0,1/A2,3)1 | 16/26 | 6/6 | 10/20 | NS |
| Platelet count (× 104/μL) | 14.0 (10.7-18.3) | 15.4 (13.0-19.5) | 13.2 (9.4-17.4) | NS |
| Prothrombin time (%) | 99 (91-111) | 102 (91-110) | 98 (92-112) | NS |
| Albumin (g/dL) | 4.2 (4.0-4.5) | 4.4 (4.2-4.6) | 4.2 (4.0-4.4) | 0.0496 |
| Total bilirubin (mg/dL) | 0.8 (0.6-1.0) | 0.8 (0.5-1.1) | 0.8 (0.6-1.0) | NS |
| AST (IU/L) | 47 (32-71) | 38 (28-63) | 48 (35-77) | NS |
| ALT (IU/L) | 50 (35-90) | 50 (30-92) | 52 (36-91) | NS |
| γ-GTP (IU/L) | 33 (22-51) | 37 (15-66) | 30 (23-50) | NS |
| ALP (U/L) | 267 (212-379) | 246 (187-262) | 314 (215-416) | 0.0053 |
| Hyaluronic acid (ng/mL) | 95 (46-173) | 51 (21-98) | 114 (68-180) | 0.0076 |
| HCV RNA (logIU/mL) | 6.4 (5.8-7.0) | 6.0 (5.2-7.1) | 6.4 (6.1-6.9) | NS |
| APRI | 0.91 (0.56-1.69) | 0.80 (0.45-1.09) | 1.05 (0.63-1.73) | NS |
| FIB-4 | 2.69 (1.64-4.46) | 1.66 (1.26-2.53) | 3.34 (2.23-4.63) | 0.0014 |
| Vs (m/s); Before treatment | 1.44 (1.17-1.75) | 1.28 (1.04-1.40) | 1.56 (1.20-1.83) | 0.0142 |
Age, fibrosis stage, ALP, hyaluronic acid, FIB-4, and Vs values were significantly lower in patients who achieved SVR compared with patients with non-SVR (P = 0.0016, P < 0.0001, P = 0.0053, P = 0.0076, P = 0.0014, and P = 0.0142, respectively). Albumin was significantly higher in patients with SVR than in those with non-SVR (P = 0.0496).
In multivariate analysis, age (P = 0.0077) was found to be associated with SVR of IFN plus RBV therapy (Table 4).
| β | Standard error | P value | 95%CI | |
| Age (yr) | -0.347 | 0.006 | 0.008 | -0.0271-(-0.0043) |
| ALP (U/L) | -0.196 | 0.001 | NS | -0.0021-(-0.0003) |
| Vs (m/s) | -0.196 | 0.162 | NS | -0.5773-(-0.0721) |
The present study examined the usefulness of Vs values measured by ARFI for the assessment of liver fibrosis change after IFN-plus-RBV therapy. It was demonstrated that Vs values were significantly reduced at EOT and 1 year after EOT in SVR patients compared with pretreatment values, and that Vs values were significantly reduced 2 years after EOT compared with pretreatment values and the values at EOT in patients who achieved SVR. Thus far, several studies with paired pre- and post-IFN therapy biopsies reported decreased fibrosis in 29% (mean times between biopsies, 1.6 years)[41], 44% (2.5 years)[42], 59% (3.7 years)[43] or 82% (5.2 years)[7] of patients with SVR. George et al[7] reported that 67% of patients with SVR and pretreatment cirrhosis or advanced fibrosis had a 2-point or greater decrease in the fibrosis score in 5.2 years. In the present study, 9 of 13 patients (69.2%) had a 2-point reduction of deduced fibrosis stage in a period of 2 years. Two of 13 patients (15.4%) had a 1-point reduction. Our previous study using TE demonstrated that 78% of SVR patients with pretreatment-deduced fibrosis stage F3 or F4 had a 2-point or greater decrease in deduced fibrosis stage in a period of 2.1 years[44]. The occurrence rates and the degree of fibrosis reduction after IFN-based treatment in the present study with ARFI were comparable with our previous study with TE and were higher than the reports based on liver biopsy. The reason for higher occurrence rates and degree of fibrosis reduction after IFN-based treatment with ARFI and TE may be attributed to the higher sensitivity of ARFI and TE to detect subtle reduction of fibrosis compared with liver biopsy.
The reduction of Vs value observed in the present study is probably the reflection of fibrosis regression. Several studies with TE, another noninvasive method for the assessment of liver fibrosis, have reported that LS is affected by ALT levels. Fraquelli et al[45] reported that, by TE, fibrosis staging is overestimated by necroinflammatory activity and steatosis. Coco et al[46] found that LS is higher in patients with an elevated ALT than in those with either spontaneous biochemical remission or after antiviral therapy. Thus, it is probable that ALT or inflammatory activity affects TE. Our previous study demonstrated that Vs is not affected by inflammatory activity. Rizzo et al[47] reported that ARFI was not associated with ALT, body mass index, Metavir score, and liver steatosis, while TE was significantly correlated with the ALT value by multivariate analysis. Bota et al[48] reported that discordance of at least two stages of fibrosis between ARFI results and histologic assessments were associated: female sex (P = 0.004), interquartile range interval (IQR) ≥ 30%, high ALT, and high AST in an univariate analysis, while in a multivariate analysis, female sex and IQR of ≥ 30% were associated with discordances. However, Yoon et al[49] reported that the optimum cut-off values for Vs measured by ARFI were 1.13 m/s for F2 or more and 1.98 m/s for F4; these decreased to 1.09 m/s for F2 or more and 1.81 m/s for F4 in patients with normal ALT levels. Thus, the faster and conspicuous reduction of Vs may be partially attributed to the reduction of inflammatory activity. Further studies are necessary to elucidate the correlation of reduction of Vs values with histological changes after SVR.
Among patients who relapsed, there were no significant changes in Vs value among the points of ARFI measurement. In NVR patients, the Vs values did not change from pretreatment to EOT, although they increased from EOT to 1 year after EOT and 2 years after EOT. These findings indicate that there was no significant improvement of fibrosis in patients who relapsed and that fibrosis progressed in NVR patients after IFN-based therapy. The deduced fibrosis stages progressed in 25% of patients who relapsed and in 12.5% of patients with NVR whose pretreatment-deduced stages were F3 or F4. In the patients with pretreatment-deduced stages of F0-2, the deduced fibrosis stage increased one point or more in 28.6% of patients who relapsed and 50% of those with NVR (data not shown).
In our previous study using TE, LS significantly reduced at EOT and at 1 year after EOT compared with baseline among those who relapsed. This finding may indicate that the inflammatory activity increases LS values of TE. In the present study using ARFI, Vs was not significantly reduced in patients who relapsed. This may indicate that the inflammatory activity does not affect Vs values of ARFI. Therefore, ARFI may be a more appropriate method for the follow-up of fibrosis regression after antiviral treatment compared with TE.
In patients with HCV genotypes 2 or 3, the combination of peg-IFN and RBV is usually given for 24 wk, achieving rates of SVR of approximately 75%-85%. In patients with HCV genotype 1, the combination of peg-IFN and RBV is usually given for 48 weeks, resulting in SVR rates of 40%-50%[50,51]. The fibrosis stage has been reported to be one of the predictive factors for SVR in HCV genotype 1. In the present study, baseline Vs was significantly lower in patients achieving SVR than in those who relapsed or had NVR. However, in multivariate analysis, age was identified as the only risk factor associated with SVR. In a previous study, older age was reported to decrease the SVR rate of IFN plus RBV therapy[52].
In the present study, there was a reduction of Vs values in patients who achieved SVR after IFN plus RBV therapy. However, IFN-free therapy has become the first-line therapy for chronic hepatitis C. In the same fashion, there is an ongoing study to determine the changes of Vs values during IFN-free therapy to then evaluate the changes of fibrosis after IFN-free therapy in our hospital.
The authors would like to thank Enago (http://www.enago.jp) for the English language review.
Interferon (IFN) treatment has been demonstrated to reduce liver fibrosis by liver biopsies and by transient elastography which can assess liver fibrosis noninvasively. The shear-wave velocity (Vs) by acoustic radiation force impulse (ARFI) is another noninvasive method for assessing liver fibrosis. Reduction of liver fibrosis after antiviral therapy has not been well evaluated by ARFI.
The Vs value by ARFI is a brand-new ultrasound technology. Since the invention of ARFI, a lot of studies demonstrated that ARFI is a useful tool to evaluate liver fibrosis in chronic liver diseases noninvasively.
The present study evaluated Vs value by ARFI before and after IFN treatment and demonstrated the reduction of liver fibrosis after IFN treatment in SVR patients with chronic hepatitis C.
This research showed that ARFI is a useful tool to evaluate the reduction of fibrosis in patients with chronic hepatitis C after IFN-plus-RBV therapy.
The Vs value measured by acoustic radiation force impulse is a new ultrasonography modality to evaluate liver stiffness by acoustic radiation force-based imaging method. The examiners can choose the place where Vs value is measured on the conventional B-mode ultrasound image.
The article is a well-designed and performed work on the changes of shear wave velocity in patients with chronic hepatitis C who received antiviral treatment. The Vs values were significantly reduced at EOT and at different time points post treatment in patients with SVR while no changes were identified in patient with treatment failure.
P- Reviewer: Alexopoulou A S- Editor: Ma YJ L- Editor: O’Neill M E- Editor: Liu XM
| 1. | Dienstag JL, McHutchison JG. American Gastroenterological Association technical review on the management of hepatitis C. Gastroenterology. 2006;130:231-264; quiz 214-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 276] [Cited by in RCA: 270] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 2. | Di Bisceglie AM. Hepatitis C. Lancet. 1998;351:351-355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 266] [Cited by in RCA: 267] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 3. | Marcellin P. Hepatitis C: the clinical spectrum of the disease. J Hepatol. 1999;31 Suppl 1:9-16. [PubMed] |
| 4. | Strader DB, Wright T, Thomas DL, Seeff LB. Diagnosis, management, and treatment of hepatitis C. Hepatology. 2004;39:1147-1171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1283] [Cited by in RCA: 1232] [Article Influence: 58.7] [Reference Citation Analysis (0)] |
| 5. | Poynard T, McHutchison J, Manns M, Trepo C, Lindsay K, Goodman Z, Ling MH, Albrecht J. Impact of pegylated interferon alfa-2b and ribavirin on liver fibrosis in patients with chronic hepatitis C. Gastroenterology. 2002;122:1303-1313. [PubMed] |
| 6. | Cammà C, Di Bona D, Schepis F, Heathcote EJ, Zeuzem S, Pockros PJ, Marcellin P, Balart L, Alberti A, Craxì A. Effect of peginterferon alfa-2a on liver histology in chronic hepatitis C: a meta-analysis of individual patient data. Hepatology. 2004;39:333-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 165] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 7. | George SL, Bacon BR, Brunt EM, Mihindukulasuriya KL, Hoffmann J, Di Bisceglie AM. Clinical, virologic, histologic, and biochemical outcomes after successful HCV therapy: a 5-year follow-up of 150 patients. Hepatology. 2009;49:729-738. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 311] [Cited by in RCA: 291] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 8. | D’Ambrosio R, Aghemo A, Rumi MG, Ronchi G, Donato MF, Paradis V, Colombo M, Bedossa P. A morphometric and immunohistochemical study to assess the benefit of a sustained virological response in hepatitis C virus patients with cirrhosis. Hepatology. 2012;56:532-543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 312] [Cited by in RCA: 319] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 9. | Alexopoulou A, Papatheodoridis GV. Current progress in the treatment of chronic hepatitis C. World J Gastroenterol. 2012;18:6060-6069. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 20] [Cited by in RCA: 23] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 10. | Poordad F, McCone J, Bacon BR, Bruno S, Manns MP, Sulkowski MS, Jacobson IM, Reddy KR, Goodman ZD, Boparai N. Boceprevir for untreated chronic HCV genotype 1 infection. N Engl J Med. 2011;364:1195-1206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1996] [Cited by in RCA: 1981] [Article Influence: 141.5] [Reference Citation Analysis (0)] |
| 11. | Jacobson IM, McHutchison JG, Dusheiko G, Di Bisceglie AM, Reddy KR, Bzowej NH, Marcellin P, Muir AJ, Ferenci P, Flisiak R. Telaprevir for previously untreated chronic hepatitis C virus infection. N Engl J Med. 2011;364:2405-2416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1905] [Cited by in RCA: 1862] [Article Influence: 133.0] [Reference Citation Analysis (0)] |
| 12. | Drafting Committee for Hepatitis Management Guidelines, the Japan Society of Hepatology. JSH Guidelines for the Management of Hepatitis C Virus Infection: A 2014 Update for Genotype 1. Hepatol Res. 2014;44 Suppl S1:59-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 47] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 13. | Gao M. Antiviral activity and resistance of HCV NS5A replication complex inhibitors. Curr Opin Virol. 2013;3:514-520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 113] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 14. | McPhee F, Sheaffer AK, Friborg J, Hernandez D, Falk P, Zhai G, Levine S, Chaniewski S, Yu F, Barry D. Preclinical Profile and Characterization of the Hepatitis C Virus NS3 Protease Inhibitor Asunaprevir (BMS-650032). Antimicrob Agents Chemother. 2012;56:5387-5396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 163] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 15. | Lok AS, Gardiner DF, Lawitz E, Martorell C, Everson GT, Ghalib R, Reindollar R, Rustgi V, McPhee F, Wind-Rotolo M. Preliminary study of two antiviral agents for hepatitis C genotype 1. N Engl J Med. 2012;366:216-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 478] [Cited by in RCA: 476] [Article Influence: 36.6] [Reference Citation Analysis (0)] |
| 16. | Chayama K, Takahashi S, Toyota J, Karino Y, Ikeda K, Ishikawa H, Watanabe H, McPhee F, Hughes E, Kumada H. Dual therapy with the nonstructural protein 5A inhibitor, daclatasvir, and the nonstructural protein 3 protease inhibitor, asunaprevir, in hepatitis C virus genotype 1b-infected null responders. Hepatology. 2012;55:742-748. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 284] [Cited by in RCA: 290] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 17. | Kumada H, Suzuki Y, Ikeda K, Toyota J, Karino Y, Chayama K, Kawakami Y, Ido A, Yamamoto K, Takaguchi K. Daclatasvir plus asunaprevir for chronic HCV genotype 1b infection. Hepatology. 2014;59:2083-2091. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 468] [Cited by in RCA: 453] [Article Influence: 41.2] [Reference Citation Analysis (0)] |
| 18. | Afdhal N, Zeuzem S, Kwo P, Chojkier M, Gitlin N, Puoti M, Romero-Gomez M, Zarski JP, Agarwal K, Buggisch P. Ledipasvir and sofosbuvir for untreated HCV genotype 1 infection. N Engl J Med. 2014;370:1889-1898. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1357] [Cited by in RCA: 1365] [Article Influence: 124.1] [Reference Citation Analysis (0)] |
| 19. | Feld JJ, Kowdley KV, Coakley E, Sigal S, Nelson DR, Crawford D, Weiland O, Aguilar H, Xiong J, Pilot-Matias T. Treatment of HCV with ABT-450/r-ombitasvir and dasabuvir with ribavirin. N Engl J Med. 2014;370:1594-1603. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 681] [Cited by in RCA: 656] [Article Influence: 59.6] [Reference Citation Analysis (0)] |
| 20. | Lawitz E, Sulkowski MS, Ghalib R, Rodriguez-Torres M, Younossi ZM, Corregidor A, DeJesus E, Pearlman B, Rabinovitz M, Gitlin N. Simeprevir plus sofosbuvir, with or without ribavirin, to treat chronic infection with hepatitis C virus genotype 1 in non-responders to pegylated interferon and ribavirin and treatment-naive patients: the COSMOS randomised study. Lancet. 2014;384:1756-1765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 626] [Cited by in RCA: 597] [Article Influence: 54.3] [Reference Citation Analysis (0)] |
| 21. | Bedossa P, Dargère D, Paradis V. Sampling variability of liver fibrosis in chronic hepatitis C. Hepatology. 2003;38:1449-1457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1193] [Cited by in RCA: 1398] [Article Influence: 63.5] [Reference Citation Analysis (0)] |
| 22. | Hui AY, Liew CT, Go MY, Chim AM, Chan HL, Leung NW, Sung JJ. Quantitative assessment of fibrosis in liver biopsies from patients with chronic hepatitis B. Liver Int. 2004;24:611-618. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 37] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 23. | Regev A, Berho M, Jeffers LJ, Milikowski C, Molina EG, Pyrsopoulos NT, Feng ZZ, Reddy KR, Schiff ER. Sampling error and intraobserver variation in liver biopsy in patients with chronic HCV infection. Am J Gastroenterol. 2002;97:2614-2618. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1504] [Cited by in RCA: 1569] [Article Influence: 68.2] [Reference Citation Analysis (0)] |
| 24. | Sandrin L, Fourquet B, Hasquenoph JM, Yon S, Fournier C, Mal F, Christidis C, Ziol M, Poulet B, Kazemi F. Transient elastography: a new noninvasive method for assessment of hepatic fibrosis. Ultrasound Med Biol. 2003;29:1705-1713. [PubMed] |
| 25. | Saito H, Tada S, Nakamoto N, Kitamura K, Horikawa H, Kurita S, Saito Y, Iwai H, Ishii H. Efficacy of non-invasive elastometry on staging of hepatic fibrosis. Hepatol Res. 2004;29:97-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 74] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 26. | Castéra L, Vergniol J, Foucher J, Le Bail B, Chanteloup E, Haaser M, Darriet M, Couzigou P, De Lédinghen V. Prospective comparison of transient elastography, Fibrotest, APRI, and liver biopsy for the assessment of fibrosis in chronic hepatitis C. Gastroenterology. 2005;128:343-350. [PubMed] |
| 27. | Osakabe K, Ichino N, Nishikawa T, Sugiyama H, Kato M, Kitahara S, Hashimoto S, Kawabe N, Harata M, Nitta Y. Reduction of liver stiffness by antiviral therapy in chronic hepatitis B. J Gastroenterol. 2011;46:1324-1334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 46] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 28. | Ogawa E, Furusyo N, Toyoda K, Takeoka H, Maeda S, Hayashi J. The longitudinal quantitative assessment by transient elastography of chronic hepatitis C patients treated with pegylated interferon alpha-2b and ribavirin. Antiviral Res. 2009;83:127-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 110] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 29. | Vergniol J, Foucher J, Castéra L, Bernard PH, Tournan R, Terrebonne E, Chanteloup E, Merrouche W, Couzigou P, de Lédinghen V. Changes of non-invasive markers and FibroScan values during HCV treatment. J Viral Hepat. 2009;16:132-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 95] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 30. | Wang JH, Changchien CS, Hung CH, Tung WC, Kee KM, Chen CH, Hu TH, Lee CM, Lu SN. Liver stiffness decrease after effective antiviral therapy in patients with chronic hepatitis C: Longitudinal study using FibroScan. J Gastroenterol Hepatol. 2010;25:964-969. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 67] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 31. | Andersen ES, Moessner BK, Christensen PB, Kjær M, Krarup H, Lillevang S, Weis N. Lower liver stiffness in patients with sustained virological response 4 years after treatment for chronic hepatitis C. Eur J Gastroenterol Hepatol. 2011;23:41-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 32. | Hézode C, Castéra L, Roudot-Thoraval F, Bouvier-Alias M, Rosa I, Roulot D, Leroy V, Mallat A, Pawlotsky JM. Liver stiffness diminishes with antiviral response in chronic hepatitis C. Aliment Pharmacol Ther. 2011;34:656-663. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 102] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 33. | Friedrich-Rust M, Wunder K, Kriener S, Sotoudeh F, Richter S, Bojunga J, Herrmann E, Poynard T, Dietrich CF, Vermehren J. Liver fibrosis in viral hepatitis: noninvasive assessment with acoustic radiation force impulse imaging versus transient elastography. Radiology. 2009;252:595-604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 476] [Cited by in RCA: 468] [Article Influence: 29.3] [Reference Citation Analysis (0)] |
| 34. | Lupsor M, Badea R, Stefanescu H, Sparchez Z, Branda H, Serban A, Maniu A. Performance of a new elastographic method (ARFI technology) compared to unidimensional transient elastography in the noninvasive assessment of chronic hepatitis C. Preliminary results. J Gastrointestin Liver Dis. 2009;18:303-310. [PubMed] |
| 35. | Takahashi H, Ono N, Eguchi Y, Eguchi T, Kitajima Y, Kawaguchi Y, Nakashita S, Ozaki I, Mizuta T, Toda S. Evaluation of acoustic radiation force impulse elastography for fibrosis staging of chronic liver disease: a pilot study. Liver Int. 2010;30:538-545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 172] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 36. | Forestier N, Gaus A, Herrmann E, Sarrazin C, Bojunga J, Poynard T, Albert J, Gerber L, Schneider MD, Dultz G. Acoustic radiation force impulse imaging for evaluation of antiviral treatment response in chronic hepatitis C. J Gastrointestin Liver Dis. 2012;21:367-373. [PubMed] |
| 37. | Yamada R, Hiramatsu N, Oze T, Morishita N, Harada N, Miyazaki M, Yakushijin T, Miyagi T, Yoshida Y, Tatsumi T. Significance of liver stiffness measurement by acoustic radiation force impulse (ARFI) among hepatitis C patients. J Med Virol. 2014;86:241-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 38. | Wai CT, Greenson JK, Fontana RJ, Kalbfleisch JD, Marrero JA, Conjeevaram HS, Lok AS. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology. 2003;38:518-526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2762] [Cited by in RCA: 3246] [Article Influence: 147.5] [Reference Citation Analysis (0)] |
| 39. | Vallet-Pichard A, Mallet V, Nalpas B, Verkarre V, Nalpas A, Dhalluin-Venier V, Fontaine H, Pol S. FIB-4: an inexpensive and accurate marker of fibrosis in HCV infection. comparison with liver biopsy and fibrotest. Hepatology. 2007;46:32-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1288] [Cited by in RCA: 1610] [Article Influence: 89.4] [Reference Citation Analysis (0)] |
| 40. | Nishikawa T, Hashimoto S, Kawabe N, Harata M, Nitta Y, Murao M, Nakano T, Mizuno Y, Shimazaki H, Kan T. Factors correlating with acoustic radiation force impulse elastography in chronic hepatitis C. World J Gastroenterol. 2014;20:1289-1297. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 28] [Cited by in RCA: 27] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 41. | Veldt BJ, Saracco G, Boyer N, Cammà C, Bellobuono A, Hopf U, Castillo I, Weiland O, Nevens F, Hansen BE. Long term clinical outcome of chronic hepatitis C patients with sustained virological response to interferon monotherapy. Gut. 2004;53:1504-1508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 129] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 42. | Toccaceli F, Laghi V, Capurso L, Koch M, Sereno S, Scuderi M. Long-term liver histology improvement in patients with chronic hepatitis C and sustained response to interferon. J Viral Hepat. 2003;10:126-133. [PubMed] |
| 43. | Shiratori Y, Imazeki F, Moriyama M, Yano M, Arakawa Y, Yokosuka O, Kuroki T, Nishiguchi S, Sata M, Yamada G. Histologic improvement of fibrosis in patients with hepatitis C who have sustained response to interferon therapy. Ann Intern Med. 2000;132:517-524. [PubMed] |
| 44. | Arima Y, Kawabe N, Hashimoto S, Harata M, Nitta Y, Murao M, Nakano T, Shimazaki H, Kobayashi K, Ichino N. Reduction of liver stiffness by interferon treatment in the patients with chronic hepatitis C. Hepatol Res. 2010;40:383-392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 55] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 45. | Fraquelli M, Rigamonti C, Casazza G, Donato MF, Ronchi G, Conte D, Rumi M, Lampertico P, Colombo M. Etiology-related determinants of liver stiffness values in chronic viral hepatitis B or C. J Hepatol. 2011;54:621-628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 101] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 46. | Coco B, Oliveri F, Maina AM, Ciccorossi P, Sacco R, Colombatto P, Bonino F, Brunetto MR. Transient elastography: a new surrogate marker of liver fibrosis influenced by major changes of transaminases. J Viral Hepat. 2007;14:360-369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 484] [Cited by in RCA: 509] [Article Influence: 28.3] [Reference Citation Analysis (0)] |
| 47. | Rizzo L, Calvaruso V, Cacopardo B, Alessi N, Attanasio M, Petta S, Fatuzzo F, Montineri A, Mazzola A, L’abbate L. Comparison of transient elastography and acoustic radiation force impulse for non-invasive staging of liver fibrosis in patients with chronic hepatitis C. Am J Gastroenterol. 2011;106:2112-2120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 154] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 48. | Bota S, Sporea I, Sirli R, Popescu A, Jurchis A. Factors which influence the accuracy of acoustic radiation force impulse (ARFI) elastography for the diagnosis of liver fibrosis in patients with chronic hepatitis C. Ultrasound Med Biol. 2013;39:407-412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 39] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 49. | Yoon KT, Lim SM, Park JY, Kim do Y, Ahn SH, Han KH, Chon CY, Cho M, Lee JW, Kim SU. Liver stiffness measurement using acoustic radiation force impulse (ARFI) elastography and effect of necroinflammation. Dig Dis Sci. 2012;57:1682-1691. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 70] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 50. | Ghany MG, Strader DB, Thomas DL, Seeff LB. Diagnosis, management, and treatment of hepatitis C: an update. Hepatology. 2009;49:1335-1374. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2320] [Cited by in RCA: 2242] [Article Influence: 140.1] [Reference Citation Analysis (1)] |
| 51. | Jacobson IM. Treatment options for patients with chronic hepatitis C not responding to initial antiviral therapy. Clin Gastroenterol Hepatol. 2009;7:921-930. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 52. | Yamada G, Iino S, Okuno T, Omata M, Kiyosawa K, Kumada H, Hayashi N, Sakai T. Virological response in patients with hepatitis C virus genotype 1b and a high viral load: impact of peginterferon-alpha-2a plus ribavirin dose reductions and host-related factors. Clin Drug Investig. 2008;28:9-16. [PubMed] |