Published online Sep 21, 2015. doi: 10.3748/wjg.v21.i35.10137
Peer-review started: March 2, 2015
First decision: April 20, 2015
Revised: May 17, 2015
Accepted: June 15, 2015
Article in press: June 15, 2015
Published online: September 21, 2015
Processing time: 200 Days and 18.3 Hours
AIM: To investigate the molecular mechanisms of the high IGF-1 level linking diabetes and cancers, which is a risk factor.
METHODS: We used cell growth, wound healing and transwell assay to evaluate the proliferation and metastasis ability of the hepatocellular carcinoma (HCC) cells. Western blot and reverse transcription polymerase chain reaction were used to assess a previously identified lysosomal protease, cathepsin B (CTSB) expression in the HCC cell lines. C57 BL/6J and KK-Ay diabetic mice are used to detect the growth and metastasis of HCC cells that were depleted with or without CTSB shRNA in vivo. Statistical significance was determined by Student’s t-test.
RESULTS: IGF-1 promoted the growth and metastasis of the HCC cell lines via its ability to enhance CTSB expression in both a time-dependent and concentration-dependent manner. HCC cells grew much faster in diabetic KK-Ay mice than in C57 BL/6J mice. Additionally, more metastatic nodules were found in the lungs of KK-Ay mice than the lungs of C57 BL/6J mice. CTSB depletion protects against the tumor-promoting actions of IGF-1 in HCC cells, as well tumor growth and metastasis both in vitro and in vivo. IGF-1 did not change the mRNA levels of CTSB but prolonged the half-life of cathepsin B in Hepa 1-6 and H22 cells. Our results showed that IGF-1 promotes the growth and metastasis of the HCC cells most likely by hindering CTSB degradation mediated by the ubiquitin-proteasome system (UPS), but not autophagy. Overexpression of proteasome activator 28, a family of activators of the 20S proteasome, could not only restore IGF-1-inhibited UPS activity but also decrease IGF-1-induced CTSB accumulation.
CONCLUSION: Our study demonstrates that IGF-1 promotes the growth and metastasis of hepatocellular carcinoma by inhibition of proteasome-mediated CTSB degradation.
Core tip: IGF-1 promoted the growth and metastasis of the hepatocellular carcinoma via its ability to enhance cathepsin B (CTSB) expression. CTSB depletion protects against the tumor-promoting actions of IGF-1 in hepatocellular carcinoma (HCC) cells, as well tumor growth and metastasis both in vitro and in vivo. Our results showed that IGF-1 promotes HCC most likely by hindering CTSB degradation mediated by the ubiquitin-proteasome system (UPS). Proteasome activator 28 overexpression could not only restore IGF-1-inhibited UPS activity but also decrease IGF-1-induced CTSB accumulation. Our study demonstrates that targeting CTSB expression may contribute to therapeutic strategies for the treatment of cancers associated with diabetes.
-
Citation: Lei T, Ling X. IGF-1 promotes the growth and metastasis of hepatocellular carcinoma
via the inhibition of proteasome-mediated cathepsin B degradation. World J Gastroenterol 2015; 21(35): 10137-10149 - URL: https://www.wjgnet.com/1007-9327/full/v21/i35/10137.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i35.10137
Hepatocellular carcinoma (HCC) is one of the most lethal cancers worldwide. Few effective treatments exist partly because the cell- and molecular-based mechanisms that contribute to the pathogenesis of this tumor type are poorly understood[1]. Although a previous study revealed that the pathogenesis of HCC was closely associated with chronic liver inflammation, fibrosis and cirrhosis[2], most studies have indicated that 5% to 30% of patients with HCC lack a readily identifiable risk factor for their cancer[3]. Notably, an increased risk for HCC has been identified in patients with metabolic syndrome or insulin resistance. Recently, IGF-1 has emerged as an important modulator in the development and progression of cancers[4,5]. The IGF signaling pathway is an important regulatory mechanism of tumorigenesis and drug resistance in many cancers. IGF-1, a member of the insulin superfamily, has growth promoting and anabolic effects that function in a dose-dependent manner. The function of IGF-1 is regulated by IGF-1 receptor (IGF-1R), a tyrosine kinase receptor that activates the PI3K/Akt and MAPK pathways[6,7]. Moreover, IGF-1R expression is upregulated in HCC tissues, and using an IGF-1R inhibitor can reduce the growth and invasion of HCC cells[8]. Indeed, IGF-1 has been confirmed to be an inducer of many types of cancer including breast cancer, colon cancer, pulmonary cancer, and HCC[9].
Cathepsins are a family of lysosomal proteases that belong to cysteine, serine, or aspartic protease classes[10]. Several cathepsins participate in inflammatory diseases such as rheumatoid arthritis, atherosclerosis, periodontitis, and pancreatitis[11,12]. Recent studies of cathepsin family functions have focused on their cancer-regulating effects. Many tumors in incipient stages have been shown to exhibit elevated levels of proteases, which are thought to play a crucial role in tumor angiogenesis, invasion and metastasis[13-16]. During cancer progression, cathepsins can not only cleave components of the extracellular matrix and basement membrane but also activate many other proteases that promote cancer invasion and progression[17]. CTSB, CTSK, and CTSS have become novel anti-cancer pharmacological targets in recent years. Moreover, increased expression of some cathepsins has diagnostic value in several types of cancer[18]. However, in HCC, the regulatory effects that may cause high cathepsin levels have not been well defined. Hence, understanding the mechanisms controlling cathepsin expression and degradation will potentially guide the development of novel diagnostic tools for early tumor detection and of disease-modifying therapeutic strategies to combat tumors.
In our current study, we found that IGF-1 has a relationship with the CTSB protein stability. IGF-I could promote HCC cell growth and metastasis by enhancing CTSB expression, and the genetic inhibition of CTSB expression in HCC cells could decrease the IGF-1-induced tumor-promoting effect in vitro and in vivo. We further analyzed the mechanism of the regulatory effect of IGF-1 on CTSB and found that IGF-1 most likely promotes HCC by hindering CTSB degradation mediated by IGF-1-impaired UPS activity.
Anti-CTSB monoclonal antibodies were obtained from Abcam (United States). Lentiviruses expressing CTSB control-shRNA, CTSB shRNA1 and CTSB shRNA2 were obtained from Transomic Technologies (United States). The plasmid UbG76V-GFP was obtained from Addgene. All other materials, unless otherwise stated, were obtained from Sigma-Aldrich (United States or China).
Hepa 1-6 and H22 cell lines were purchased from the Cell Culture Center of Peking Union Medical College or Chinese Academy of Sciences (Beijing, China). The cell lines were cultured under standard conditions. To establish stably transfected cell lines expressing CTSB-shRNA sequences, Hepa 1-6 cells were infected with lentiviruses expressing CTSB control-shRNA, CTSB-shRNA1 and CTSB-shRNA2 and selected in the presence of 1 μg/mL puromycin for 4 wk. The puromycin-resistant clones were individually expanded into cell lines and screened for the extent of CTSB protein expression by Western blot analysis.
For bioluminescent cell sorting, Hepa 1-6 cells expressing control-shRNA, CTSB-shRNA1 or CTSB-shRNA2 were transduced with a lentiviral vector expressing the genes encoding firefly luciferase and green fluorescent protein (Invabio, Shanghai, China). Single cells were seeded in individual wells of 96-well plates. High bioluminescent clones were selected for further expansion and grown to develop an in vivo bioluminescence imaging model of growth and metastasis.
Hepa 1-6 and H22 cell monolayers were wounded with a pipette tip, washed with PBS and cultured in 1% FBS medium or 1% FBS medium with IGF-1 (100 nmol/L) for 24 h. Images were captured at 0 and 24 h after wounding with an microscope and the lesion area was measured.
Transwell invasion assays were performed using Transwell chambers with 8 μm pore size filter membranes (Millipore). Chambers were precoated with 10 μg/mL fibronectin on the lower surface, and the polycarbonate filter was coated with Matrigel (30 μg/well) (BD MatrigelTM Matrix). Then, the chambers were inserted into 24-well culture plates. The cells were starved overnight in assay media (DMEM media containing 0.1% FBS), and then single cell suspensions were seeded into the upper chamber (5 × 104 cells per well in 0.1% FBS in DMEM or 1640 medium). After 24 h, the non-invaded cells on the upper side of the filter were removed with a cotton swab. The invaded cells were fixed in 4% paraformaldehyde in PBS, stained with 0.5% toluidine in 2% Na2CO3, and counted in eight random fields by brightfield microscopy at magnification × 200.
Total RNA was isolated from Hepa 1-6 cells (RNeasy Kit; Qiagen) according to the manufacturer’s protocol. cDNA was synthesized by reverse transcription using an AMV-RT kit (Promega). Actin was used as the loading control. reverse transcription polymerase chain reaction was performed using a Taq polymerase MIX (Tiangen), according to the manufacturer’s protocols. Samples were mixed with CATB or actin primers, denatured at 95 °C for 5 min, and subjected to 30 amplification cycles (94 °C for 1 min, 60 °C for 30 s, and 72 °C for 1 min), followed by a final 5 min extension at 72 °C, using a Thermo scientific PCR System. PCR products were analyzed on 2% agarose gels in TAE buffer.
C57 BL/6J and KK-Ay diabetic mice were purchased from Vital River Lab Animal Technology, Co., Ltd. (Beijing, China) and housed under standard conditions of humidity, room temperature, and light-dark cycles. KK-Ay diabetic mice have higher level of blood glucose (27-31 mmol/L) and plasma IGF-1 (710-730 ng/mL) than normal C57 BL/6J mice. All mice were given free access to food and water throughout the study. The study protocol was approved by the Laboratory Animal Care and Use Committee of the First Affiliated Hospital of Liaoning Medical University.
To analyze protein expression, the cells were lysed in RIPA buffer [50 mmol/L Tris-HCl (pH 7.4), 1% NP-40, 0.25% Na deoxycholate, 150 mmol/L NaCl, and 1 mmol/L EDTA] supplemented with 50 mmol/L NaF, 20 mmol/L β-glycerophosphate and a complete protease inhibitor cocktail (Roche). Protein concentrations were determined by bicinchoninic acid reagent. Proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE; 12%, 10%, or 8% acrylamide) and transferred to polyvinylidene fluoride (PVDF) membranes. The blots were incubated overnight at 4 °C with primary antibodies. After the blots were washed, they were incubated with the appropriate HRP-conjugated secondary antibody and processed to detect electrochemiluminescence signals.
Hepa 1-6 cells expressing control-shRNA or CTSB-shRNA1 (2 × 106 cells per tumor) were harvested and resuspended in 100 μL of PBS. The cells were injected s.c. into the backs of 5-wk-old C57 BL/6J and KK-Ay mice. Tumor volume (TV) was calculated using the following formula: TV = W2× L × 0.5.
For experimental metastasis, 3 × 106 tumor cells in 200 μL PBS were injected into the lateral tail vein of each mouse. Metastases were counted in a genotype-blinded fashion under a dissection scope. Tumor growth and metastasis were also quantified using an in vivo imaging system.
In vivo optical imaging was performed using an IVIS Spectrum optical imaging system. Before imaging, each mouse was administered an ip injection of 125 mg/kg luciferin (Caliper Life Science). The optical signal was expressed as photon flux. To account for inter-individual variability, the data were normalized by determining the proliferation index [as reflected by the ratio of bioluminescence imaging (BLI) activity between two time points] for each animal.
All data are presented as the mean ± SEM. The sample size of most experiments was chosen empirically following previous experience in the assessment of experimental variability. Generally, all experiments were performed with n≥ 3 biological replicates. P < 0.05 was statistically significant. Levels of significance are indicated in the figure legends.
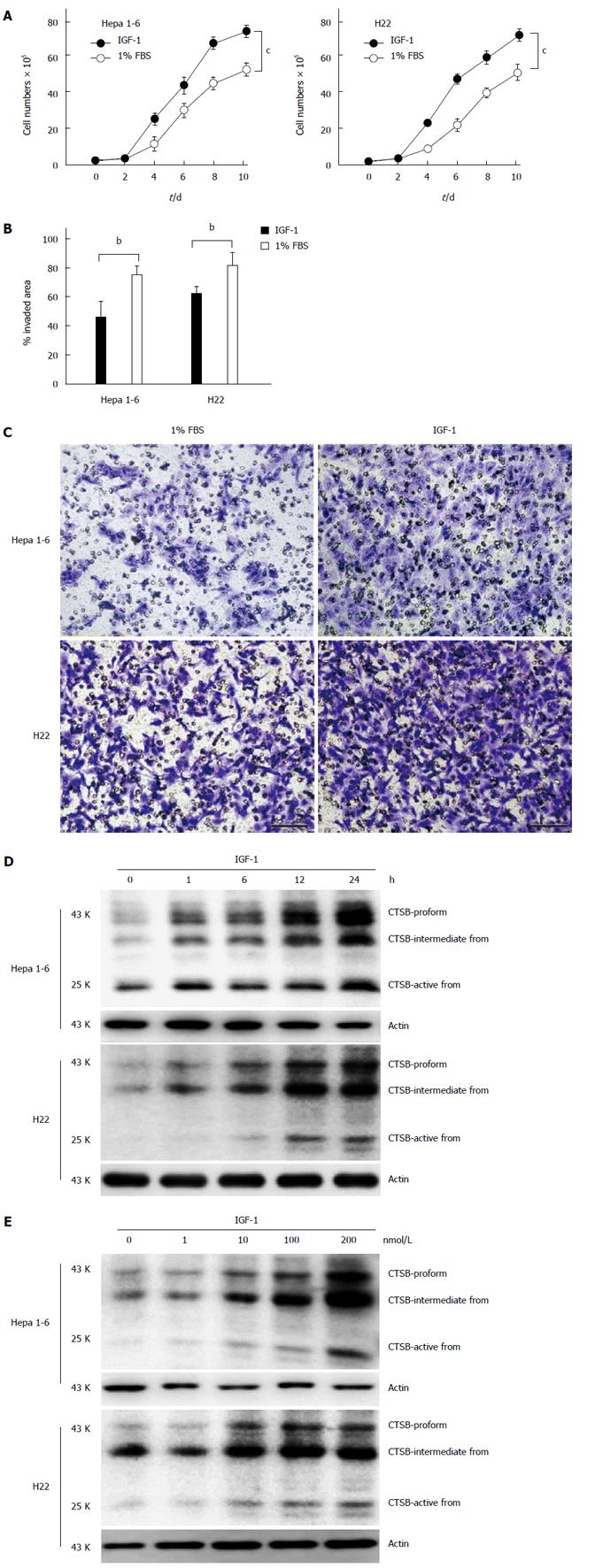
Previous investigations have shown that the IGF-1/insulin system possesses potent mitogenic and pro-migratory properties and have extensively implicated this system in many malignancies. In the current study, we found that IGF-1 could not only promote the proliferation of hepatocellular carcinoma cell lines (Hep 1-6 and H22 cells) (Figure 1A) but also induce the invasion and migration of these cell lines (Figure 1B and 1C). High expression levels of CTSB have been linked to aggressiveness and poor prognosis in several cancers, such as colorectal, hepatocellular and ovarian carcinomas[19]. Although both the IGF-1/insulin system and CTSB are important risk factors for the development of hepatocellular carcinoma, the relationship between IGF-1 and high expression levels of CTSB in the development of HCC has not yet been reported. In this study, we found that IGF-1 could upregulate the expression of CTSB in HCC cell lines (Hepa 1-6 and H22 cells) in both a time-dependent and concentration-dependent manner (Figure 1D and 1E).
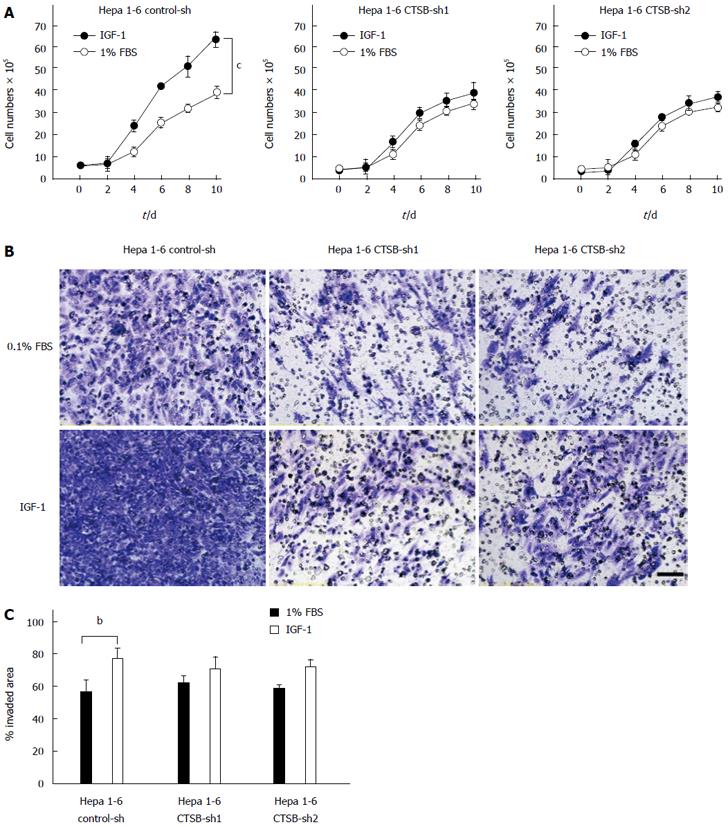
To investigate the role of CTSB in IGF-1-induced tumor proliferation and metastasis, Hepa 1-6 cells stably transfected with control-shRNA, CTSB-shRNA1 or CTSB-shRNA2 were cultured in the absence or presence of IGF-1. Indeed, CTSB depletion impeded the ability of IGF-1 to induce the proliferation (Figure 2A), invasion and migration (Figure 2B and 2C) of Hepa 1-6 cells, suggesting a potential role of CTSB in IGF-1-induced HCC progression.
The genetically diabetic KK-Ay mouse has been widely used as a relevant model of human type 2 diabetes (T2D)[20]. Adult KK-Ay mice exhibited hyperglycemia, hyperinsulinemia. and high plasma IGF-1 levels compared with C57 BL/6J mice[21]. We found that Hepa 1-6 cells grew much faster in diabetic KK-Ay mice than in C57 BL/6J mice after inoculation (Figure 3A). Additionally, a greater number of metastatic nodules were found in the lungs of KK-Ay mice, whereas fewer metastases were found in the lungs of C57 BL/6J mice (Figure 3B). To validate the role of CTSB in IGF-1-accelerated tumor development, we compared the tumor growth and metastasis of cathepsin B knockdown Hepa 1-6 cells in KK-Ay mice with that in C57 BL/6J mice. We found that cathepsin B knockdown rarely repressed tumor growth and metastasis in C57 BL/6J mice but that cathepsin B knockdown could clearly reduce tumor growth and metastasis in KK mice compared to normal CTSB expression (Figure 3C and D). Taken together, these results suggest that CTSB plays a crucial role in tumor growth and metastasis in mice, particularly in mice with T2D.
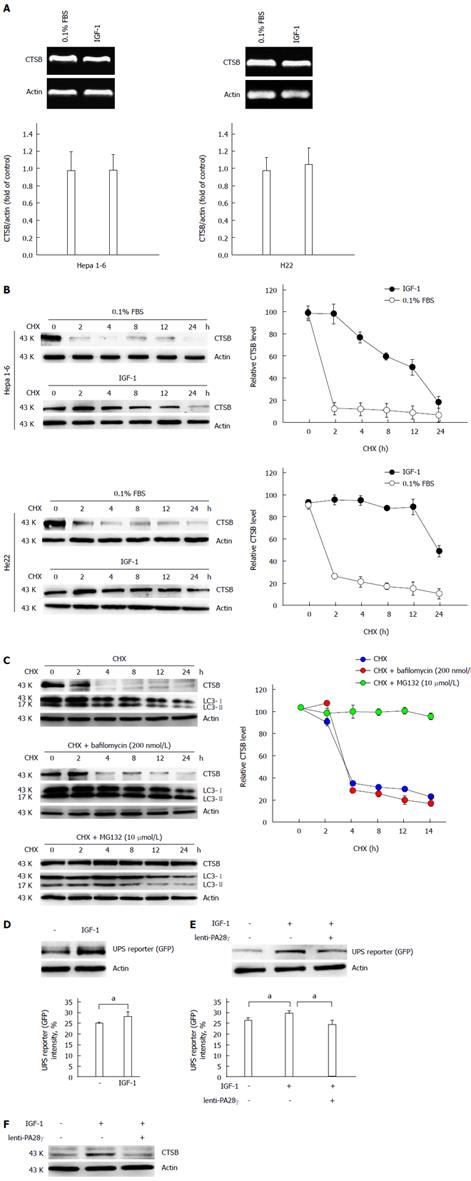
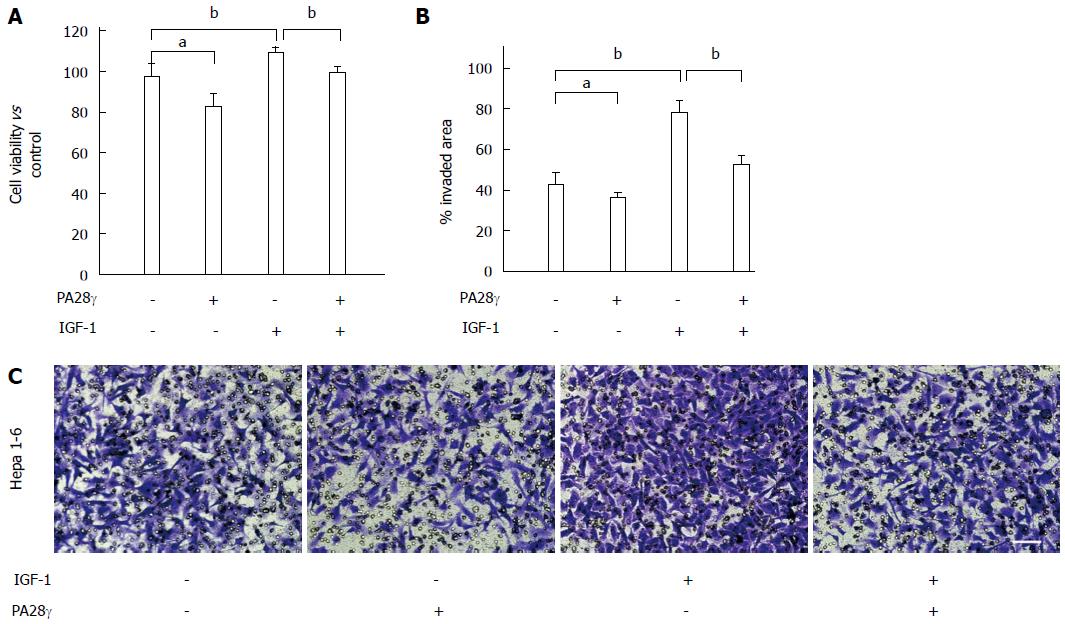
Thus, we examined how IGF-1 caused CTSB accumulation, and we found that IGF-1 did not change the mRNA levels of CTSB (Figure 4A) but prolonged the half-life of cathepsin B in Hepa 1-6 and H22 cells (Figure 4B). Autophagy and UPS, the two primary proteolytic systems in cells, along with molecular chaperones, constitute essential components of the cellular quality control systems[22]. To determine mechanism of cathepsin B degradation, turnover assays were conducted in the presence and absence of bafilomycin, an inhibitor of later phase autophagy. The turnover of LC3 protein could act as a control to show the effects of bafilomycin. We found that cathepsin B turnover was not inhibited by bafilomycin but was inhibited by MG132, a proteasome inhibitor (Figure 4C), suggesting that IGF-1 interferes with cathepsin B clearance by compromising the ubiquitin-proteasome system (UPS). Insulin/IGF-1 signaling regulates UPS activity via a deubiquitinating enzyme[23]. We used an UbG76V-GFP reporter that contains a UFD signal composed of a single, uncleavable, N-terminally linked ubiquitin attached to GFP. The resulting fluorescent chimera is a substrate for polyubiquitination and proteasome-mediated proteolysis[24]. In our study, we found that IGF-1 impeded UPS activity in Hepa 1-6 cells (Figure 4D). Proteasome activator 28 (PA28γ), which belongs to a family of activators of the 20S proteasome, binds to the 20S proteasome and stimulates peptidase activity in an ATP-independent manner[25]. We further investigated whether PA28γ overexpression could rescue UPS-mediated degradation, and we found that PA28γ overexpression not only restored the UPS activity inhibited by IGF-1 (Figure 4E) but also decreased the CTSB accumulation induced by IGF-1 (Figure 4F). The proliferation and metastasis assays showed that PA28γ overexpression inhibited IGF-1-induced HCC cell growth and invasion (Figure 5). Taken together, these results verify that IGF-1 promoted the growth and metastasis of hepatocellular carcinoma by inhibiting proteasome-mediated CTSB degradation.
Although insulin and IGF-1 have long been considered to have a biological connection between energy metabolic disorders and cancers, the potential mechanism has remained only partially understood. Insulin/IGF receptor activation is generally thought to trigger the PI3K-AKT and MAPK/ERK pathways, thus promoting mitogenesis and antagonizing apoptosis during tumor development. Pro-angiogenesis and pro-migration also account for insulin/IGF-1-induced tumor development. However, clinical trials show that targeting the insulin/IGF-1 signal does not produce satisfactory efficacy against cancers.
Many insults including hepatic microbial infection, hepatitis B and/or C, genotoxic agents, oxidative stress-inducing DNA damage, and immune response imbalance can trigger tumor metastasis in the liver. Proteases, particularly the matrix metalloproteinase and cathepsin families, clearly play critical roles in tumor cell migration and invasion[26]. Cathepsins have crucial functions in protein turnover in lysosomes due to their different protease activities. In general, cathepsins function in intercellular protein catabolism. However, in tumor cells and other specific situations, some cathepsins transit to tissue spaces and play an extracellular role that results in tissue damage. While persistent reports regarding the association between cathepsins and cancer have been published for several decades, the mechanism by which cathepsins are involved in cancer genesis remains unclear.
Currently, the most important and researched members of cathepsins associated with cancer genesis are cathepsins B, G, C, and K. To date, dozens of compounds targeting cathepsins have entered clinical studies to treat various types of cancer. Numerous studies have demonstrated that CTSB overexpression correlates with invasion and metastasis in different cancers. Similar to other reports, we observed increased CTSB expression in cancer tissues compared to that in normal tissues (data not shown), and we also found that IGF-1 could increase CTSB protein levels in different HCC cell lines but had no effect on its mRNA levels. This finding illustrated that IGF-1 only enhanced CTSB protein stability without changing its mRNA level.
Two different degradation pathways exist in human cells; one pathway is the ubiquitin-proteasome system, and the other is autophagy. In this study, CTSB degradation occurred primarily via the UPS pathway as confirmed using an inhibitor of UPS or autophagy. The UPS pathway plays an important role in the regulation of cellular proteins involved in transcription, apoptosis, adhesion, angiogenesis and tumor cell growth[27-30]. Thus, knowing the function of UPS under different situations is important for cancer research. In our study, IGF-1 could inhibit UPS pathway activity, which resulted in CTSB accumulation in different HCC cells. Increased CTSB expression mediated the malignancy-promoting actions of IGF-1, whereas CTSB knockdown reversed the IGF-induced cancer cell proliferation and invasion. We also observed that CTSB knockdown in Hepa 1-6 cells markedly decreased the tumor size and metastasis number in KK-Ay mice; however, this effect was weaker in C57 BL/6J mice. This phenomenon illustrated that CTSB is more important for cancer development and progression in metabolic syndrome patients. Taken together, our study demonstrates that targeting CTSB expression may contribute to therapeutic strategies for the treatment of cancers associated with diabetes.
Hepatocellular carcinoma (HCC) is one of the most lethal cancers worldwide. Few effective treatments exist partly because the cell- and molecular-based mechanisms that contribute to the pathogenesis of this tumor type are poorly understood. Although a previous study revealed that the pathogenesis of HCC was closely associated with chronic liver inflammation, fibrosis and cirrhosis, most studies have indicated that 5% to 30% of patients with HCC lack a readily identifiable risk factor for their cancer. Notably, an increased risk for HCC has been identified in patients with metabolic syndrome or insulin resistance. Recently, IGF-1 has emerged as an important modulator in the development and progression of cancers. In this study, we investigated the how IGF-1 participated in the progression of HCC.
Although insulin and IGF-1 have long been considered to have a biological connection between energy metabolic disorders and cancers, the potential mechanism has remained only partially understood. Insulin/IGF receptor activation is generally thought to trigger the PI3K-AKT and MAPK/ERK pathways, thus promoting mitogenesis and antagonizing apoptosis during tumor development. Pro-angiogenesis and pro-migration also account for insulin/IGF-1-induced tumor development. However, clinical trials show that targeting the insulin/IGF-1 signal does not produce satisfactory efficacy against cancers.
Cathepsins are a family of lysosomal proteases that belong to cysteine, serine, or aspartic protease classes. Recent studies of cathepsin family functions have focused on their cancer-regulating effects. Many tumors in incipient stages have been shown to exhibit elevated levels of proteases, which are thought to play a crucial role in tumor angiogenesis, invasion and metastasis. During cancer progression, cathepsins can not only cleave components of the extracellular matrix and basement membrane but also activate many other proteases that promote cancer invasion and progression.
However, in HCC, the regulatory effects that may cause high cathepsin levels have not been well defined. Hence, understanding the mechanisms controlling cathepsin expression and degradation will potentially guide the development of novel diagnostic tools for early tumor detection and of disease-modifying therapeutic strategies to combat tumors.
This study found that IGF-1 has a relationship with the cathepsin B (CTSB) protein stability. IGF-I could promote HCC cell growth and metastasis by enhancing CTSB expression, and the genetic inhibition of CTSB expression in HCC cells could decrease the IGF-1-induced tumor-promoting effect in vitro and in vivo. This study further analyzed the mechanism of the regulatory effect of IGF-1 on CTSB and found that IGF-1 most likely promotes HCC by hindering CTSB degradation mediated by IGF-1-impaired UPS activity.
This study suggested that targeting CTSB may be a useful strategy for HCC patient with diabetes.
Cathepsins are a family of lysosomal proteases that belong to cysteine, serine, or aspartic protease classes.
In general, this is a good paper describing the effect of IGF-1 on HCC proliferation, migration, and metastasis. The authors found CTSB as a downstream target of IGF-1 controlling the mechanism above. The mechanism of IGF-1 controlling CTSB level through protein degradation is pretty well demonstrated.
P- Reviewer: Lu WY, Plattner R S- Editor: Yu J L- Editor: A E- Editor: Liu XM
| 1. | Arzumanyan A, Reis HM, Feitelson MA. Pathogenic mechanisms in HBV- and HCV-associated hepatocellular carcinoma. Nat Rev Cancer. 2013;13:123-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 581] [Cited by in RCA: 634] [Article Influence: 52.8] [Reference Citation Analysis (0)] |
| 2. | Thorgeirsson SS, Grisham JW. Molecular pathogenesis of human hepatocellular carcinoma. Nat Genet. 2002;31:339-346. [PubMed] |
| 3. | Siegel AB, Zhu AX. Metabolic syndrome and hepatocellular carcinoma: two growing epidemics with a potential link. Cancer. 2009;115:5651-5661. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 296] [Cited by in RCA: 302] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 4. | Renehan AG, Zwahlen M, Minder C, O’Dwyer ST, Shalet SM, Egger M. Insulin-like growth factor (IGF)-I, IGF binding protein-3, and cancer risk: systematic review and meta-regression analysis. Lancet. 2004;363:1346-1353. [PubMed] |
| 5. | Cho YL, Hur SM, Kim JY, Kim JH, Lee DK, Choe J, Won MH, Ha KS, Jeoung D, Han S. Specific activation of insulin-like growth factor-1 receptor by ginsenoside Rg5 promotes angiogenesis and vasorelaxation. J Biol Chem. 2015;290:467-477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 48] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 6. | Zhu C, Qi X, Chen Y, Sun B, Dai Y, Gu Y. PI3K/Akt and MAPK/ERK1/2 signaling pathways are involved in IGF-1-induced VEGF-C upregulation in breast cancer. J Cancer Res Clin Oncol. 2011;137:1587-1594. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 105] [Cited by in RCA: 99] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 7. | Singh P, Alex JM, Bast F. Insulin receptor (IR) and insulin-like growth factor receptor 1 (IGF-1R) signaling systems: novel treatment strategies for cancer. Med Oncol. 2014;31:805. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 151] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 8. | Shan J, Shen J, Liu L, Xia F, Xu C, Duan G, Xu Y, Ma Q, Yang Z, Zhang Q. Nanog regulates self-renewal of cancer stem cells through the insulin-like growth factor pathway in human hepatocellular carcinoma. Hepatology. 2012;56:1004-1014. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 244] [Cited by in RCA: 252] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 9. | Baserga R, Peruzzi F, Reiss K. The IGF-1 receptor in cancer biology. Int J Cancer. 2003;107:873-877. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 475] [Cited by in RCA: 484] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 10. | Reiser J, Adair B, Reinheckel T. Specialized roles for cysteine cathepsins in health and disease. J Clin Invest. 2010;120:3421-3431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 402] [Cited by in RCA: 461] [Article Influence: 30.7] [Reference Citation Analysis (0)] |
| 11. | Sulpizio S, Franceschini N, Piattelli A, Di Sebastiano P, Innocenti P, Selvaggi F. Cathepsins and pancreatic cancer: the 2012 update. Pancreatology. 2012;12:395-401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 20] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 12. | Ichimaru E, Tanoue M, Tani M, Tani Y, Kaneko T, Iwasaki Y, Kunimatsu K, Kato I. Cathepsin B in gingival crevicular fluid of adult periodontitis patients: identification by immunological and enzymological methods. Inflamm Res. 1996;45:277-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 13. | Schwartz MK. Tissue cathepsins as tumor markers. Clin Chim Acta. 1995;237:67-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 54] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 14. | Sulpizio S, Franceschini N, Piattelli A, Di Sebastiano P, Innocenti P, Selvaggi F. Cathepsins and pancreatic cancer: The 2012 update. Pancreatology. 2012;12:395-401. [RCA] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 20] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 15. | Joyce JA, Hanahan D. Multiple roles for cysteine cathepsins in cancer. Cell Cycle. 2004;3:1516-1619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 119] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 16. | Gole B, Huszthy PC, Popović M, Jeruc J, Ardebili YS, Bjerkvig R, Lah TT. The regulation of cysteine cathepsins and cystatins in human gliomas. Int J Cancer. 2012;131:1779-1789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 41] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 17. | Gocheva V, Joyce JA. Cysteine cathepsins and the cutting edge of cancer invasion. Cell Cycle. 2007;6:60-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 304] [Cited by in RCA: 313] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 18. | Haris M, Singh A, Mohammed I, Ittyerah R, Nath K, Nanga RP, Debrosse C, Kogan F, Cai K, Poptani H. In vivo magnetic resonance imaging of tumor protease activity. Sci Rep. 2014;4:6081. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 47] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 19. | Aggarwal N, Sloane BF. Cathepsin B: multiple roles in cancer. Proteomics Clin Appl. 2014;8:427-437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 250] [Cited by in RCA: 278] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 20. | Fajardo RJ, Karim L, Calley VI, Bouxsein ML. A review of rodent models of type 2 diabetic skeletal fragility. J Bone Miner Res. 2014;29:1025-1040. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 129] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 21. | Berryman DE, Glad CA, List EO, Johannsson G. The GH/IGF-1 axis in obesity: pathophysiology and therapeutic considerations. Nat Rev Endocrinol. 2013;9:346-356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 164] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 22. | Schreiber A, Peter M. Substrate recognition in selective autophagy and the ubiquitin-proteasome system. Biochim Biophys Acta. 2014;1843:163-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 120] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 23. | Matilainen O, Arpalahti L, Rantanen V, Hautaniemi S, Holmberg CI. Insulin/IGF-1 signaling regulates proteasome activity through the deubiquitinating enzyme UBH-4. Cell Rep. 2013;3:1980-1995. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 53] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 24. | Korolchuk VI, Mansilla A, Menzies FM, Rubinsztein DC. Autophagy inhibition compromises degradation of ubiquitin-proteasome pathway substrates. Mol Cell. 2009;33:517-527. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 551] [Cited by in RCA: 535] [Article Influence: 33.4] [Reference Citation Analysis (0)] |
| 25. | Sun Y, Sijts AJ, Song M, Janek K, Nussbaum AK, Kral S, Schirle M, Stevanovic S, Paschen A, Schild H. Expression of the proteasome activator PA28 rescues the presentation of a cytotoxic T lymphocyte epitope on melanoma cells. Cancer Res. 2002;62:2875-2882. [PubMed] |
| 26. | DeClerck YA, Mercurio AM, Stack MS, Chapman HA, Zutter MM, Muschel RJ, Raz A, Matrisian LM, Sloane BF, Noel A. Proteases, extracellular matrix, and cancer: a workshop of the path B study section. Am J Pathol. 2004;164:1131-1139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 171] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 27. | Crawford LJ, Irvine AE. Targeting the ubiquitin proteasome system in haematological malignancies. Blood Rev. 2013;27:297-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 62] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 28. | Chen FZ, Zhao XK. Ubiquitin-proteasome pathway and prostate cancer. Onkologie. 2013;36:592-596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 29. | Ding F, Xiao H, Wang M, Xie X, Hu F. The role of the ubiquitin-proteasome pathway in cancer development and treatment. Front Biosci (Landmark Ed). 2014;19:886-895. [PubMed] |
| 30. | Crosas B. Deubiquitinating enzyme inhibitors and their potential in cancer therapy. Curr Cancer Drug Targets. 2014;14:506-516. [PubMed] |