Published online Sep 21, 2015. doi: 10.3748/wjg.v21.i35.10113
Peer-review started: January 30, 2015
First decision: May 18, 2015
Revised: May 30, 2015
Accepted: July 15, 2015
Article in press: July 15, 2015
Published online: September 21, 2015
Processing time: 242 Days and 12.3 Hours
AIM: To study whether transfer of blood between the right gastroepiploic artery and gastroduodenal artery could lessens the damage to bile canaliculi.
METHODS: Forty male Bama miniature pigs were divided into four groups as follows: a control group, two hepatic artery ischemia groups (1 h and 2 h), and a hepatic artery bridging group. The hemodynamics of the hepatic artery in the hepatic artery bridging group was measured using color Doppler ultrasound. Morphological changes in the bile canaliculus were observed by transmission electron microscopy. Cofilin, heat shock protein 27 and F-actin expression was detected by immunohistochemistry, Western blot, and real-time polymerase chain reaction. Terminal deoxynucleotidyl transferase-mediated nick end-labeling method was used to evaluate liver injury.
RESULTS: The hemodynamics was not changed in the hepatic artery bridging group. The microvilli in the bile canaliculus were impaired in the two hepatic artery ischemia groups. The down-regulation of cofilin and F-actin and up-regulation of heat shock protein 27 were observed in the two hepatic artery ischemia groups, while there were no significant differences between the control group and hepatic artery bridging group.
CONCLUSION: Hepatic artery ischemia aggravates damage to bile canaliculi, and this damage can be diminished by a hepatic artery bridging duct.
Core tip: The incidence of nonanastomotic biliary strictures ranges from 1% to 20% after liver transplantation (LT). The manner by which arterial ischemic injury to the bile ducts may be reduced in LT is an urgent problem to be addressed. The aim of this study was to validate whether a bridging duct between the right gastroepiploic artery and gastroduodenal artery could lessen the damage to the biliary tract. We have observed that hepatic artery ischemia aggravates damage to bile canaliculi, and this damage can be diminished by a hepatic artery bridging duct.
- Citation: Wang JZ, Liu Y, Wang JL, Lu L, Zhang YF, Lu HW, Li YM. Hepatic artery bridging lessens temporary ischemic injury to bile canaliculi. World J Gastroenterol 2015; 21(35): 10113-10125
- URL: https://www.wjgnet.com/1007-9327/full/v21/i35/10113.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i35.10113
Improvements in surgical techniques, immunosuppressant therapy and reductions in perioperative complications in the past few decades have significantly improved patient outcomes after liver transplantation (LT)[1]. Nevertheless, LT remains associated with various postoperative complications that account for patient morbidity and mortality[2]. Biliary strictures, which are classified into anastomotic biliary strictures and nonanastomotic biliary strictures (i.e., ischemic-type biliary lesions), are the most frequent type of biliary complication and are typically due to ischemia/reperfusion injury, vascular insufficiency, or fibrotic healing caused by improper technique[3,4]. Among the above-mentioned causes, hepatic ischemic injury to the bile duct is a major clinical problem during the preoperative period and frequently occurs after major hepatic resection or LT[5]. With the progress of surgical techniques in LT, anastomotic biliary strictures are becoming increasingly less important, while nonanastomotic biliary strictures remain prominent. The pathogenesis of nonanastomotic biliary strictures is associated with nonfunctioning F-actin microfilaments in the bile canaliculus[6,7]. Bile canaliculus epithelial cells play crucial roles in the formation and secretion of bile as well as in the excretion of circulating xenobiotic substances[8]. Microvilli in bile canaliculus epithelial cells consist of F-actin microfilaments, which are very important for bile excretion[7]. The continuous assembly and disassembly of F-actin microfilaments promote bile duct contraction and bile excretion; therefore, the loss of F-actin microfilaments leads to bile secretion disorder[9,10].
Some researchers have confirmed that cofilin and heat shock protein 27 (HSP27) are very important in the assembly and disassembly of F-actin microfilaments[11,12]. Cofilin is a member of the actin depolymerizing factor (ADF)/cofilin family of proteins. It plays a key role in actin dynamics by promoting the disassembly and assembly of actin filaments[13]. HSP27 is a member of a small HSP family that is constitutively expressed in cells[14]. The main functions of HSP27, as a molecular chaperone, include the facilitation of the refolding of partially denatured proteins into active conformations, and the modulation of F-actin and cell movement[15]. However, the over-expression of HSP27 may be detrimental when it occurs in extracellular spaces[16]. Some researchers demonstrate that extracellular HSP27 mediates inflammatory response through signaling toll-like receptors (TLRs) to activate nuclear factor-κB (NF-κB) in mononuclear cells and induce dendritic cell maturation[17].
Various protocols for revascularization in LT have been performed in clinical studies. Sequential portal and arterial revascularization (SeqR) and simultaneous portal and arterial revascularization (SimR) have been advocated to improve patient outcomes after LT[18-22]. In SeqR, the liver graft is sequentially reperfused by portal and arterial reperfusion; however, the primary disadvantage of this protocol is the potential increased risk of arterial ischemic injury to bile ducts, which depend solely on the arterial blood supply[23]. By contrast, in SimR, the liver graft is simultaneously reperfused by the portal vein and the hepatic artery. Nonetheless, the disadvantage of SimR is that it prolongs the warm ischemia time (WIT) and the anhepatic phase, which can be detrimental to patient mortality and morbidity related to the graft[24]. These protocols of revascularization in LT have their own shortcomings in reducing arterial ischemic injury to the bile ducts. Hence, the manner by which arterial ischemic injury to the bile ducts may be reduced in LT is an urgent problem to be addressed.
With the development of LT, some arteries have been advocated as alternative for hepatic arterial anastomosis, such as the left gastric, right gastric, middle colic, gastroduodenal and right gastroepiploic artery. Still, the right gastroepiploic artery remains the optimum, when the hepatic artery cannot be used[25]. The aim of this study was to validate whether the transfer of blood through a bridging duct between the right gastroepiploic artery and the gastroduodenal artery could lessen the damage to F-actin caused by hepatic artery ischemia.
Animals: Forty male Bama miniature pigs aged 3-4 mo were obtained from the laboratory animal center of the Third Military Medical University in China. All pigs were given access to water ad libitum. The diet was formulated according to the recommended nutrient allowances for this pig breed. All appropriate measures were taken to minimize pain or discomfort. This study had been approved by the Ethics Committee of Xi’an Jiaotong University School of Medicine and was performed in accordance with the Guide for the Care and Use of Laboratory Animals of the Chinese National Institutes of Health.
Groups: All animals were divided randomly into four groups. The pigs in group A (control group) did not undergo hepatic artery ischemia and were simultaneously reperfused through the portal vein and hepatic artery after cold perfusion. The pigs in group B underwent hepatic artery ischemia for 1 h, while those in group C underwent hepatic artery ischemia for 2 h. The pigs in group D (hepatic artery bridging group) underwent blood transfer through a bridging duct between the right gastroepiploic artery and the gastroduodenal artery for 2 h.
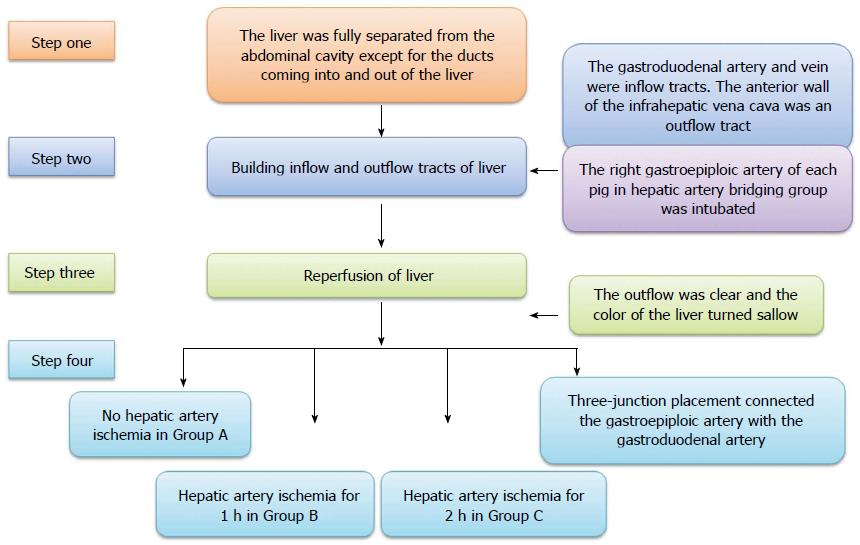
Animal model building: All operations were performed under general anesthesia (propofol 1.0 mL/kg per hour, intravenous drip) and a right subcostal incision was made in the upper abdominal region. The ligaments around the liver were disconnected. The liver was fully separated from the abdominal cavity except for the ducts coming into and out of the liver. Both the gastroduodenal artery and vein were intubated as inflow tracts and the proximal parts were ligated. The anterior wall of the infrahepatic vena cava was split as an outflow tract. The other blood inflow and outflow of the liver (such as the portal vein, suprahepatic vena cava, infrahepatic vena cava, and common hepatic artery) were interrupted by clamps. Lactated Ringer’s solution (500 mL at 4 °C, containing heparin 12.5 U/mL) was infused into the liver from the gastroduodenal artery and vein, respectively, until the outflow was clear and the color of the liver turned sallow, and then, the infrahepatic vena cava was repaired. After perfusion, the clamps on the portal vein, suprahepatic vena cava, and infrahepatic vena cava were released, and the release of the common hepatic artery differed in all groups. The common hepatic artery of each pig in group A was released at the same time as the portal vein, suprahepatic vena cava, and infrahepatic vena cava, while the pigs in groups B and C underwent hepatic artery ischemia for 1 h and 2 h, respectively, after the portal vein, suprahepatic vena cava and infrahepatic vena cava were released. The right gastroepiploic artery of each pig in group D was intubated before perfusion to the liver, and the tube was connected to the gastroduodenal artery using three-junction placement so that blood could be transferred to the liver and biliary duct even though the common hepatic artery was not released (The inside diameter and length of the tube between the right gastroepiploic and gastroduodenal artery were 2 mm and 20 cm, respectively), and the other operation was the same as that performed for group C (Figure 1).
Specimen collection: A small piece of hepatic tissue, which was used as a control, was resected immediately at the edge of the liver after laparotomy, and another hepatic tissue was resected at 2 h after reperfusion. Reoperations were performed under general anesthesia at 2 wk, and 1, 2 and 3 mo after the model was built. During the reoperation, a subcostal incision of approximately 4 cm was made, and a liver tissue slice was resected. Blood samples (approximately 5 mL) were harvested preoperatively and postoperatively at 1, 3, 5, 7, 9, 11, 13, 15, 18, 21, 24, and 27 d and 1, 1.5, 2, 2.5, and 3 mo after surgery via precava vein puncture. All the animals were sacrificed after 3 mo.
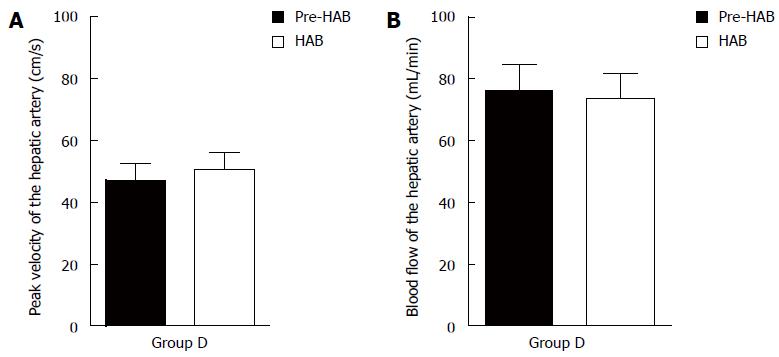
Peak velocity and blood flow of the hepatic artery in group D were measured using color Doppler ultrasound before and after the bridging duct was placed.
Total bilirubin (TB), alanine aminotransferase (ALT), alkaline phosphatase (ALP) and γ-glutamyl transpeptidase (γ-GT) were measured in the serum in duplicate using commercial kits according to the manufacturers’ protocols with an Olympus AU5421 biochemistry analyzer.
Liver fragments of approximately 2 mm3 were fixed in 2.5% glutaraldehyde for 3 h, rinsed in PBS and then postfixed in 1% osmium tetroxide for 2 h. Samples were dehydrated in graded alcohols, embedded in Epon 812, cut on an ultramicrotome, and then stained with uranyl acetate and lead citrate. Ultrathin sections were viewed using transmission electron microscopy (Hitachi 7650, Hitachi High-Technologies Corporation, Japan).
Specimens were fixed in 4% paraformaldehyde, embedded in paraffin, sliced 4 mm thick, and then prepared on glass slides. Some sections were observed by hematoxylin-eosin staining (HE) and some were stained for cofilin using a rabbit polyclonal antibody (Santa Cruz Biotechnology, sc-33779) and HSP27 using a goat polyclonal antibody (Santa Cruz Biotechnology, sc-1049). This procedure was followed by a second reaction with biotin-labeled anti-rabbit IgG against cofilin, anti-goat IgG against HSP27, and anti- mouse IgG against F-actin (Zhongshan Goldenbrige Biotechnology Co., Ltd., Beijing, China). An avidin-biotin coupling reaction was performed on the sections using an SP Kit (Zhongshan Goldenbrige Biotechnology Co., Ltd., Beijing, China).
All slide images were captured with a Leica SCN400 slide scanner, and density was analyzed using Image-Pro Plus 6.0 software.
The apoptosis of hepatocytes was identified by detecting DNA fragmentation in situ. DNA fragmentation was detected by transferase-mediated nick end-labeling (TUNEL) assay, which was performed on deparaffinized and dehydrated sections using an In Situ Cell Death Detection Kit (Zhongshan Goldenbrige Biomedical Technology Co., Beijing, China) according to the manufacturer’s instructions. TUNEL-positive hepatocytes displayed a characteristic apoptotic morphology, including chromatin condensation, cell fragmentation, and apoptotic bodies. Apoptotic hepatocytes were examined in 10 randomly selected areas per section. The apoptotic index was calculated as the percentage of apoptotic cells in the total number of hepatocytes.
For the immunoblotting of cofilin and HSP27, liver tissue was homogenized in 50 mmol/L Tris buffer (pH 7.4), 150 mmol/L NaCl, 1% NP-40, 0.5% sodium deoxycholate and 0.1% SDS containing protease inhibitors. Aliquots containing 40 μg of protein were subjected to 10% sodium dodecyl sulfate-polyamide gel electrophoresis and transferred to PVDF transfer membranes (Trans-Blot Cell, Hercules, CA, 3-4 h, 85 mA). Immunoblotting was performed to detect cofilin and HSP27 (overnight at 4 °C). After the procedure involving the secondary antibodies (2 h at 20 °C), the specimens were washed and subjected to chemiluminescence using a commercially available blotting kit.
The integrated density of the scanned immunoblotting signals was measured using the “GeneTools” program on a personal computer.
Total RNA was extracted using Trizol reagent (Invitrogen, United States). The RNA was reverse-transcribed to cDNA using a reverse transcriptase kit (PrimeScript RT Reagent Kit; TaKaRa). The relative abundance of each mRNA sample was quantitated by Q-PCR using specific primers and SYBR Premix Ex Taq (TaKaRa). Primers for cofilin, HSP27, F-actin and β-actin were designed and synthesized by TaKaRa Biotechnology (Table 1). Real-time PCR was conducted using an iQ Multicolor Real-Time PCR Detection System (Bio-Rad, Hercules, CA). Cycle threshold values were obtained from Bio-Rad iQ5 2.0 Standard Edition optical system software (Bio-Rad). The data were analyzed using the ΔΔCt method, and β-actin served as an internal control.
| Gene | Forward (5'-3') | Reverse (5'-3') |
| Cofilin | CAAGAAGGACCTGGTGTTC | TTGGAGCTGGCGTAGATCATT |
| HSP27 | CGTTGCCAATAACACAAACG | GGCTTCTACTTGGCTCCAGA |
| F-actin | GCTAAGAAGGCGATACAA | AGA ATG AGG ACTGGGTGA |
| β-actin | CCAGGTCATCACCATCGG | CCGTGTTGGCGTAGAGGT |
The statistical methods of this study were reviewed by Deyu Meng from Institute for Information and System Sciences, Faculty of Mathematics and Statistics, Xi’an Jiaotong University. The data are presented as the mean ± SD and were processed with SPSS 19.0 statistical software (IL, United States). Comparisons were performed between groups by one-way ANOVA. P < 0.05 was considered statistically significant.
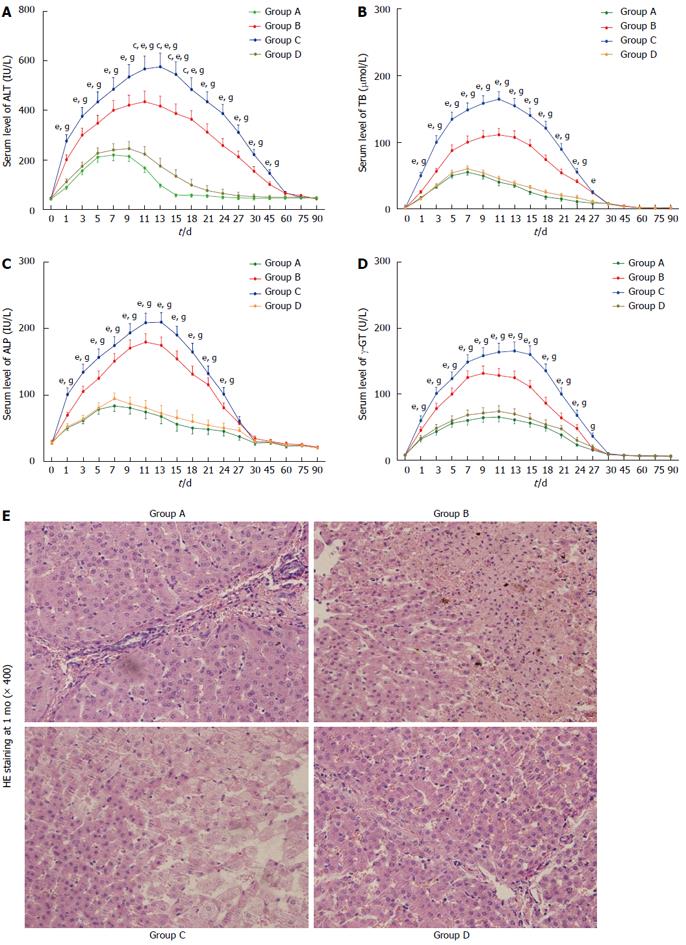
Following the surgery, we monitored serum ALT, TB, ALP and γ-GT levels. Serum ALT, TB, ALP and γ-GT levels were within the normal ranges immediately after surgery but progressively increased during the first few days for all groups, reaching peak values on days 7 to 13. ALT fully recovered to normal ranges within 2 mo. In addition, the levels of TB, ALP and γ-GT gradually declined and fully recovered to normal ranges within 1 mo. During the entire observation period, the ALT in group C was the highest of all the groups within 45 d (P < 0.05), and it was higher in group D than in group A from days 11 to 18 (P < 0.05) (Figure 2A). The TB in group C was the highest of all the groups within 24 d (P < 0.05) (Figure 2B). The ALP was significantly higher in groups B and C than in groups A and D from 1 to 24 d (P < 0.05) (Figure 2C). The level of γ-GT in group C was the highest of all the groups from days 1 to 27 (P < 0.05) (Figure 2D). There were no significant differences in the levels of TB, ALP or γ-GT between groups A and D within 3 mo (P > 0.05). HE staining indicated that the hepatic cord was disarranged and that some hepatocytes disappeared in groups B and C (Figure 2E).
There was no significant difference in peak velocity or blood flow before or after the bridging duct was placed for group D (P > 0.05) (Figure 3).
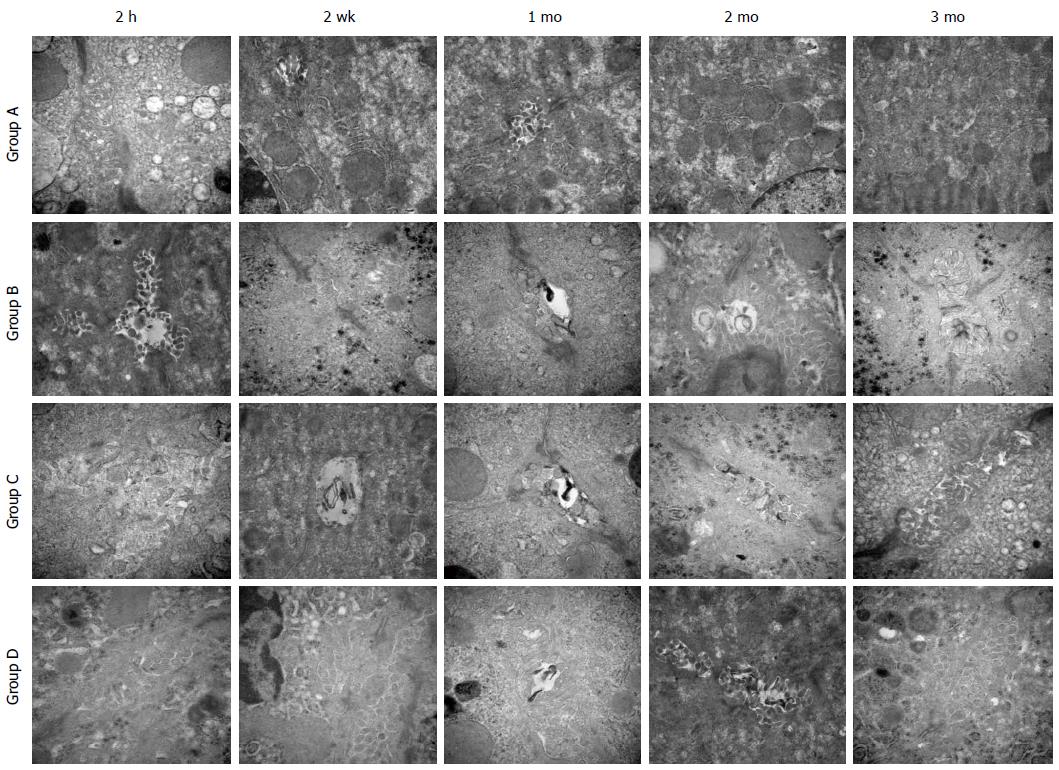
Transmission electron microscopy showed that at 2 h after reperfusion, the microvilli exhibited slight edema in groups A and D, whereas some microvilli disappeared in groups B and C. After 2 wk, the microvilli also showed edema in groups A and D, the bile thrombus could be observed in groups B and C and some of the microvilli were absent. After 2 mo, the microvilli recovered to normal states in groups A and D, and a few of them disappeared in groups B and C. By the third month, the microvilli in all groups were normal (Figure 4).
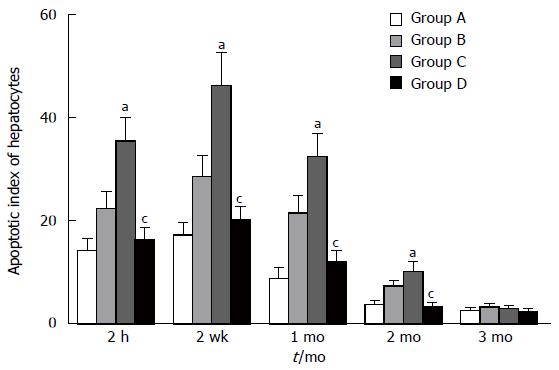
The apoptosis of hepatocytes increased 2 h after reperfusion in all groups and lasted for 2 mo. The second week was the peak time point. Compared with groups A and D, groups B and C exhibited significant increases in the apoptosis index at 2 h, 2 wk, 1 mo, and 2 mo (P < 0.05), while there was no significant difference between groups A and D within 3 mo (P > 0.05) (Figure 5).
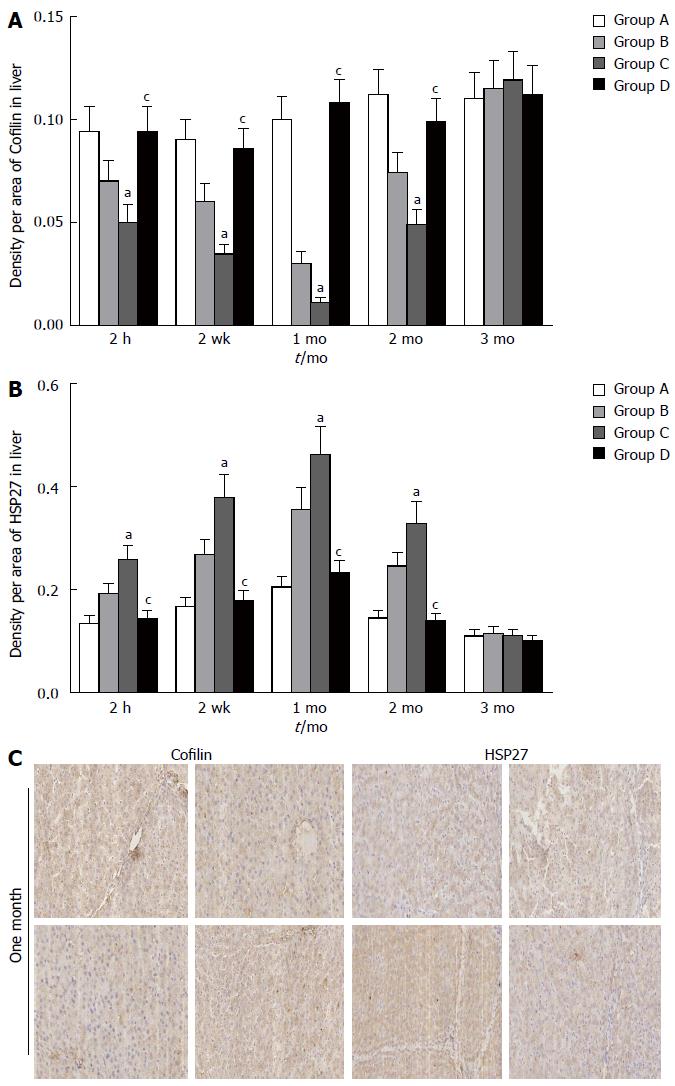
To further evaluate the function of the bile canaliculus, we examined cofilin and HSP27 expression. Immunohistochemical staining of the hepatocytes showed the down-regulation of cofilin and up-regulation of HSP27 at 2 h, 2 wk, 1 mo, and 2 mo in groups B and C compared with groups A and D (P < 0.05) (Figure 6A, B and C).
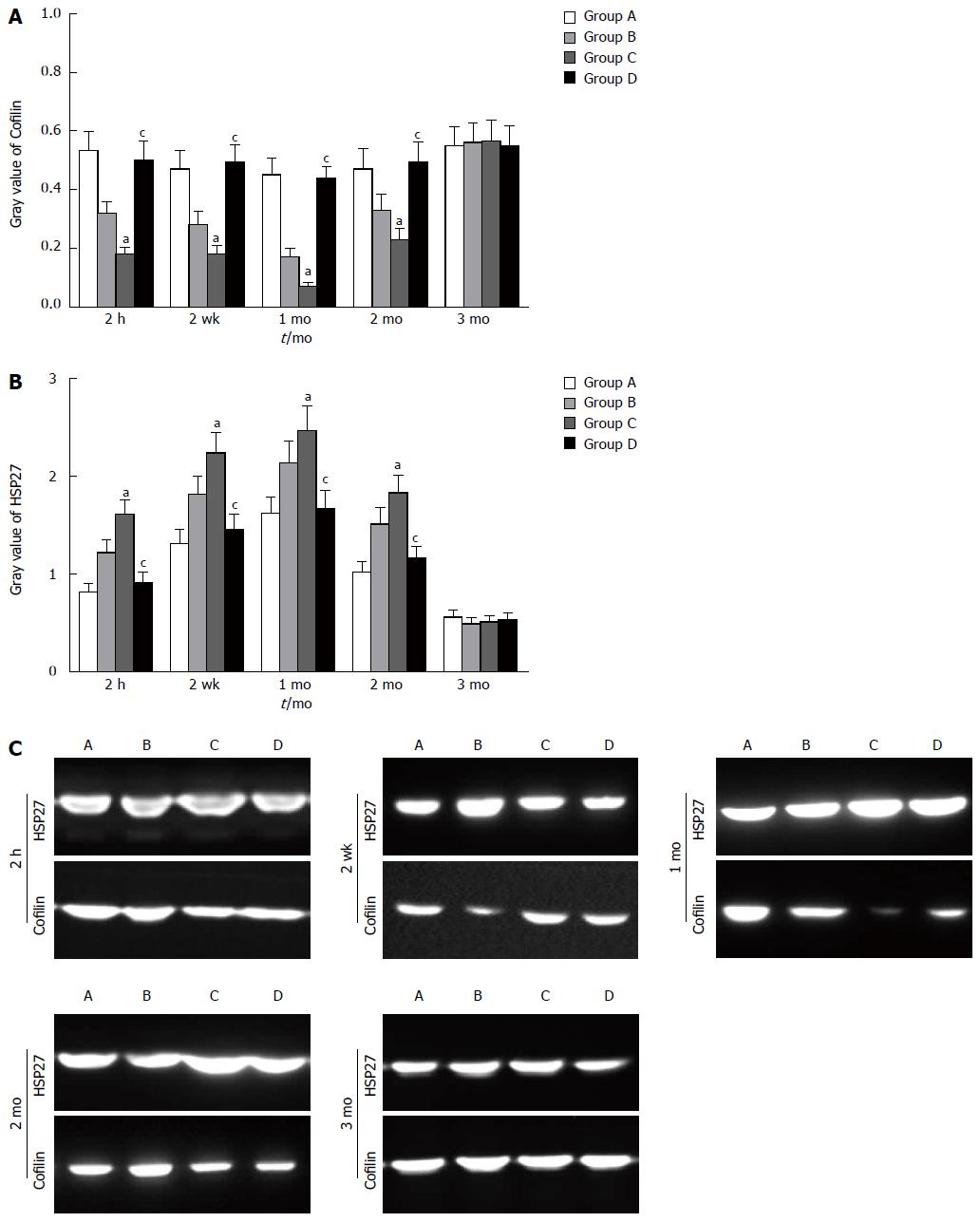
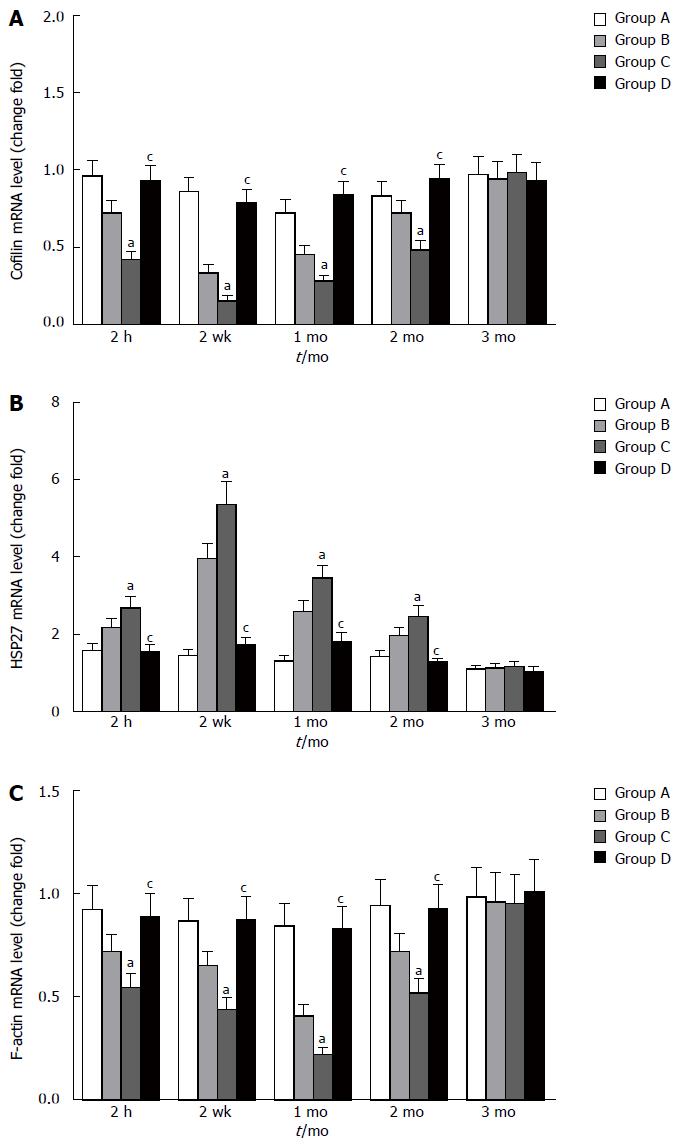
Similar changes were observed in Western blot analysis and real-time quantitative polymerase chain reaction. Cofilin expression decreased at 2 h and 2 wk, reaching the lowest level at 1 mo. Compared with the other groups, group C exhibited a significant decrease in cofilin expression at 2 h, 2 wk, 1 mo, and 2 mo (P < 0.05). There was no significant difference between groups A and D within 3 mo (P > 0.05) (Figure 7A). HSP27 expression increased at 2 h and 2 wk, reaching the highest level at 1 mo. Compared with groups A and D, group C exhibited significant increases at 2 h, 2 wk, 1 mo, and 2 mo (P < 0.05). There was no significant difference between groups A and D within 3 mo (P > 0.05) (Figure 7B). The four groups showed differential expression of cofilin and HSP27 within 3 mo (Figure 7C). The mRNA level of cofilin was decreased at 2 h after reperfusion, the lowest level was observed at the second week before recovery, and the preoperative level was gradually reached within 3 mo. Compared with groups A and D, significant differences were revealed at 2 h, 2 wk, 1 mo and 2 mo for group C (P < 0.05) (Figure 8A). The mRNA level of HSP27 was increased at 2 h after reperfusion; the highest level was obtained at the second week, and the preoperative level was reached within 3 mo. Compared with the other groups, significant differences were revealed at 2 h, 2 wk, 1 mo, and 2 mo for group C (P < 0.05) (Figure 8B). The mRNA level of F-actin was decreased at 2 h, 2 wk, 1 mo and 2 mo in group C. However, there were no significant differences between groups A and D within 3 mo (P > 0.05) (Figure 8C).
Nonanastomotic biliary strictures account for a major proportion of patient morbidity and mortality after LT and are characterized by progressive jaundice, fever, and abdominal discomfort, accompanied by increases in blood bilirubin, ALP and γ-GT[24,26]. Pathological examination may identify bile thrombus and intrahepatic cholestasis, and intra- and/or extrahepatic bile duct strictures may be revealed by an imaging test[27]. A classification of nonanastomotic biliary strictures has been proposed based on the localization of the abnormalities, distinguishing between type I (extrahepatic lesions), type II (intrahepatic lesions), and type III (intra- and extrahepatic alterations)[28,29].
To lessen the damage to F-actin caused by hepatic artery ischemia, we designed a hepatic artery bridge animal model. The clamping and releasing of the vessels in this model were performed to simulate disconnection and reconstruction. This approach could avoid potential surgical technical complications, making the operation easier to perform. Although conventional LT procedures are simplified, the key sequences of LT, such as graft perfusion, cold preservation, cold ischemia-reperfusion, warm ischemia-reperfusion, and the anhepatic phase, still exist. Thus, this modified model could adequately imitate the hepatic artery ischemia-reperfusion procedure in LT. Most confounding factors related to nonanastomotic biliary strictures were excluded or well controlled in this model. Considering all these facts, we placed a bridging duct between the right gastroepiploic artery and the gastroduodenal artery.
Under normal circumstances, ALP and γ-GT are bound to the canaliculus membranes of hepatocytes and the apical cell surfaces of cholangiocytes. The increased release of these enzymes occurs under pathologic conditions when cell membranes are injured[30,31]. Serological tests showed that ALT, TB, ALP and γ-GT were elevated in groups B and C during the early postoperative period in our study; however, there were no significant differences in the levels of TB, ALP or γ-GT between groups A and D within 3 mo. These findings imply that hepatic artery ischemia can induce biliary tract injury and that a bridging duct between the right gastroepiploic artery and the gastroduodenal artery can lessen the liver damage.
Bile canaliculi, which are connected to the canal of Hering and bile ducts, are formed by grooves on some of the lateral faces of hepatocytes[32,33]. Our observations showed that hepatic artery ischemia led to the loss of many microvilli from the bile canaliculi and that a longer duration of hepatic artery ischemia was associated with a more serious loss; however, the presence of a bridging duct decreased the damage. The function of microvilli relies on the continual polymerization and depolymerization of actin microfilaments, and both cofilin and HSP27 play important roles in F-actin dynamics[12,34,35]. The down-regulation of cofilin and F-actin indicated that the function of bile canaliculi was also impaired and over-expression of HSP27 was detrimental to the assembly and disassembly of F-actin microfilaments after hepatic artery ischemia. Some studies have suggested that HSP27 is an endogenous activator of toll-like receptor 2 (TLR2) and toll-like receptor 4 (TLR4), and that TLR4 is one of the signaling pathways involved in mediating the proinflammatory effects of extracellular HSP27[36]. Fortunately, the bridging duct did not impair the function of bile canaliculi, and we speculate that it did not change the hemodynamics in group D and that it circumvented the damage due to ischemia reperfusion injury, which is common in LT.
Intrahepatic cholangiocytes are only nourished by the peribiliary vascular plexus (PVP), which represents the terminal branches of the hepatic artery[37]. The PVP communicates with the branches of the portal vein, and portal vein blood may then reflow into the PVP during the warm ischemia. Microthrombus may further form in PVP because of the slow flow rate[38]. This phenomenon is assumed to be the cause of nonanastomotic biliary strictures. Our research revealed that the hepatic artery bridging duct did not decrease the peak velocity or blood flow in the hepatic artery, which might explain how a hepatic artery bridging duct could lessen the damage to microvilli caused by hepatic artery ischemia. The advantage of the hepatic artery bridging duct was that it lessened the injury to bile canaliculi caused by hepatic artery ischemia and reduced blood flow to the liver and biliary tract, which are inevitable in LT.
Our research has its limitations. Only clamping and releasing of the vessels in this model were performed to simulate disconnection and reconstruction. Another limitation is that this modified model merely imitates the hepatic artery ischemia-reperfusion procedure in LT, and most confounding factors related to nonanastomotic biliary strictures are excluded. These limitations can be overcome in the future by conducting animal allergenic LT studies.
In conclusion, hepatic artery ischemia aggravates damage to bile canaliculi, and this damage can be lessened by the presence of a bridging duct between the right gastroepiploic artery and the gastroduodenal artery within 3 mo. Whether a bridging duct can be used in animal allergenic LT will be determined in a follow-up study.
Biliary complications are a serious concern after liver transplantation (LT). Non-anastomotic strictures (NAS) have become the main type of biliary complication in recent years. The reasons behind the NAS are complicated and involve several factors. These factors include the use of reperfusion, postoperative cytomegalovirus infection, hepatic artery ischemia, and poor HLA-match.
Some researchers have confirmed that cofilin and heat shock protein 27 (HSP27) are very important in the assembly and disassembly of F-actin microfilaments. Cofilin plays a key role in actin dynamics by promoting the disassembly and assembly of actin filaments. The main functions of HSP27, as a molecular chaperone, include the facilitation of the refolding of partially denatured proteins into active conformations, and the modulation of F-actin and cell movement.
To lessen the damage to F-actin caused by hepatic artery ischemia, we designed a hepatic artery bridge animal model. This approach could avoid potential surgical technical complications, making the operation easier to perform, and the key sequences of LT, such as graft perfusion, cold preservation, cold ischemia-reperfusion, warm ischemia-reperfusion, and the anhepatic phase, still existed. Thus, this modified model could adequately imitate the hepatic artery ischemia-reperfusion procedure in LT. The authors placed a bridging duct between the right gastroepiploic artery and the gastroduodenal artery and found that the bridging duct could lessen the damage to F-actin caused by hepatic artery ischemia.
The study results suggest that hepatic artery ischemia aggravates damage to bile canaliculi, and this damage can be lessened by the presence of a bridging duct between the right gastroepiploic artery and the gastroduodenal artery within 3 mo.
The current methods of revascularization in liver transplantation can be divided into two main groups according to whether the liver graft is reperfused sequentially or simultaneously. The most commonly used procedure for the revascularization of liver grafts is sequential portal and arterial revascularization. However, sequential portal and arterial revascularization increases warm ischemic injury to the bile ducts, which depends on the hepatic artery. The disadvantage of simultaneous portal and arterial revascularization is that it prolongs the warm ischemia time and the anhepatic phase, which can be detrimental to patient mortality and morbidity related to the graft.
It is an interesting technique to prevent hepatic artery ischemia. This animal model study is well-designed and the results are interesting and important.
P- Reviewer: Chen H, Darius T, Sherid M S- Editor: Yu J L- Editor: Wang TQ E- Editor: Zhang DN
| 1. | Porrett PM, Hsu J, Shaked A. Late surgical complications following liver transplantation. Liver Transpl. 2009;15 Suppl 2:S12-S18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 47] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 2. | Lekerika N, Gutiérrez Rico RM, Arco Vázquez J, Prieto Molano L, Arana-Arri E, Martínez Indart L, Martínez Ruiz A, Ortiz de Urbina López J. Predicting fluid responsiveness in patients undergoing orthotopic liver transplantation: effects on intraoperative blood transfusion and postoperative complications. Transplant Proc. 2014;46:3087-3091. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 3. | Sharma S, Gurakar A, Jabbour N. Biliary strictures following liver transplantation: past, present and preventive strategies. Liver Transpl. 2008;14:759-769. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 263] [Cited by in RCA: 275] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 4. | Reichman TW, Sandroussi C, Grant DR, Cattral MS, Greig PD, Levy G, McGilvray ID. Surgical revision of biliary strictures following adult live donor liver transplantation: patient selection, morbidity, and outcomes. Transpl Int. 2012;25:69-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 5. | Valero V, Amini N, Spolverato G, Weiss MJ, Hirose K, Dagher NN, Wolfgang CL, Cameron AA, Philosophe B, Kamel IR. Sarcopenia adversely impacts postoperative complications following resection or transplantation in patients with primary liver tumors. J Gastrointest Surg. 2015;19:272-281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 174] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 6. | Corbani A, Burroughs AK. Intrahepatic cholestasis after liver transplantation. Clin Liver Dis. 2008;12:111-129, ix. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 22] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 7. | Tsukada N, Ackerley CA, Phillips MJ. The structure and organization of the bile canalicular cytoskeleton with special reference to actin and actin-binding proteins. Hepatology. 1995;21:1106-1113. [PubMed] |
| 8. | Rao RK, Samak G. Bile duct epithelial tight junctions and barrier function. Tissue Barriers. 2013;1:e25718. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 47] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 9. | Nieuwenhuijs VB, De Bruijn MT, Padbury RT, Barritt GJ. Hepatic ischemia-reperfusion injury: roles of Ca2+ and other intracellular mediators of impaired bile flow and hepatocyte damage. Dig Dis Sci. 2006;51:1087-1102. [PubMed] |
| 10. | Sudo R, Kohara H, Mitaka T, Ikeda M, Tanishita K. Coordinated movement of bile canalicular networks reconstructed by rat small hepatocytes. Ann Biomed Eng. 2005;33:696-708. [PubMed] |
| 11. | Arany I, Clark JS, Reed DK, Ember I, Juncos LA. Cisplatin enhances interaction between p66Shc and HSP27: its role in reorganization of the actin cytoskeleton in renal proximal tubule cells. Anticancer Res. 2012;32:4759-4763. [PubMed] |
| 12. | Doshi BM, Hightower LE, Lee J. The role of Hsp27 and actin in the regulation of movement in human cancer cells responding to heat shock. Cell Stress Chaperones. 2009;14:445-457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 68] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 13. | Chen CK, Benchaar SA, Phan M, Grintsevich EE, Loo RR, Loo JA, Reisler E. Cofilin-induced changes in F-actin detected via cross-linking with benzophenone-4-maleimide. Biochemistry. 2013;52:5503-5509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 14. | Kostenko S, Moens U. Heat shock protein 27 phosphorylation: kinases, phosphatases, functions and pathology. Cell Mol Life Sci. 2009;66:3289-3307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 284] [Cited by in RCA: 285] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 15. | Acunzo J, Katsogiannou M, Rocchi P. Small heat shock proteins HSP27 (HspB1), αB-crystallin (HspB5) and HSP22 (HspB8) as regulators of cell death. Int J Biochem Cell Biol. 2012;44:1622-1631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 224] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 16. | Graceffa P. Hsp27-actin interaction. Biochem Res Int. 2011;2011:901572. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 17. | Jin C, Cleveland JC, Ao L, Li J, Zeng Q, Fullerton DA, Meng X. Human myocardium releases heat shock protein 27 (HSP27) after global ischemia: the proinflammatory effect of extracellular HSP27 through toll-like receptor (TLR)-2 and TLR4. Mol Med. 2014;20:280-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 99] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 18. | Gurusamy KS, Naik P, Abu-Amara M, Fuller B, Davidson BR. Techniques of flushing and reperfusion for liver transplantation. Cochrane Database Syst Rev. 2012;3:CD007512. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 19. | Manzini G, Kremer M, Houben P, Gondan M, Bechstein WO, Becker T, Berlakovich GA, Friess H, Guba M, Hohenberger W. Reperfusion of liver graft during transplantation: techniques used in transplant centres within Eurotransplant and meta-analysis of the literature. Transpl Int. 2013;26:508-516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 20. | Polak WG, Porte RJ. The sequence of revascularization in liver transplantation: it does make a difference. Liver Transpl. 2006;12:1566-1570. [PubMed] |
| 21. | Sanchez-Perez B, Aranda-Narvaez JM, Suarez-Munoz MA, Fernandez-Aguilar JL, Perez-Daga JA, Gonzalez-Sanchez AJ, Montiel-Casado C, Becerra-Ortiz RM, Pulido-Roa I, Santoyo J. Simultaneous (Arterial/Portal) or Sequential (Portal) Revascularization of the Liver Graft: Does It Make Any Difference? Liver Trans. 2011;17:S236-S236. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 22. | Brockmann JG, August C, Wolters HH, Hömme R, Palmes D, Baba H, Spiegel HU, Dietl KH. Sequence of reperfusion influences ischemia/reperfusion injury and primary graft function following porcine liver transplantation. Liver Transpl. 2005;11:1214-1222. [PubMed] |
| 23. | Moreno C, Sabaté A, Figueras J, Camprubí I, Dalmau A, Fabregat J, Koo M, Ramos E, Lladó L, Rafecas A. Hemodynamic profile and tissular oxygenation in orthotopic liver transplantation: Influence of hepatic artery or portal vein revascularization of the graft. Liver Transpl. 2006;12:1607-1614. [PubMed] |
| 24. | Guichelaar MM, Benson JT, Malinchoc M, Krom RA, Wiesner RH, Charlton MR. Risk factors for and clinical course of non-anastomotic biliary strictures after liver transplantation. Am J Transplant. 2003;3:885-890. [PubMed] |
| 25. | Steinbrück K, Fernandes R, Enne M, Vasconcelos R, Bento G, Stoduto G, Auel T, Pacheco-Moreira LF. Right gastroepiploic artery as an alternative for arterial reconstruction in living donor liver transplantation. Case Reports Hepatol. 2014;2014:616251. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 26. | Heidenhain C, Pratschke J, Puhl G, Neumann U, Pascher A, Veltzke-Schlieker W, Neuhaus P. Incidence of and risk factors for ischemic-type biliary lesions following orthotopic liver transplantation. Transpl Int. 2010;23:14-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 131] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 27. | Seehofer D, Eurich D, Veltzke-Schlieker W, Neuhaus P. Biliary complications after liver transplantation: old problems and new challenges. Am J Transplant. 2013;13:253-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 215] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 28. | Spicak J, Adamec M, Janousek L, Oliverius M, Trunecka P, Peregrin J. Biliary complications after orthotopic liver transplantation (olt): the role of endoscopic management in the consecutive 400 patients. J Gastroen Hepatol. 2006;21:A47-A47. [DOI] [Full Text] |
| 29. | Hintze RE, Adler A, Veltzke W, Abou-Rebyeh H, Felix R, Neuhaus P. Endoscopic management of biliary complications after orthotopic liver transplantation. Hepatogastroenterology. 1997;44:258-262. [PubMed] |
| 30. | Held P, Harding CO. L-2-oxothiazolidine-4-carboxylate supplementation in murine gamma-GT deficiency. Free Radic Biol Med. 2003;34:1482-1487. [PubMed] |
| 31. | Martins MJ, Negrão MR, Hipólito-Reis C, Azevedo I. Physiologic concentrations of bile salts inhibit rat hepatic alkaline phosphatase but not the intestinal isoenzyme. Clin Biochem. 2000;33:611-617. [PubMed] |
| 32. | Boyer JL. Bile formation and secretion. Compr Physiol. 2013;3:1035-1078. [PubMed] |
| 33. | Saxena R, Theise N. Canals of Hering: recent insights and current knowledge. Semin Liver Dis. 2004;24:43-48. [PubMed] |
| 34. | Doctor RB, Fouassier L. Emerging roles of the actin cytoskeleton in cholangiocyte function and disease. Semin Liver Dis. 2002;22:263-276. [PubMed] |
| 35. | During RL, Gibson BG, Li W, Bishai EA, Sidhu GS, Landry J, Southwick FS. Anthrax lethal toxin paralyzes actin-based motility by blocking Hsp27 phosphorylation. EMBO J. 2007;26:2240-2250. [PubMed] |
| 36. | Binder RJ, Vatner R, Srivastava P. The heat-shock protein receptors: some answers and more questions. Tissue Antigens. 2004;64:442-451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 171] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 37. | op den Dries S, Westerkamp AC, Karimian N, Gouw AS, Bruinsma BG, Markmann JF, Lisman T, Yeh H, Uygun K, Martins PN. Injury to peribiliary glands and vascular plexus before liver transplantation predicts formation of non-anastomotic biliary strictures. J Hepatol. 2014;60:1172-1179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 152] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 38. | Fisher A, Miller CH. Ischemic-type biliary strictures in liver allografts: the Achilles heel revisited? Hepatology. 1995;21:589-591. [PubMed] |