Published online Sep 7, 2015. doi: 10.3748/wjg.v21.i33.9749
Peer-review started: March 2, 2015
First decision: March 26, 2015
Revised: April 11, 2015
Accepted: June 16, 2015
Article in press: June 16, 2015
Published online: September 7, 2015
Processing time: 190 Days and 0.3 Hours
AIM: To validate the utility of Annexin A10 as a surrogate marker of the serrated neoplasia pathway in invasive colorectal cancers (CRCs).
METHODS: A total of 1133 primary CRC patients who underwent surgical resection at Seoul National University Hospital between January 2004 and December 2007 were enrolled. Expression of Annexin A10 was evaluated by immunohistochemistry using tissue microarray and paired to our findings on clinicopathologic and molecular characteristics of each individual. CpG island methylator phenotype was determined by MethyLight assay and microsatellite instability was determined by high performance liquid chromatography. KRAS and BRAF mutation status was evaluated by direct sequencing and allele-specific PCR. Univariate and stage-specific survival analyses were performed to reveal the prognostic value of Annexin A10 expression.
RESULTS: Annexin A10 expression was observed in 66 (5.8%) of the 1133 patients. Annexin A10 expression was more commonly found in females and was associated with proximal location, ulcerative gross type, advanced T category, N category and TNM stage. CRCs with Annexin A10 expression showed an absence of luminal necrosis, luminal serration and mucin production. CRCs with Annexin A10 expression were associated with CpG island methylator phenotype, microsatellite instability and BRAF mutation. In survival analysis, Annexin A10 expression was associated with poor overall survival and progression-free survival, especially in stage IV CRCs.
CONCLUSION: Annexin A10 expression is associated with poor clinical behavior and can be used a supportive surrogate marker of the serrated neoplasia pathway in invasive CRCs.
Core tip: Annexin A10 is considered a surrogate immunohistochemical marker for sessile serrated adenomas/polyps. We validated the utility of Annexin A10 as a surrogate marker of the serrated neoplasia pathway in invasive colorectal cancers (CRCs). Annexin A10 expression was associated with female sex, proximal location, ulcerative gross type, advanced TNM stage, serration and mucin production. CRCs with Annexin A10 expression were associated with CpG island methylator phenotype, microsatellite instability and BRAF mutation. In stage-specific survival analysis, Annexin A10 expression was associated with poor clinical outcome in stage IV CRCs. Annexin A10 can be used a supportive surrogate marker of the serrated neoplasia pathway.
- Citation: Bae JM, Kim JH, Rhee YY, Cho NY, Kim TY, Kang GH. Annexin A10 expression in colorectal cancers with emphasis on the serrated neoplasia pathway. World J Gastroenterol 2015; 21(33): 9749-9757
- URL: https://www.wjgnet.com/1007-9327/full/v21/i33/9749.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i33.9749
Colorectal cancer (CRC) shows heterogeneity in terms of its molecular carcinogenesis, and this heterogeneity contributes to clinical and histomorphological variation[1]. Currently, there are three widely accepted colorectal carcinogenic pathways, the chromosomal instability (CIN) pathway, the microsatellite instability (MSI) pathway, and the epigenetic instability pathway, which corresponds to the CpG island methylator phenotype (CIMP). The CIN pathway is characterized by alterations in the number and structure of chromosomes, as well as by the accumulation of somatic mutations in genes including proto-oncogenes and tumor suppressor genes[2]. MSI is caused by a defective mismatch repair system and is characterized by alterations in the number of repeat nucleotide(s), leading to frame-shift mutations in the corresponding genes[3]. CIMP is characterized by widespread cancer-specific hypermethylation of numerous promoter CpG island loci[4]. Initially, these pathways were considered to be mutually exclusive, but recent comparative studies have reported that molecular alterations in these pathways can partially overlap[5].
CRC is one of the best models for studying multistep carcinogenesis. Virtually all CRCs originate from premalignant polyps, which can be detected by colonoscopy. Colorectal premalignant polyps are divided into two groups: adenomatous polyps (conventional adenomas), which are precursor lesions of CRCs with CIN, and serrated polyps, which are now considered to be precursor lesions of CRCs with CIMP and sporadic MSI[6]. Serrated polyps are series of polyps that share sawtooth-like glandular morphology. Serrated polyps are divided into hyperplastic polyps, sessile serrated adenomas/polyps (SSA/P) and traditional serrated adenomas (TSA). Serrated polyps are highly associated with CIMP, sporadic MSI and the BRAF mutation.
Annexin A10 is a member of the annexin family, a large multigene family of calcium- and phospholipid-binding proteins. It plays important roles in physiologic processes including differentiation and proliferation[7-9]. Annexin A10 is expressed in the foveolar cells and glandular cells of the normal antral or body-type gastric mucosa. In addition, Annexin A10 is expressed in Brunner gland cells of the duodenum and urothelial cells of the renal pelvis and urinary bladder[10]. However, aberrant expression of Annexin A10 was found in malignant tumors of other tissue types, including oral cancer, pancreatic cancer, and lung cancer[10,11]. Recently, Gonzalo et al[12] proposed Annexin A10 as a marker for the colorectal serrated neoplasia pathway. They observed increased expression of Annexin A10 in SSA/P compared with normal colonic epithelia and microvesicular hyperplastic polyps. However, little is known about Annexin A10 expression in invasive CRCs.
Previously, we reported the correlation of Annexin A10 expression with the serrated neoplasia pathway using 168 microsatellite-unstable CRCs[13]. However, the evaluation of Annexin A10 expression in a large population is required to characterize the clinicopathological and molecular characteristics of Annexin A10, because of its low prevalence in CRCs[10,14]. In this study, we evaluated the clinicopathological characteristics and prognostic value of Annexin A10 expression in 1133 primary CRCs and compared them with molecular profiles including CIMP, MSI, KRAS and BRAF mutation status. Finally, we evaluated whether Annexin A10 can be used as a surrogate marker for CRCs with CIMP.
A total of 1527 patients with CRC underwent curative surgery at Seoul National University Hospital, Seoul, South Korea, from January 2004 to December 2007. After the exclusion of 394 patients with CRC [refusal of molecular study (n = 136), non-invasive cancers (n = 50), familial adenomatous polyposis (n = 13), multiple occurrence (n = 78), neoadjuvant chemo- and/or radiotherapy (n = 89), recurrent tumors (n = 28)], formalin-fixed paraffin-embedded tissue samples from 1133 patients with CRC were selected for this study. This study was approved by the Institutional Review Board.
Clinicopathological characteristics including age, sex, tumor location, and TNM stage were obtained from electronic medical records. Through microscopic examination of representative sections of the tumors, we evaluated the following parameters without knowledge of the CIMP, MSI, KRAS and BRAF mutation status of the specimen: tumor differentiation, luminal necrosis, tumor budding, Crohn-like lymphoid reaction, number of tumor-infiltrating lymphocytes, luminal serration and extraglandular mucin production. Overall survival and progression-free survival data were extracted from the patient’s medical records, direct interviews with surviving patients or members of patients’ families or death registry offices.
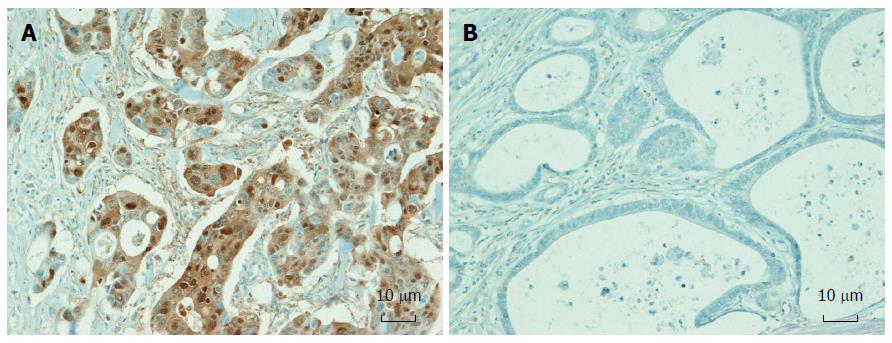
Tissue microarray (TMA) construction using formalin-fixed, paraffin-embedded (FFPE) tissues from 1133 CRCs was performed. Three different tumor areas in the FFPE tissue of individual CRCs were extracted as three tissue cores (2mm in diameter) for each case and were transferred to TMA blocks. Immunohistochemical analysis was performed with commercially available antibodies against Annexin A10 (1:300, NBP1-90156, Novus Biologicals). Expression of Annexin A10 was assessed independently by two pathologists (Bae JM and Kang GH). The presence of Annexin A10 nuclear staining in more than 5% of the tumor area in any TMA core was classified as expression of Annexin A10[13]. Tumors showing less than 5% nuclear staining of the tumor area or cytoplasmic staining without nuclear staining were classified as exhibiting no-expression of Annexin A10 (Figure 1).
Through histological examination, representative tumor portions were marked and then subjected to manual microdissection. Dissected tissues were collected into microtubes containing lysis buffer and proteinase K and were incubated at 55 °C for 2 d. DNA from paraffin-embedded tissue was extracted, and polymerase chain reaction (PCR) was performed. Direct sequencing of KRAS codons 12 and 13, and allele-specific PCR for BRAF codon 600 were performed as previously described[15]. MSI status was determined by 5 NCI markers including BAT25, BAT26, D2S123, D5S346 and D17S250. MSI was defined when two or more markers were unstable, and microsatellite stable (MSS) was defined when only one marker was unstable or when all five markers were stable.
Bisulfite DNA modification and real-time methylation specific PCR (MethyLight) assays were performed as described previously[16]. We quantified methylation of eight CIMP-specific markers (CACNA1G, CDKN2A, CRABP1, IGF2, MLH1, NEUROG1, RUNX3 and SOCS1). CIMP-positive (CIMP-P) was defined by a tumor showing methylation in ≥ five markers of the 8-marker CIMP panel and CIMP-negative (CIMP-N) as tumors showing methylation in ≤ 4 markers (0 to 4 of 8 promoters).
SAS system (version 9.3, SAS Institute, Cary, NC, United States) and R software were used for statistical analyses. The age of each group was compared using Student’s t-test. The other clinicopathological characteristics between groups were compared using χ2 test or Fisher’s exact test for categorical variables and Wilcoxon’s rank-sum test for ordinal variables. The survival curves after surgery were estimated by Kaplan-Meier method and the differences in the survival curves were tested by log-rank test. Cox proportional hazards models were used to estimate hazard ratios and corresponding 95% confidence intervals (CIs) for the overall survival. The assumption of proportional hazards was checked by plotting the log[-log[S(t)]] against time on study. All statistical tests were two-sided, and statistical significance was defined as P < 0.05.
The statistical methods of this study were reviewed by Myoung Jin Jang from Medical Research Collaborating Center, Seoul National University Hospital.
Detailed clinicopathological features and histological features according to Annexin A10 expression are summarized in Table 1. A total of 1133 patients with CRC (mean age ± SD, 60.8 ± 11.2) were included in the immunohistochemical analysis. The male to female ratio was 1.48:1 (677 males and 456 females). Tumor location was proximal colon (proximal to the splenic flexure) in 279 patients, distal colon in 441 patients and rectum in 413 patients. Median follow-up duration was 58.1 mo (0.3-89.8 mo). 785 patients received 5-fluorouracil based adjuvant chemotherapy.
| Parameter | Annexin A10no-expression(n = 1067, 94.2%) | Annexin A10expression(n = 66, 5.8%) | P value |
| Age (mean ± SD) | 61.0 ± 11.1 | 58.1 ± 12.6 | 0.0381 |
| Sex | < 0.001 | ||
| Male | 650 (60.9) | 27 (40.9) | |
| Female | 417 (39.1) | 39 (59.1) | |
| Location | < 0.001 | ||
| Proximal colon | 237 (22.2) | 42 (63.6) | |
| Distal colon | 430 (40.3) | 11 (16.7) | |
| Rectum | 400 (37.5) | 13 (19.7) | |
| Gross type | 0.027 | ||
| Fungating | 708 (66.3) | 35 (53.0) | |
| Ulcerative | 359 (33.7) | 31 (47.0) | |
| T category | < 0.0012 | ||
| 1 | 47 (4.4) | 1 (1.5) | |
| 2 | 164 (15.4) | 0 (0.0) | |
| 3 | 756 (70.8) | 52 (78.8) | |
| 4 | 100 (9.4) | 13 (19.7) | |
| N category | < 0.0012 | ||
| 0 | 558 (52.3) | 16 (24.2) | |
| 1 | 290 (27.2) | 23 (34.9) | |
| 2 | 219 (20.5) | 27 (40.9) | |
| M category | 0.049 | ||
| 0 | 892 (83.6) | 49 (74.2) | |
| 1 | 175 (16.4) | 17 (25.8) | |
| Stage | < 0.0012 | ||
| I | 173 (16.2) | 0 (0.0) | |
| II | 351 (32.9) | 15 (22.7) | |
| III | 368 (34.5) | 34 (51.5) | |
| IV | 175 (16.4) | 17 (25.7) | |
| Differentiation | 0.002 | ||
| Well Differentiated | 64 (6.0) | 2 (3.0) | |
| Moderately Differentiated | 970 (90.9) | 56 (84.9) | |
| Poorly Differentiated | 33 (3.1) | 8 (12.1) | |
| Luminal necrosis | < 0.001 | ||
| Absent | 83 (7.8) | 18 (27.3) | |
| Present | 984 (92.2) | 48 (72.7) | |
| Tumor budding | 0.2643 | ||
| Absent | 36 (3.4) | 0 (0.0) | |
| Present | 1031 (96.6) | 66 (100.0) | |
| Tumor-infiltrating Lymphocytes | 0.316 | ||
| Low TILs (< 8/HPF) | 802 (75.2) | 39 (59.1) | |
| High TILs (≥ 8/HPF) | 265 (24.8) | 27 (40.9) | |
| Crohn’s-like Lymphoid reaction | 0.470 | ||
| Absent | 908 (85.1) | 54 (81.8) | |
| Present | 159 (14.9) | 12 (18.2) | |
| Luminal serration | < 0.0013 | ||
| Absent | 1039 (97.4) | 50 (75.8) | |
| Present | 28 (2.6) | 16 (24.2) | |
| Mucin production | |||
| Absent | 958 (89.8) | 43 (65.1) | < 0.001 |
| Present | 109 (10.2) | 23 (34.9) |
Annexin A10 expression was observed in 66 (5.8%) patients. Annexin A10 expression was associated with lower age at diagnosis (P = 0.038), female sex (P < 0.001), proximal tumor location (P < 0.001), advanced T category (P < 0.001), N category (P < 0.001), M category (P = 0.049) and more advanced TNM stage (P < 0.001). As shown with microscopic examination, Annexin A10 expression was associated with absence of luminal necrosis (P < 0.001), increased number of tumor-infiltrating lymphocytes (P = 0.003), luminal serration (P < 0.001) and mucin production (P < 0.001).
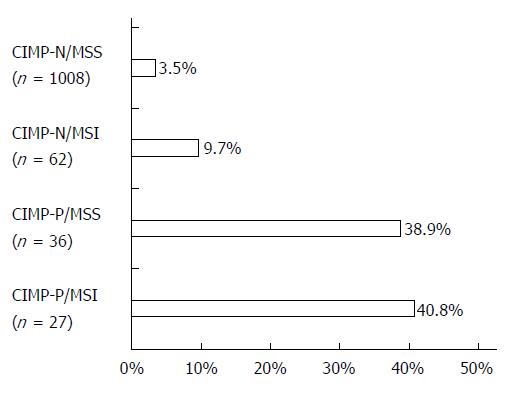
Table 2 shows molecular characteristics of CRCs according to Annexin A10 expression. Among the 1133 CRCs, CIMP-P CRCs were detected in 63 (5.6%) patients and MSI CRCs were found in 88 (7.8%) patients. Annexin A10 expression was associated with CIMP-P CRCs (P < 0.001) and MSI CRCs (P < 0.001). Sensitivity and specificity of Annexin A10 expression for detection of CIMP-P CRCs were 39.7% and 96.2%, respectively. Positive predictive value (PPV) and negative predictive value (NPV) of Annexin A10 expression for detection of CIMP-P CRCs were 0.38 and 0.96, respectively. In four molecular subtypes which were generated by the combined status of CIMP and MSI, Annexin A10 expression was observed in 3.5% of CIMP-N/MSS CRCs, 9.7% of CIMP-N/MSI CRCs, 38.9% of CIMP-P/MSS CRCs and 40.8% of CIMP-P/MSI CRCs (Figure 2). In the mutation studies, CRCs with Annexin A10 expression showed a higher frequency of the BRAF mutation than did CRCs with Annexin A10 no-expression (P < 0.001).
| Parameter | Annexin A10 | Annexin A10 | P value |
| no-expression | expression | ||
| (n = 1067, 94.2%) | (n = 66, 5.8%) | ||
| CIMP | < 0.0011 | ||
| CIMP-N | 1029 (96.4) | 41 (62.1) | |
| CIMP-P | 38 (3.6) | 25 (37.9) | |
| MSI | < 0.0011 | ||
| MSS | 995 (93.2) | 49 (74.2) | |
| MSI | 72 (6.8) | 17 (25.8) | |
| KRAS mutation | 0.399 | ||
| (n = 1071) | |||
| Wild type | 745 (73.8) | 42 (68.8) | |
| Mutant type | 265 (26.2) | 19 (31.2) | |
| BRAF mutation | < 0.0011 | ||
| (n = 1005) | |||
| Wild type | 912 (96.4) | 50 (84.7) | |
| Mutant type | 34 (3.6) | 9 (15.3) |
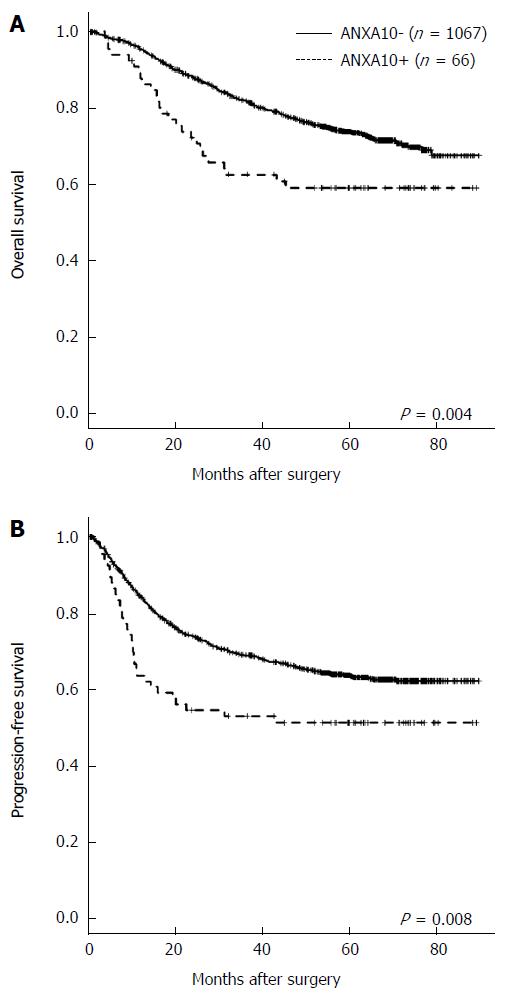
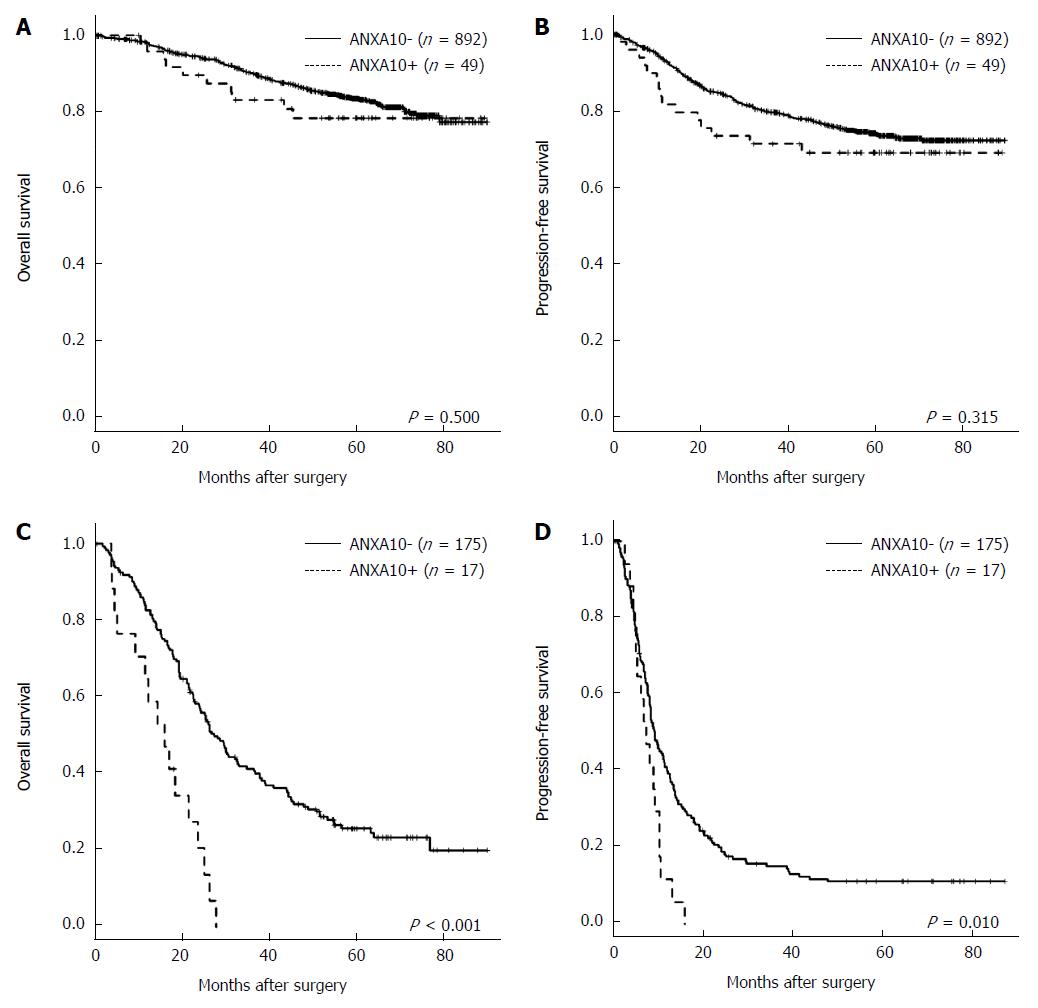
As shown using univariate survival analysis with Kaplan-Meier survival curves, patients with Annexin A10 expression showed worse overall survival (P = 0.004) and progression-free survival (P = 0.008) than patients with Annexin A10 no-expression (Figure 3). Although sample size did not get enough power to predict clinical outcome, in stage IV CRCs, the Annexin A10 expression group showed shorter median overall survival (OS) (17.0 mo vs 25.3 mo, P < 0.001) and shorter progression-free survival (PFS) (7.5 mo vs 9.2 mo, p=0.010) compared with the Annexin A10 no-expression group (Figure 4). However, stages I-III CRCs did not show a significant difference in clinical outcome according to Annexin A10 expression status (P = 0.500 for OS and P = 0.315 for PFS, median survival: not reached) (Figure 4). Multivariate survival analysis using Cox proportional hazard model in stage IV CRCs suggested that Annexin A10 expression could be an independent prognostic marker for overall survival in stage IV CRCs, despite limitation of insufficient sample size (Table 3).
| Univariate analysis | Multivariate analysis | |||
| Variable | HR (95%CI) | P value | HR (95%CI) | P value |
| Differentiation (undifferentiated/differentiated) | 2.20 (1.31-3.72) | 0.003 | 1.95 (1.07-3.56) | 0.030 |
| Tumor location (proximal colon/distal colon, rectum) | 1.66 (1.16-2.38) | 0.005 | 1.60 (1.06-2.42) | 0.025 |
| Adjuvant chemotherapy (treatment/no-treatment) | 0.42 (0.27-0.66) | < 0.001 | 0.47 (0.27-0.81) | 0.006 |
| Annexin A10 (expression/no-expression) | 3.13 (1.83-5.38) | < 0.001 | 2.38 (1.18-4.83) | 0.016 |
| Age (yr) (≥ 65/< 65) | 1.54 (1.10-2.15) | 0.012 | 1.37 (0.92-2.03) | 0.123 |
| Gross pattern (ulcerative/fungating) | 1.41 (1.01-1.96) | 0.042 | 1.18 (0.81-1.71) | 0.387 |
| CIMP (CIMP-P/CIMP-N) | 2.24 (1.24-4.06) | 0.008 | 1.13 (0.44-2.89) | 0.801 |
| MSI (MSI/MSS) | 2.46 (1.08-5.63) | 0.033 | 1.46 (0.44-4.86) | 0.540 |
| BRAF mutation (Mt/Wt) | 2.42 (1.12-5.19) | 0.024 | 1.67 (0.71-3.91) | 0.239 |
| Sex (male/female) | 1.03 (0.74-1.45) | 0.858 | - | - |
| KRAS mutation (Mt/Wt) | 1.09 (0.75-1.58) | 0.653 | - | - |
CIMP is one of the molecular subtypes of CRC and is characterized by concurrent hypermethylation of promoter CpG islands in tumor-suppressor genes and tumor-associated genes. To characterize CIMP, we must measure the methylation status of several panels of multiple genes using methylation-specific PCR or the MethyLight assay[17-19]. However, these assays are not easily used in daily clinical practice because these assays require complicated work and show low cost-effectiveness and inconsistent results[20]. Therefore, an easily applicable strong surrogate marker is required to characterize CIMP clinically. Clear association of the BRAF mutation and CIMP led us to consider a recently developed BRAF V600E-specific antibody (clone VE1)[21]. Some studies showed excellent concordance of immunohistochemical staining results of clone VE1 and the BRAF mutation determined by sequence analysis[22-24]. However, other studies reported poor sensitivity of clone VE1 immunostaining owing to its vulnerability to pretreatment conditions[25,26].
In a recent study, Annexin A10 was proposed as a surrogate marker for SSA/P[12] and found to be expressed even in traditional serrated adenomas[27]. Because SSA/P or traditional serrated adenomas are considered to be precursor lesions of CIMP-P CRCs, Annexin A10 expression could be another surrogate marker for CIMP-P CRCs. In a study by Tsai et al[28], Annexin A10 was found to be expressed in 28% of CIMP-P/MSI CRCs and 67% of CIMP-P/MSS CRCs. Our present study showed that Annexin A10 was expressed in 40.8% of CIMP-P/MSI CRCs and 38.9% of CIMP-P/MSS CRCs. These results imply that nuclear expression of Annexin A10 might be lost or reduced during multistep carcinogenesis of some SSA/P or another carcinogenic pathway can contribute to the development of CIMP-P CRCs.
The clinicopathological characteristics of CRCs in which Annexin A10 is expressed are not well known. In this study, Annexin A10 expression was observed in 5.8% of 1133 primary surgically resected CRCs. This result is similar to a previous study which reported that Annexin A10 was expressed in 6% of CRCs[10]. Tsai et al[28] reported that Annexin A10 expression is associated with right-side tumor location, moderate to poor differentiation, Crohn-like lymphoid reaction and lack of dirty necrosis, but there was no correlation with mucinous differentiation, medullary histology or tumor-infiltrating lymphocytes. Sajanti et al[29] reported that Annexin A10 expression was associated with proximal tumor location, mucin production and serrated histology, but there was no correlation with stage and grade. In our present study, CRCs with Annexin A10 expression were associated with lower age at diagnosis, female sex, proximal tumor location, advanced T, N, M category, advanced TNM stage, absence of luminal necrosis, increased number of tumor-infiltrating lymphocytes, luminal serration and mucin production. Ethnic difference and different proportion of molecular subtypes such as CIMP and MSI might contribute to discrepancies in clinicopathologic characteristics of Annexin A10 expression between studies. However, previous studies had several limitations. The study of Tsai et al[28] might have selection bias because they included entire CRCs which had CIMP, MSI and BRAF mutation as a case group, however CRCs with conventional pathway were randomly selected as a control group. The study of Sajanti et al[29] analyzed only 42.4% (146/344) of patients who underwent surgical resection in the enrollment period. Our present study showed clinicopathologic characteristics of Annexin A10 expression in a large and consecutively collected CRC patient population.
The prognostic value of Annexin A10 expression in CRCs has not yet been reported. However, association of Annexin A10 with poor prognostic molecular features such as CIMP and the BRAF mutation led us to assume that Annexin A10 was a poor prognostic indicator[30-32]. In this study, stage-specific survival analysis results showed that Annexin A10 expression is associated with poor OS and PFS in stage IV CRCs. Multivariate survival analysis confined to stage IV CRCs suggested that Annexin A10 expression could be an independent prognostic marker in advanced stage CRCs.
This study has several limitations. First, Annexin A10 expression was measured using TMA. Regional heterogeneity of Annexin A10 expression was reported, so we could not exclude the possibility of false-negativity in Annexin A10 expression[28]. Second, exclusivity of Annexin A10 for sporadic MSI CRCs is inconclusive, but we could not evaluate germline mutation status of MMR genes (hMLH1, hMSH2, hMSH6 and hPMS2)[28,33,34]. Third, the proportion of CIMP-P, MSI and BRAF mutations in this study was low compared to Western population[31,35] .
In conclusion, Annexin A10 expression has a supportive but inconclusive role as a surrogate marker of CIMP-P CRCs. Further studies focusing on the molecular mechanisms of Annexin A10 expression and its oncogenic functions in CRCs are required.
The serrated neoplasia pathway is an explanatory model of multistep carcinogenesis in CRC displaying the CpG island methylator phenotype (CIMP). Recently, Annexin A10, a eukaryotic calcium- and phospholipid-binding protein, was proposed to be a surrogate marker for sessile serrated adenomas/polyps.
In this study, the authors attempted to validate the utility of Annexin A10 as a surrogate marker of the serrated neoplasia pathway in invasive CRCs.
Annexin A10 expression was observed in 66 (5.8%) patients. CRCs with Annexin A10 expression were associated with CIMP, microsatellite instability and BRAF mutation. Annexin A10 expression was associated with poor overall survival and progression-free survival in stage IV CRCs.
The study results suggest that Annexin A10 expression has a supportive but inconclusive role as a surrogate marker of CIMP-P CRCs
Annexin A10 is a member of the annexin family, a large multigene family of calcium- and phospholipid-binding proteins and plays important roles in physiologic processes including differentiation and proliferation. Serrated neoplasia pathway is a model of multistep carcinogenesis for CRCs which share serrated morphology, CIMP and BRAF mutation.
This is an interesting study investigating the association between Annexin A10 expression and clinical behavior in CRC.
P- Reviewer: Hsu LS, Sakata Y S- Editor: Wang JL L- Editor: Wang TQ E- Editor: Liu XM
| 1. | Jass JR. Classification of colorectal cancer based on correlation of clinical, morphological and molecular features. Histopathology. 2007;50:113-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 955] [Cited by in RCA: 1004] [Article Influence: 55.8] [Reference Citation Analysis (0)] |
| 2. | Fearon ER, Vogelstein B. A genetic model for colorectal tumorigenesis. Cell. 1990;61:759-767. [PubMed] |
| 3. | Boland CR, Goel A. Microsatellite instability in colorectal cancer. Gastroenterology. 2010;138:2073-2087.e3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1677] [Cited by in RCA: 1548] [Article Influence: 103.2] [Reference Citation Analysis (0)] |
| 4. | Hinoue T, Weisenberger DJ, Lange CP, Shen H, Byun HM, Van Den Berg D, Malik S, Pan F, Noushmehr H, van Dijk CM. Genome-scale analysis of aberrant DNA methylation in colorectal cancer. Genome Res. 2012;22:271-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 455] [Cited by in RCA: 489] [Article Influence: 34.9] [Reference Citation Analysis (0)] |
| 5. | Cancer Genome Atlas Network. Comprehensive molecular characterization of human colon and rectal cancer. Nature. 2012;487:330-337. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6773] [Cited by in RCA: 6674] [Article Influence: 513.4] [Reference Citation Analysis (0)] |
| 6. | Rex DK, Ahnen DJ, Baron JA, Batts KP, Burke CA, Burt RW, Goldblum JR, Guillem JG, Kahi CJ, Kalady MF. Serrated lesions of the colorectum: review and recommendations from an expert panel. Am J Gastroenterol. 2012;107:1315-1329; quiz 1314, 1330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 825] [Cited by in RCA: 830] [Article Influence: 63.8] [Reference Citation Analysis (0)] |
| 7. | Mussunoor S, Murray GI. The role of annexins in tumour development and progression. J Pathol. 2008;216:131-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 223] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 8. | Rescher U, Gerke V. Annexins--unique membrane binding proteins with diverse functions. J Cell Sci. 2004;117:2631-2639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 430] [Cited by in RCA: 474] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 9. | Gerke V, Creutz CE, Moss SE. Annexins: linking Ca2+ signalling to membrane dynamics. Nat Rev Mol Cell Biol. 2005;6:449-461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1036] [Cited by in RCA: 1124] [Article Influence: 56.2] [Reference Citation Analysis (0)] |
| 10. | Lu SH, Yuan RH, Chen YL, Hsu HC, Jeng YM. Annexin A10 is an immunohistochemical marker for adenocarcinoma of the upper gastrointestinal tract and pancreatobiliary system. Histopathology. 2013;63:640-648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 11. | Shimizu T, Kasamatsu A, Yamamoto A, Koike K, Ishige S, Takatori H, Sakamoto Y, Ogawara K, Shiiba M, Tanzawa H. Annexin A10 in human oral cancer: biomarker for tumoral growth via G1/S transition by targeting MAPK signaling pathways. PLoS One. 2012;7:e45510. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 44] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 12. | Gonzalo DH, Lai KK, Shadrach B, Goldblum JR, Bennett AE, Downs-Kelly E, Liu X, Henricks W, Patil DT, Carver P. Gene expression profiling of serrated polyps identifies annexin A10 as a marker of a sessile serrated adenoma/polyp. J Pathol. 2013;230:420-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 66] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 13. | Kim JH, Rhee YY, Kim KJ, Cho NY, Lee HS, Kang GH. Annexin A10 expression correlates with serrated pathway features in colorectal carcinoma with microsatellite instability. APMIS. 2014;122:1187-1195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 14. | Ogino S, Chan AT, Fuchs CS, Giovannucci E. Molecular pathological epidemiology of colorectal neoplasia: an emerging transdisciplinary and interdisciplinary field. Gut. 2011;60:397-411. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 430] [Cited by in RCA: 452] [Article Influence: 32.3] [Reference Citation Analysis (0)] |
| 15. | Kim JH, Shin SH, Kwon HJ, Cho NY, Kang GH. Prognostic implications of CpG island hypermethylator phenotype in colorectal cancers. Virchows Arch. 2009;455:485-494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 110] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 16. | Yoo EJ, Park SY, Cho NY, Kim N, Lee HS, Kang GH. Helicobacter pylori-infection-associated CpG island hypermethylation in the stomach and its possible association with polycomb repressive marks. Virchows Arch. 2008;452:515-524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 17. | Toyota M, Ahuja N, Ohe-Toyota M, Herman JG, Baylin SB, Issa JP. CpG island methylator phenotype in colorectal cancer. Proc Natl Acad Sci USA. 1999;96:8681-8686. [PubMed] |
| 18. | Ogino S, Cantor M, Kawasaki T, Brahmandam M, Kirkner GJ, Weisenberger DJ, Campan M, Laird PW, Loda M, Fuchs CS. CpG island methylator phenotype (CIMP) of colorectal cancer is best characterised by quantitative DNA methylation analysis and prospective cohort studies. Gut. 2006;55:1000-1006. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 273] [Cited by in RCA: 290] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 19. | Weisenberger DJ, Siegmund KD, Campan M, Young J, Long TI, Faasse MA, Kang GH, Widschwendter M, Weener D, Buchanan D. CpG island methylator phenotype underlies sporadic microsatellite instability and is tightly associated with BRAF mutation in colorectal cancer. Nat Genet. 2006;38:787-793. [PubMed] |
| 20. | Balic M, Pichler M, Strutz J, Heitzer E, Ausch C, Samonigg H, Cote RJ, Dandachi N. High quality assessment of DNA methylation in archival tissues from colorectal cancer patients using quantitative high-resolution melting analysis. J Mol Diagn. 2009;11:102-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 47] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 21. | Capper D, Preusser M, Habel A, Sahm F, Ackermann U, Schindler G, Pusch S, Mechtersheimer G, Zentgraf H, von Deimling A. Assessment of BRAF V600E mutation status by immunohistochemistry with a mutation-specific monoclonal antibody. Acta Neuropathol. 2011;122:11-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 369] [Cited by in RCA: 377] [Article Influence: 26.9] [Reference Citation Analysis (0)] |
| 22. | Affolter K, Samowitz W, Tripp S, Bronner MP. BRAF V600E mutation detection by immunohistochemistry in colorectal carcinoma. Genes Chromosomes Cancer. 2013;52:748-752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 47] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 23. | Sinicrope FA, Smyrk TC, Tougeron D, Thibodeau SN, Singh S, Muranyi A, Shanmugam K, Grogan TM, Alberts SR, Shi Q. Mutation-specific antibody detects mutant BRAFV600E protein expression in human colon carcinomas. Cancer. 2013;119:2765-2770. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 55] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 24. | Toon CW, Walsh MD, Chou A, Capper D, Clarkson A, Sioson L, Clarke S, Mead S, Walters RJ, Clendenning M. BRAFV600E immunohistochemistry facilitates universal screening of colorectal cancers for Lynch syndrome. Am J Surg Pathol. 2013;37:1592-1602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 111] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 25. | Adackapara CA, Sholl LM, Barletta JA, Hornick JL. Immunohistochemistry using the BRAF V600E mutation-specific monoclonal antibody VE1 is not a useful surrogate for genotyping in colorectal adenocarcinoma. Histopathology. 2013;63:187-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 67] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 26. | Kuan SF, Navina S, Cressman KL, Pai RK. Immunohistochemical detection of BRAF V600E mutant protein using the VE1 antibody in colorectal carcinoma is highly concordant with molecular testing but requires rigorous antibody optimization. Hum Pathol. 2014;45:464-472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 51] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 27. | Wiland HO, Shadrach B, Allende D, Carver P, Goldblum JR, Liu X, Patil DT, Rybicki LA, Pai RK. Morphologic and molecular characterization of traditional serrated adenomas of the distal colon and rectum. Am J Surg Pathol. 2014;38:1290-1297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 56] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 28. | Tsai JH, Lin YL, Cheng YC, Chen CC, Lin LI, Tseng LH, Cheng ML, Liau JY, Jeng YM. Aberrant expression of annexin A10 is closely related to gastric phenotype in serrated pathway to colorectal carcinoma. Mod Pathol. 2015;28:268-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 38] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 29. | Sajanti SA, Väyrynen JP, Sirniö P, Klintrup K, Mäkelä J, Tuomisto A, Mäkinen MJ. Annexin A10 is a marker for the serrated pathway of colorectal carcinoma. Virchows Arch. 2015;466:5-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 30. | Kalady MF, Dejulius KL, Sanchez JA, Jarrar A, Liu X, Manilich E, Skacel M, Church JM. BRAF mutations in colorectal cancer are associated with distinct clinical characteristics and worse prognosis. Dis Colon Rectum. 2012;55:128-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 111] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 31. | Ogino S, Nosho K, Kirkner GJ, Kawasaki T, Meyerhardt JA, Loda M, Giovannucci EL, Fuchs CS. CpG island methylator phenotype, microsatellite instability, BRAF mutation and clinical outcome in colon cancer. Gut. 2009;58:90-96. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 633] [Cited by in RCA: 642] [Article Influence: 40.1] [Reference Citation Analysis (0)] |
| 32. | Juo YY, Johnston FM, Zhang DY, Juo HH, Wang H, Pappou EP, Yu T, Easwaran H, Baylin S, van Engeland M. Prognostic value of CpG island methylator phenotype among colorectal cancer patients: a systematic review and meta-analysis. Ann Oncol. 2014;25:2314-2327. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 139] [Cited by in RCA: 135] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 33. | Pai RK, Shadrach BL, Carver P, Heald B, Moline J, Church J, Kalady MF, Burke CA, Plesec TP, Lai KK. Immunohistochemistry for annexin A10 can distinguish sporadic from Lynch syndrome-associated microsatellite-unstable colorectal carcinoma. Am J Surg Pathol. 2014;38:518-525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 34. | Kim JH, Kang GH. Annexin A10 expression in microsatellite-unstable colorectal cancers: is it specific to sporadic tumors? Am J Surg Pathol. 2014;38:1577-1579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 35. | Dahlin AM, Palmqvist R, Henriksson ML, Jacobsson M, Eklöf V, Rutegård J, Oberg A, Van Guelpen BR. The role of the CpG island methylator phenotype in colorectal cancer prognosis depends on microsatellite instability screening status. Clin Cancer Res. 2010;16:1845-1855. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 136] [Article Influence: 9.1] [Reference Citation Analysis (0)] |