Published online Aug 28, 2015. doi: 10.3748/wjg.v21.i32.9671
Peer-review started: December 28, 2014
First decision: January 22, 2015
Revised: January 30, 2015
Accepted: June 15, 2015
Article in press: June 16, 2015
Published online: August 28, 2015
Processing time: 246 Days and 3.1 Hours
A 58-year-old man was admitted to our hospital. Laboratory data showed severe liver injury and that the patient was positive for immunoglobulin M anti-hepatitis A virus (HAV) antibodies. He was also complicated with severe renal dysfunction and had an extremely high level of serum hepatocyte growth factor (HGF). Therefore, he was diagnosed with severe acute liver failure with acute renal failure (ARF) caused by HAV infection. Prognosis was expected to be poor because of complications by ARF and high serum HGF. However, liver and renal functions both improved rapidly without intensive treatment, and he was subsequently discharged from our hospital on the 21st hospital day. Although complication with ARF and high levels of serum HGF are both important factors predicting poor prognosis in acute liver failure patients, the present case achieved a favorable outcome. Endogenous HGF might play an important role as a regenerative effector in injured livers and kidneys.
Core tip: Renal involvement with hepatitis B and C is well described. However, the mechanism of hepatitis A-associated acute renal failure (ARF) is uncertain. Although the prognosis of hepatitis A is generally good, complication with ARF can have a negative impact. Hepatocyte growth factor (HGF) is a predictive factor for acute liver failure. Fulminant hepatic failure patients with high serum HGF have high mortality. By contrast, HGF is also an important factor accelerating tissue regeneration of injured organs, including the liver and kidneys. Here, we describe a patient with acute hepatitis A who achieved a favorable outcome despite complications with both ARF and high serum HGF.
- Citation: Oe S, Shibata M, Miyagawa K, Honma Y, Hiura M, Abe S, Harada M. Hepatitis A complicated with acute renal failure and high hepatocyte growth factor: A case report. World J Gastroenterol 2015; 21(32): 9671-9674
- URL: https://www.wjgnet.com/1007-9327/full/v21/i32/9671.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i32.9671
Acute hepatitis A is usually a mild to moderate illness, but in rare cases it can lead to severe complications, such as fulminant hepatitis, acute renal failure (ARF), blood dyscrasias, including hemolytic and aplastic anemia, and autoimmune hepatitis[1]. Although ARF can develop in more than 80% of patients with fulminant hepatitis with massive hepatic necrosis[2], the development of ARF is not a common complication of nonfulminant hepatitis A. Although the prognosis of hepatitis A is generally good, complication with ARF can have a negative impact.
Hepatocyte growth factor (HGF) is predictive factor of acute liver failure. Tsubouchi et al[3] reported that fulminant hepatic failure patients with high serum HGF showed high mortality. By contrast, HGF is also an important factor accelerating tissue regeneration of injured organs, including the liver and kidney[4].
Here, we describe a patient with acute hepatitis A who achieved a favorable outcome despite complications with both ARF and high serum HGF.
A 58-year-old man was admitted to our hospital in 2010 with fever, malaise, loss of appetite and jaundice for 3 d. Although he had consumed about 180 g/d of alcohol for 38 years, he had been in good health and had no history of abnormality in annual medical checkups, including urinalysis.
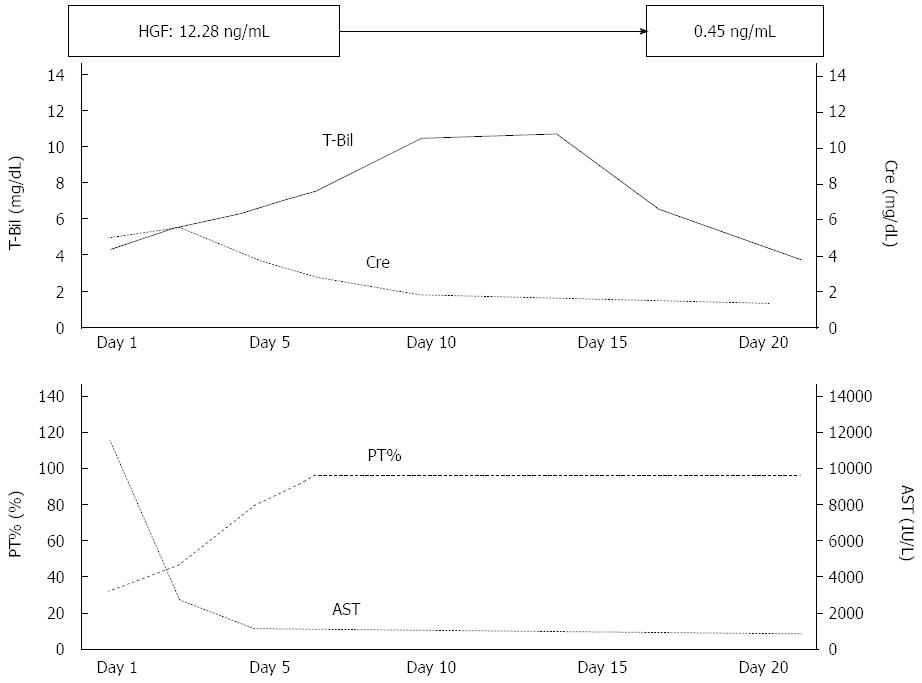
A physical examination showed icteric skin and hepatomegaly, but no signs of dehydration. Laboratory findings revealed severe liver injury and coagulopathy; white blood cells were 10200/μL [reference value (RV); 3100-9.1/μL)], red blood cells were 503 × 104/μL (RV; 4.27 × 104-5.58 × 104/μL), hemoglobin was 16.2 g/dL (RV; 13.5-17.2 g/dL), platelets were 98000/μL (RV; 157000-340000/μL), total protein was 5.8 g/dL (RV; 6.7-8.3 g/dL), albumin was 3.0 g/dL (RV; 4.0-5.0 g/dL), total bilirubin was 4.7 mg/dL (RV; 0.2-1.5 mg/dL), direct bilirubin was 3.9 mg/dL (RV; 0.1-0.4 mg/dL), aspartate aminotransferase was 12217 IU/L (RV; 13-33 IU/L), alanine aminotransferase was 5725 IU/L (RV; 8-42 IU/L), gamma glutamyltranspeptidase was 878 IU/L (RV; 10-47 IU/L), lactate dehydrogenase was 9536 IU/L (RV; 119-229 IU/L), blood urea nitrogen was 51 mg/dL (RV; 8-22 mg/dL), creatinine was 5.40 mg/dL (RV; 0.6-1.1 mg/dL), prothrombin time percentage was 28.2% (RV; more than 74%), and markers of hepatitis B virus, hepatitis C virus, Epstein-Barr virus and cytomegalovirus were negative. A chemiluminescent immunoassay showed that his serum immunoglobulin M anti-hepatitis A virus (HAV) antibody was strongly positive at 11.4 Index (RV; below 0.8 Index). The serum level of hepatocyte growth factor (HGF) was extremely high at 12.28 ng/mL (RV; below 0.4 ng/mL). Moreover, laboratory data showed renal dysfunction with abnormal urinalysis, such as macroproteineuria and many granular casts. Serum level of complement (C) 3 was 25 mg/dL (RV; 78-128 mg/dL), C4 was less than 5 mg/dL (RV; 12-31 mg/dL) and C1q-binding immune complex in sera was within normal limits. Hence, he was diagnosed with HAV-related acute liver failure complicated with ARF. His laboratory findings were very severe. His general condition and appetite were not good. However, both his general condition and laboratory data rapidly improved after supportive treatment, such as administration of proton pump inhibitors to prevent gastrointestinal bleeding and lactulose for enterotoxins, such as ammonia. On the 10th hospital day, ALT, prothrombin activity and creatinine had improved to 372 IU/L, 100% and 1.79 mg/dL, respectively (Figure 1). Proteinuria had also disappeared. On the 21st hospital day, HGF decreased to 0.45 ng/mL and the patient was subsequently discharged from our hospital. One month later, his liver and renal function test had improved to within normal limits.
ARF is a common complication in patients with fulminant hepatitis, but it is also found in 1.5%-4.7% of nonfulminant hepatitis A[5]. Since the first case of ARF with hepatitis A reported by Wilkinson et al[6] in 1978, about 50 cases of hepatitis A-associated ARF have been described in the literature[5,7]. In an analysis of 208 hepatitis A patients, 15 of 205 non-fulminant and all three fulminant patients were complicated with ARF[8]. Moreover, hepatitis A patients with ARF had higher ALT, peak total bilirubin and more prolonged prothrombin time compared with patients without ARF. Excessive alcohol consumption and diabetes were more prevalent in the ARF patients. In these patients, development of ARF might be related to the degree of acute hepatic injury, because both long-term alcohol abuse and diabetes are associated with renal dysfunction[9,10]. His history of heavy alcohol consumption might have contributed to ARF.
Renal involvement with hepatitis B and C is well described. These mechanisms are thought to be associated with immune complex deposition[11]. The deposition of immune complexes containing hepatitis B surface antigen to glomeruli is the key pathological process of diffuse membranous glomerulonephritis. Hepatitis C virus (HCV) infection is associated with cryoglobulinemia and membranoproliferative glomerulonephritis. Deposition of immune complexes involving HCV and anti-HCV IgG is also important in HCV-related renal disease. However, the mechanism of hepatitis A associated ARF is uncertain, and several possible mechanisms have been considered. First, because most hepatitis A patients complain of nausea, vomiting and diarrhea, circulatory insufficiency caused by dehydration may activate the renin-angiotensin system and may induce prerenal renal failure[5]. In our case, the patient had severe nausea, vomiting and poor oral intake. Second, immune complex-mediated nephritis is possible, because hepatitis A patients have been found to have several types of glomerular disorders, including membranous nephropathy, mesangial proliferative glomerulonephritis and membranoproliferative glomerulonephritis[12]. Although the present case also had proteinuria suggestive of glomerulonephritis, the level of C1q-binding immune complex in their sera was within normal limits. Third, endotoxemia induced by acute hepatitis may cause renal injury. Systemic hypotension, renal vasoconstriction, release of cytokines and activation of neutrophils, may all contribute to the development of renal injury from endotoxemia. Endotoxemia is often observed within 10 d of onset in patients with acute hepatitis A[13]. Although the present case was hospitalized at the fifth day after onset, serum endotoxin levels were within the normal range.
HGF is a Kringle-containing polypeptide growth factor originally identified as a potent mitogen for mature hepatocytes in primary culture[14]. HGF is produced and secreted as pro-HGF by stroma cells, such as fibroblasts, macrophages and renal mesangium, and predominantly acts on a variety of epithelial cells to regulate cell growth, cell motility and morphogenesis[15]. HGF is predictive factor of acute liver failure. Tsubouchi et al[3] reported that 14 of 15 fulminant hepatic failure patients with a maximum serum HGF over 10 ng/mL died. By contrast, in patients with acute liver failure, serum levels of both growth and growth-inhibitory factors are elevated[3,16]. It was reported that HGF and its receptor, the c-met system, was activated, but was hampered by elevated growth-inhibitory factors, such as TGF-β in patients with liver failure[17]. The reciprocal action of these factors is thought to be a result of impaired liver regeneration. The effectiveness of recombinant HGF as a therapeutic drug for acute liver failure has been reported[4]. Similarly, HGF is also effective for renal regeneration. HGF stimulates proliferation of renal epithelial cells and induces tubular formation in vitro[17]. HGF may function as a renotropic factor for regeneration with ARF[18]. Expression of HGF mRNA and HGF activity in the kidney increased in rat models with renal ischemia or HgCl2 administration. This phenomenon suggested the possibility that HGF is a renotropic factor for renal regeneration following acute renal injury. Hence, recombinant HGF has been administered clinically as therapeutic drug for ARF.
In conclusion, although the present case with severe acute liver failure because of hepatitis A complicated with ARF and high serum HGF level, the patient recovered rapidly without intensive treatment. Endogenous HGF may play an important role in recovery from injuries of liver and kidney.
A 58-year-old man with fever, malaise, loss of appetite and jaundice.
The patient had icteric skin and hepatomegaly, but no signs of dehydration.
Acute hepatitis B, acute hepatitis C, biliary tract disease.
Laboratory findings revealed severe liver injury, renal dysfunction and coagulopathy. The patient’s serum was positive for serum immunoglobulin M anti-hepatitis A virus antibody. Serum level of hepatocyte growth factor (HGF) was extremely high (12.28 ng/mL).
An abdominal computed tomography scan revealed an enlarged liver and normal kidneys.
The patient was treated with supportive therapy, such as administration of proton pump inhibitors and lactulose.
ARF has been found in relatively few cases of nonfulminant hepatitis A. About 50 cases of hepatitis A associated ARF have been described in the literature. HGF was a predictive factor of acute liver failure. Fulminant hepatic failure patients with high serum HGF had a high mortality.
The present case had severe acute liver failure caused by hepatitis A that was complicated with ARF and high serum HGF level. However, the patient recovered rapidly with supportive treatment. HGF might play an important role as a regenerative effector in injured livers and kidneys.
This article reports a rare case of severe hepatitis A complicated with ARF and high serum HGF level.
P- Reviewer: Sagnelli E, Trevisan A S- Editor: Yu J L- Editor: Stewart G E- Editor: Liu XM
| 1. | Cuthbert JA. Hepatitis A: old and new. Clin Microbiol Rev. 2001;14:38-58. [PubMed] |
| 2. | Wilkinson SP, Blendis LM, Williams R. Frequency and type of renal and electrolyte disorders in fulminant hepatic failure. Br Med J. 1974;1:186-189. [PubMed] |
| 3. | Tsubouchi H, Kawakami S, Hirono S, Miyazaki H, Kimoto M, Arima T, Sekiyama K, Yoshiba M, Arakaki N, Daikuhara Y. Prediction of outcome in fulminant hepatic failure by serum human hepatocyte growth factor. Lancet. 1992;340:307. [PubMed] |
| 4. | Ido A, Moriuchi A, Numata M, Murayama T, Teramukai S, Marusawa H, Yamaji N, Setoyama H, Kim ID, Chiba T. Safety and pharmacokinetics of recombinant human hepatocyte growth factor (rh-HGF) in patients with fulminant hepatitis: a phase I/II clinical trial, following preclinical studies to ensure safety. J Transl Med. 2011;9:55. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 50] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 5. | Lin CC, Chang CH, Lee SH, Chiang SS, Yang AH. Acute renal failure in non-fulminant hepatitis A. Nephrol Dial Transplant. 1996;11:2061-2066. [PubMed] |
| 6. | Wilkinson SP, Davies MH, Portmann B, Williams R. Renal failure in otherwise uncomplicated acute viral hepatitis. Br Med J. 1978;2:338-341. [PubMed] |
| 7. | Shroff GR, Badve SV, Joshi AG, Desai DC, Abraham P, Sirsat RA. Acute renal tubular injury with acute hepatitis A infection: is it just a coincidence? Nephrology (Carlton). 2004;9:44-46. [PubMed] |
| 8. | Jung YJ, Kim W, Jeong JB, Kim BG, Lee KL, Oh KH, Yoon JH, Lee HS, Kim YJ. Clinical features of acute renal failure associated with hepatitis A virus infection. J Viral Hepat. 2010;17:611-617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 9. | Cecchin E, De Marchi S. Alcohol misuse and renal damage. Addict Biol. 1996;1:7-17. [PubMed] |
| 10. | Weisberg LS, Kurnik PB, Kurnik BR. Risk of radiocontrast nephropathy in patients with and without diabetes mellitus. Kidney Int. 1994;45:259-265. [PubMed] |
| 11. | McCann UG, Rabito F, Shah M, Nolan CR, Lee M. Acute renal failure complicating nonfulminant hepatitis A. West J Med. 1996;165:308-310. [PubMed] |
| 12. | Morita M, Kitajima K, Yoshizawa H, Itoh Y, Iwakiri S, Shibata C, Mayumi M. Glomerulonephritis associated with arteritis in marmosets infected with hepatitis A virus. Br J Exp Pathol. 1981;62:103-113. [PubMed] |
| 13. | Nakano H. [Study on the cause of elevated total serum immunoglobulin M in patients with acute hepatitis A]. Nihon Shokakibyo Gakkai Zasshi. 1987;84:1755-1763. [PubMed] |
| 14. | Nakamura T, Nishizawa T, Hagiya M, Seki T, Shimonishi M, Sugimura A, Tashiro K, Shimizu S. Molecular cloning and expression of human hepatocyte growth factor. Nature. 1989;342:440-443. [PubMed] |
| 15. | Nakamura T. Structure and function of hepatocyte growth factor. Prog Growth Factor Res. 1991;3:67-85. [PubMed] |
| 16. | Eguchi S, Okudaira S, Azuma T, Ohno Y, Fujioka H, Furui J, Tanaka K, Kanematsu T. Changes in liver regenerative factors in a case of living-related liver transplantation. Clin Transplant. 1999;13:536-544. [PubMed] |
| 17. | Matsumoto K, Nakamura T. Hepatocyte growth factor: molecular structure, roles in liver regeneration, and other biological functions. Crit Rev Oncog. 1992;3:27-54. [PubMed] |
| 18. | Igawa T, Matsumoto K, Kanda S, Saito Y, Nakamura T. Hepatocyte growth factor may function as a renotropic factor for regeneration in rats with acute renal injury. Am J Physiol. 1993;265:F61-F69. [PubMed] |