Published online Aug 28, 2015. doi: 10.3748/wjg.v21.i32.9666
Peer-review started: December 12, 2015
First decision: January 8, 2015
Revised: February 20, 2015
Accepted: April 9, 2015
Article in press: April 9, 2015
Published online: August 28, 2015
Processing time: 259 Days and 23.1 Hours
Resectability of hepatocellular carcinoma in patients with chronic liver disease is dramatically limited by the need to preserve sufficient remnant liver in order to avoid postoperative liver insufficiency. Preoperative treatments aimed at downsizing the tumor and promoting hypertrophy of the future remnant liver may improve resectability and reduce operative morbidity. Here we report the case of a patient with a large hepatocellular carcinoma arising from chronic liver disease. Preoperative treatment, including tumor downsizing with transarterial radioembolization and induction of future remnant liver hypertrophy with right portal vein embolization, resulted in a 53% reduction in tumor volume and compensatory hypertrophy in the contralateral liver. The patient subsequently underwent extended right hepatectomy with no postoperative signs of liver decompensation. Pathological examination demonstrated a margin-free resection and major tumor response. This new therapeutic sequence, combining efficient tumor targeting and subsequent portal vein embolization, could improve the feasibility and safety of major liver resection for hepatocellular carcinoma in patients with liver injury.
Core tip: Surgical treatment of hepatocellular carcinoma in patients with chronic liver disease is challenging due to the contradictory need to perform a radical tumor resection while preserving a maximal amount of tumor-free remnant liver. Preoperative treatment may be indicated for tumor downsizing and to promote hypertrophy of the future remnant liver. We report the case of a cirrhotic patient undergoing extended right hepatectomy for a large hepatocellular carcinoma after transarterial radioembolization and right portal vein embolization. Our results suggest that this approach is feasible and safe and may represent a new therapeutic option before major hepatectomy in patients with liver injury.
- Citation: Bouazza F, Poncelet A, Garcia CA, Delatte P, Engelhom JL, Galdon MG, Deleporte A, Hendlisz A, Vanderlinden B, Flamen P, Donckier V. Radioembolisation and portal vein embolization before resection of large hepatocellular carcinoma. World J Gastroenterol 2015; 21(32): 9666-9670
- URL: https://www.wjgnet.com/1007-9327/full/v21/i32/9666.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i32.9666
Surgical resection or local destruction remain the only potentially curative options in patients with hepatocellular carcinoma (HCC) and chronic liver disease (CLD) who are not candidates for liver transplantation. However, in cases of liver injury, and particularly of cirrhosis, the need to preserve sufficient liver parenchyma to minimize the risk of postoperative hepatic insufficiency dramatically limits the options for radical liver resection. Accordingly, resection of HCC in patients with CLD is usually only recommended in patients with compensated cirrhosis and small uninodular tumors[1]. A recent report indicated that extension of this strict indication might be feasible in some cases involving large tumors, leading to satisfactory long-term results in selected patients[2]. In this context, the safety and efficacy of major liver resection for large HCC could be improved by preoperative treatments designed to downsize the tumor and allow safer parenchyma-sparing resection. In the tumor-free portion of the liver, preoperative treatments aimed at promoting compensatory hypertrophy of the anticipated future remnant liver (FRL) could reduce the risk for post-hepatectomy liver failure. To target the tumor, intra-arterial therapies are well-established treatments based on the fact that HCCs mainly rely on arterial vascularization. Transarterial chemoembolization (TACE) has been shown to be an efficient palliative treatment for unresectable HCC[3], but, in a preoperative setting, neoadjuvant TACE failed to demonstrate a clear oncological long-term benefit as compared with surgical resection alone[4]. Transarterial radioembolization (TARE), consisting of intra-arterial injection of microspheres labelled with yttrium-90 (90Y), has recently gained ground in the treatment of HCC with several reports suggesting the efficacy of TARE for induction of tumor necrosis[5]. In combination with surgery, preoperative TARE has been shown to be feasible[6] while no clear clinical benefit has been demonstrated yet. Portal vein embolization (PVE) is the standard technique used to promote liver hypertrophy in patients who are not initially amenable to surgery due to anticipated insufficient FRL. In cases involving major liver resection in patients with cirrhosis, preoperative PVE significantly reduces the risk of postoperative liver failure[7]. It has also been shown that sequential TACE and PVE may improve the safety of major surgical resection through synergistic effects that increase the rate of compensatory hypertrophy of the FRL[8]. A similar sequence, but including TARE instead of TACE, followed by PVE before surgical resection has not yet been reported but could potentially represent a new therapeutic option. We describe herein the case of a patient with a large HCC who underwent an extended right hepatectomy after TARE followed by PVE and discuss the potential advantages of this new approach.
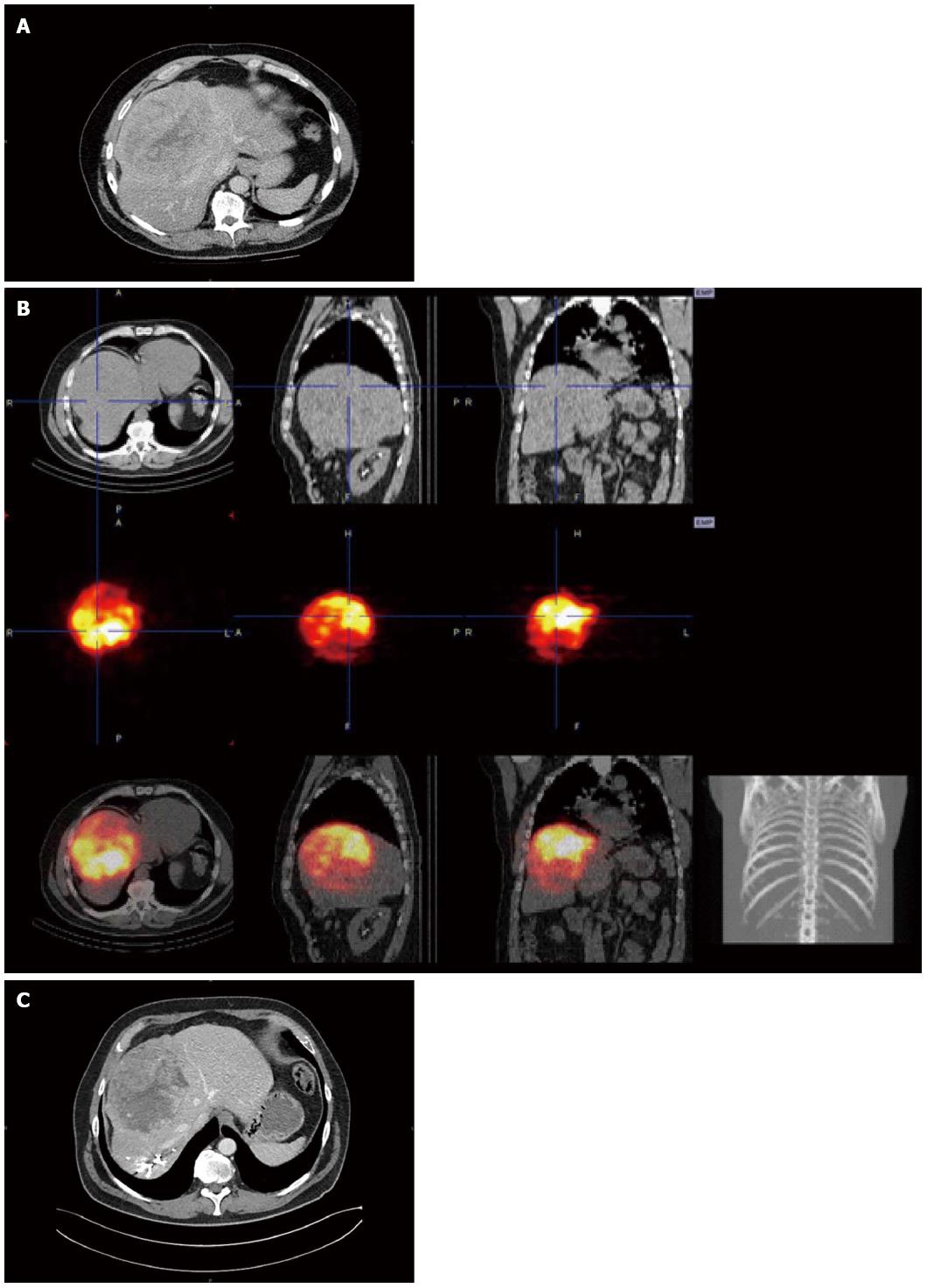
A 62 year-old man with a past medical history of mild to severe alcohol consumption presented with a liver tumor. Contrast-enhanced abdominal computed tomography (CT) scan demonstrated the presence of a 167 mm mass in segment VIII of the liver with HCC characteristics as indicated by arterial wash-in and portal phase wash-out (Figure 1A). This corresponded to a LI-RADS 5 lesion and a stage B tumor in the BCLC classification. Additionally, the presence of cirrhosis was suggested on the basis of the irregular aspect of the liver surface and the relative hypertrophy of segment I. Blood tests, including liver function and alpha-fetoprotein, were normal. Complete work-up did not demonstrate extra-hepatic tumor dissemination. Due to tumor size and location, a right hepatectomy extended to the middle hepatic vein was indicated. Analysis of liver volumes on CT scan showed a total liver volume (TLV) of 3100 mL, a tumor volume of 1548 mL, and an FRL (segments I, II, III, IV) of 470 mL, corresponding to an FRL/TLV ratio of 15%, an FRL/TLV tumor volume of 30%, and an FRL/body weight ratio of 0.48. Therefore, a 2-step preoperative strategy was proposed, consisting of tumor-targeted TARE followed by right PVE. After simulation of TARE with technetium-99 (99Tc) macroaggregated albumin showed no extra-hepatic deposition and excellent tumor targeting, 90Y hyperselective radioembolization of the segment VIII artery to the tumor was performed allowing the delivery of 97 mCi of 90Y microspheres (Figure 1B). No side effects related to this procedure were observed. A right PVE was performed 11 wk after TARE treatment, without complications. Preoperative CT scan at 6 wk after PVE, showed a tumor volume of 717 mL, a TLV of 2770 mL, and an FRL of 752 mL, corresponding to an FRL/TLV ratio of 27%, FRL/TLV tumor volume of 37%, and FRL/body weight ratio of 0.77 (Figure 1C). Accordingly, considering the fact that such compensatory hypertrophy indicated a preserved regenerative capacity of the future remnant liver, an extended right hepatectomy was judged to be feasible. Right hepatectomy, extended to the middle hepatic vein and partially to segment IV, was performed 6 wk after PVE. During surgery, no adhesions between tumor and diaphragm were found. No portal triad clamping or perioperative transfusions were required and intraoperative blood loss was 600 mL. The patient’s postoperative course was unremarkable, clinically and biologically. No signs of liver decompensation were observed (minimal values of prothrombin time, peak international normalized ratio, and total bilirubin were 64%, 1.2, and 1.2 mg/dL on postoperative days 1, 1, and 2, respectively) and the patient was discharged on day 9. Pathological examination of the specimen demonstrated a margin-free resection and a major tumor response, as indicated by approximately 30% of residual cancer cells, and confirmed the presence of cirrhosis in tumor-free liver. Twelve months after surgery the patient is disease free and has achieved full rehabilitation.
The dual pathology in patients with large HCC and CLD imposes contradictory therapeutic objectives, requiring a radical oncological resection on the one hand, and the preservation of a maximal amount of liver parenchyma on the other hand. In this context, preoperative treatments could improve the feasibility and safety of surgical resection by tumor downsizing and expansion of the FRL. In addition, neoadjuvant treatments can contribute to patient selection by allowing preoperative measurement of response to treatment, evaluation of the biological behavior of the tumor, and assessment of liver functional reserve as indicated by regenerative capacity. The case reported here indicates that a therapeutic sequence combining TARE and PVE before major liver resection could be feasible and may potentially represent a new strategy for treatment of large HCC in patients with CLD. The anti-tumor efficacy of TARE was demonstrated by significant tumor downsizing, as indicated by a 53% reduction of tumor volume 17 wk after TARE and by significant tumor necrosis at pathology. After TARE, PVE led to 37.5% compensatory hypertrophy in the FRL, allowing uneventful margin-free extended right hepatectomy. As compared with the combination of TACE and PVE that was proven feasible but not associated with oncological benefit, preoperative TARE and PVE could have several advantages. From a safety point of view, it can be postulated that TARE before PVE could be less harmful to the liver than TACE, as particles used for radioembolization are smaller than those used for chemoembolization, allowing a more distal and selective distribution. Furthermore, the safety of sequential TARE and PVE is suggested by the feasibility of TARE in patients with portal vein thrombosis[5], while this condition contraindicates TACE. In addition, as compared with a strategy using preoperative PVE only, this sequence in which TARE precedes PVE could potentially limit the risk of tumor growth while waiting for liver regeneration. The capacity of TARE to have a synergistic effect on PVE-induced FRL hypertrophy is hypothetical but, interestingly in this context, TARE could also induce atrophy of the irradiated-parenchyma and promote compensatory regeneration of the non-irradiated segments[5]. The potential synergy of this effect with liver hypertrophy promoted by PVE and its influence on the safety of subsequent surgery remains to be investigated but may offer new therapeutic perspectives. Of note, in contrast with previous reports[6], preoperative TARE in the present case did not create post-irradiation adhesions which may complicate surgery. Aside from its feasibility and safety, the oncological benefit of preoperative TARE remains hypothetical. Yet, in this setting, recent studies have suggested an advantage of TARE over TACE in terms of efficacy for treatment of HCC, as indicated by increased tumor downstaging and prolonged time to progression[9]. In conclusion, the combination of TARE followed by PVE could represent a new option in patients with CLD and large HCC that was not initially considered to be resectable due to insufficient RLV. Due to the unique capacity of TARE to induce tumor necrosis and contralateral hypertrophy of the non-embolized liver at the same time, this sequence may offer new therapeutic perspectives and requires further investigation.
A sixty year old male patient presented with a large liver mass in the right lobe.
Due to the presence of alcohol-related chronic liver disease, a diagnosis of hepatocellular carcinoma was suspected.
Differential diagnoses included other solid liver tumors, primary or secondary.
Laboratory data, including alpha-fetoprotein, were not contributive.
Contrast-enhanced computed tomography scan revealed characteristic hepatocellular features such as arterial wash-in and portal phase wash-out.
On the operative specimen, pathology confirmed the diagnosis of hepatocellular carcinoma and a major response to preoperative radioembolization as indicated by tumor necrosis greater than 50%.
Extended right hepatectomy was preceded by transarterial radioembolisation to induce tumor downsizing and portal vein embolization to promote future remnant liver hypertrophy.
In such cases of large hepatocellular carcinoma arising from diseased liver, the resectability is dramatically limited by the need to preserve sufficient tumor-free liver to avoid postoperative liver insufficiency.
This case indicates that new a preoperative strategy, combining radioembolization and portal vein embolization, could be feasible and safe and allow major resection for hepatocellular carcinoma in patients with fibrotic liver.
The feasibility and safety of this approach should be confirmed in further series, while the oncological benefit remains to be determined.
P- Reviewer: Cheung TT, Kim HJ, Schey R S- Editor: Ma YJ L- Editor: A E- Editor: Zhang DN
| 1. | Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020-1022. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5972] [Cited by in RCA: 6562] [Article Influence: 468.7] [Reference Citation Analysis (1)] |
| 2. | Torzilli G, Belghiti J, Kokudo N, Takayama T, Capussotti L, Nuzzo G, Vauthey JN, Choti MA, De Santibanes E, Donadon M. A snapshot of the effective indications and results of surgery for hepatocellular carcinoma in tertiary referral centers: is it adherent to the EASL/AASLD recommendations?: an observational study of the HCC East-West study group. Ann Surg. 2013;257:929-937. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 333] [Cited by in RCA: 415] [Article Influence: 34.6] [Reference Citation Analysis (0)] |
| 3. | Llovet JM, Real MI, Montaña X, Planas R, Coll S, Aponte J, Ayuso C, Sala M, Muchart J, Solà R. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial. Lancet. 2002;359:1734-1739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2502] [Cited by in RCA: 2608] [Article Influence: 113.4] [Reference Citation Analysis (0)] |
| 4. | Lau WY, Yu SC, Lai EC, Leung TW. Transarterial chemoembolization for hepatocellular carcinoma. J Am Coll Surg. 2006;202:155-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 57] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 5. | Salem R, Mazzaferro V, Sangro B. Yttrium 90 radioembolization for the treatment of hepatocellular carcinoma: biological lessons, current challenges, and clinical perspectives. Hepatology. 2013;58:2188-2197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 141] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 6. | Vouche M, Lewandowski RJ, Atassi R, Memon K, Gates VL, Ryu RK, Gaba RC, Mulcahy MF, Baker T, Sato K. Radiation lobectomy: time-dependent analysis of future liver remnant volume in unresectable liver cancer as a bridge to resection. J Hepatol. 2013;59:1029-1036. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 217] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 7. | Azoulay D, Castaing D, Krissat J, Smail A, Hargreaves GM, Lemoine A, Emile JF, Bismuth H. Percutaneous portal vein embolization increases the feasibility and safety of major liver resection for hepatocellular carcinoma in injured liver. Ann Surg. 2000;232:665-672. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 291] [Cited by in RCA: 283] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 8. | Yoo H, Kim JH, Ko GY, Kim KW, Gwon DI, Lee SG, Hwang S. Sequential transcatheter arterial chemoembolization and portal vein embolization versus portal vein embolization only before major hepatectomy for patients with hepatocellular carcinoma. Ann Surg Oncol. 2011;18:1251-1257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 94] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 9. | Lewandowski RJ, Kulik LM, Riaz A, Senthilnathan S, Mulcahy MF, Ryu RK, Ibrahim SM, Sato KT, Baker T, Miller FH. A comparative analysis of transarterial downstaging for hepatocellular carcinoma: chemoembolization versus radioembolization. Am J Transplant. 2009;9:1920-1928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 408] [Cited by in RCA: 434] [Article Influence: 27.1] [Reference Citation Analysis (0)] |