Published online Aug 28, 2015. doi: 10.3748/wjg.v21.i32.9638
Peer-review started: March 15, 2015
First decision: April 13, 2015
Revised: May 10, 2015
Accepted: June 9, 2015
Article in press: June 10, 2015
Published online: August 28, 2015
Processing time: 166 Days and 6.6 Hours
AIM: To summarize our single-center experience with liver transplantation (LT) for biliary atresia (BA).
METHODS: From October 2006 to December 2012, 188 children with BA were analyzed retrospectively. The stage I group (from October 2006 to December 2010) comprised the first 74 patients, and the stage II group (from January 2011 to December 2012) comprised the remaining 114 patients. Finally, 123 liver transplants were performed in 122 (64.9%) patients, whereas 66 patients did not undergo LT due to denial by their parents or lack of suitable liver grafts. The selection of graft types depended on the patients’ clinical status and whether a suitable living donor was available. The characteristics of patients in stages I and II were described, and the surgical outcomes of LT recipients were compared between the two stages. The Kaplan-Meier method was used to estimate the cumulative patient and graft survival rates, and the equality of survival distributions was evaluated using the log-rank test.
RESULTS: The 188 children consisted of 102 boys and 86 girls. Their ages ranged from 3 to 144 mo with a median of 8 mo. One hundred and fifteen (61.2%) patients were born in rural areas. Comparing stage I and stage II patients, the proportion of patients referred by pediatricians (43.2% vs 71.1%, respectively; P < 0.001) and the proportion of patients who previously received a Kasai procedure (KP) (32.4% vs 44.7%, respectively; P = 0.092) obviously increased, and significantly more parents were willing to treat their children with LT (73% vs 86%, respectively; P = 0.027). Grafts from living donors (102/122, 83.6%) were the most commonly used graft type. Surgical complications (16/25, 64.0%) were the main reason for posttransplant mortality. Among the living donor liver transplantation recipients (n = 102), the incidence of surgical complications was significantly reduced (34.1% vs 15.5%, respectively; P = 0.029) and survival rates of patients and grafts were greatly improved (81.8% vs 89.7%, respectively, at 1 year; 75.0% vs 87.8%, respectively, at 3 years; P = 0.107) from stage I to stage II.
CONCLUSION: The status of surgical treatments for BA has been changing in mainland China. Favorable midterm outcomes after LT were achieved as centers gained greater technical experience.
Core tip: Biliary atresia (BA) accounts for at least 50% of the liver transplants performed in pediatric patients. However, in mainland China, various social, cultural and financial factors are responsible for a low diagnostic rate or a delayed Kasai procedure for children with BA. Pediatric liver transplantation has been progressing immensely in mainland China. In this study, we analyzed our single-center data of children with BA between 2006 and 2012, representing the largest series of BA patients in mainland China ever reported. Based on these data, socioeconomic backgrounds that impact the current status of surgical treatments for BA in mainland China were introduced.
- Citation: Li QG, Wan P, Zhang JJ, Chen QM, Chen XS, Han LZ, Xia Q. Liver transplantation for biliary atresia: A single-center study from mainland China. World J Gastroenterol 2015; 21(32): 9638-9647
- URL: https://www.wjgnet.com/1007-9327/full/v21/i32/9638.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i32.9638
Biliary atresia (BA) is the most common cause of chronic cholestasis in infants, accounting for at least 50% of the liver transplants performed in pediatric patients. BA occurs in approximately 1:5000 to 1:19000 live births[1-6]. In mainland China, large-scale epidemiological data are still not available; however, a huge population of children with BA can be presumed based on the 20 million newborns per year. Although the study by Alexopoulos et al[7] suggested that patients who underwent salvage liver transplantation (LT) following a Kasai procedure (KP) might have a lower survival rate than those who directly proceeded to a transplant, the sequential surgical treatment with a KP followed by LT is currently accepted to be a conventional treatment strategy for most cases[8,9]. However, in mainland China, various social, cultural and financial factors are responsible for a low diagnostic rate or a delayed KP for children with BA. Thus, many pediatric BA patients without a prior KP or with a failed KP are potential LT candidates in our country.
In recent years, pediatric LT has been progressing immensely in mainland China. However, to our knowledge, there are very few English language literature sources from mainland China concerning the diagnosis and surgical treatment of BA. It is essential to introduce these real things happening to this population and to help other peers understand these backgrounds and trends in China. Ren Ji Hospital (Shanghai) has been a pioneer for pediatric LT in mainland China since the first liver transplant for infantile patients was performed in October 2006 with the assistance of Professor Chao-Long Chen from Chang Gung Memorial Hospital (Taiwan). Here, we will retrospectively analyze our single-center data of children with BA between 2006 and 2012 and introduce the overall profile of surgical treatments for BA in mainland China.
All pediatric transplant candidates at the Department of Liver Surgery, Ren Ji Hospital (Shanghai) have been enrolled in our prospective database under the supervision of a full-time coordinator. The data of those who underwent LT were synchronized with the China Liver Transplant Registry (CLTR) online (http://www.cltr.org/), and those who did not receive a transplant were kept in our database. The clinical characteristics and surgical data of the patients were retrospectively reviewed from the database. From October 2006 to December 2012, 220 children with BA visited our clinic. Thirty-two cases were considered ineligible due to transfers to other centers or incomplete data. The remaining 188 cases were included in the analysis of this study, most of whom were confirmed with BA through liver biopsy or intraoperative cholangiography. Stage I (from October 2006 to December 2010) comprised the first 74 patients, while stage II (from January 2011 to December 2012) comprised the remaining 114 patients. The following patient information was described according to the different transplant stages: demographic characteristics, geographical location, place of birth (urban or rural), hospital class for the initial diagnosis, previous surgical history, et al. Finally, 123 liver transplants were performed in 122 (64.9%) patients, and the patient characteristics and follow-up results were analyzed. Moreover, the annual caseloads were calculated to describe the time trends of transplants from 2006 to 2012.
Patients received a series of laboratory and imaging tests for the assessment of the clinical status, and pediatric end-stage liver disease (PELD) scores and Child-Pugh scores were calculated to measure the illness severity. Selection of graft types depended on the patients’ clinical status and whether a suitable living donor was available. The suitable age for a living donor ranged from 18 to 55 years. The quality, volume and anatomy of the donor liver were carefully evaluated through computed tomography (CT) angiography, and the liver-to-spleen ratio of CT values was used to evaluate the fatty degeneration degree of the liver. If both the donor and recipient were considered to be in suitable conditions for living donor liver transplantation (LDLT), an ethical review would be arranged.
All of the surgical procedures were performed by specialists with experience in the pediatric LT technique at the Department of Liver Surgery, Ren Ji Hospital. For LDLT, intraoperative real-time cholangiography was indispensable and the cut-ultrasound aspiration was used in the donor operation, and then the graft was implanted into the recipient’s abdominal cavity using the piggyback technique. The ex situ splitting technique was used for split liver transplantation (SLT), and classic orthotopic LT was performed for whole liver recipients. All patients underwent Roux-en-Y hepaticojejunostomy for bile duct reconstruction. Intraoperative color Doppler ultrasonography was performed to measure the blood flow velocity and pattern after the vascular anastomosis and abdominal wall closure. The severity of postoperative complications of the living donors was evaluated using the modified Clavien-Dindo classification[10]. Organ donation or transplantation in the study was strictly implemented under the regulation of the Shanghai Organ Transplant Committee and Declaration of Helsinki. All of the living organs were donated with informed consent. Deceased donors involved in the study were obtained from brain-dead or non-heartbeating donors. The postoperative immunosuppression regimen was described previously[11]. Briefly, the initial immunosuppressive therapy after LT consisted of a dual drug regimen of tacrolimus (0.15 mg/kg per day)/cyclosporine (8 mg/kg per day) combined with methylprednisolone (4 mg/kg per day). The target trough level for tacrolimus was 8-12 ng/mL during the first 30 d. The target C0 and C2 levels for cyclosporine were 150-200 ng/mL and 800-1200 ng/mL, respectively. The dose of methylprednisolone was gradually tapered by 4 mg per day and maintained with prednisone 2.5 mg daily taken orally. Prednisone was withdrawn within 3-6 mo after LT. Additional mycophenolate mofetil was used when necessary.
After discharge of the initial hospital stay, the patients were regularly followed in the clinic weekly during the first 3 mo after LT, biweekly from the 4th to the 6th mo, monthly from the 7th to the 12th mo, and every 3 mo thereafter. The following tests were performed at each follow-up visit: measurements of the height and body weight, serum liver and renal function tests, serological viral tests (cytomegalovirus and Epstein-Barr virus) and measurements of serum levels of tacrolimus/cyclosporine. Abdominal sonography was performed every 3 mo during the first 2 years and annually thereafter. Serum tests for hepatitis B virus were performed for patients who received a hepatitis B core antibody (anti-HBc)-positive graft[11]. Because children came from various parts of the country, some children had these tests performed at the local hospitals; however, the reports were regularly sent to us by facsimile or electronic mail, and any medication adjustments were made after a consultation with physicians at our department. The duration of survival was calculated from the time of LT until death or the last follow-up contact, and the cut-off date of follow-up was April 30, 2014. The follow-up period ranged from 0.1 to 90.3 mo, with a median of 28.6 mo.
Statistical analyses were performed using SPSS 18.0 for Windows (SPSS Inc. Chicago, IL, United States). Categorical data are expressed as numbers with percentages, and continuous data are expressed as medians with a range. The survival rates of patients and grafts were plotted using Kaplan-Meier curves, and the differences in selected factors were evaluated using the log-rank test. P-values less than 0.05 were considered to indicate statistical significance.
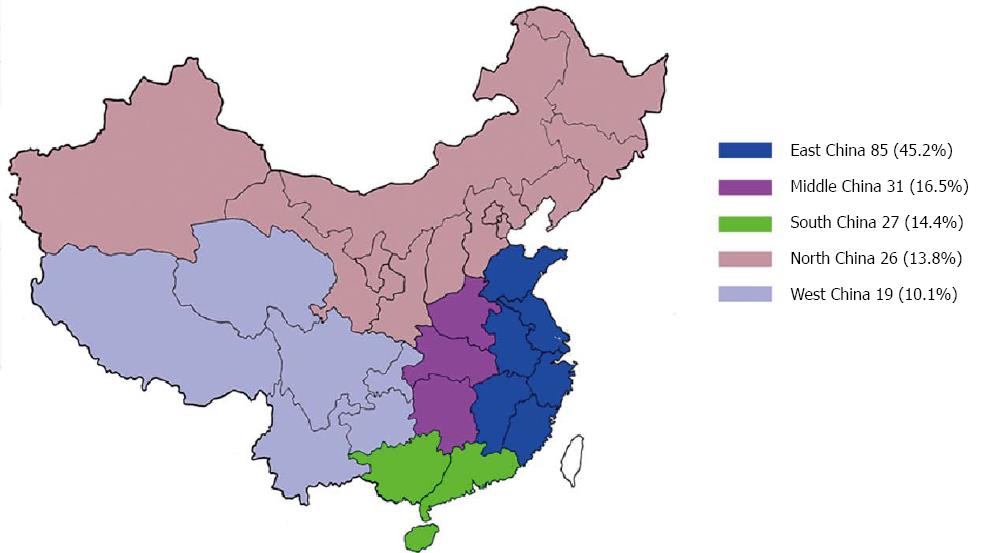
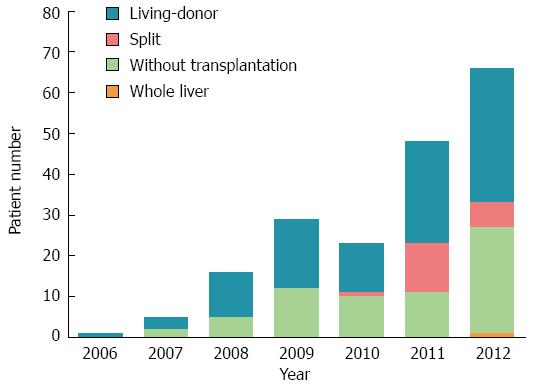
The 188 BA patients comprised 102 boys and 86 girls, with a median age of 8 mo (range: 3 to 144 mo) at the initial consultation at our hospital. Patients were distributed throughout the mainland, and most of them (45.2%) came from provinces close to Shanghai (East China) (Figure 1). One hundred and fifteen (61.2%) patients were born in rural areas, and 73 (38.8%) patients came from urban areas. The caseload of LT for BA showed a trend of an annual increase. During the last two years, the rapid growth of case numbers and the increase in donation types were obvious (Figure 2). The first SLT and whole liver transplantation (WLT) for BA patients were performed in December 2010 and January 2012, respectively. In this cohort, grafts from living donors were the only graft type used before 2010, and LDLT accounted for 93.2% (12 cases), 67.6% (25 cases), and 82.5% (33 cases) of liver transplants for BA in 2010, 2011, and 2012, respectively.
One hundred and thirty-eight (73.4%) patients were initially seen at a class I or II hospital, and 34.8% (48/138) of them were transferred to class III hospitals to receive KP; on the other hand, 50 (26.6%) patients were initially treated at a class III hospital, with 54.0% (27/50) of them directly proceeding to a KP. Thus, a total of 75 (39.9%) patients in this study had a history of a prior KP, and patients who initially visited a class III hospital showed a significantly higher proportion undergoing KP than those initially seen at a class I or II hospital (P = 0.017). Comparing patients from rural areas with those from urban areas, urban patients were more inclined to directly go to a class III hospital for medical support (13.9% vs 46.6%, respectively; P < 0.001), and KP was more frequently performed in urban children than in rural children (43.8% vs 37.4%, respectively; P = 0.379). Additionally, rural parents were more likely to refuse LT treatment than urban parents (62.8% vs 39.1%, respectively; P = 0.066).
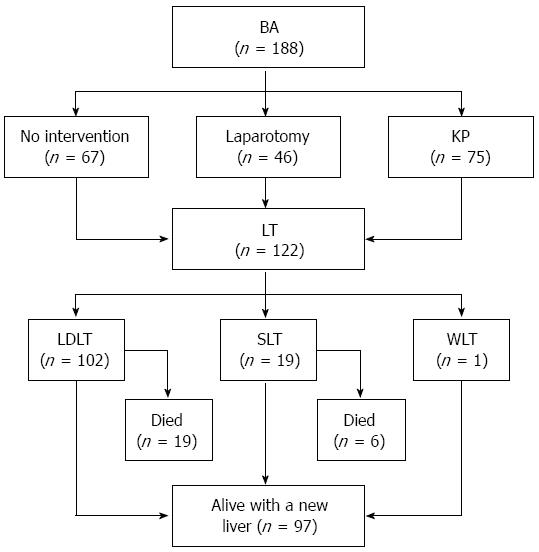
The changes and trends of the patient characteristics between stages I and II are summarized in Table 1. In stage II, the proportion of BA patients referred by pediatricians was significantly higher than that in stage I (71.1% vs 43.2%, respectively; P < 0.001). Parents who refused LT treatment for their children decreased significantly from stage I to stage II (27.0% vs 14.0%, respectively; P = 0.027), and the lack of organ sources became the predominant cause for pretransplant deaths of patients in stage II. Furthermore, patients who had a prior history of KP showed a slightly higher proportion in stage II than in stage I (44.7% vs 32.4%, respectively; P = 0.092). Figure 3 shows the flowchart providing outcomes of the 188 BA patients.
| Variable | Stage I (n = 74) | Stage II (n = 114) | P-value |
| Gender | 0.146 | ||
| Boys | 45 (60.8) | 57 (50.0) | |
| Girls | 29 (39.2) | 57 (50.0) | |
| Age | 0.315 | ||
| ≤ 12 mo | 53 (71.6) | 89 (78.1) | |
| > 12 mo | 21 (28.4) | 25 (21.9) | |
| Place of birth | 0.698 | ||
| Rural | 44 (59.5) | 71 (62.3) | |
| Urban | 30 (40.5) | 43 (37.7) | |
| Hospital class for initial treatments | 0.072 | ||
| I or II | 49 (66.2) | 89 (78.1) | |
| III | 25 (33.8) | 25 (21.9) | |
| Previous surgical intervention | |||
| None | 28 (37.9) | 39 (34.2) | 0.612 |
| Laparotomy | 22 (29.7) | 24 (21.1) | 0.176 |
| KP | 24 (32.4) | 51 (44.7) | 0.092 |
| Referral for transplantation | < 0.001 | ||
| Referred | 32 (43.2) | 81 (71.1) | |
| Non-referred | 42 (56.8) | 33 (28.9) | |
| Reason for no transplantation | |||
| Refusal by the parents | 20 (27.0) | 16 (14.0) | 0.027 |
| Lack of a suitable graft | 9 (12.2) | 21 (18.4) | 0.252 |
A total of 122 patients finally underwent LT, including 102 (83.6%) LDLT recipients, 19 (15.6%) SLT recipients and 1 (0.8%) WLT recipient. The median age when the transplant occurred was 9.4 mo (range: 4.5 to 118.4 mo). Seventy-four (60.7%) recipients did not undergo KP before LT, and their median age at the consultation at our hospital was 8.2 mo (range: 4.1 to 31.0 mo). For the 48 recipients with a prior KP (39.3%), the median age at KP was 73.5 d (range: 27 to 845 d), and only 13 (10.7%) patients underwent KP within 60 d of age. The median ages at LT for patients with a KP before 60 d (n = 13), patients with a KP after 60 d (n = 35) and patients without a prior KP (n = 74) were 20.4 mo (range: 6.3 to 118.4 mo), 14.2 mo (range: 5.7 to 82.0 mo), and 8.5 mo (range: 4.5 to 31.1 mo), respectively (P < 0.001).
The baseline characteristics of the recipients and grafts are shown in Table 2. In the SLT group, 17 infants shared 17 whole livers with adult recipients, and a 920-g whole liver was shared by a 6-year-old girl and a 2-year-old boy. The characteristics and outcomes of the 102 living donors are summarized in Table 3. No deaths or major complications occurred in the living donors after surgery, but 4 (3.9%) donors experienced minor complications, including wound infections and pulmonary infections.
| Variable | Living-donor (n = 102) | Split (n = 19) | Whole liver(n = 1) |
| Gender | |||
| Boys | 57 (55.9) | 7 (36.8) | 1 |
| Girls | 45 (44.1) | 12 (63.2) | 0 |
| Age (mo) | 9.3 (4.5-70.1) | 10.5 (5.6-118.4) | 7.8 |
| Body weight (kg) | 8.0 (5-19) | 8.0 (6-28) | 10.0 |
| Height (cm) | 67 (56-108) | 67 (62-115) | 70 |
| PELD score | 17 (-9-36) | 16 (-7-36) | 21 |
| Surgical history | |||
| KP | 42 (41.2) | 6 (31.6) | 0 |
| Laparotomy | 29 (28.4) | 6 (31.6) | 0 |
| ABO blood group | |||
| A | 34 (33.3) | 4 (21.1) | 0 |
| B | 27 (26.5) | 4 (21.1) | 0 |
| AB | 11 (10.8) | 5 (26.3) | 1 |
| O | 30 (29.4) | 6 (31.5) | 0 |
| Graft type | |||
| Whole liver | 0 | 0 | 1 |
| LLS | 99 | 17 | 0 |
| Left lobe without MHV | 2 | 1 | 0 |
| Left lobe with MHV | 1 | 0 | 0 |
| Extended right lobe | 0 | 1 | 0 |
| GRWR (%) | 2.7 (1.5-5.4) | 2.7 (1.1-4.2) | 4.0 |
| Variable | |
| Age (yr) | 30 (20-56) |
| Gender | |
| Male | 43 (42.2) |
| Female | 59 (57.8) |
| BMI (kg/m2) | 21.4 (16.9-27.5) |
| D/R ABO compatibility | |
| Identical | 78 (76.5) |
| Compatible | 24 (23.5) |
| D/R relationship | |
| Parent | 94 (92.1) |
| Grandparent | 6 (5.9) |
| Uncle/Aunt | 2 (2.0) |
| Graft weight (g) | 247.5 (145-420) |
| Postoperative hospital stay (d) | 7 (4-19) |
| Postoperative complications | |
| Wound infection (grade I) | 2 |
| Pulmonary infection (grade II) | 2 |
The management and outcomes of the posttransplant complications are listed in Table 4. By the last follow-up contact, 12 (26.7%) stage I recipients and 13 (16.9%) stage II recipients died of postoperative complications, and 1 stage I patient underwent retransplantation 61 mo after the primary LT due to a severe biliary complication. Among them, 16 (16/25, 64.0%) deaths were caused by surgical complications. Therefore, surgical complications were the main reason for posttransplant mortality, but the incidence of surgical complications was greatly reduced with greater technical experience. In this cohort, 15 (33.3%) stage I patients and 14 (18.2%) stage II patients experienced one or more surgical complications (P = 0.058). Furthermore, the incidence of surgical complications was significantly decreased from 34.1% (15/44) in stage I to 15.5% (9/58) in stage II within the LDLT group (n = 102; P = 0.029). Regarding non-surgical complications, stage II recipients also had greatly improved outcomes compared with stage I recipients.
| Complications | Patient number1 | Managements | Outcomes2 | |
| Stage I (n = 45) | Stage II (n = 77) | |||
| Surgical complications | ||||
| HAT | 4 | 2 | DSA (3 pts); thrombectomy and reconstruction (6 pts) | 3 pts died |
| PVT | 5 | 5 | Reoperation (5 pts); metal stent placement (1 pt) | 7 pts died |
| Biliary leakage | 3 | 2 | Drainage or reoperation | 3 pts died |
| Biliary stricture | 2 | 1 | PTCD (1 pt) | 2 pts died |
| Biliary sludge | 1 | 0 | Retransplantation | Alive |
| Wound dehiscence | 3 | 1 | Debridement and re-closure | 2 pts died |
| Digestive tract perforation | 2 | 2 | Reoperation | 4 pts died |
| Intra-abdominal bleeding | 0 | 1 | Reoperation | Alive |
| Wound infection | 1 | 0 | Regular wound dressing | 1 pt died |
| Small-for-size syndrome | 0 | 1 | - | 1 pt died |
| Large-for-size syndrome | 0 | 1 | - | 1 pt died |
| Non-surgical complications | ||||
| Pulmonary infection | 14 | 16 | Antibiotics (30 pts); mechanical ventilation (5 pts) | 11 pts died |
| CMV infection | 17 | 13 | Antivirus therapy | 7 pts died |
| EBV infection | 3 | 10 | Antivirus therapy | 1 pt died |
| De novo HBV infection | 7 | 6 | Antivirus therapy | 2 pts died |
| Drug-induced liver injury | 0 | 1 | Withdrawal of the drug | 1 pt died |
| Tuberculous pleurisy | 1 | 0 | Anti-tuberculosis | Alive |
| Acute rejection | 15 | 13 | Increased the dosage of the immunosuppressant; bolus doses of steroids | 5 pts died |
| PTLD | 0 | 1 | - | 1 pt died |
| Hirsutism | 7 | 0 | Replacement of cyclosporine with tacrolimus | 1 pt died |
| Intravascular hemolysis | 1 | 1 | Steroid therapy; withdrawal of blood transfusion | 1 pt died |
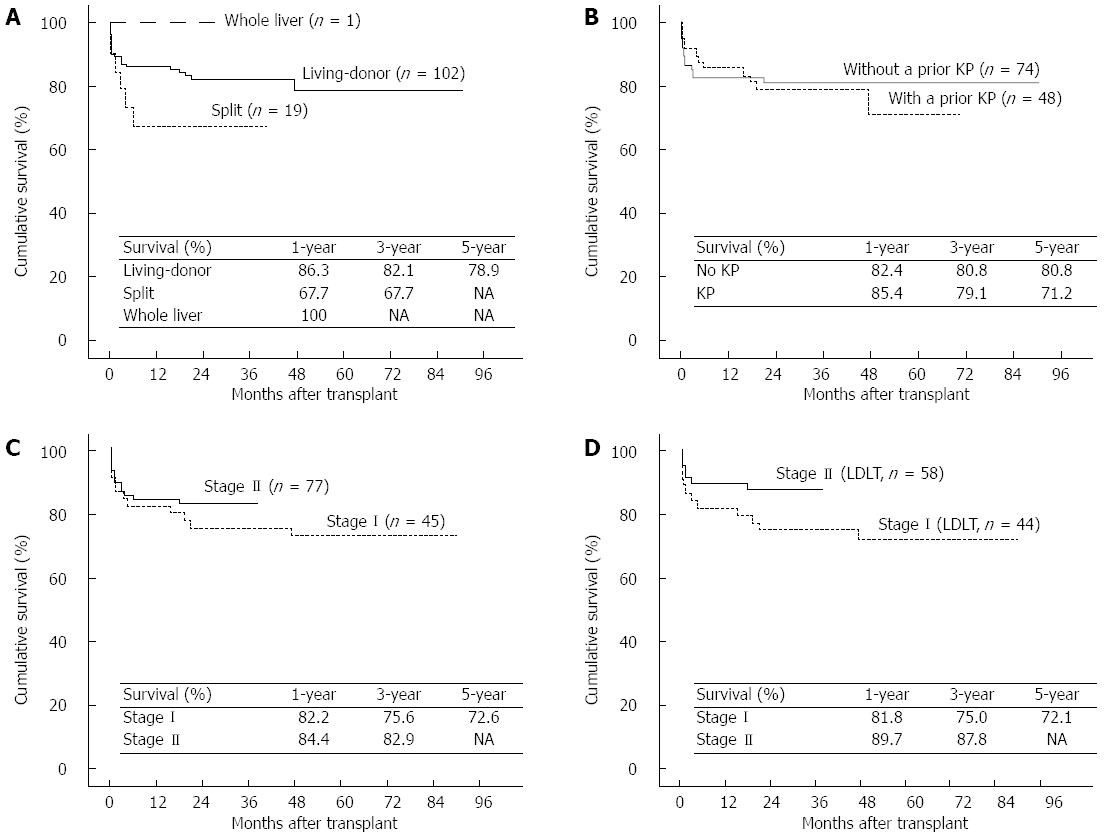
The 1-, 3-, and 5-year patient and graft survival rates of the 122 LT recipients were 83.6%, 80.0%, and 76.9%, respectively. Although the difference between the survival rates after LDLT and SLT did not reach statistical significance (P = 0.133), LDLT conferred a 14.4% survival benefit in the 3-year survival rate compared with SLT (82.1% vs 67.7%); thus, the LDLT recipients were expected to achieve a more favorable prognosis than those who underwent SLT (Figure 4A). The survival rates of patients who proceeded directly to LT (n = 74) were comparable to those with a prior KP (n = 48) (Figure 4B; 82.4% vs 85.4% at 1 year, respectively; 80.8% vs 71.2% at 5 years, respectively; P = 0.701). Because most cases of SLT (18 of 19) were in stage II, the survival benefit from stage II was impaired (Figure 4C; P = 0.358). However, the patient and graft survival rates after LDLT were greatly improved from stage I to stage II because our center gained greater experience with LDLT (Figure 4D; 81.8% vs 89.7% at 1 year, respectively; 75.0% vs 87.8% at 3 years, respectively; P = 0.107).
China is a vast country consisting of 28 provinces and 4 municipalities. There are large discrepancies in the socioeconomic development between coastal areas in the east and inland areas in the west. Presently, more than half of the Chinese population live in rural areas[12]. On the other hand, pediatric congenital diseases occur much more commonly in rural populations because neonatal screening for congenital diseases is not conducted in most rural areas[13-15]. Medical services provided by the hospitals in different areas are unequal, and well-equipped healthcare facilities are usually not available in rural areas. Moreover, the Chinese household registration system (known as “huji”) officially identifies a person as a resident of a certain area, and residents from different areas are enrolled in different medical insurance coverage, which depends on the financial condition of the local area. For most rural families, the parents are financially responsible for their children’s medical expenses. When facing a high medical expense, very few of these parents can afford the treatment cost in the hospital. As a result, a large proportion of children with BA could not get timely diagnoses and surgical interventions when necessary medical care is required. In mainland China, at least 1500 new cases of BA occur every year as calculated by the recognized incidence of BA. However, the recognition rate of BA is less than 50%, and most children with BA in mainland China die without any surgical interventions. These factors have led to a low rate of BA diagnoses, a low rate of the KP performance and a low rate of post-KP jaundice clearance.
Currently, Kasai’s portoenterostomy has gained worldwide acceptance as the initial surgical therapy for BA infants[16]. However, it was reported that only 17% of BA patients who were treated with KP could achieve long-term transplant-free survival, and even these patients require assiduous lifelong care[17]. Therefore, KP is considered a transitional treatment for BA before LT because the transplant operation is not well-tolerated for most infants aged less than 6 mo. Data from the Netherlands Study Group of Biliary Atresia and Registry (NeSBAR) indicated that KP should be performed before 60 d of age to obtain an acceptable transplant-free survival[18], and a late referral for KP was associated with poor outcomes. However, in mainland China, specialized children’s hospitals that are qualified to perform KP are available in only several well-developed cities such as Beijing, Shanghai, Guangzhou, Hangzhou, and Chongqing. Delayed referral for a KP produced a phenomenon that BA patients in mainland China had their transplantations fairly early. Our data showed that only 10.7% of children with BA were treated with KP before 60 d of age and that 60.7% of children had their liver functions irreversibly deteriorated and lost the chance to receive a KP before LT.
In developed countries and regions, the pretransplant conditions of BA patients are completely different, and more than 80% of LT recipients had a prior KP before LT. Nonetheless, the patient age at KP is considered the key determinant for the post-KP patient survival with their native liver[9,19-21]. In Taiwan, a universal stool color screening system was established for the neonatal population since 2004, which has greatly reduced the proportion of late referrals for infants with BA[22-24]. The success rate of KP could be improved by enhancing the early referral, and better postoperative outcomes of children with BA could be obtained by the timely performance of KP[25]. In the United Kingdom, surgical outcomes have been improved by the centralization of care to supra-regional centers[26]. Moreover, a French study reported that the caseload experience of KP influenced the patient prognosis with centers managing more than 20 cases per year associated with better outcomes[27].
In mainland China, the development of pediatric LT has lagged behind that of adult LT during the past two decades[28]. Pediatric transplants are performed at large transplant centers which mainly engage in adult transplantation, and most children’s hospitals are not authorized to perform LT. There is little communication between pediatricians and transplant surgeons. Additionally, most families prefer to bear another child rather than choose transplantation when they are confronted with the high cost and the “one child policy”. However, recent changes in these situations are encouraging and gratifying with increasingly more attention from society being paid to this group of patients. Pediatric LT in our country has undergone immense progress in recent years. Our hospital is currently the largest transplant center for pediatric LT in mainland China. We work in close collaboration with Shanghai Children’s Medical Center to enable children to maximize the benefit gained from surgery. In this study, the annual caseload was hugely increased, the postoperative outcomes were greatly improved in stage II patients, particularly for LDLT recipients, and the 3-year patient and graft survival rates after LDLT reached 87.8%, which was comparable to those of developed countries[29,30]. This progress was mainly attributed to the following factors: (1) our results in stage I enhanced the understanding of transplantation by parents and pediatricians and promoted their willingness for referral or acceptance; (2) some charitable organizations voluntarily provided financial support during stage II; (3) grafts from deceased donors have been used since December 2010 to expand the donor pool for recipients without a suitable living donor; and (4) improvements in surgical techniques and posttransplant management with feedback on the long-term outcomes significantly decreased the incidence of posttransplant complications.
Although the shortage of deceased donors is a universal problem, the situation is particularly serious in Asia for various social, cultural, and historical reasons. Thus, the living donor was the only graft type available for most recipients in this study, and grafts from cadaveric organ donations were mostly used by patients without a suitable living donor. However, the LDLT recipients would have priority in acquiring financial support. It was shown in our previous work that the LDLT benefit was magnified with respect to hospital mortality, postoperative hospitalization rates, and midterm survival as centers gained greater surgical experience[31]. Thus, the postoperative outcomes in stage I were relatively unfavorable due to the effect of the learning curve. Specifically, the retransplantation rate is extremely low in mainland China, which is also influenced by the aforementioned socioeconomic factors, and only 1 patient underwent retransplantation in this study. This report provides a general description of surgical treatments for BA in mainland China based on our single-center experience. Conceivably, some effective steps ought to be taken in the future: (1) a nationwide BA screening system should be established; (2) medical insurance should cover all children from different areas; (3) timely referrals must be executed between junior and senior hospitals; and (4) close communication and cooperation should be promoted between pediatricians and transplant surgeons.
In conclusion, many children with BA in mainland China could not receive a timely KP due to various socioeconomic factors, but the situation has been changing. LT for BA could yield favorable outcomes through the accumulation of experience. Grafts from living donors are currently the most commonly used graft type for children with BA, and the 3-year patient and graft survival rates of 87.8% could be achieved by LDLT recipients. However, efforts should be directed to enhance the disease screening and insurance coverage for children with BA.
Biliary atresia (BA) is the most frequent cause of chronic cholestasis in infants and accounts for at least 50% of the liver transplants performed in pediatric patients. The sequential surgical treatment comprising the Kasai procedure followed by liver transplantation (LT) is currently accepted to be the conventional treatment strategy for most cases. However, in mainland China, various social, cultural and financial factors are responsible for a low diagnostic rate or a delayed Kasai procedure for children with BA.
In mainland China, pediatric LT has been progressing immensely in recent years. However, there are very few English language literature sources from mainland China concerning the diagnosis and surgical treatment of BA. The research hotspot is to introduce these real things happening to this population and to help other peers understand these backgrounds and trends in China.
In recent years, the status of surgical treatments for BA has been changing immensely in mainland China. The present study represents the largest series of BA patients in mainland China ever reported, showing that proportions of patients referred by pediatricians and those of patients who previously received a Kasai procedure were increasing obviously and that an increasing number of parents were willing to treat their children with LT in recent years. On the other hand, the current data also suggested that the incidence of surgical complications could be significantly reduced and the survival rates of patients and grafts could greatly improve as the transplant center gained greater technical experience.
The data in this study suggested that LT for BA could yield favorable outcomes through the accumulation of technical experience. Furthermore, this study also provided readers with important information regarding the socioeconomic obstacles to BA treatment and the substantial progress of pediatric LT in mainland China.
BA is a birth defect in newborn infants that is characterized by extrahepatic ductopenia and progressive cholestasis. The only effective treatments for BA are the Kasai procedure and LT. The Kasai procedure, also known as hepatoportoenterostomy, is a surgical technique performed in children with BA to allow bile drainage by attaching part of the small intestine to the porta hepatis.
Available papers concerning pediatric LT in mainland China are scarce. The authors in this study analyzed the characteristics and outcomes of children with BA based on a large single-center series. This study showed that favorable midterm outcomes after LT could be achieved as the transplant center gained greater technical experience. The results were interesting and provided important information concerning the background and trends of surgical treatments for BA in mainland China.
P- Reviewer: Suominen JS S- Editor: Ma YJ L- Editor: Wang TQ E- Editor: Zhang DN
| 1. | Balistreri WF, Grand R, Hoofnagle JH, Suchy FJ, Ryckman FC, Perlmutter DH, Sokol RJ. Biliary atresia: current concepts and research directions. Summary of a symposium. Hepatology. 1996;23:1682-1692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 260] [Cited by in RCA: 211] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 2. | McKiernan PJ, Baker AJ, Kelly DA. The frequency and outcome of biliary atresia in the UK and Ireland. Lancet. 2000;355:25-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 298] [Cited by in RCA: 277] [Article Influence: 11.1] [Reference Citation Analysis (1)] |
| 3. | Chardot C, Carton M, Spire-Bendelac N, Le Pommelet C, Golmard JL, Auvert B. Epidemiology of biliary atresia in France: a national study 1986-96. J Hepatol. 1999;31:1006-1013. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (1)] |
| 4. | Chardot C, Buet C, Serinet MO, Golmard JL, Lachaux A, Roquelaure B, Gottrand F, Broué P, Dabadie A, Gauthier F. Improving outcomes of biliary atresia: French national series 1986-2009. J Hepatol. 2013;58:1209-1217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 177] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 5. | Yoon PW, Bresee JS, Olney RS, James LM, Khoury MJ. Epidemiology of biliary atresia: a population-based study. Pediatrics. 1997;99:376-382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 152] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 6. | Livesey E, Cortina Borja M, Sharif K, Alizai N, McClean P, Kelly D, Hadzic N, Davenport M. Epidemiology of biliary atresia in England and Wales (1999-2006). Arch Dis Child Fetal Neonatal Ed. 2009;94:F451-F455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 87] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 7. | Alexopoulos SP, Merrill M, Kin C, Matsuoka L, Dorey F, Concepcion W, Esquivel C, Bonham A. The impact of hepatic portoenterostomy on liver transplantation for the treatment of biliary atresia: early failure adversely affects outcome. Pediatr Transplant. 2012;16:373-378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 32] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 8. | Otte JB, de Ville de Goyet J, Reding R, Hausleithner V, Sokal E, Chardot C, Debande B. Sequential treatment of biliary atresia with Kasai portoenterostomy and liver transplantation: a review. Hepatology. 1994;20:41S-48S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 9. | Altman RP, Lilly JR, Greenfeld J, Weinberg A, van Leeuwen K, Flanigan L. A multivariable risk factor analysis of the portoenterostomy (Kasai) procedure for biliary atresia: twenty-five years of experience from two centers. Ann Surg. 1997;226:348-53; discussion 353-5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 10. | Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18532] [Cited by in RCA: 24805] [Article Influence: 1181.2] [Reference Citation Analysis (0)] |
| 11. | Xi ZF, Xia Q, Zhang JJ, Chen XS, Han LZ, Zhu JJ, Wang SY, Qiu de K. De novo hepatitis B virus infection from anti-HBc-positive donors in pediatric living donor liver transplantation. J Dig Dis. 2013;14:439-445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 12. | Rudan I, Chan KY, Zhang JS, Theodoratou E, Feng XL, Salomon JA, Lawn JE, Cousens S, Black RE, Guo Y. Causes of deaths in children younger than 5 years in China in 2008. Lancet. 2010;375:1083-1089. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 160] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 13. | Wang Y, Zhu J, He C, Li X, Miao L, Liang J. Geographical disparities of infant mortality in rural China. Arch Dis Child Fetal Neonatal Ed. 2012;97:F285-F290. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 32] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 14. | Ma Y, Guo S, Wang H, Xu T, Huang X, Zhao C, Wang Y, Scherpbier RW, Hipgrave DB. Cause of death among infants in rural western China: a community-based study using verbal autopsy. J Pediatr. 2014;165:577-584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 15. | Feng XL, Guo S, Hipgrave D, Zhu J, Zhang L, Song L, Yang Q, Guo Y, Ronsmans C. China’s facility-based birth strategy and neonatal mortality: a population-based epidemiological study. Lancet. 2011;378:1493-1500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 86] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 16. | Oh M, Hobeldin M, Chen T, Thomas DW, Atkinson JB. The Kasai procedure in the treatment of biliary atresia. J Pediatr Surg. 1995;30:1077-180; discussion 1077-180;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 39] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 17. | Lykavieris P, Chardot C, Sokhn M, Gauthier F, Valayer J, Bernard O. Outcome in adulthood of biliary atresia: a study of 63 patients who survived for over 20 years with their native liver. Hepatology. 2005;41:366-371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 225] [Cited by in RCA: 197] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 18. | de Vries W, de Langen ZJ, Groen H, Scheenstra R, Peeters PM, Hulscher JB, Verkade HJ. Biliary atresia in the Netherlands: outcome of patients diagnosed between 1987 and 2008. J Pediatr. 2012;160:638-644.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 88] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 19. | Tessier ME, Harpavat S, Shepherd RW, Hiremath GS, Brandt ML, Fisher A, Goss JA. Beyond the Pediatric end-stage liver disease system: solutions for infants with biliary atresia requiring liver transplant. World J Gastroenterol. 2014;20:11062-11068. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 17] [Cited by in RCA: 17] [Article Influence: 1.5] [Reference Citation Analysis (2)] |
| 20. | Serinet MO, Wildhaber BE, Broué P, Lachaux A, Sarles J, Jacquemin E, Gauthier F, Chardot C. Impact of age at Kasai operation on its results in late childhood and adolescence: a rational basis for biliary atresia screening. Pediatrics. 2009;123:1280-1286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 275] [Cited by in RCA: 286] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 21. | Schreiber RA, Barker CC, Roberts EA, Martin SR, Alvarez F, Smith L, Butzner JD, Wrobel I, Mack D, Moroz S. Biliary atresia: the Canadian experience. J Pediatr. 2007;151:659-65, 665.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 184] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 22. | Hsiao CH, Chang MH, Chen HL, Lee HC, Wu TC, Lin CC, Yang YJ, Chen AC, Tiao MM, Lau BH. Universal screening for biliary atresia using an infant stool color card in Taiwan. Hepatology. 2008;47:1233-1240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 237] [Cited by in RCA: 190] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 23. | Tseng JJ, Lai MS, Lin MC, Fu YC. Stool color card screening for biliary atresia. Pediatrics. 2011;128:e1209-e1215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 43] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 24. | Chiu CY, Chen PH, Chan CF, Chang MH, Wu TC. Biliary atresia in preterm infants in Taiwan: a nationwide survey. J Pediatr. 2013;163:100-3.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 64] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 25. | Gu YH, Yokoyama K, Mizuta K, Tsuchioka T, Kudo T, Sasaki H, Nio M, Tang J, Ohkubo T, Matsui A. Stool color card screening for early detection of biliary atresia and long-term native liver survival: a 19-year cohort study in Japan. J Pediatr. 2015;166:897-902.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 26. | Davenport M, De Ville de Goyet J, Stringer MD, Mieli-Vergani G, Kelly DA, McClean P, Spitz L. Seamless management of biliary atresia in England and Wales (1999-2002). Lancet. 2004;363:1354-1357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 192] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 27. | Serinet MO, Broué P, Jacquemin E, Lachaux A, Sarles J, Gottrand F, Gauthier F, Chardot C. Management of patients with biliary atresia in France: results of a decentralized policy 1986-2002. Hepatology. 2006;44:75-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 114] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 28. | Zhou J, Shen Z, He Y, Zheng S, Fan J. The current status of pediatric liver transplantation in Mainland China. Pediatr Transplant. 2010;14:575-582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 19] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 29. | Barshes NR, Lee TC, Balkrishnan R, Karpen SJ, Carter BA, Goss JA. Orthotopic liver transplantation for biliary atresia: the U.S. experience. Liver Transpl. 2005;11:1193-1200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 78] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 30. | Fouquet V, Alves A, Branchereau S, Grabar S, Debray D, Jacquemin E, Devictor D, Durand P, Baujard C, Fabre M. Long-term outcome of pediatric liver transplantation for biliary atresia: a 10-year follow-up in a single center. Liver Transpl. 2005;11:152-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 142] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 31. | Wan P, Yu X, Xia Q. Operative outcomes of adult living donor liver transplantation and deceased donor liver transplantation: a systematic review and meta-analysis. Liver Transpl. 2014;20:425-436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 90] [Article Influence: 8.2] [Reference Citation Analysis (0)] |