Published online Aug 28, 2015. doi: 10.3748/wjg.v21.i32.9577
Peer-review started: December 11, 2014
First decision: January 22, 2015
Revised: February 9, 2015
Accepted: April 3, 2015
Article in press: April 3, 2015
Published online: August 28, 2015
Processing time: 261 Days and 11 Hours
AIM: To determine whether the decreased density of duodenal endocrine cells in irritable bowel syndrome (IBS) is associated with abnormalities in stem cell differentiation.
METHODS: The study sample comprised 203 patients with IBS (180 females and 23 males with a mean age of 36 years) and a control group of 86 healthy subjects without gastrointestinal complaints (77 females and 9 males with a mean age of 38 years). The patients included 80 with mostly diarrhoea (IBS-D), 47 with both diarrhoea and constipation (IBS-M), and 76 with mostly constipation (IBS-C). Both the patients and controls underwent gastroscopy and four biopsy samples were taken from the descending part of the duodenum, proximal to the papilla of Vater. The biopsy samples were sectioned and immunostained for Musashi 1 (Msi-1), neurogenin 3 (NEUROG3), secretin, cholecystokinin (CCK), gastric inhibitory peptide (GIP), somatostatin and serotonin. Immunostaining was performed with an ultraView Universal DAB Detection Kit (v1.02.0018, Venata Medical Systems, Basal, Switzerland) using the BenchMark Ultra immunohistochemistry/in situ hybridization staining module (Venata Medical Systems). Endocrine cell densities were quantified by computerized image analysis using the Olympus cellSens imaging program.
RESULTS: The densities of Msi-1 and NEUROG3 cells were significantly lower in IBS patients, regardless of the subtype, than in the controls (77 ± 17 vs 8 ± 2; P = 0.0001, and 351 ± 33 vs 103 ± 22; P = 0.00002, respectively). Furthermore, the densities of secretin, and CCK cells were significantly lower in patients with diarrhoea as the predominant IBS symptom (IBS-D) than in the controls (161 ± 11 vs 88 ± 8; P = 0.00007, and 325 ± 41 vs 118 ± 10; P = 0.00006, respectively), but not in patients with constipation as the predominant IBS symptom (IBS-C) or those with both diarrhoea and constipation (IBS-M). The GIP cell density was significantly reduced in both IBS-D (152 ± 12 vs 82 ± 7; P = 0.00003), and IBS-C (152 ± 12 vs 107 ± 8; P = 0.01), but not in IBS-M. The densities of somatostatin cells in the controls and the IBS-total, IBS-D, IBS-M and IBS-C patients were 81 ± 8, 28 ± 3, 20 ± 4, 37 ± 5 and 28 ± 4 cells/mm2 epithelium, respectively. The density of somatostatin cells was lower in IBS-total, IBS-D, IBS-M and IBS-C patients than in the controls (P = 0.00009, 0.00006, 0.009 and 0.00008, respectively). The density of serotonin cells did not differ between IBS patients and controls.
CONCLUSION: The reduction in duodenal endocrine cells in IBS patients found in this study is probably attributable to the reduction in cells expressing Msi-1 and NEUROG3.
Core tip: Musashi 1 (Msi-1) is a marker for both intestinal stem cells and their early progeny, and neurogenin 3 (NEUROG3) is a marker for early intestinal endocrine cell progenitors. The densities of Msi-1 and NEUROG3 cells were reduced in the duodenum of patients with irritable bowel syndrome (IBS), regardless of the subtype, indicating disturbances in both the clonogenic renewal of small intestine stem cells and their proliferation toward endocrine cells. It is most likely that the reduction in the duodenal endocrine cells in patients with IBS is caused by an abnormality in the stem cell clonogenic and proliferation activities.
- Citation: El-Salhy M, Hatlebakk JG, Hausken T. Reduction in duodenal endocrine cells in irritable bowel syndrome is associated with stem cell abnormalities. World J Gastroenterol 2015; 21(32): 9577-9587
- URL: https://www.wjgnet.com/1007-9327/full/v21/i32/9577.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i32.9577
Irritable bowel syndrome (IBS) is considered to be a functional disorder of the colon[1,2]. There is no test or examination for the diagnosis of IBS, which is therefore a diagnosis of exclusion, whereby examinations and tests are conducted to exclude organic diseases that could explain the patient’s symptoms[3-5]. Although attempts have been made to achieve a positive diagnosis based on symptom assessments[6-10], this system is not widely used in everyday clinical practice[4,11,12]. IBS does not develop into a serious disease or cause death, but it does significantly decrease the quality of life in patients[1].
Several abnormalities have been described in the endocrine cells of the stomach, duodenum, ileum and large intestine of IBS patients[13-29]. These abnormalities are believed to play a major role in the pathophysiology of the disorder and could represent a potential tool for its treatment[2,30-33]. The duodenum contains five endocrine cell types secreting secretin, cholecystokinin (CCK), gastric inhibitory polypeptide (GIP), somatostatin, and serotonin[34]. The functions and mode of actions of these hormones are described in detail elsewhere[32-34]. Briefly, secretin, CCK, GIP and serotonin inhibit gastric emptying. Whereas CCK and serotonin stimulate intestinal motility, and secretin, GIP and somatostatin inhibit its motility. Secretin stimulates pancreatic bicarbonate and fluid secretion and CCK stimulates pancreatic exocrine secretion. CCK also stimulates gallbladder contraction and regulates food intake. Somatostatin inhibits gut exocrine and neuroendocrine secretion and serotonin conveys sensation from the gut by activating submucosal sensory neurons.
In congenital malabsorptive diarrhoea, a paucity of intestinal endocrine cells is caused by a loss-of-function mutation in the gene encoding the protein neurogenin 3 (NEUROG3), which is expressed in the endocrine progenitor cells required for intestinal endocrine development[35]. Furthermore, a decrease in the number of intestinal endocrine cells following small intestine allograft rejection is associated with a reduction in the progenitors of intestinal endocrine cells that express NEUROG3 and NeuroD[36]. It is therefore logical to assume that the abnormalities encountered in the small intestine of IBS patients are associated with disturbance(s) in the small intestine stem cells and/or their progenitors. In order to test this assumption, cells expressing Musashi 1 (Msi-1, expressed in both stem cells and in their early progeny) and NEUROG3 (expressed in early endocrine cell progenitors)[37] were investigated in the duodenum of IBS patients and were compared with those in healthy volunteers. Furthermore, the endocrine cell types known to occur in the duodenum were investigated.
Patients with IBS according to Rome III criteria were recruited from those referred to Stord Hospital[9,10]. Thus, 203 patients with IBS were included in the study. They were 180 females and 23 males with a mean age of 36 years (range: 18-66 years). These patients included 80 with mostly diarrhoea (IBS-D), 47 with diarrhoea and constipation (IBS-M), and 76 with mostly constipation (IBS-C). All of the patients had a long duration of IBS symptoms and a symptom onset that was not associated with any gastrointestinal infections. The patients were examined physically, and blood tests were taken to exclude inflammation, and liver, kidney and thyroid diseases. Microscopic colitis was excluded by examining tissue obtained by colonoscopy with segmental biopsy sampling.
Eighty sex-matched healthy subjects were included as controls. They were 77 females and 9 males (mean age, 38 years; age range: 18-67 years). Of these subjects, 59 were healthy volunteers recruited at Stord Hospital, Haukeland University Hospital, and the University of Bergen. Fifteen were recruited from the population of Stord city and 44 were university students or hospital employees. A further 27 were healthy subjects who underwent gastroscopy because of health worries due to a relative being diagnosed with cancer.
The local Committee for Medical and Health Research Ethics West, Norway approved the study. Both patients and healthy volunteers gave oral and written consent.
Both the patients and controls underwent standard gastroscopy after an overnight fast, during which four biopsy samples were taken from the descending part of the duodenum, proximal to the papilla of Vater. Biopsy samples were also taken from the antral part of the stomach and used to identify the presence of Helicobacter pylori (H. pylori) (HelicotecUT Plus, Strong Biotech, Taipei, Taiwan).
After fixation in 4% buffered paraformaldehyde, paraffin-embedded biopsies were cut into sections 5 μm thick. The sections were stained with hematoxylin-eosin and immunostained with an ultraView Universal DAB Detection Kit (v1.02.0018, Venata Medical Systems, Basal, Switzerland) using the BenchMark Ultra immunohistochemistry/in situ hybridization staining module (Venata Medical Systems). The sections were incubated with primary antibodies for 32 min at 37 °C. The primary antibodies, which were diluted as per the specific suppliers’ instructions, were polyclonal rabbit anti-synthetic peptide conjugated to keyhole limpet haemocyanin derived from within residues 1-100 of human Msi-1 (code ab21628, Abcam, Cambridge, United Kingdom), monoclonal mouse-anti-protein expressed in 293T cells transfected with human NEUROG3 expression vector (code ab87108, Abcam), polyclonal rabbit anti-human secretin (code sc-20938, Santa Cruz Biotechnology, Santa Cruz, CA, United States), rabbit antibodies against human synthetic gastrin-17, which cross reacts with CCK (code A0568, Dako, Glostrup, Denmark), mouse antibodies against human synthetic GIP (code Sc-57162, Santa Cruz Biotechnology), rabbit antibodies against synthetic cyclic somatostatin (code A0566, Dako) and mouse antibodies against serotonin (code R87104, Dako).
Cell densities were quantified using the Olympus cellSens imaging program (version 1.7). A microscope (BX 43, Olympus, Tokyo, Japan) equipped with a digital camera (DP 26, Olympus) was used. The number of immunoreactive cells, the number of crypts and the area containing epithelial cells were measured. A × 40 objective was used, and each frame (field) represented a tissue area of 0.035 mm2. Immunoreactive cells were measured in ten fields, which were chosen randomly. Immunostained sections from the IBS patients and controls were coded, and measurements were made by the same person (M.E.), who was not aware of the identity of the sections. Cell density is expressed as the number of cells per 100 crypts (for Msi-1 and NEUROG3) or the number of cells per square millimetre of epithelium (for endocrine cells).
Differences in gender between the patients and controls were determined using the χ2 test, and the incidence of H. pylori infection with Fisher’s exact test. The Mann-Whitney non-parametric test was used to establish the difference in age between the patients and controls. The Kruskal-Wallis non-parametric test with Dunn’s post-test was used to identify the differences between controls, all IBS patients (IBS-total), and IBS-D, IBS-M, and IBS-C patients. The data are given as mean ± SE values, and P < 0.05 was considered statistically significant.
Neither gender nor age distribution differed significantly between the patients and the controls (P = 1.0 and 0.6, respectively). H. pylori was found in 12 patients and in 8 healthy subjects, which was not statistically significant (P = 0.6).
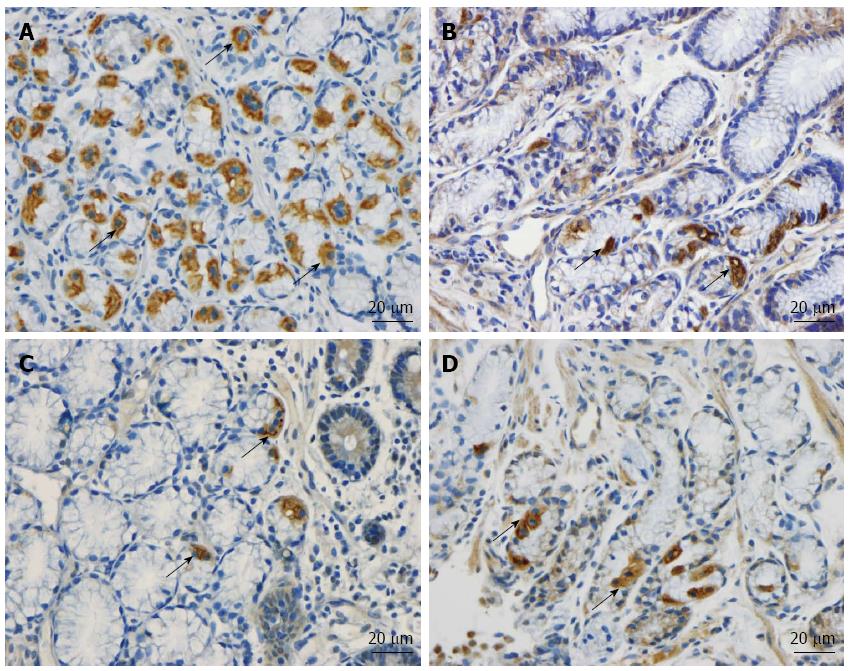
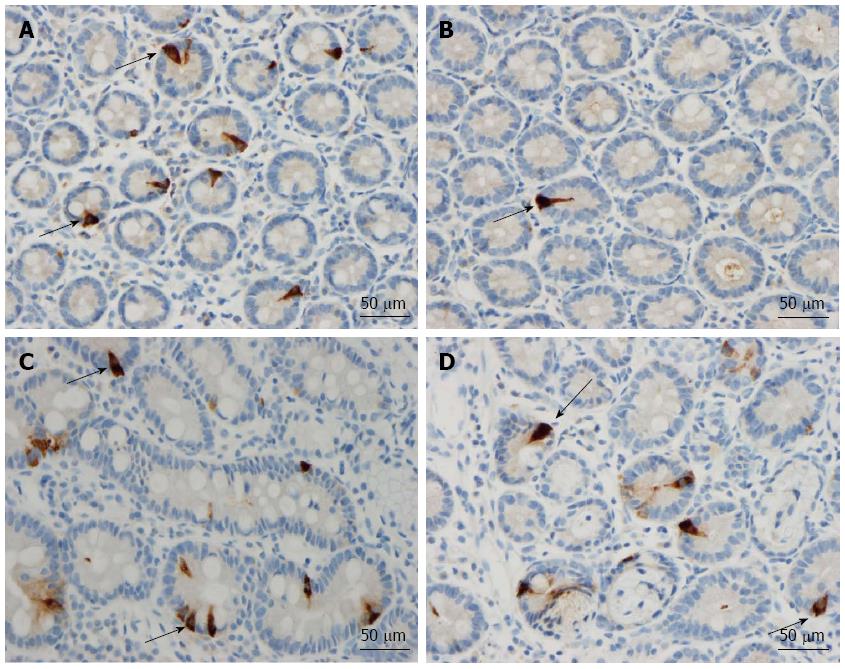
The duodenum of both the patients and controls was normal both endoscopically and microscopically. Msi-1 immunoreactivity was observed in both the cytoplasm and nucleus, and immunoreactive cells were found in the crypts of the duodenum of both the patients and the controls. NEUROG3 immunoreactivity was found exclusively in the nuclei of cells, which were observed in both the crypts and alongside the villi; secretin, CCK, GIP, somatostatin and serotonin cells were localized mostly in the crypts.
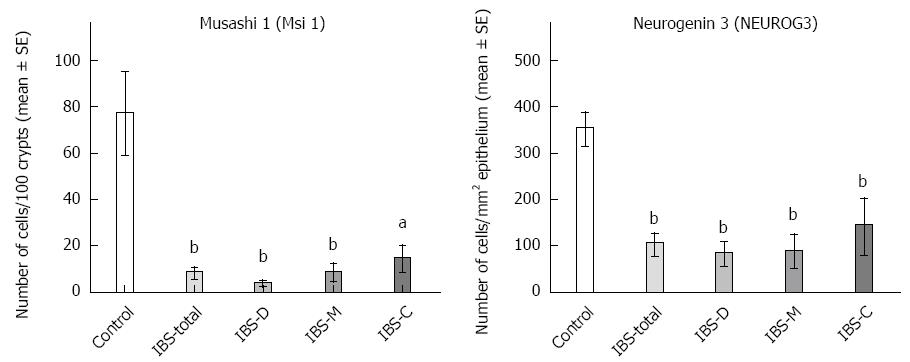
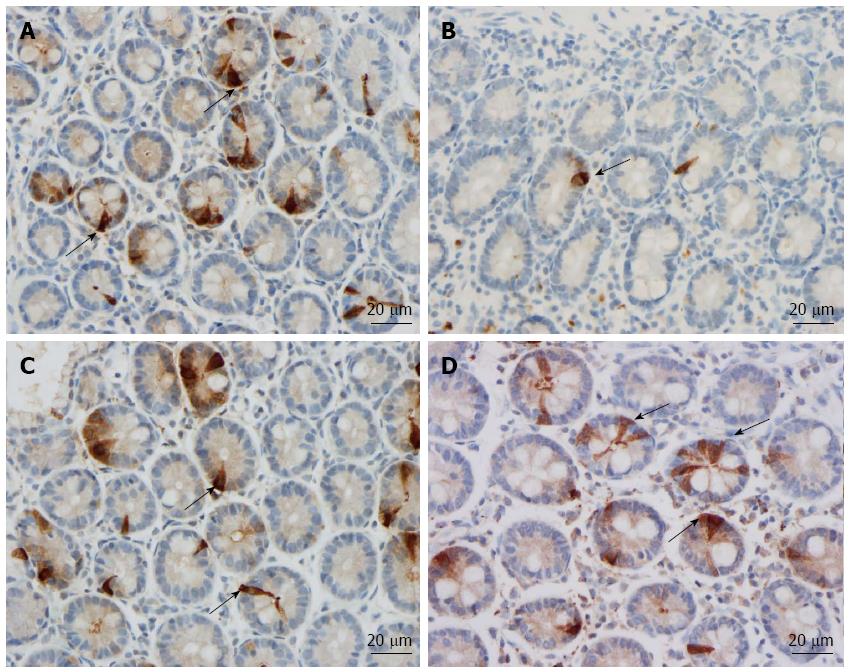
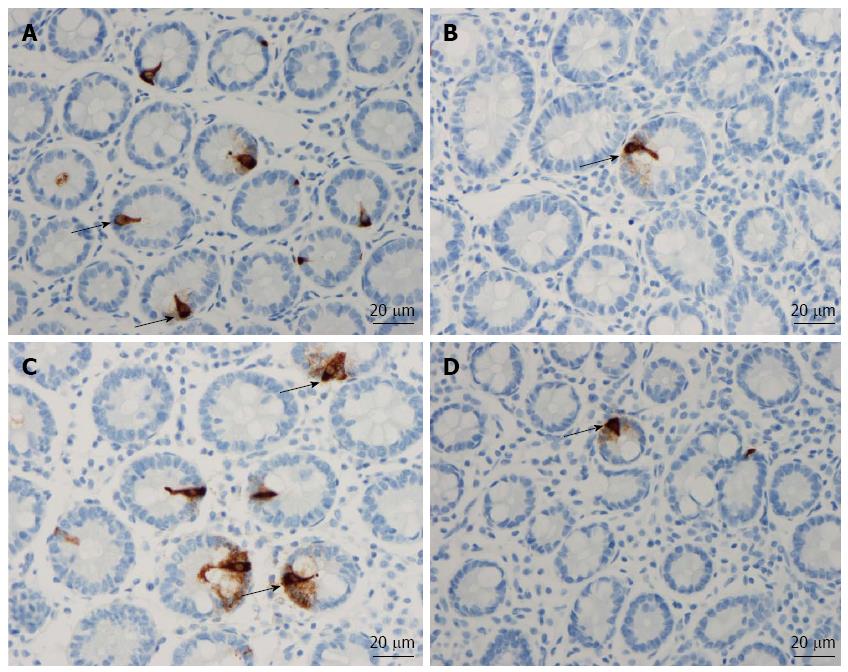
Msi-1: The numbers of Msi-1 cells were 77 ± 17, 8 ± 2, 4 ± 0.7, 8 ± 3 and 15 ± 5 cells/100 crypts in the controls, IBS-total, IBS-D, IBS-M, and IBS-C patients, respectively (Figures 1 and 2). The Kruskal-Wallis test showed that these results were significant (P = 0.002). Dunn’s post-test revealed that the density of Msi-1 cells was lower in IBS-total, IBS-D, IBS-M, and IBS-C than in the controls (P = 0.0001, 0.0005, 0.002, and 0.04, respectively).
NEUROG3: The densities of NEUROG3 cells in the controls and IBS-total, IBS-D, IBS-M and IBS-C patients were 351 ± 33, 103 ± 22, 83 ± 24, 87 ± 34,142 ± 58 and 149 ± 17 cells/100 crypts, respectively (Figure 1 and 3). The Kruskal-Wallis test showed that these results were significant (P = 0.00004). The density of NEUROG3 cells was lower in IBS-total, IBS-D, IBS-M and IBS-C than in the controls (P = 0.00002, 0.00003, 0.001 and 0.0009, respectively).
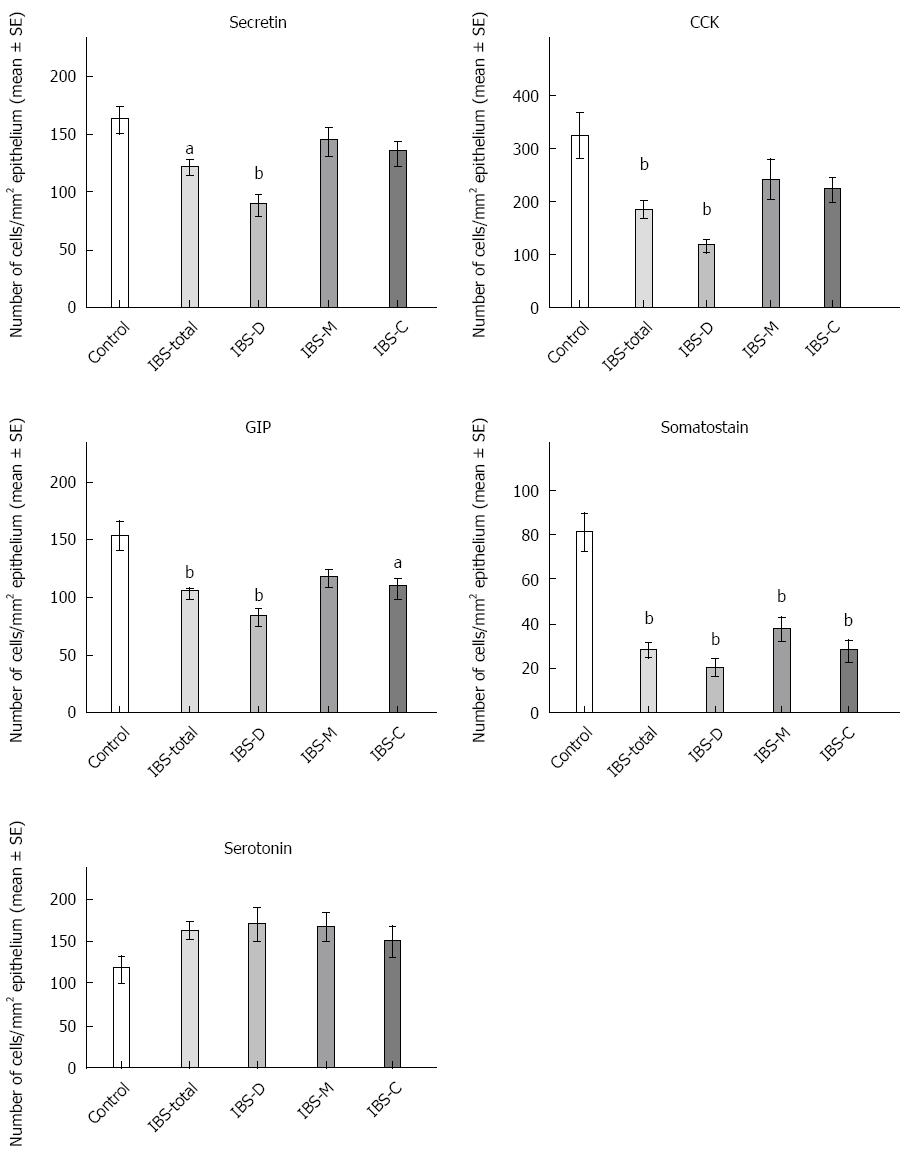
Secretin: The densities of secretin cells were 161 ± 11, 121 ± 7, 88 ± 8, 56 ± 13 and 133 ± 10 cells/mm2 epithelium in the controls and the IBS-total, IBS-D, IBS-M and IBS-C patients, respectively (Figures 4 and 5). A comparison between the controls, IBS-total and IBS subgroups using the Kruskal-Wallis test revealed significant differences (P = 0.00003). The density of secretin cells was lower in both the IBS-total and IBS-D patients than in the controls (P = 0.001 and 0.00007, respectively).
CCK: The densities of CCK cells were 325 ± 41, 186 ± 14, 118 ± 10, 242 ± 37 and 224 ± 20 cells/mm2 epithelium in the controls and the IBS-total, IBS-D, IBS-M and IBS-C patients, respectively (Figures 4 and 6). The Kruskal-Wallis test showed that these results were significant (P < 0.00008). Post-testing revealed that the density of CCK cells was significantly lower in IBS-total and IBS-D patients than in the controls (P = 0.00007 and 0.00006).
GIP: The densities of GIP cells in the controls and IBS-total, IBS-D, IBS-M and IBS-C patients were 152 ± 12, 103 ± 5, 82 ± 7, 116 ± 6, and 107 ± 8 cells/mm2 epithelium, respectively (Figures 4 and 7). The Kruskal-Wallis test showed that these results were significant (P = 0.00008). The GIP cell density was significantly lower in IBS-total, IBS-D and IBS-C patients than in the controls (P = 0.0006, 0.00003 and 0.01, respectively).
Somatostatin: The densities of somatostatin cells in the controls and the IBS-total, IBS-D, IBS-M and IBS-C patients were 81 ± 8, 28 ± 3, 20 ± 4, 37 ± 5 and 28 ± 4 cells/mm2 epithelium, respectively (Figure 4). The Kruskal-Wallis test showed that these results were significant (P = 0.00004). The density of somatostatin cells was lower in IBS-total, IBS-D, IBS-M and IBS-C patients than in the controls (P = 0.00009, 0.00006, 0.009 and 0.00008, respectively).
Serotonin: The densities of serotonin cells were 117 ± 15, 160 ± 10, 169 ± 18, 167 ± 16 and 149 ± 17 cells/mm2 epithelium in the controls and the IBS-total, IBS-D, IBS-M and IBS-C patients, respectively (P = 0.06, Kruskal-Wallis test). There were no significant differences in the densities of serotonin cells between the controls and the IBS-total and IBS subtypes (Figure 4).
Msi-1 is a marker for both intestinal stem cells and their early progeny[37-40]. The present study found that the Msi-1 cell density was reduced in the duodenum of all IBS patients, regardless of the subtype. This finding indicates that the clonogenic renewal of small intestine stem cells is reduced in patients with IBS. Furthermore, cells expressing NEUROG3, which is a marker for early intestinal cell progenitors[36,41,42], was reduced in the duodenum of all patients with IBS, again regardless of the subtype. A reduction in NEUROG3-expressing cells has been found in congenital malabsorptive diarrhoea[35], and a reduction in intestinal endocrine cells has been noted in small intestine allograft rejection[36]. Moreover, NEUROG3-knockout mice failed to develop any intestinal endocrine cells[43]. It is therefore logical to assume that the reduction in duodenal endocrine cells in IBS patients observed herein is attributable to the reduction in cells expressing Msi-1 and NEUROG3.
The abnormalities in duodenal endocrine cells observed in this study are in line with those reported elsewhere[22]. Thus, the densities of secretin and CCK cells were reduced in IBS-D, and those of GIP and somatostatin cells were reduced in IBS-D and IBS-C. The present study also showed that the only cell type in which the density was reduced in IBS-M, an IBS subtype that has not been investigated previously in this regard, was somatostatin cells. The present finding of unaffected serotonin cells in the small intestine of IBS patients is in agreement with previously reported observations[22,27,29].
It is interesting that changes in the density of duodenal endocrine cells differ with IBS subtype, being highest in IBS-D and lowest in IBS-M. The impact of the reduction in endocrine cell types in each IBS subtype on symptom development has been discussed previously[22]. It is believed that the reduction in secretin- and CCK-cell densities in IBS-D may lead to low levels of secretion of bicarbonate, pancreatic enzymes and bile salts, resulting in diarrhoea. Furthermore, as secretin inhibits intestinal motility and both secretin and CCK inhibit gastric emptying[33], any reduction in the population of these cells would contribute to the development of diarrhoea. Secretin, GIP and somatostatin inhibit gastric acid secretion[33], and as all of the IBS subtypes are associated with a reduction in the density of one or more of the cells secreting these hormones, they may also exhibit a high level of gastric acid secretion. It has been demonstrated that the antral gastrin cell density is increased and somatostatin cell density decreased in the stomach of IBS patients[17]. Given that gastric acid secretion is stimulated by gastrin and inhibited by somatostatin, it has been suggested that gastric acid secretion is increased in IBS. The present findings, together with those reported previously[17], may explain the high incidence of dyspepsia and gastro-oesophageal reflux found in IBS patients[44-52].
Intestinal stem cell self-renewal (clonogeny) and proliferation are regulated by several signalling pathways[37]. Several factors, such as hereditary, diet, intestinal bacterial flora and low-grade inflammation, have been demonstrated to play an important role in the pathophysiology of IBS. Changes in diet, intestinal bacterial flora and low-grade inflammation have been reported to affect the density of gut endocrine cells[34,53,54]. It is tempting to speculate that the factors that have been demonstrated to play a major role in the pathophysiology of IBS can affect the signalling pathways for stem cell clonogenic renewal and proliferation, resulting in abnormalities in gastrointestinal endocrine cells with the development of IBS symptoms.
Irritable bowel syndrome (IBS) is a common gastrointestinal disorder in which a reduced density of small intestinal endocrine cells has been reported. In some pathological conditions such as congenital malabsorptive diarrhoea, a decreased number of stem cell endocrine progenitors and a decreased number of intestinal endocrine cells have been reported. The present study was conducted in order to determine whether the decreased density of duodenal endocrine cells in IBS patients is associated with abnormalities in stem cell differentiation.
This study showed, for the first time, abnormal intestinal stem cell clonogenic and proliferation activities in IBS. These abnormalities in duodenal stem cells appear to account for the reduction in duodenal endocrine cell density reported in IBS patients.
The pathogenesis of IBS is not completely understood, but abnormalities in the gastrointestinal endocrine cells are believed to play a major role in the pathophysiology of IBS. The cause of the reduction in intestinal endocrine cells in patients with IBS is unknown. In the present study, Musashi 1 (Msi 1), which is a marker for both intestinal stem cells and their early progeny, and neurogenin 3 (NEUROG3), which is a marker for early intestinal endocrine cell progenitors were investigated in the duodenum of IBS patients. The densities of Msi-1 and NEUROG3 cells were reduced in the duodenum of patients with IBS, regardless of the subtype, indicating disturbances in both clonogenic renewal and proliferation activities of stem cells. These disturbances in duodenal stem cells were accompanied by a reduction in endocrine cells indicating an association between the abnormalities in stem cells and the reduction in duodenal endocrine cells in IBS patients.
The identification of abnormalities in intestinal stem cells offers a new approach in the research of IBS pathogenesis and may provide an effective tool for the treatment of IBS. Thus, research concerning the cause of the abnormalities in stem cells in IBS should be carried out, and stem cell stimulation/transplantation may be an option for the treatment of IBS in the near future.
Intestinal stem cells: each intestinal crypt contains 4 to 6 stem cells; stem cell clonogeny: represents self-renewal, in which stem cells divide into a new identical cells; stem cell differentiation progeny activity: stem cells differentiate into two lineages: the secretory lineage and absorptive lineage. The secretory lineage gives rise to goblet, endocrine and Paneth cells and the absorptive lineage to absorptive enterocytes.
In this article, the authors found that the reduction in the duodenal endocrine cells in patients with IBS is caused by an abnormality in the stem-cell clonogenic and proliferation activities. This is a well-written paper containing interesting results.
P- Reviewer: Luo HS, Ma XP, Rahimi R S- Editor: Ma YJ L- Editor: Webster JR E- Editor: Liu XM
| 1. | El-Salhy M, Gundersen D, Hatlebakk JG, Hausken T. Irritable bowel syndrome: diagnosis, pathogenesis and treatment options. New York: Nova Science Publishers, Inc 2012; . |
| 2. | El-Salhy M, Hatlebakk JG, Gilja OH, Hausken T. Irritable bowel syndrome: recent developments in diagnosis, pathophysiology, and treatment. Expert Rev Gastroenterol Hepatol. 2014;8:435-443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 36] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 3. | Camilleri M. Do the Symptom-Based, Rome Criteria of Irritable Bowel Syndrome Lead to Better Diagnosis and Treatment Outcomes? The Con Argument. Clin Gastroenterol Hepatol. 2009;8:129. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 4. | Quigley EM. The ‘con’ case. The Rome process and functional gastrointestinal disorders: the barbarians are at the gate! Neurogastroenterol Motil. 2007;19:793-797. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 24] [Article Influence: 1.3] [Reference Citation Analysis (1)] |
| 5. | Jellema P, van der Windt DA, Schellevis FG, van der Horst HE. Systematic review: accuracy of symptom-based criteria for diagnosis of irritable bowel syndrome in primary care. Aliment Pharmacol Ther. 2009;30:695-706. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 70] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 6. | Manning AP, Thompson WG, Heaton KW, Morris AF. Towards positive diagnosis of the irritable bowel. Br Med J. 1978;2:653-654. [PubMed] |
| 7. | Drossman DA. Rome III: the new criteria. Chin J Dig Dis. 2006;7:181-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 122] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 8. | Drossman DA. The functional gastrointestinal disorders and the Rome II process. Gut. 1999;45 Suppl 2:II1-II5. [PubMed] |
| 9. | Longstreth GF, Thompson WG, Chey WD, Houghton LA, Mearin F, Spiller RC. Functional bowel disorders. Gastroenterology. 2006;130:1480-1491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3413] [Cited by in RCA: 3381] [Article Influence: 177.9] [Reference Citation Analysis (1)] |
| 10. | Spiller R, Aziz Q, Creed F, Emmanuel A, Houghton L, Hungin P, Jones R, Kumar D, Rubin G, Trudgill N. Guidelines on the irritable bowel syndrome: mechanisms and practical management. Gut. 2007;56:1770-1798. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 609] [Cited by in RCA: 538] [Article Influence: 29.9] [Reference Citation Analysis (1)] |
| 11. | Gladman LM, Gorard DA. General practitioner and hospital specialist attitudes to functional gastrointestinal disorders. Aliment Pharmacol Ther. 2003;17:651-654. [PubMed] |
| 12. | Barbara G, Stanghellini V. Biomarkers in IBS: when will they replace symptoms for diagnosis and management? Gut. 2009;58:1571-1575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 32] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 13. | Dizdar V, Spiller R, Singh G, Hanevik K, Gilja OH, El-Salhy M, Hausken T. Relative importance of abnormalities of CCK and 5-HT (serotonin) in Giardia-induced post-infectious irritable bowel syndrome and functional dyspepsia. Aliment Pharmacol Ther. 2010;31:883-891. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 38] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 14. | El-Salhy M, Gilja OH, Gundersen D, Hatlebakk JG, Hausken T. Duodenal chromogranin a cell density as a biomarker for the diagnosis of irritable bowel syndrome. Gastroenterol Res Pract. 2014;2014:462856. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 15. | El-Salhy M, Gilja OH, Gundersen D, Hatlebakk JG, Hausken T. Endocrine cells in the ileum of patients with irritable bowel syndrome. World J Gastroenterol. 2014;20:2383-2391. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 33] [Cited by in RCA: 32] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 16. | El-Salhy M, Gilja OH, Gundersen D, Hausken T. Endocrine cells in the oxyntic mucosa of the stomach in patients with irritable bowel syndrome. World J Gastrointest Endosc. 2014;6:176-185. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 21] [Cited by in RCA: 23] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 17. | El-Salhy M, Gilja OH, Hatlebakk JG, Hausken T. Stomach antral endocrine cells in patients with irritable bowel syndrome. Int J Mol Med. 2014;34:967-974. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 18. | El-Salhy M, Gilja OH, Hausken T. Chromogranin A cells in the stomachs of patients with sporadic irritable bowel syndrome. Mol Med Rep. 2014;10:1753-1757. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 19. | El-Salhy M, Gundersen D, Hatlebakk JG, Gilja OH, Hausken T. Abnormal rectal endocrine cells in patients with irritable bowel syndrome. Regul Pept. 2014;188:60-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 41] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 20. | El-Salhy M, Gundersen D, Ostgaard H, Lomholt-Beck B, Hatlebakk JG, Hausken T. Low densities of serotonin and peptide YY cells in the colon of patients with irritable bowel syndrome. Dig Dis Sci. 2012;57:873-878. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 54] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 21. | El-Salhy M, Hatlebakk JG, Gilja OH, Hausken T. Densities of rectal peptide YY and somatostatin cells as biomarkers for the diagnosis of irritable bowel syndrome. Peptides. 2015;67:12-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 22. | El-Salhy M, Vaali K, Dizdar V, Hausken T. Abnormal small-intestinal endocrine cells in patients with irritable bowel syndrome. Dig Dis Sci. 2010;55:3508-3513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 40] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 23. | El-Salhy M, Lomholt-Beck B, Hausken T. Chromogranin A as a possible tool in the diagnosis of irritable bowel syndrome. Scand J Gastroenterol. 2010;45:1435-1439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 58] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 24. | El-Salhy M, Wendelbo I, Gundersen D. Serotonin and serotonin transporter in the rectum of patients with irritable bowel disease. Mol Med Rep. 2013;8:451-455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 34] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 25. | El-Salhy M, Wendelbo IH, Gundersen D. Reduced chromogranin A cell density in the ileum of patients with irritable bowel syndrome. Mol Med Rep. 2013;7:1241-1244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 29] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 26. | Wendelbo I, Mazzawi T, El-Salhy M. Increased serotonin transporter immunoreactivity intensity in the ileum of patients with irritable bowel disease. Mol Med Rep. 2014;9:180-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 27. | Park JH, Rhee PL, Kim G, Lee JH, Kim YH, Kim JJ, Rhee JC, Song SY. Enteroendocrine cell counts correlate with visceral hypersensitivity in patients with diarrhoea-predominant irritable bowel syndrome. Neurogastroenterol Motil. 2006;18:539-546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 63] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 28. | Coates MD, Mahoney CR, Linden DR, Sampson JE, Chen J, Blaszyk H, Crowell MD, Sharkey KA, Gershon MD, Mawe GM. Molecular defects in mucosal serotonin content and decreased serotonin reuptake transporter in ulcerative colitis and irritable bowel syndrome. Gastroenterology. 2004;126:1657-1664. [PubMed] |
| 29. | Wang SH, Dong L, Luo JY, Gong J, Li L, Lu XL, Han SP. Decreased expression of serotonin in the jejunum and increased numbers of mast cells in the terminal ileum in patients with irritable bowel syndrome. World J Gastroenterol. 2007;13:6041-6047. [PubMed] |
| 30. | Camilleri M. Physiological underpinnings of irritable bowel syndrome: neurohormonal mechanisms. J Physiol. 2014;592:2967-2980. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 77] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 31. | El-Salhy M, Gilja OH, Gundersen D, Hatlebakk JG, Hausken T. Interaction between ingested nutrients and gut endocrine cells in patients with irritable bowel syndrome (review). Int J Mol Med. 2014;34:363-371. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 27] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 32. | El-Salhy M, Gundersen D, Gilja OH, Hatlebakk JG, Hausken T. Is irritable bowel syndrome an organic disorder? World J Gastroenterol. 2014;20:384-400. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 71] [Cited by in RCA: 72] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 33. | El-Salhy M, Seim I, Chopin L, Gundersen D, Hatlebakk JG, Hausken T. Irritable bowel syndrome: the role of gut neuroendocrine peptides. Front Biosci (Elite Ed). 2012;4:2783-2800. [PubMed] |
| 34. | El-Salhy M. Irritable bowel syndrome: diagnosis and pathogenesis. World J Gastroenterol. 2012;18:5151-5163. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 77] [Cited by in RCA: 125] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 35. | Wang J, Cortina G, Wu SV, Tran R, Cho JH, Tsai MJ, Bailey TJ, Jamrich M, Ament ME, Treem WR. Mutant neurogenin-3 in congenital malabsorptive diarrhea. N Engl J Med. 2006;355:270-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 245] [Cited by in RCA: 226] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 36. | Fishbein TM, Novitskiy G, Lough DM, Matsumoto C, Kaufman SS, Shetty K, Zasloff M. Rejection reversibly alters enteroendocrine cell renewal in the transplanted small intestine. Am J Transplant. 2009;9:1620-1628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 37. | Montgomery RK, Breault DT. Small intestinal stem cell markers. J Anat. 2008;213:52-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 73] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 38. | Potten CS, Booth C, Tudor GL, Booth D, Brady G, Hurley P, Ashton G, Clarke R, Sakakibara S, Okano H. Identification of a putative intestinal stem cell and early lineage marker; musashi-1. Differentiation. 2003;71:28-41. [PubMed] |
| 39. | Kayahara T, Sawada M, Takaishi S, Fukui H, Seno H, Fukuzawa H, Suzuki K, Hiai H, Kageyama R, Okano H. Candidate markers for stem and early progenitor cells, Musashi-1 and Hes1, are expressed in crypt base columnar cells of mouse small intestine. FEBS Lett. 2003;535:131-135. [PubMed] |
| 40. | He XC, Yin T, Grindley JC, Tian Q, Sato T, Tao WA, Dirisina R, Porter-Westpfahl KS, Hembree M, Johnson T. PTEN-deficient intestinal stem cells initiate intestinal polyposis. Nat Genet. 2007;39:189-198. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 371] [Cited by in RCA: 368] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 41. | Schonhoff SE, Giel-Moloney M, Leiter AB. Minireview: Development and differentiation of gut endocrine cells. Endocrinology. 2004;145:2639-2644. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 236] [Cited by in RCA: 236] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 42. | Schonhoff SE, Giel-Moloney M, Leiter AB. Neurogenin 3-expressing progenitor cells in the gastrointestinal tract differentiate into both endocrine and non-endocrine cell types. Dev Biol. 2004;270:443-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 223] [Cited by in RCA: 239] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 43. | Jenny M, Uhl C, Roche C, Duluc I, Guillermin V, Guillemot F, Jensen J, Kedinger M, Gradwohl G. Neurogenin3 is differentially required for endocrine cell fate specification in the intestinal and gastric epithelium. EMBO J. 2002;21:6338-6347. [PubMed] |
| 44. | Pourhoseingholi A, Vahedi M, Pourhoseingholi MA, Ashtari S, Moghimi-Dehkordi B, Safaee A, Zali MR. Irritable bowel syndrome, gastro-oesophageal reflux disease and dyspepsia: overlap analysis using loglinear models. Arab J Gastroenterol. 2012;13:20-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 45. | Kim HG, Lee KJ, Lim SG, Jung JY, Cho SW. G-Protein Beta3 Subunit C825T Polymorphism in Patients With Overlap Syndrome of Functional Dyspepsia and Irritable Bowel Syndrome. J Neurogastroenterol Motil. 2012;18:205-210. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 46. | Suzuki H, Hibi T. Overlap syndrome of functional dyspepsia and irritable bowel syndrome - are both diseases mutually exclusive? J Neurogastroenterol Motil. 2011;17:360-365. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 43] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 47. | Nakajima S, Takahashi K, Sato J, Fukuda M, Yamamoto K, Inoue T, Okumura Y, Fujiyama Y. Spectra of functional gastrointestinal disorders diagnosed by Rome III integrative questionnaire in a Japanese outpatient office and the impact of overlapping. J Gastroenterol Hepatol. 2010;25 Suppl 1:S138-S143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 38] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 48. | Olafsdottir LB, Gudjonsson H, Jonsdottir HH, Thjodleifsson B. Stability of the irritable bowel syndrome and subgroups as measured by three diagnostic criteria - a 10-year follow-up study. Aliment Pharmacol Ther. 2010;32:670-680. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 21] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 49. | Kaji M, Fujiwara Y, Shiba M, Kohata Y, Yamagami H, Tanigawa T, Watanabe K, Watanabe T, Tominaga K, Arakawa T. Prevalence of overlaps between GERD, FD and IBS and impact on health-related quality of life. J Gastroenterol Hepatol. 2010;25:1151-1156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 180] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 50. | Noh YW, Jung HK, Kim SE, Jung SA. Overlap of Erosive and Non-erosive Reflux Diseases With Functional Gastrointestinal Disorders According to Rome III Criteria. J Neurogastroenterol Motil. 2010;16:148-156. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 74] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 51. | Hori K, Matsumoto T, Miwa H. Analysis of the gastrointestinal symptoms of uninvestigated dyspepsia and irritable bowel syndrome. Gut Liver. 2009;3:192-196. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 36] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 52. | Ford AC, Marwaha A, Lim A, Moayyedi P. Systematic review and meta-analysis of the prevalence of irritable bowel syndrome in individuals with dyspepsia. Clin Gastroenterol Hepatol. 2010;8:401-409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 125] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 53. | Mazzawi T, Gundersen D, Hausken T, El-Salhy M. Increased gastric chromogranin A cell density following changes to diets of patients with irritable bowel syndrome. Mol Med Rep. 2014;10:2322-2326. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 54. | El-Salhy M, Mazzawi T, Gundersen D, Hatlebakk JG, Hausken T. Changes in the symptom pattern and the densities of large-intestinal endocrine cells following Campylobacter infection in irritable bowel syndrome: a case report. BMC Res Notes. 2013;6:391. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |