Published online Aug 28, 2015. doi: 10.3748/wjg.v21.i32.9466
Peer-review started: April 24, 2015
First decision: May 18, 2015
Revised: June 5, 2015
Accepted: July 18, 2015
Article in press: July 18, 2015
Published online: August 28, 2015
Processing time: 127 Days and 21.8 Hours
Cirrhosis is associated with marked abnormalities in the circulatory function that involve a reduction in systemic vascular resistance. An important cause of this vasodilatation is the increased production or activity of nitric oxide (NO) in the splanchnic circulation. During portal hypertension and cirrhosis an increased endothelial NO synthase (eNOS) activity is demonstrated in splanchnic vessels. In contrast, the activity of eNOS in the cirrhotic liver is decreased, which suggests a different regulation of eNOS in the liver and in the splanchnic vessels. Asymmetric dimethylarginine (ADMA) is an endogenous NO inhibitor and higher plasma levels of ADMA are related to increased cardiovascular risk in both the general population and among patients with cirrhosis. It has been demonstrated that the liver is a key player in the metabolism of ADMA. This observation was further supported by investigations in human patients, showing a close correlation between ADMA plasma levels and the degree of hepatic dysfunction. ADMA is degraded to citrulline and dimethylamine by dimethylarginine dimethylaminohydrolases (DDAHs). DDAHs are expressed as type 1 and 2 isoforms and are widely distributed in various organs and tissues, including the liver. In this review, we discuss experimental and clinical data that document the effects of dimethylarginines on vascular function in cirrhosis. Our increasing understanding of the routes of synthesis and metabolism of methylarginines is beginning to provide insights into novel mechanisms of liver disease and allowing us to identify potential therapeutic opportunities.
Core tip: Several lines of evidence point out that the liver is an important organ clearing asymmetric dimethylarginine (ADMA), an endogenous inhibitor of nitric oxide synthase and a mediator of elevated intrahepatic vascular tone in cirrhosis. ADMA is degraded by dimethylarginine dimethylaminohydrolases that are expressed widely in the liver. Therefore, liver dysfunction could lead to alterations in the levels of ADMA and modifies nitric oxide bioavailability. Our increasing understanding of the routes of synthesis and metabolism of methylarginines is beginning to provide insights into novel mechanisms of liver disease and allowing us to identify potential therapeutic opportunities.
- Citation: Lluch P, Segarra G, Medina P. Asymmetric dimethylarginine as a mediator of vascular dysfunction in cirrhosis. World J Gastroenterol 2015; 21(32): 9466-9475
- URL: https://www.wjgnet.com/1007-9327/full/v21/i32/9466.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i32.9466
Nitric oxide (NO) is generated from the metabolism of L-arginine by three isoforms of NO synthase (NOS), namely endothelial NOS (eNOS), inducible NOS (iNOS) and neuronal NOS (nNOS)[1]. Endothelial NO accounts for the powerful vasodilator effects of endothelium-derived vasodilator factor[2-4] and consequently plays a decisive role in determining vasomotor tone. Upon its generation in endothelial cells, NO acts in part by stimulating soluble guanylate cyclase to produce second-messenger cyclic guanosine monophosphate (cGMP), which in turn mediates vasodilatation[5].
Free guanidino-methylated (NG) arginine residues occur endogenously as a result of proteolysis of methylated proteins[6,7]. The L-arginine analogues include NG-methyl-L-arginine (L-NMMA), NG, NG-dimethyl-L-arginine (asymmetric dimethylarginine; ADMA) and NG, N’G-dimethyl-L-arginine (symmetric dimethylarginine; SDMA). ADMA and L-NMMA are competitive inhibitors of the NOS enzymes[8-10]. SDMA, a stereoisomer of ADMA, has no inhibitory effect on NOS, but may interfere with NO synthesis by competing with L-arginine for transport across cell membranes[11,12]. More recent data demonstrate that SDMA, but not ADMA, stimulates reactive oxygen species production of monocytes by acting on Ca2+ entry via store-operated calcium channels[13]. Since plasma concentrations of ADMA are approximately ten times higher than that of L-NMMA, it was postulated that ADMA is the main endogenous regulator of the L-arginine/NO pathway[11].
Protein methylation is a post-translational modification of intracellular proteins involving the addition of a methyl group, provided by S-adenosyl methionine, to arginine residues in the polypeptide chain by the action of protein arginine methyltransferases (PRMTs)[14]. PRMTs catalyze monomethylation, which initially leads to the synthesis of L-NMMA[14] or transfer two methyl groups in either a symmetric (leading to SDMA) or an asymmetric configuration (leading to ADMA)[15,16]. PRMTs are divided in two groups: types I and II. Type I PRMTs produce ADMA and type II produce SDMA. Both types are responsible for the production of L-NMMA[15,17].
Alterations in expression of PRMTs are associated with corresponding changes in ADMA release, thus suggesting that rates of ADMA formation in the vessel wall may be regulated in part through alteration in PRMTs expression[18]. The gene expression of PRMTs is increased in cultured endothelial cells after administration of both native low-density lipoprotein and oxidized low-density lipoprotein[18]. These compounds, similarly to glucose and homocysteine, are also responsible for a higher activity of PRMTs, which results in increased ADMA concentration[16,18-20].
Plasma levels of ADMA in healthy subjects are in the range of 0.3-0.5 μmol/L[11], but in some pathological states increase two- or even ten-fold, contributing to inhibit NO synthesis[21]. It is noteworthy that the concentration of ADMA inside cells is higher than outside. The intracellular levels of ADMA in endothelial cells obtained from rabbit carotid artery is ten times higher than in plasma[22].
ADMA is excreted in part by the kidneys, but the main elimination pathway is throught the enzyme dimethylarginine dimethylaminohydrolase (DDAH I and II) to citrulline and dimethylamine[11,23]. DDAH is widely distributed in tissues, including the liver[24-26]. In contrast to ADMA, SDMA is eliminated by renal clearance and cannot be degraded by DDAH[23,27-29]. There has been increasing interest in the studies concerning plasma levels of NO, ADMA and SDMA in patients with liver dysfunction[26,30-34]. In experiments in Wistar rats, Nijveldt et al[26] demonstrated the potential role of the liver in the metabolism of ADMA as evidenced by a high net uptake and a considerable fractional extraction rate. In contrast to ADMA, SDMA was hardly affected by the liver[26]. Further studies showed markedly increased plasma levels of ADMA in multiple organ failure patients[31] and in patients developing hepatic failure after major hepatectomy[35]. Interestingly, Siroen and co-workers have shown that the human liver not only takes up ADMA, but also SDMA from the portal and systemic circulation, and suggested that high plasma levels of SDMA may have hemodynamic consequences similar to those reported by ADMA[36]. This finding is in contrast with the results in the rat in which SDMA was not affected by the liver[26]. This discrepancy between human and rat liver has been ascribed to the presence of other, relatively unknown alternative metabolic pathways for both dimethylarginines[27], which may be more significant in the human liver in comparison with the rat liver[36].
DDAH I appears to be the major isoform responsible for ADMA degradation[37], being the major isoform expressed in the hepatocytes[38]. Loss of DDAH I activity, using specific inhibitors, leads to accumulation of ADMA. Both plasma and tissue levels of ADMA increase in Ddah+/- mice with deletion of the Ddah1 gene. In Ddah+/- mice, some symptoms of endothelial dysfunction, including increased contraction in response to phenylephrine, reduced relaxation in response to acetylcholine or calcium ionophore A23187, and increased relaxation in response to sodium nitroprusside, are observed[39]. Hemodynamic effects such as increased mean arterial blood pressure, decreased cardiac output and heart rate, and elevated right ventricular pressure are also revealed. No increase in the expression of DDAH II in these mice is noted[39].
Elevated ADMA plasma levels have been found to be associated with impaired endothelium-dependent vasodilatation[40]. Elevated plasma levels of ADMA were detected in patients with cardiovascular diseases[41-44]. Oxidative stress is also responsible for increased synthesis and/or inhibition of catabolism of ADMA[45] that are observed in patients with hypercholesterolemia, hyperglycemia, hyperhomocysteinemia, diabetes, hypertension, and heart failure[18,43,46,47]. A high level of ADMA independently predicts future cardiovascular risk in patients with coronary artery disease[48] and adverse cardiovascular events in patients undergoing percutaneous coronary intervention[49]. The level of this compound also increases with aging[40]. In critically ill patients with clinical evidence of more than two organ failures, ADMA is a strong and independent risk factor of mortality[31]. Renal failure[11] and liver cirrhosis[30] are further examples of disorders with increased levels of ADMA as both organs are responsible for the elimination of ADMA. Significant correlation was observed between the concentration of ADMA in graft and liver function after transplantation[50].
Several lines of evidence indicate that elevated inflammatory biomarkers are closely associated with endothelial dysfunction and NO synthesis inhibition[51,52]. Systemic inflammation is linked to enhanced ADMA plasma levels and endothelial dysfunction, both in low-grade inflammation, such as atherosclerosis[53,54] and in chronic inflammatory diseases, such as rheumatoid arthritis[55], inflammatory bowel disease[56], and asthma[57].
Excessive NO production[58] and cGMP plasma levels[59] seem to play a key role in determining the decrease in splanchnic vascular resistance observed in decompensated cirrhosis[58]. Conversely, the levels of NO in hepatic tissue are decreased, and this is probably due to reduced hepatic eNOS activity shown in experimental animals[60,61] and humans[62]. A decrease in NO production by sinusoidal endothelial cells in the cirrhotic liver is an important factor in the development and maintenance of portal hypertension[52,61-64].
Patients with decompensated alcoholic cirrhosis (class B and C according to Pugh classification)[65] exhibit greater plasma levels of ADMA and NO when compared with both patients with compensated alcoholic cirrhosis (class A of Pugh score) and healthy subjects, but SDMA and L-arginine levels are not different between groups[30]. There is a positive correlation between the clinical score of the patients and concentrations of ADMA and NO and a negative correlation between plasma ADMA and NO concentrations in the control group and compensated patients[30]. However, no correlation is observed between ADMA and NO in decompensated cirrhotic patients[30]. Therefore, the increase in ADMA and NO concentrations in decompensated patients may reflect a response to hepatocellular damage[30]. Since SDMA concentrations are not significantly different between groups, a high ratio ADMA/SDMA is observed in the decompensated group of patients, thus suggesting a decreased activity in DDAH[66,67]. It is conceivable that a rise in ADMA in the liver would decrease local production of NO and intrahepatic vascular resistance would increase. Elevated ADMA, attributable to reduced renal excretion, is unlikely because creatinine plasma levels in the three groups of patients were within normal values[30].
The increase in plasma levels of ADMA observed in alcoholic cirrhosis[30] and in acute decompensation of alcoholic liver disease[68] is moderate (twofold elevation in ADMA). The increase in plasma levels of ADMA in individuals with vascular risk factors[69] and in patients with coronary artery disease[70] is also small. Therefore it has been proposed that small changes in the plasma levels of ADMA may be sufficient to alter significantly NO production by endothelial cells[71]. Cellular studies demonstrate that ADMA accumulates inside endothelial cells reaching values 5-10 times higher than outside the cells[22]. Therefore, small changes in plasma levels of ADMA would be expected to have a large effect on the intracellular levels of this NOS inhibitor and cause inhibition of NO formation[22]. However, the intracellular levels of ADMA in endothelial cells under cirrhosis are not known.
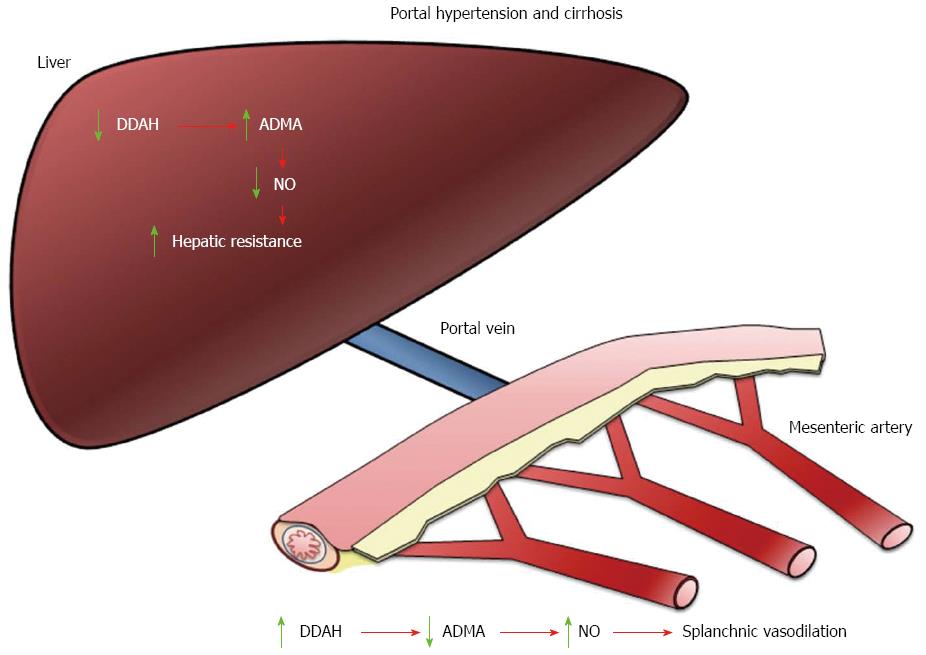
In a rat carotid model of balloon injury it was observed that 28 d post injury there was an impairment of endothelium-dependent vascular relaxation that was associated with a significant increase in intracellular levels of methylarginines (ADMA and L-NMMA)[22]. These results demonstrated for the first time that under these pathological conditions intracellular methylarginines reach concentrations sufficient to inhibit NO production and endothelium-dependent relaxation. Whether these observations can be applied to other pathological conditions is still unknown. The course of ADMA concentrations has been investigated in patients undergoing liver transplantation[32]. The results showed that preoperative plasma levels were significantly elevated, and ADMA plasma concentrations decreased on the day after transplantation, thus indicating that the liver graft is quickly capable of clearing ADMA[32]. Furthermore, in patients who experienced acute rejection, ADMA concentrations were higher compared with nonrejectors, indicating a reduced activity of the degrading enzyme DDAH in the liver[32]. A recent report has shown a significant reduction in hepatic DDAH-1 expression in cirrhotic livers that is associated with increased hepatic ADMA, reduced hepatic eNOS activity and elevated portal hypertension[38] (Figure 1).
A paradoxical situation has been described in the setting of alcoholic cirrhosis where an increased plasma concentration of ADMA, endogenous inhibitor of NOS, is linked to an increase of NO. Interpretation of these results is difficult but some possible explanations have been proposed. It has been demonstrated that DDAH activity is inhibited by high concentrations of NO[72]. This NO-induced DDAH inhibition would lead to accumulation of ADMA[72]. If this DDAH inhibition also occurs in cirrhosis, it may clarify why the enhanced synthesis of NO in cirrhosis can be associated with an inhibition of the DDAH activity and an increase in ADMA levels.
It has been shown that ADMA inhibits the basal release of NO from the endothelium and increases the tone of peripheral vessels[73]. This inhibitory effect on NO synthesis corrects the hyporeactivity to vasoconstrictors demonstrated in experimental models of portal hypertension[74]. Therefore, it is possible that a rise in ADMA plasma levels in cirrhosis might represent a compensatory mechanism to decrease NO overproduction and, accordingly, to counterbalance excessive peripheral vasodilatation[30].
The paradox is extended to the study of hyperdynamic circulation in cirrhosis where the mesenteric vasodilation established during cirrhosis is associated with an increase in systemic ADMA levels[30,68,75,76]. The increase in such levels does not inhibit NOS nor the excess NO in the splanchnic endothelial cells in contrast with the decreased generation of NO in the liver[77]. Most studies have paid attention to NOS activity and NO overproduction in splanchnic vessels disregarding the role of DDAHs in these vessels. In small mesenteric arteries from portal hypertensive and cirrhotic rats the low ability of ADMA to inhibit NOS is related to a higher expression of DDAHs and a larger ADMA degradation[78]. It has been proposed that this greater DDAH expression could be a new mechanism involved in the increased basal release of NO and enhanced mesenteric vasodilation observed in the hyperdynamic circulation[78] (Figure 1).
In patients with acute alcoholic hepatitis ADMA, SDMA and their combined sum, which has been termed dimethylarginine score (DAS = ADMA + SDMA), are increased[68]. The calculated DAS value is significantly higher in nonsurviving patients with acute hepatic decompensation compared to survivors. Therefore the DAS value has been proposed as a clinically relevant predictive indicator when evaluating survival in acute hepatic decompensation[68]. DDAH protein expression is reduced and PRMT-1 increased in alcoholic hepatitis livers, thus indicating that the increase in ADMA may result from both decreased breakdown (decreased hepatic DDAH) and/or increased production (increased PRMT). The increase in SDMA is probably secondary to impairment of the renal circulatory bed. Increased ADMA has been associated with a decrease in renal plasma flow and increased renovascular resistance[79] which would lead to increased renal retention of SDMA.
The role of NO in the pathophysiology of HCV infection still remains controversial. Clinical studies have failed to provide association between the NO plasma levels and chronic hepatitis C[80-82]. Considering data from the literature, patients with chronic hepatitis C have shown NO plasma levels decreased[81] or enhanced[82]. Furthermore, no significant differences were observed between NO, ADMA, and SDMA plasma levels in patients with chronic hepatitis C and in patients with sustained viral response after treatment with interferon (IFN) plus ribavirin[83]. One explanation for the discrepancy may be the grade of hepatocellular damage. Certainly, a previous study performed in our laboratory has demonstrated that in patients with compensated alcoholic cirrhosis (i.e., Child-Pugh score below 7), no significant differences in ADMA, SDMA and NO plasma concentrations were observed as compared to healthy subjects[30]. However, in patients with decompensated alcoholic cirrhosis (i.e., Child-Pugh score equal or above 7) or hepatorenal syndrome, ADMA and NO concentrations were significantly higher when compared to healthy subjects[30,84].
It remains unclear the mechanisms by which IFN mediates its anti-HCV effect in vivo. Mononuclear cells obtained from patients with hepatitis C treated with IFN-α show increased iNOS activity and NO synthesis, pointing out that induced NO may be associated to the antiviral actions of IFN in hepatitis C[85]. Although it has been demonstrated a widespread expression of iNOS in hepatocyte from patients with chronic hepatitis C, this increased expression of the enzyme is not accompanied by increased NO serum concentration[86]. Furthermore, in patients with hepatitis C after treatment with IFN for two weeks the NO serum levels were increased if HCV-RNA was eradicated, but if HCV-RNA was present the NO levels were not different from those in healthy subjects[82]. In another study, NO plasma levels were decreased in four patients treated with pegylated IFN-α-2b or IFN-α-2a plus ribavirin for 48 wk[87]. Consistent with these results, a previous study demonstrated that NO plasma levels in patients with chronic hepatitis C treated with INF-α for 18 mo did not differ from controls[88]. It remains to be clarified whether NO and ADMA levels change throughout the period of treatment of the HCV infection with IFN or another therapy for hepatitis C.
Hepatorenal syndrome (HRS), a major complication of end-stage cirrhosis, is characterized by functional renal failure and severe alterations in the systemic circulation (for review see[89]). Impairment of kidney function is a consequence of marked reduction of renal blood flow probably caused by activation of specific vasoconstrictor systems including sympathetic nerves, renin-angiotensin, and arginine-vasopressin to counteract the vasodilatation of splanchnic circulation[89]. Nijveldt et al[90] hypothesized a causal role for ADMA in the progression of renal failure in advanced cirrhosis. They proposed that accumulation of ADMA, probably caused by impaired hepatic removal, may have detrimental effects on renal function by inhibiting NO synthesis, thereby interfering with renal blood flow and glomerular filtration[90]. ADMA elicits contractile effects on human renal arteries[91] and increased plasma levels of ADMA has been associated with a decrease in renal plasma flow and increase in renovascular resistance in humans[79]. Therefore, it has been proposed that increased ADMA might be involved in the decrease of renal perfusion and the initiation of functional renal failure in advanced cirrhosis[84].
In a previous study we investigated whether plasma levels of SDMA, ADMA and NO are elevated in patients with hepatorenal syndrome, compared with patients with cirrhosis without renal failure[84]. In the group of patients with hepatorenal syndrome, SDMA concentration was about fourfold higher compared to patients with cirrhosis with normal kidney function or healthy subjects. ADMA and NO concentrations were significantly higher in both groups of patients when compared to controls. In patients with hepatorenal syndrome, a significant positive correlation was observed between SDMA and serum creatinine but not between ADMA and creatinine.
SDMA plasma levels remain within normal values in alcoholic cirrhosis with normal renal function[30] and in patients with end-stage liver failure prior to liver transplantation[33]. This strongly suggests that the high levels of SDMA observed in patients with HRS are caused by impairment of renal function. As a consequence, SDMA is not correlated with NO but it is significantly correlated with serum creatinine. A positive correlation between plasma SDMA concentrations and serum creatinine has been previously demonstrated in patients with chronic renal failure due to primary renal disease in the absence of liver dysfunction[11,66,92]. After kidney transplantation, the concentrations of SDMA returned to baseline values[92]. Kielstein et al[93] collected and analyzed data from 18 studies involving 2136 patients; their results showed that SDMA concentration correlated highly with inulin clearance as well as with serum creatinine. These results confirmed previous studies in humans[94-96] and experimental animals[97,98] showing a close relationship between SDMA and renal function. On the other hand, in our study we did not find correlation between plasma levels of ADMA and serum creatinine which indicates that the increase in plasma ADMA in patients with HRS was not due to a decrease in renal clearance of ADMA. In contrast to ADMA, SDMA is eliminated by renal clearance and cannot be degraded by DDAH[23]. The significance of high plasma levels of SDMA in HRS is uncertain because there is no evidence that it may inhibit NOS[11]. However, SDMA at high concentrations may interfere with NO production by blocking cellular L-arginine uptake[11,12,99]. These studies suggest that SDMA could have an inhibitory effect of NO synthesis by limiting arginine availability to NOS[12]. Indeed, in primary chronic renal failure, Fleck and coworkers pointed out the potential importance of SDMA and concluded that not only ADMA but also SDMA levels are likely responsible for hypertension in these patients, possibly by competition for reabsorption between SDMA and arginine in the kidney[92]. Therefore, SDMA is likely to be another factor responsible for the increased intrarenal vascular resistance observed in HRS.
The pathogenesis of renal vasoconstriction in cirrhosis is multifactorial[89]. Several lines of evidence suggest that splanchnic arterial vasodilatation due to an increased synthesis of NO plays a main role in the initiation of reduced renal perfusion. Intravenous administration of NOS inhibitors to cirrhotic patients increases renal blood flow and glomerular filtration rate, probably due to the increase in renal perfusion caused by an increment in systemic arterial pressure in these patients[100]. These findings led to the suggestion that NOS inhibitors might be useful in the treatment of ascites in cirrhosis[101]. However, experiments in cirrhotic rats raise the possibility that NO blockade may have deleterious effects by increasing intrahepatic vascular resistance[60]. Moreover, it is possible that the plasma concentrations of ADMA achieved in cirrhosis could be biologically effective in renal vessels. With regard to this, it has been proposed that increased ADMA in hepatic dysfunction plays an important role in the development of renal failure in patients with cirrhosis[90]. Indeed, ADMA increases renovascular resistance in humans[79] and elicits contractile effects on human renal[91] and cerebral[102] arteries. In addition, NOS inhibitors enhance vascular contractile responses to adrenergic agonists and sympathetic stimulation of human arteries[103-106]. Thus, elevated ADMA levels could promote renal vasoconstriction by blocking NO synthesis in the endothelium of renal vessels as well as potentiating the effects of perivascular sympathetic nerves. This would lead to impairment of renal function and SDMA retention.
Several basic and clinical studies have established that the liver is an important organ in the metabolism of ADMA. As ADMA is an endogenous inhibitor of NOS, changes in the liver function could affect ADMA levels and interfere with the NO synthesis. Despite this, there is still a concerning gap in the knowledge, understanding, and general awareness of mechanisms involved in the vascular dysfunction associated to cirrhosis. The regulation of methylarginine metabolism by modulating cellular PRMT or DDAH activity will therefore likely present a potential therapeutic option for the treatment of vascular dysfunction associated to liver diseases.
P- Reviewer: Owczarek D S- Editor: Ma YJ L- Editor: A E- Editor: Zhang DN
| 1. | Sessa WC. The nitric oxide synthase family of proteins. J Vasc Res. 1994;31:131-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 343] [Cited by in RCA: 302] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 2. | Furchgott RF, Zawadzki JV. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980;288:373-376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8681] [Cited by in RCA: 8095] [Article Influence: 179.9] [Reference Citation Analysis (1)] |
| 3. | Ignarro LJ, Byrns RE, Buga GM, Wood KS. Endothelium-derived relaxing factor from pulmonary artery and vein possesses pharmacologic and chemical properties identical to those of nitric oxide radical. Circ Res. 1987;61:866-879. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 821] [Cited by in RCA: 783] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 4. | Palmer RM, Ferrige AG, Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987;327:524-526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7858] [Cited by in RCA: 7386] [Article Influence: 194.4] [Reference Citation Analysis (0)] |
| 5. | Waldman SA, Murad F. Biochemical mechanisms underlying vascular smooth muscle relaxation: the guanylate cyclase-cyclic GMP system. J Cardiovasc Pharmacol. 1988;12 Suppl 5:S115-S118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 73] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 6. | Kakimoto Y, Akazawa S. Isolation and identification of N-G,N-G- and N-G,N’-G-dimethyl-arginine, N-epsilon-mono-, di-, and trimethyllysine, and glucosylgalactosyl- and galactosyl-delta-hydroxylysine from human urine. J Biol Chem. 1970;245:5751-5758. [PubMed] |
| 7. | Cooke JP. Asymmetrical dimethylarginine: the Uber marker? Circulation. 2004;109:1813-1818. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 307] [Cited by in RCA: 303] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 8. | Palmer RM, Ashton DS, Moncada S. Vascular endothelial cells synthesize nitric oxide from L-arginine. Nature. 1988;333:664-666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3437] [Cited by in RCA: 3373] [Article Influence: 91.2] [Reference Citation Analysis (0)] |
| 9. | Rees DD, Palmer RM, Schulz R, Hodson HF, Moncada S. Characterization of three inhibitors of endothelial nitric oxide synthase in vitro and in vivo. Br J Pharmacol. 1990;101:746-752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1468] [Cited by in RCA: 1485] [Article Influence: 42.4] [Reference Citation Analysis (0)] |
| 10. | Vallance P, Leone A, Calver A, Collier J, Moncada S. Endogenous dimethylarginine as an inhibitor of nitric oxide synthesis. J Cardiovasc Pharmacol. 1992;20 Suppl 12:S60-S62. [PubMed] |
| 11. | Vallance P, Leone A, Calver A, Collier J, Moncada S. Accumulation of an endogenous inhibitor of nitric oxide synthesis in chronic renal failure. Lancet. 1992;339:572-575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1547] [Cited by in RCA: 1541] [Article Influence: 46.7] [Reference Citation Analysis (0)] |
| 12. | Bode-Böger SM, Scalera F, Kielstein JT, Martens-Lobenhoffer J, Breithardt G, Fobker M, Reinecke H. Symmetrical dimethylarginine: a new combined parameter for renal function and extent of coronary artery disease. J Am Soc Nephrol. 2006;17:1128-1134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 225] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 13. | Schepers E, Glorieux G, Dhondt A, Leybaert L, Vanholder R. Role of symmetric dimethylarginine in vascular damage by increasing ROS via store-operated calcium influx in monocytes. Nephrol Dial Transplant. 2009;24:1429-1435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 109] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 14. | Gary JD, Clarke S. RNA and protein interactions modulated by protein arginine methylation. Prog Nucleic Acid Res Mol Biol. 1998;61:65-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 404] [Cited by in RCA: 406] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 15. | Vallance P, Leiper J. Cardiovascular biology of the asymmetric dimethylarginine: dimethylarginine dimethylaminohydrolase pathway. Arterioscler Thromb Vasc Biol. 2004;24:1023-1030. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 406] [Cited by in RCA: 420] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 16. | Ito A, Tsao PS, Adimoolam S, Kimoto M, Ogawa T, Cooke JP. Novel mechanism for endothelial dysfunction: dysregulation of dimethylarginine dimethylaminohydrolase. Circulation. 1999;99:3092-3095. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 443] [Cited by in RCA: 451] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 17. | Tran CT, Leiper JM, Vallance P. The DDAH/ADMA/NOS pathway. Atheroscler Suppl. 2003;4:33-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 223] [Cited by in RCA: 230] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 18. | Böger RH, Sydow K, Borlak J, Thum T, Lenzen H, Schubert B, Tsikas D, Bode-Böger SM. LDL cholesterol upregulates synthesis of asymmetrical dimethylarginine in human endothelial cells: involvement of S-adenosylmethionine-dependent methyltransferases. Circ Res. 2000;87:99-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 343] [Cited by in RCA: 345] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 19. | Lin KY, Ito A, Asagami T, Tsao PS, Adimoolam S, Kimoto M, Tsuji H, Reaven GM, Cooke JP. Impaired nitric oxide synthase pathway in diabetes mellitus: role of asymmetric dimethylarginine and dimethylarginine dimethylaminohydrolase. Circulation. 2002;106:987-992. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 506] [Cited by in RCA: 508] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 20. | Stühlinger MC, Tsao PS, Her JH, Kimoto M, Balint RF, Cooke JP. Homocysteine impairs the nitric oxide synthase pathway: role of asymmetric dimethylarginine. Circulation. 2001;104:2569-2575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 446] [Cited by in RCA: 447] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 21. | Sydow K, Münzel T. ADMA and oxidative stress. Atheroscler Suppl. 2003;4:41-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 307] [Cited by in RCA: 322] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 22. | Cardounel AJ, Cui H, Samouilov A, Johnson W, Kearns P, Tsai AL, Berka V, Zweier JL. Evidence for the pathophysiological role of endogenous methylarginines in regulation of endothelial NO production and vascular function. J Biol Chem. 2007;282:879-887. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 187] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 23. | Ogawa T, Kimoto M, Sasaoka K. Purification and properties of a new enzyme, NG,NG-dimethylarginine dimethylaminohydrolase, from rat kidney. J Biol Chem. 1989;264:10205-10209. [PubMed] |
| 24. | Kimoto M, Tsuji H, Ogawa T, Sasaoka K. Detection of NG,NG-dimethylarginine dimethylaminohydrolase in the nitric oxide-generating systems of rats using monoclonal antibody. Arch Biochem Biophys. 1993;300:657-662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 86] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 25. | Kimoto M, Whitley GS, Tsuji H, Ogawa T. Detection of NG,NG-dimethylarginine dimethylaminohydrolase in human tissues using a monoclonal antibody. J Biochem. 1995;117:237-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 120] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 26. | Nijveldt RJ, Teerlink T, Siroen MP, van Lambalgen AA, Rauwerda JA, van Leeuwen PA. The liver is an important organ in the metabolism of asymmetrical dimethylarginine (ADMA). Clin Nutr. 2003;22:17-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 126] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 27. | Ogawa T, Kimoto M, Watanabe H, Sasaoka K. Metabolism of NG,NG-and NG,N’G-dimethylarginine in rats. Arch Biochem Biophys. 1987;252:526-537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 116] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 28. | Ogawa T, Kimoto M, Sasaoka K. Dimethylarginine: pyruvate aminotransferase in rats. Purification, properties, and identity with alanine: glyoxylate aminotransferase 2. J Biol Chem. 1990;265:20938-20945. [PubMed] |
| 29. | Leiper J, Vallance P. Biological significance of endogenous methylarginines that inhibit nitric oxide synthases. Cardiovasc Res. 1999;43:542-548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 338] [Cited by in RCA: 341] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 30. | Lluch P, Torondel B, Medina P, Segarra G, Del Olmo JA, Serra MA, Rodrigo JM. Plasma concentrations of nitric oxide and asymmetric dimethylarginine in human alcoholic cirrhosis. J Hepatol. 2004;41:55-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 81] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 31. | Nijveldt RJ, Teerlink T, Van Der Hoven B, Siroen MP, Kuik DJ, Rauwerda JA, van Leeuwen PA. Asymmetrical dimethylarginine (ADMA) in critically ill patients: high plasma ADMA concentration is an independent risk factor of ICU mortality. Clin Nutr. 2003;22:23-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 185] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 32. | Siroen MP, Warlé MC, Teerlink T, Nijveldt RJ, Kuipers EJ, Metselaar HJ, Tilanus HW, Kuik DJ, van der Sijp JR, Meijer S. The transplanted liver graft is capable of clearing asymmetric dimethylarginine. Liver Transpl. 2004;10:1524-1530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 36] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 33. | Tsikas D, Rode I, Becker T, Nashan B, Klempnauer J, Frölich JC. Elevated plasma and urine levels of ADMA and 15(S)-8-iso-PGF2alpha in end-stage liver disease. Hepatology. 2003;38:1063-1064. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 34. | Vizzutti F, Romanelli RG, Arena U, Rega L, Brogi M, Calabresi C, Masini E, Tarquini R, Zipoli M, Boddi V. ADMA correlates with portal pressure in patients with compensated cirrhosis. Eur J Clin Invest. 2007;37:509-515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 32] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 35. | Nijveldt RJ, Teerlink T, Siroen MP, van der Hoven B, Prins HA, Wiezer MJ, Meijer C, van der Sijp JR, Cuesta MA, Meijer S. Elevation of asymmetric dimethylarginine (ADMA) in patients developing hepatic failure after major hepatectomy. JPEN J Parenter Enteral Nutr. 2004;28:382-387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 36. | Siroen MP, van der Sijp JR, Teerlink T, van Schaik C, Nijveldt RJ, van Leeuwen PA. The human liver clears both asymmetric and symmetric dimethylarginine. Hepatology. 2005;41:559-565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 77] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 37. | Pope AJ, Karrupiah K, Kearns PN, Xia Y, Cardounel AJ. Role of dimethylarginine dimethylaminohydrolases in the regulation of endothelial nitric oxide production. J Biol Chem. 2009;284:35338-35347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 74] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 38. | Mookerjee RP, Mehta G, Balasubramaniyan V, Mohamed Fel Z, Davies N, Sharma V, Iwakiri Y, Jalan R. Hepatic dimethylarginine-dimethylaminohydrolase1 is reduced in cirrhosis and is a target for therapy in portal hypertension. J Hepatol. 2015;62:325-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 58] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 39. | Leiper J, Nandi M, Torondel B, Murray-Rust J, Malaki M, O’Hara B, Rossiter S, Anthony S, Madhani M, Selwood D. Disruption of methylarginine metabolism impairs vascular homeostasis. Nat Med. 2007;13:198-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 342] [Cited by in RCA: 310] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 40. | Asagami T, Abbasi F, Stuelinger M, Lamendola C, McLaughlin T, Cooke JP, Reaven GM, Tsao PS. Metformin treatment lowers asymmetric dimethylarginine concentrations in patients with type 2 diabetes. Metabolism. 2002;51:843-846. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 137] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 41. | Böger RH, Bode-Böger SM, Szuba A, Tsao PS, Chan JR, Tangphao O, Blaschke TF, Cooke JP. Asymmetric dimethylarginine (ADMA): a novel risk factor for endothelial dysfunction: its role in hypercholesterolemia. Circulation. 1998;98:1842-1847. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 805] [Cited by in RCA: 826] [Article Influence: 30.6] [Reference Citation Analysis (0)] |
| 42. | Surdacki A, Nowicki M, Sandmann J, Tsikas D, Boeger RH, Bode-Boeger SM, Kruszelnicka-Kwiatkowska O, Kokot F, Dubiel JS, Froelich JC. Reduced urinary excretion of nitric oxide metabolites and increased plasma levels of asymmetric dimethylarginine in men with essential hypertension. J Cardiovasc Pharmacol. 1999;33:652-658. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 296] [Cited by in RCA: 298] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 43. | Usui M, Matsuoka H, Miyazaki H, Ueda S, Okuda S, Imaizumi T. Increased endogenous nitric oxide synthase inhibitor in patients with congestive heart failure. Life Sci. 1998;62:2425-2430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 168] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 44. | Hermenegildo C, Medina P, Peiró M, Segarra G, Vila JM, Ortega J, Lluch S. Plasma concentration of asymmetric dimethylarginine, an endogenous inhibitor of nitric oxide synthase, is elevated in hyperthyroid patients. J Clin Endocrinol Metab. 2002;87:5636-5640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 54] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 45. | Cooke JP. Does ADMA cause endothelial dysfunction? Arterioscler Thromb Vasc Biol. 2000;20:2032-2037. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 384] [Cited by in RCA: 382] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 46. | Böger RH, Bode-Böger SM, Tsao PS, Lin PS, Chan JR, Cooke JP. An endogenous inhibitor of nitric oxide synthase regulates endothelial adhesiveness for monocytes. J Am Coll Cardiol. 2000;36:2287-2295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 179] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 47. | Böger RH, Bode-Böger SM, Sydow K, Heistad DD, Lentz SR. Plasma concentration of asymmetric dimethylarginine, an endogenous inhibitor of nitric oxide synthase, is elevated in monkeys with hyperhomocyst(e)inemia or hypercholesterolemia. Arterioscler Thromb Vasc Biol. 2000;20:1557-1564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 176] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 48. | Schnabel R, Blankenberg S, Lubos E, Lackner KJ, Rupprecht HJ, Espinola-Klein C, Jachmann N, Post F, Peetz D, Bickel C. Asymmetric dimethylarginine and the risk of cardiovascular events and death in patients with coronary artery disease: results from the AtheroGene Study. Circ Res. 2005;97:e53-e59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 278] [Cited by in RCA: 286] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 49. | Lu TM, Ding YA, Lin SJ, Lee WS, Tai HC. Plasma levels of asymmetrical dimethylarginine and adverse cardiovascular events after percutaneous coronary intervention. Eur Heart J. 2003;24:1912-1919. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 143] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 50. | Martín-Sanz P, Olmedilla L, Dulin E, Casado M, Callejas NA, Pérez-Peña J, Garutti I, Sanz J, Calleja J, Barrigón S. Presence of methylated arginine derivatives in orthotopic human liver transplantation: relevance for liver function. Liver Transpl. 2003;9:40-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 21] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 51. | Tripepi G, Mattace Raso F, Sijbrands E, Seck MS, Maas R, Boger R, Witteman J, Rapisarda F, Malatino L, Mallamaci F. Inflammation and asymmetric dimethylarginine for predicting death and cardiovascular events in ESRD patients. Clin J Am Soc Nephrol. 2011;6:1714-1721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 86] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 52. | Vairappan B. Endothelial dysfunction in cirrhosis: Role of inflammation and oxidative stress. World J Hepatol. 2015;7:443-459. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 74] [Cited by in RCA: 70] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 53. | Miyazaki H, Matsuoka H, Cooke JP, Usui M, Ueda S, Okuda S, Imaizumi T. Endogenous nitric oxide synthase inhibitor: a novel marker of atherosclerosis. Circulation. 1999;99:1141-1146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 497] [Cited by in RCA: 500] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 54. | Antoniades C, Demosthenous M, Tousoulis D, Antonopoulos AS, Vlachopoulos C, Toutouza M, Marinou K, Bakogiannis C, Mavragani K, Lazaros G. Role of asymmetrical dimethylarginine in inflammation-induced endothelial dysfunction in human atherosclerosis. Hypertension. 2011;58:93-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 72] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 55. | Kwaśny-Krochin B, Głuszko P, Undas A. Plasma asymmetric dimethylarginine in active rheumatoid arthritis: links with oxidative stress and inflammation. Pol Arch Med Wewn. 2012;122:270-276. [PubMed] |
| 56. | Owczarek D, Cibor D, Mach T. Asymmetric dimethylarginine (ADMA), symmetric dimethylarginine (SDMA), arginine, and 8-iso-prostaglandin F2alpha (8-iso-PGF2alpha) level in patients with inflammatory bowel diseases. Inflamm Bowel Dis. 2010;16:52-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 50] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 57. | Carraro S, Giordano G, Piacentini G, Kantar A, Moser S, Cesca L, Berardi M, Di Gangi IM, Baraldi E. Asymmetric dimethylarginine in exhaled breath condensate and serum of children with asthma. Chest. 2013;144:405-410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 44] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 58. | Martin PY, Ginès P, Schrier RW. Nitric oxide as a mediator of hemodynamic abnormalities and sodium and water retention in cirrhosis. N Engl J Med. 1998;339:533-541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 310] [Cited by in RCA: 272] [Article Influence: 10.1] [Reference Citation Analysis (33)] |
| 59. | Siqueira C, de Moura MC, Pedro AJ, Rocha P. Elevated nitric oxide and 3’,5’ cyclic guanosine monophosphate levels in patients with alcoholic cirrhosis. World J Gastroenterol. 2008;14:236-242. [PubMed] |
| 60. | Gupta TK, Toruner M, Chung MK, Groszmann RJ. Endothelial dysfunction and decreased production of nitric oxide in the intrahepatic microcirculation of cirrhotic rats. Hepatology. 1998;28:926-931. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 294] [Cited by in RCA: 261] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 61. | Rockey DC, Chung JJ. Reduced nitric oxide production by endothelial cells in cirrhotic rat liver: endothelial dysfunction in portal hypertension. Gastroenterology. 1998;114:344-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 301] [Cited by in RCA: 268] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 62. | Sarela AI, Mihaimeed FM, Batten JJ, Davidson BR, Mathie RT. Hepatic and splanchnic nitric oxide activity in patients with cirrhosis. Gut. 1999;44:749-753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 80] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 63. | Tome S, Lucey MR. Review article: current management of alcoholic liver disease. Aliment Pharmacol Ther. 2004;19:707-714. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 71] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 64. | Bolognesi M, Di Pascoli M, Verardo A, Gatta A. Splanchnic vasodilation and hyperdynamic circulatory syndrome in cirrhosis. World J Gastroenterol. 2014;20:2555-2563. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 133] [Cited by in RCA: 151] [Article Influence: 13.7] [Reference Citation Analysis (3)] |
| 65. | Pugh RN, Murray-Lyon IM, Dawson JL, Pietroni MC, Williams R. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg. 1973;60:646-649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5490] [Cited by in RCA: 5735] [Article Influence: 110.3] [Reference Citation Analysis (2)] |
| 66. | Kielstein JT, Böger RH, Bode-Böger SM, Frölich JC, Haller H, Ritz E, Fliser D. Marked increase of asymmetric dimethylarginine in patients with incipient primary chronic renal disease. J Am Soc Nephrol. 2002;13:170-176. [PubMed] |
| 67. | Savvidou MD, Hingorani AD, Tsikas D, Frölich JC, Vallance P, Nicolaides KH. Endothelial dysfunction and raised plasma concentrations of asymmetric dimethylarginine in pregnant women who subsequently develop pre-eclampsia. Lancet. 2003;361:1511-1517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 369] [Cited by in RCA: 349] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 68. | Mookerjee RP, Malaki M, Davies NA, Hodges SJ, Dalton RN, Turner C, Sen S, Williams R, Leiper J, Vallance P. Increasing dimethylarginine levels are associated with adverse clinical outcome in severe alcoholic hepatitis. Hepatology. 2007;45:62-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 85] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 69. | Böger RH, Bode-Böger SM, Thiele W, Junker W, Alexander K, Frölich JC. Biochemical evidence for impaired nitric oxide synthesis in patients with peripheral arterial occlusive disease. Circulation. 1997;95:2068-2074. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 323] [Cited by in RCA: 319] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 70. | Wang J, Sim AS, Wang XL, Salonikas C, Naidoo D, Wilcken DE. Relations between plasma asymmetric dimethylarginine (ADMA) and risk factors for coronary disease. Atherosclerosis. 2006;184:383-388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 68] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 71. | Wilcken DE, Sim AS, Wang J, Wang XL. Asymmetric dimethylarginine (ADMA) in vascular, renal and hepatic disease and the regulatory role of L-arginine on its metabolism. Mol Genet Metab. 2007;91:309-17; discussion 308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 55] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 72. | Leiper J, Murray-Rust J, McDonald N, Vallance P. S-nitrosylation of dimethylarginine dimethylaminohydrolase regulates enzyme activity: further interactions between nitric oxide synthase and dimethylarginine dimethylaminohydrolase. Proc Natl Acad Sci USA. 2002;99:13527-13532. [PubMed] |
| 73. | Vallance P, Collier J, Moncada S. Effects of endothelium-derived nitric oxide on peripheral arteriolar tone in man. Lancet. 1989;2:997-1000. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1105] [Cited by in RCA: 1108] [Article Influence: 30.8] [Reference Citation Analysis (0)] |
| 74. | Sieber CC, Groszmann RJ. Nitric oxide mediates hyporeactivity to vasopressors in mesenteric vessels of portal hypertensive rats. Gastroenterology. 1992;103:235-239. [PubMed] |
| 75. | Laleman W, Omasta A, Van de Casteele M, Zeegers M, Vander Elst I, Van Landeghem L, Severi T, van Pelt J, Roskams T, Fevery J. A role for asymmetric dimethylarginine in the pathophysiology of portal hypertension in rats with biliary cirrhosis. Hepatology. 2005;42:1382-1390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 59] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 76. | Tain YL, Hsieh CS, Chen CC, Sheen JM, Lee CT, Huang LT. Melatonin prevents increased asymmetric dimethylarginine in young rats with bile duct ligation. J Pineal Res. 2010;48:212-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 31] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 77. | Langer DA, Shah VH. A gas, an amino acid, and an imposter: the story of nitric oxide, L-arginine, and ADMA in portal hypertension. Hepatology. 2005;42:1255-1257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 78. | Serna E, Mauricio MD, Lluch P, Segarra G, Cortina B, Lluch S, Medina P. Basal release of nitric oxide in the mesenteric artery in portal hypertension and cirrhosis: role of dimethylarginine dimethylaminohydrolase. J Gastroenterol Hepatol. 2013;28:880-886. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 79. | Kielstein JT, Böger RH, Bode-Böger SM, Martens-Lobenhoffer J, Lonnemann G, Frölich JC, Haller H, Fliser D. Low dialysance of asymmetric dimethylarginine (ADMA)--in vivo and in vitro evidence of significant protein binding. Clin Nephrol. 2004;62:295-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 28] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 80. | Majano PL, Garcia-Monzon C. Does nitric oxide play a pathogenic role in hepatitis C virus infection? Cell Death Differ. 2003;10 Suppl 1:S13-S15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 81. | Amaro MJ, Bartolomé J, Pardo M, Cotonat T, López-Farré A, Carreño V. Decreased nitric oxide production in chronic viral hepatitis B and C. J Med Virol. 1997;51:326-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 82. | Hokari A, Zeniya M, Esumi H, Ishikawa T, Kurasima Y, Toda G. Role of nitric oxide (NO) in interferon-alpha therapy for hepatitis C. J Infect. 2005;51:47-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 83. | Lluch P, Cortina B, Vila JM, Segarra G, Mauricio MD, del Olmo JA, Serra MA, Lluch S, Rodrigo JM. Unchanged plasma levels of dimethylarginines and nitric oxide in chronic hepatitis C. Scand J Gastroenterol. 2009;44:224-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 84. | Lluch P, Mauricio MD, Vila JM, Segarra G, Medina P, Del Olmo JA, Rodrigo JM, Serra MA. Accumulation of symmetric dimethylarginine in hepatorenal syndrome. Exp Biol Med (Maywood). 2006;231:70-75. [PubMed] |
| 85. | Sharara AI, Perkins DJ, Misukonis MA, Chan SU, Dominitz JA, Weinberg JB. Interferon (IFN)-alpha activation of human blood mononuclear cells in vitro and in vivo for nitric oxide synthase (NOS) type 2 mRNA and protein expression: possible relationship of induced NOS2 to the anti-hepatitis C effects of IFN-alpha in vivo. J Exp Med. 1997;186:1495-1502. [PubMed] |
| 86. | Mihm S, Fayyazi A, Ramadori G. Hepatic expression of inducible nitric oxide synthase transcripts in chronic hepatitis C virus infection: relation to hepatic viral load and liver injury. Hepatology. 1997;26:451-458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 53] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 87. | Ersoy Y, Bayraktar NM, Mizrak B, Ozerol IH, Gunal S, Aladag M, Bayindir Y. The level of endothelin-1 and nitric oxide in patients with chronic viral hepatitis B and C and correlation with histopathological grading and staging. Hepatol Res. 2006;34:111-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 88. | George M, Baluch M, Van Thiel DH. Plasma and hepatic tissue levels of thrombomodulin, tissue factor, NFkappaB and nitric oxide in responders and nonresponders to IFNalpha therapy. J Viral Hepat. 2003;10:360-366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 89. | Ginès P, Guevara M, Arroyo V, Rodés J. Hepatorenal syndrome. Lancet. 2003;362:1819-1827. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 443] [Cited by in RCA: 372] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 90. | Nijveldt RJ, Teerlink T, van Leeuwen PA. The asymmetrical dimethylarginine (ADMA)-multiple organ failure hypothesis. Clin Nutr. 2003;22:99-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 65] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 91. | Segarra G, Medina P, Vila JM, Chuan P, Domenech C, Torondel B, Lluch A. Inhibition of nitric oxide activity by arginine analogs in human renal arteries. Am J Hypertens. 2001;14:1142-1148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 92. | Fleck C, Janz A, Schweitzer F, Karge E, Schwertfeger M, Stein G. Serum concentrations of asymmetric (ADMA) and symmetric (SDMA) dimethylarginine in renal failure patients. Kidney Int Suppl. 2001;78:S14-S18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 49] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 93. | Kielstein JT, Salpeter SR, Bode-Boeger SM, Cooke JP, Fliser D. Symmetric dimethylarginine (SDMA) as endogenous marker of renal function--a meta-analysis. Nephrol Dial Transplant. 2006;21:2446-2451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 256] [Cited by in RCA: 284] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 94. | Al Banchaabouchi M, Marescau B, Possemiers I, D’Hooge R, Levillain O, De Deyn PP. NG, NG-dimethylarginine and NG, NG-dimethylarginine in renal insufficiency. Pflugers Arch. 2000;439:524-531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 22] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 95. | Goonasekera CD, Rees DD, Woolard P, Frend A, Shah V, Dillon MJ. Nitric oxide synthase inhibitors and hypertension in children and adolescents. J Hypertens. 1997;15:901-909. [PubMed] |
| 96. | Marescau B, Nagels G, Possemiers I, De Broe ME, Becaus I, Billiouw JM, Lornoy W, De Deyn PP. Guanidino compounds in serum and urine of nondialyzed patients with chronic renal insufficiency. Metabolism. 1997;46:1024-1031. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 122] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 97. | Carello KA, Whitesall SE, Lloyd MC, Billecke SS, D’Alecy LG. Asymmetrical dimethylarginine plasma clearance persists after acute total nephrectomy in rats. Am J Physiol Heart Circ Physiol. 2006;290:H209-H216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 41] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 98. | Pedersen LG, Tarnow I, Olsen LH, Teerlink T, Pedersen HD. Body size, but neither age nor asymptomatic mitral regurgitation, influences plasma concentrations of dimethylarginines in dogs. Res Vet Sci. 2006;80:336-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 99. | Tojo A, Welch WJ, Bremer V, Kimoto M, Kimura K, Omata M, Ogawa T, Vallance P, Wilcox CS. Colocalization of demethylating enzymes and NOS and functional effects of methylarginines in rat kidney. Kidney Int. 1997;52:1593-1601. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 161] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 100. | La Villa G, Barletta G, Pantaleo P, Del Bene R, Vizzutti F, Vecchiarino S, Masini E, Perfetto F, Tarquini R, Gentilini P. Hemodynamic, renal, and endocrine effects of acute inhibition of nitric oxide synthase in compensated cirrhosis. Hepatology. 2001;34:19-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 63] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 101. | Martin PY, Ohara M, Gines P, Xu DL, St John J, Niederberger M, Schrier RW. Nitric oxide synthase (NOS) inhibition for one week improves renal sodium and water excretion in cirrhotic rats with ascites. J Clin Invest. 1998;101:235-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 82] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 102. | Segarra G, Medina P, Ballester RM, Lluch P, Aldasoro M, Vila JM, Lluch S, Pelligrino DA. Effects of some guanidino compounds on human cerebral arteries. Stroke. 1999;30:2206-210; discussion 2206-210;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 54] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 103. | Martínez C, Vila JM, Aldasoro M, Medina P, Chuan P, Lluch S. The human deferential artery: endothelium-mediated contraction in response to adrenergic stimulation. Eur J Pharmacol. 1994;261:73-78. [PubMed] |
| 104. | Martínez C, Cases E, Vila JM, Aldasoro M, Medina P, Marco V, Lluch S. Influence of endothelial nitric oxide on neurogenic contraction of human pulmonary arteries. Eur Respir J. 1995;8:1328-1332. [PubMed] |
| 105. | Aldasoro M, Martínez C, Vila JM, Medina P, Lluch S. Influence of endothelial nitric oxide on adrenergic contractile responses of human cerebral arteries. J Cereb Blood Flow Metab. 1996;16:623-628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 106. | Torondel B, Vila JM, Segarra G, Lluch P, Medina P, Martínez-León J, Ortega J, Lluch S. Endothelium-dependent responses in human isolated thyroid arteries from donors. J Endocrinol. 2004;181:379-384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |