Published online Aug 21, 2015. doi: 10.3748/wjg.v21.i31.9420
Peer-review started: October 1, 2014
First decision: November 26, 2014
Revised: December 21, 2014
Accepted: April 3, 2015
Article in press: April 3, 2015
Published online: August 21, 2015
Processing time: 323 Days and 19.9 Hours
AIM: To present a systematic review of techniques and clinical results.
METHODS: A systematic review of published literature was performed. Only studies reporting patient outcome after radiosurgery (single fraction) delivered with robotic devices [i.e., robotic radiosurgery (RRS)] have been analyzed.
RESULTS: A total of 96 patients from 5 studies were included. The studies are characterized by small series and different methods in terms of dose, target definition, combination with chemotherapy and/or standard fractionated radiotherapy and evaluation modalities. Preliminary results are positive in terms of tumor response (ORR = 56%) and local control of the tumor (crude rate of local progressions: 19.5%). Results for median overall survival (11.4 mo) seem comparable with the ones of prolonged chemoradiation (range: 8.6-13.0 mo). However, gastrointestinal toxicity seems to be the main limitation of RRS, especially at the duodenal level.
CONCLUSION: RRS allows for local treatment in a shortened time (1 fraction) compared to traditional treatments (about 1 mo), providing the possibility for an easy integration with systemic therapies. Preliminary results did not show any outcome differences compared to standard chemoradiation. Thus, further efforts to reduce gastrointestinal toxicity are strongly needed.
Core tip: Robotic radiosurgery, a type of stereotactic body radiotherapy, has been applied in a few experiences as an alternative to long course, conventional radiotherapy. As described in this systematic review, results suggest a good profile of efficacy. Its use in further trials appears justified to treat pancreatic lesions. Particular attention is needed to manage acute and late toxicity. Its potential is highly interesting for the opportunity of integration with chemotherapy and surgery.
- Citation: Buwenge M, Cellini F, Silvestris N, Cilla S, Deodato F, Macchia G, Mattiucci GC, Valentini V, Morganti AG. Robotic radiosurgery in pancreatic cancer: A systematic review. World J Gastroenterol 2015; 21(31): 9420-9429
- URL: https://www.wjgnet.com/1007-9327/full/v21/i31/9420.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i31.9420
The prognosis of pancreatic cancer is dismal. Even in patients with non-metastatic disease at diagnosis, recurrences after primary therapy are very common both as local relapse/progression and as distant metastases. Local recurrence rates even for patients who have undergone surgery reach percentages of 70%-80%[1,2]. In addition, local disease progression can produce severe symptoms (pain, biliary and/or intestinal obstruction, malnutrition) significantly worsening the quality of life of patients.
Traditionally, radiotherapy (RT) has been used to obtain a local control of the disease. RT, usually associated with concurrent and adjuvant chemotherapy, is potentially useful to improve the resection rate[3], control symptoms in locally advanced carcinomas[4] and to reduce the risk of recurrence in resected patients[5]. The main limitation of RT is the presence of radiosensitive organs in the upper abdomen in close proximity with the pancreas. In fact, due to these anatomic relationships, RT can produce severe side effects especially at the level of the duodenum. Therefore, a strong interest has arisen in the use of RT techniques gaining a higher level of precision with the aim of administering effective doses to the target while reducing the irradiation of surrounding healthy organs.
One of the most promising newer techniques is robotic radiosurgery (RRS). This is a particular technique of stereotactic body radiotherapy (SBRT). Based on American Society of Radiation Oncology definition, SBRT is an external beam technique able to deliver high radiation dose to an extra-cranial body target with high precision in a single or few fractions[6]. RRS is based on the delivery of a single large fraction of radiation using a robotic linear accelerator. The reduced volume of irradiated normal tissue achieved by improving the treatment precision allows the delivery of a single fraction of radiation (with RRS), which can potentially ablate all tissue in the treated area. However, in literature there is currently only limited evidence on SBRT, represented by preliminary studies generally performed on small patient populations[7-16]. Evidence on RRS is even more limited[17-21]. Therefore, the purpose of this analysis is to present a systematic review of the techniques and clinical results of RRS in pancreatic cancer.
Type of studies: In this review were included all studies (case studies or clinical trials) reporting outcome and toxicity of patients treated with RSS.
Type of participants: Only studies enrolling patients suffering from unresectable and/or locally advanced adenocarcinoma of the pancreas were included in this analysis.
Type of interventions: (1) radiotherapy - eligible interventions were single fractionated radiation therapy performed with a robotic machine; (2) chemotherapy - all systematic treatments based on chemotherapy, regardless of the type of antineoplastic agent and the use of single or combination chemotherapy were eligible; and (3) supportive care - studies were included in the analysis regardless of the type of supportive therapy or other palliative treatments, including blood transfusions, analgesic treatments, bypass palliative interventions or stents placement.
Type of outcome measures: Primary endpoint of the analysis was overall survival after RRS and secondary endpoints were: clinical response, local control, and treatment-related toxicity.
A bibliographic research was performed based on the PRISMA methodology[22] using PubMed. In the database, a search was carried out using the Medical Subject Heading (MeSH) database; the search algorithm was ‘‘Radiotherapy” [MeSH] AND ‘‘Pancreatic” [MeSH] AND (“robotic” OR “cyberknife”). The research in Pubmed was complemented by an additional screening of the references of publication identified through the database. The search was not limited to a particular time interval. It was restricted to English-language peer-reviewed journal publications. The found papers were independently selected and evaluated by two different authors (Buwenge M and Cellini F). Any discrepancies in the selection of papers and data collection were managed by the senior author (AGM). Potentially eligible studies were retrieved and a full-text evaluation was performed as to whether it satisfied both the inclusion and exclusion criteria. Only clinical studies on RRS delivered with robotic devices in patients with pancreatic carcinoma were included in the review process. Studies including patients with metastatic disease were not excluded.
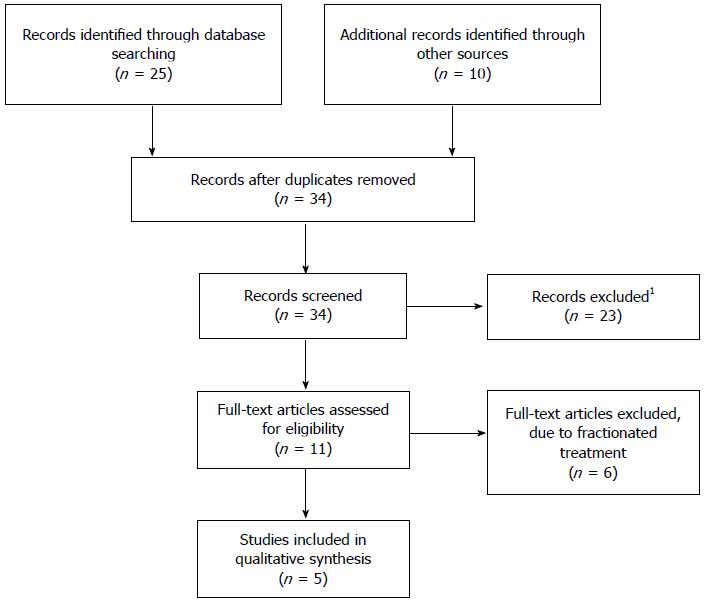
Through the literature search, performed as previously described, 35 papers were identified. Figure 1 describes the process of paper selection. Five studies fulfilled the inclusion criteria and were included in this review.
Koong et al[17] designed a phase I trial for patients with locally advanced carcinoma of the pancreas. The purpose of the study was to define the maximum tolerated dose of RRS. The authors enrolled 15 patients at three subsequent dose levels: 15 Gy, 20 Gy, and 25 Gy. Clinical response was evaluated using high resolution CT and acute toxicity was scored with the RTOG scale. Local tumor control was recorded in all 6 patients who received 25 Gy without cases of grade > 3 gastrointestinal toxicity. Given the result in terms of tumor response, the trial was stopped even if no cases of dose-limiting toxicity were recorded. Based on these results, the authors concluded that the recommended dose for the RRS is 25 Gy.
Koong et al[18] enrolled 19 patients with locally advanced pancreatic cancer in a subsequent trial. Treatment was based on IMRT (45 Gy with concurrent 5-fluorouracil) followed by RRS boost (25 Gy). Sixteen of 19 patients completed the treatment and only one of these developed local progression of the disease while 2 patients showed grade 3 toxicity. Considering a median survival of only 33 wk, the authors concluded that this treatment regimen although feasible, seems not able to improve survival.
Seo et al[20] performed a retrospective analysis of 30 patients with similar stage of disease. These patients underwent conformal radiotherapy (40 Gy) followed by RRS boost (14-17 Gy). Twenty-one patients had received chemotherapy. One-year local progression-free survival (LPFS) was 70.2% and 1-year overall survival (OS) was 60.0%. Given an incidence of grade 4 toxicity of only 3%, the authors concluded that this regimen is feasible and deserves further evaluation in prospective studies.
Goyal et al[21] reported their experience with RRS in patients with unresectable pancreatic cancer. Twenty patients received a RRS dose of 22-30 Gy (median 25 Gy). Chemotherapy was administered in 68% of patients. One-year LPFS was 65% and 1-year OS was 56%. The incidence of grade 3 and grade 4 toxicity was 16% and 0%, respectively. The authors concluded that RRS treatment is tolerable and allows satisfactory local control of the disease.
The details on definition of “locally advanced inoperable lesions” criteria were not specified for all the above previously mentioned reports.
Schellenberg et al[19] designed a prospective trial to test a combined modality treatment including RRS. Sixteen patients with locally advanced disease (defined as “> 50% involvement of the superior mesenteric vein/superior mesenteric artery or any involvement of the celiac axis”) received 1-9 cycles (median: 4) of gemcitabine-based chemotherapy. Patients underwent RRS (25 Gy) between the 1st and 2nd chemotherapy cycles. Local progression of the disease was observed in 19% of patients and 1-year OS was 50% (median: 11.4 mo). The incidence of grade 3 acute toxicity was only 6%. However, 7 patients showed late toxicity: 5 ulcers, 1 duodenal stenosis, and 1 duodenal perforation. In this group of 7 patients there was a trend toward a greater irradiated volume of the duodenum. The authors concluded that using this regimen, the clinical outcome is similar to standard concurrent chemoradiation but with an alarming incidence of intestinal toxicity.
Methods: Table 1 shows study and treatment characteristics of the analyzed series. Of the 5 studies included in this analysis, 2 were phase I trials, 2 were case series and 1 was a phase II trial. The number of patients enrolled was 15-30 (median: 19). In a study in which RRS was used as a boost, only 16/19 patients (84.2%) received the RRS treatment[18]. In another study, 14 patients (73.7%) received RRS treatment, while another 5 patients received a fractionated (3 fractions) treatment[21]. All studies enrolled patients with locally advanced disease. In one study, patients who had received prior radiotherapy were explicitly excluded[20], while in another 2 studies some patients were previously irradiated in the same site[17,21]. In addition, in one study, patients with tumor diameter > 7.5 cm were excluded[17], while in another study patients with more than 3 metastatic lymph nodes or tumor invasion of the duodenum were excluded[20]. The definition of the target was based on a pancreatic protocol CT in 2 studies[17,18], while in other 3 studies even 18F-FDG-PET was used[19-21]. In 3 studies the margins between tumor and PTV were not described[17,18,21]. In the study of Schellenberg a margin of 2-3 mm between GTV and PTV was used[19], while in the study of Seo the PTV was defined as GTV + 2 mm in radial direction and as GTV + 4 mm in cranio-caudal direction[20]. In all studies, an image-guided technique based on fiducial tracking was used. The dose was prescribed to different points. In 3 studies, the dose was prescribed to the isodose completely surrounding the tumor[17-19]. In 1 study, the dose was prescribed to the isodose that covered at least 97% of the PTV[20], while in the last study it was prescribed to 70% isodose[21]. In the two studies in which RRS was used as a boost, the median prescribed dose to the target was 25 Gy in 1 study[18] and 16.5 Gy in the other study[20]. In the studies based on RRS alone, the median prescribed dose to the target was 20 Gy in one study[17] and 25 Gy in 2 other studies[19,21]. All patients received chemotherapy in 2 studies[18,19], while in the other 3 studies the percentage of patients treated with chemotherapy was variable (6.6%-70.0%)[17,20,21]. In the 2 studies reporting tumor response, the RECIST criteria were used[20,21]. Toxicity evaluation was performed using the RTOG scale in 3 studies[17,18,21] and the CTC scale in the other 2 studies[19,21].
| Ref. | Study design | Inclusion criteria | Simulation | PTV | Image guidance | Dose prescription | Radiotherapy dose, median (range) | % of patients receiving chemotherapy |
| Koong et al[17], 2004 | Phase I | LA; < 7.5 cm1 | Pancreatic protocol CT | NR | Fiducials tracking | To isodose surrounding PTV | 20 Gy SF RRS | 6.6 before RRS |
| Koong et al[18], 2005 | Phase II | LA | Pancreatic protocol CT | NR | Mid-breath-hold or fiducials tracking | To isodose surrounding PTV | 45 Gy in 1.8 Gy/fr (IMRT) +; 25 Gy SF RRS boost | 100.0 concurrent to IMRT: 5-FU or CAP |
| Schellenberg et al[19], 2008 | Case serie | LA | End-expiration biphasic CT + respiratory gated CT + PET-CT | PTV: GTV + 2-3 mm | Fiducials tracking | To isodose surrounding PTV | 25 Gy SF RRS | 100.0 1 cycle GEM pre-RRS; 0-8 cycles GEM post-RRS |
| Seo et al[20], 2009 | Phase I | LA; no duodenal invasion; < 3 N+ | CT + PET-CT (restricted respiratory motion) | PTV: GTV + 2 mm, or 4 mm cranio-caudally | Fiducials tracking | To isodose covering 97% of PTV | 40 Gy in 2 Gy/fr (3D-CRT) + 16.5 Gy (14-17) SF RRS boost | 70.0 (6 before RT; 15 concurrent to 3D-CRT) |
| Goyal et al[21], 2012 | Case serie | LA | CT + MRI ± PET-CT | NR | Fiducial tracking | To 70% isodose | 14 pts: 25 (20-25) SF RRS 5 pts: 3 fractions (24-30) | 68% before RRS (various schedules) |
Results: Table 2 reports patient and tumor characteristics of the selected studies. All studies enrolled patients with locally advanced cancer. However, in 3 trials the clinical stage was not reported[17-19], in 1 study all patients had cT4 tumor stage[20], while in the last study even metastatic patients (4/19: 21.1%) were enrolled[21]. Four studies[17-20] reported the GTV size, which varied between 29.0 and 57.2 cm3 (median: 45.5 cm3). In 4 studies, the tumor site in the pancreas was reported[17-20] with a percentage of tumors in the head of the pancreas between 56.7% and 87.5% (median: 67.3%). In 4 papers reporting the median follow-up[17,18,20,21], this was between 5 and 14.5 mo (median: 7.2 mo). Table 3 shows the results of the selected studies.
| Ref. | Patients | Stage | Median GTV size (cm3); mean (range) | Site | Median follow-up (mo), range |
| Koong et al[17], 2004 | 15 | NR | 29 (19-72) | H: 66.6%, B: 26.6%, T: 6.6% | 5.0 |
| Koong et al[18], 2005 | 191 | NR | 50 (14-92) | H: 68%, B: 32% | 5.4 |
| Schellenberg et al[19], 2008 | 16 | NR | PTV: 48 (21-84) | H: 87.5%, B: 12.5% | NR |
| Seo et al[20], 2009 | 30 | T4: 100.0% N1: 30.0% | 41 (21-96) | H: 56.7% B/T: 43.3% | 14.5 |
| Goyal et al[21], 2012 | 19 | M1: 4 pts | 57 (10-118) | NR | 9 (5.8-23.1) |
| Ref. | Tumor response criteria | Tumor response (%) | Median overall survival (mo) | Local control | Toxicity scale | Grade 3-4toxicity |
| Koong et al[17], 2004 | - | NR | 11.0 | LP: 20%1 | RTOG | 0% |
| Koong et al[18], 2005 | - | NR | 7.7; 1-yr: 15% | LP: 6.2%2 | RTOG | Acute: Gastroparesis:10.5%3 |
| Schellenberg et al[19], 2008 | - | NR | 11.4; 1 yr: 50% | LP: 19% | CTC 3.0 | Acute: gastric ulcer: 6.2%; Late: duodenal stenosis: 6.2%; Duodenal perforation: 6.2%4 |
| Seo et al[20], 2009 | RECIST | 68 (PR: 68) | 14.0; 1 yr: 60% | LP: 44% LPFS (1-yr): 70.2 %; | RTOG | Acute: duodenal obstruction: 3.3%; Late: 0% |
| Goyal et al[21], 2012 | RECIST | 44 (CR: 13, PR: 31) | 14.45; 1 yr: 56% | LPFS (1-yr): 65% LPFS (median): 11.4 mo | CTC 3.0 | GI ulcer: 16%6 |
Only 2 studies showed the results in terms of tumor response. In 1 study, a partial response rate of 68% was reported[20], while in another study a partial response rate of 31% and a complete response rate of 13% (overall response rate: 44%) were reported[21]. The crude percentage of local progressions was reported in 4 studies[17-20] with values ranging from 6.2% to 44.0% (median: 19.5%). Two studies[20,21] also reported 1-year local progression-free survival with values of 65.0% and 70.2%, respectively. All studies reported median survival, ranging from 7.7 mo to 14.4 mo (median 11.4 mo)[17-21]. In addition, 4 studies[18-21] presented the results in terms of 1-year survival, with values ranging from 15% to 60% (median: 53%).
In all studies, the only severe toxicity (grade > 3) recorded was gastrointestinal. Three studies reported cases of obstructive damage: 10.5% of acute gastroparesis[18], 12.5% duodenal stenosis[19] and 3.3% of duodenal obstruction[20]. The other type of gastrointestinal toxicity was ulcerative: 6.2% of gastric ulcer and 6.2% of duodenal perforation[19] and 16% of gastrointestinal ulcer[21]. It should be noted that, in some cases, other cases of ulcerative damages were reported but classified as grade < 3 toxicity. More specifically, Koong et al[18] reported an unknown number of late duodenal ulcers, Schellenberg et al[19] reported a percentage 31.2% of G2 ulcers and Goyal et al[20] also described 1 case (5.7%) of asymptomatic pyloric ulcer.
Pancreatic cancer is highly aggressive, and very little space to provide cure for patients is currently available. Significant improvements in diagnostics for staging, and both local (i.e., surgery and radiotherapy) and systemic treatments (i.e., full dose chemotherapy, molecular and immune response targeted therapies) have been reported, however, during recent years[23]. Prognosis is in general dismal, with overall 5-year survival rates inferior to 20% even for favorable presentations[24,25]. Resections with microscopically-free margins (R0) still represent the optimal chance to achieve best survival rates[26], but apart from upfront resectable presentations (generally representing 10%-20% of cases), rates of R0 resection after neoadjuvant treatments are still suboptimal for locally advanced unresectable (LA) and borderline resectable (BR) lesions[23,27,28]. Radiochemotherapy (RTCT) has the potential to convert both LA (in around 23% to 40%)[3,29] and BR lesions (40% to 54%) to resectable[30,31]. The role played by integrating RTCT into the neoadjuvant schedules was questioned by some recent Phase III trials, although with non-definitive results[32,33]. The new integration of modern drugs and modern radiotherapy techniques could enhance the efficacy of RTCT[34,35]. For instance, intensity modulated radiotherapy (IMRT) provided better clinical results in terms of both limitations of treatment toxicity[36] and dose escalation[37]. In this frame, the intriguing potential of stereotatic body radiotherapy (SBRT) to deliver a biologically high effective dose to the tumor, in a much shorter interval (1-5 fractions vs 25-30 fractions), almost without interference with full dose chemotherapy opens new perspectives in both research and routine clinical activities[11]. SBRT obtained clinical outcome for survival at least comparable to the literature for LA lesions in some preliminary experiments, and excellent rates of local control over 90%[7]. Moreover, SBRT gained resection rates of up to 56% for BR lesions[38].
SBRT requires less time to be delivered and is easily integrated with systemic therapies, therefore it also has great potential in the palliative use of radiotherapy for such tumors[39].
RRS of pancreatic cancer presents several theoretical advantages. From the radiobiological point of view, the extreme concentration of the dose in the short time could improve the antineoplastic effect by avoiding the risk of tumor repopulation during the treatment. From the technical point of view, the use of robotic equipment is able to produce an extreme spatial concentration of the dose potentially able to reduce the risk of side effects. In addition, the brevity of the treatment favors the integration with systemic treatments, currently considered as a standard therapeutic option in pancreatic cancer. In order to analyze the results available in the scientific literature, a systematic review was performed. Only little evidence was found to be available on this topic. In particular, only five studies were retrieved within the last decade. These studies are methodologically heterogeneous in terms of inclusion criteria, target definition, dose prescription, chemotherapy usage and criteria for toxicity assessment.
The various studies also present obvious methodological limitations: missing study design in 2 trials[19,21], lack of justification of the sample size in the phase II study[18], no description of the definition of the target in 3 studies[17,18,21], inclusion of patients with metastases in one study[21], inclusion of patients previously irradiated in two studies[17,21], and shortness of the follow-up (< 10 mo) in 3 studies[17,18,21], which severely limits the reliability of the results, especially in terms of late toxicity and rate of local progression. Regarding the latter, it can be observed that the study with higher incidence of local progression (44%) is the one with the longer follow-up period (14.5 mo)[20]. Even the reporting of results shows obvious shortcomings: lack of description of the case series in terms of tumor stage, lymph nodes in 4 studies[17-20] and tumor site in 1 study[21], no description of follow-up observation time in 1 study[19], no description of tumor response in 3 studies[17-19], no description of local control with actuarial analysis in 3 studies[17-19], and no description of the number of cases of duodenal ulceration in 1 study[18].
With these limitations it is not possible to perform some analyses on the studies evaluated in this review. Indeed, the lack of description of target definition prevents assessment of whether this issue affects toxicity and local control. In addition, the lack of description of tumor response or actuarial local control, along with the variable use of chemotherapy, the inhomogeneity of dose prescription methods and of the doses administered within the different case series, prevents analysis of dose response. However, some considerations may be proposed. The few data available on the response rate (ORR = 44%-68%) appear at least comparable to those on standard treatment based on concurrent chemoradiation with conventional fractionation (0%-36%)[40-42]. Even more interesting are the results in terms of survival (median, 11.4 mo) - quite similar to those recorded with standard treatment (range: 8.6%-13.0%)[32,43-50].
The most negative aspect reported in the analyzed studies is represented by gastrointestinal toxicity, especially in terms of ulcerations. In particular, the frequency of such complications has reached in some cases very high rates, mostly for the trials adding chemotherapy, while previous irradiation (for treatments applying RRS as boost of dose) did not clearly enhance toxicity. For example, in the series by Schellenberg and colleagues, when grouping all cases of ulceration regardless of the degree assigned by the authors, the rate of ulcerations reached a percentage of 43.7% of the patients[19]. In contrast, there have been no reports of severe hematological toxicity, frequently observed in studies of concurrent chemoradiation (G3-4: 30.9%)[32].
The use of a standard radiation treatment before RRS does not appear to produce significant benefits. In fact, of the 2 studies using RRS as a boost, one is the paper showing the absolute worst results in terms of local progression (44%)[20] and the other one is the study reporting the worst survival (median: 7.7 mo)[18]. There is no clear relationship between dose and survival. The study showing the better survival (median, 14.4 mo) employed relatively low doses compared to other studies (20-25 Gy), with some patients receiving fractionated treatment[21]. On the contrary, in the study with worse survival (median 7.7 mo) the highest dose (IMRT + RRS: 70 Gy) was delivered[18]. As previously noted, it is particularly difficult to identify factors predictive of toxicity. For example, in the study using the highest dose[18] the rate of late ulcerations was not reported. However, it may be noted that in another study using RRS as a boost[20], the complication rate was relatively low (3.3%), with no cases of ulceration. This fact might be related to the relatively low dose of RRS (median: 16.5 Gy) or to the exclusion of patients with tumor invasion of the duodenum.
On the other hand, the study with the highest rate of ulcers (43.7%) and the only described case of duodenal perforation was the one in which all patients received a dose of 25 Gy RRS[19]. The results in terms of survival seem to suggest the usefulness of integrating RRS with chemotherapy. In fact, in the 3 studies with best results in terms of survival (median: 11.4-14.4 mo) chemotherapy was prescribed in 68%-100% of patients[19-21] while in the 2 studies with worse results (median: 7.7-11.0 mo) chemotherapy was used only in 6.6% of patients[17] or only as concomitant therapy to IMRT[18]. Furthermore, it is also possible that the imaging methods used in staging and planning affected treatment outcome due to a better patient selection. In fact, it can be noted that in the same 3 studies with improved survival[19-21] RRS simulation and planning were based both on pancreatic protocol CT and on 18F-FDG-PET.
The overall conclusion is that future studies of RRS or SBRT appear justified in cancers of the pancreas, considering the practical benefits and the preliminary outcomes, similar to those of standard radiotherapy regimens. It would be helpful if these studies could report the dose-volume histogram parameters to allow correlation analysis between dosimetric data and toxicity. In addition, it would be useful if these studies were planned as part of combined modality treatments based on homogeneous and standard chemotherapy regimens. Furthermore, treatment techniques should be optimized to reduce the incidence of gastrointestinal toxicity. Possible strategies for this purpose may be based on the technique of simultaneous integrated boost (SIB), with a possible reduction of the dose in sites most at risk of ulceration (duodenal wall).
One of the most interesting perspectives of concomitant chemoradiation is the possibility of improving the resectability rate in locally advanced tumors[3]. Therefore, further studies might be aimed at assessing the role of RRS for the same purpose, possibly in combination with neoadjuvant chemotherapy. In fact, the brevity of the RRS would be a useful element to integrate local radiotherapy with systemic therapy and to reduce the delay of the surgery. Recent reports suggest achievement of R0 resection rates up to 55% and 10% for BR and LA presentations, respectively, by the use of modern regimens like FOLFIRINOX[51]. To explore the potential of these schedules some trials are ongoing testing the integration of stereotatic treatments with new drugs like Nab-Paclitaxel (Identifier: NCT02241551) or multidrug regimens like FOLFIRINOX (Identifier: NCT01992705) to understand if even better results are achievable and how toxic such combinations could be.
Finally, it is well known that the treatment of pancreatic tumors at an advanced stage has in most cases a palliative aim and that most of these patients suffer from abdominal and lumbar pain. Therefore, it would be useful to evaluate the role of this technique in pain control, even considering the advantage of using a very brief treatment in patients with poor prognosis.
We thank Caroline Oakley (Scientific Direction - National Cancer Research Centre “Giovanni Paolo II”) for the English Language Assistance.
Pancreatic cancer is one of the most aggressive neoplastic lesions and its prognosis is usually dismal even for more favorable presentations. In addition, local disease progression can produce severe symptoms (pain, biliary and/or intestinal obstruction, malnutrition) significantly worsening the quality of life of patients. Traditionally, radiotherapy (usually associated with concurrent and/or adjuvant chemotherapy) has been used to obtain a local control of the disease. A conventional treatment course of radiotherapy requires on average 1-1.5 mo. This can sometimes be difficult to integrate with full-dose courses of chemotherapy; thus, it is usually required to choose what approach to perform first. One of the most promising and innovative techniques of radiotherapy is robotic radiosurgery. Many technical features of this type of treatment delivery make it appealing in the subset of primary treatments for pancreatic cancers. Clinical evidence is still preliminary, but extremely promising.
The major impact in the treatment of pancreatic cancers is nowadays represented by any attempt to improve the resection rates providing microscopically-negative margins. That includes both the development of more effective chemotherapeutic schedules and the optimization of more effective, less toxic radiotherapy treatment courses. The introduction of a radiotherapy technique allowing delivery of an efficient treatment in few or even one single application could potentially lead to an ideal integration of radiotherapy with full-dose chemotherapy.
The described radiotherapy technique (i.e., robotic radiosurgery), from a technological point of view represents the most advanced form of radiotherapy treatment delivery. It requires particular treatment facilities, providing the highest level of precision in targeting the lesions to irradiate. It allows the highest levels of treatment conformation to the target, thus reducing the amount of normal tissue involved in the radiotherapy fields. Moreover, it applies the delivery of high radiotherapy doses in a short time that biologically increases the damage to the tumor.
The increased level of awareness of the potential efficacy of the described technique will lead to further investigation in the near future of what represents one of the most promising and revolutionary treatment approaches, both regarding its intrinsic efficacy and the possibility of widespread use of a most effective regimen of integration with systemic treatments.
Stereotactic body radiotherapy (SBRT): this is an external beam radiotherapy technique able to deliver high radiation dose to an extra-cranial body target with high precision in a single or few fractions. Robotic radiosurgery: this is a specific type of SBRT treating patients by the delivery of a single large fraction of radiation using a robotic linear accelerator. Robotic linear accelerator: this is a particular type of linear accelerator (for radiotherapy), characterized by the highest level of precision obtainable in the visualization of the target, image-guidance of the treatment delivery and device precision to conform the dose.
The authors systematically review currently available evidence on robotic radiosurgery in the treatment of locally advanced pancreatic cancer. The topic is interesting and timely and the literature review has been well conducted, providing interesting points for discussion and inspiration for performing prospective clinical trials with such novel RT delivery approaches.
P- Reviewer: Milella M, Nakano H, Seicean A S- Editor: Ma YJ L- Editor: Logan S E- Editor: Zhang DN
| 1. | Hishinuma S, Ogata Y, Tomikawa M, Ozawa I, Hirabayashi K, Igarashi S. Patterns of recurrence after curative resection of pancreatic cancer, based on autopsy findings. J Gastrointest Surg. 2006;10:511-518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 243] [Cited by in RCA: 244] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 2. | Kayahara M, Nagakawa T, Ueno K, Ohta T, Takeda T, Miyazaki I. An evaluation of radical resection for pancreatic cancer based on the mode of recurrence as determined by autopsy and diagnostic imaging. Cancer. 1993;72:2118-2123. [PubMed] |
| 3. | Morganti AG, Massaccesi M, La Torre G, Caravatta L, Piscopo A, Tambaro R, Sofo L, Sallustio G, Ingrosso M, Macchia G. A systematic review of resectability and survival after concurrent chemoradiation in primarily unresectable pancreatic cancer. Ann Surg Oncol. 2010;17:194-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 113] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 4. | Morganti AG, Trodella L, Valentini V, Barbi S, Macchia G, Mantini G, Turriziani A, Cellini N. Pain relief with short-term irradiation in locally advanced carcinoma of the pancreas. J Palliat Care. 2003;19:258-262. [PubMed] |
| 5. | Valentini V, Morganti AG, Macchia G, Mantini G, Mattiucci GC, Brizi MG, Alfieri S, Bossola M, Pacelli F, Sofo L. Intraoperative radiation therapy in resected pancreatic carcinoma: long-term analysis. Int J Radiat Oncol Biol Phys. 2008;70:1094-1099. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 6. | Potters L, Kavanagh B, Galvin JM, Hevezi JM, Janjan NA, Larson DA, Mehta MP, Ryu S, Steinberg M, Timmerman R. American Society for Therapeutic Radiology and Oncology (ASTRO) and American College of Radiology (ACR) practice guideline for the performance of stereotactic body radiation therapy. Int J Radiat Oncol Biol Phys. 2010;76:326-332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 293] [Cited by in RCA: 336] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 7. | Didolkar MS, Coleman CW, Brenner MJ, Chu KU, Olexa N, Stanwyck E, Yu A, Neerchal N, Rabinowitz S. Image-guided stereotactic radiosurgery for locally advanced pancreatic adenocarcinoma results of first 85 patients. J Gastrointest Surg. 2010;14:1547-1559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 85] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 8. | Hoyer M, Roed H, Sengelov L, Traberg A, Ohlhuis L, Pedersen J, Nellemann H, Kiil Berthelsen A, Eberholst F, Engelholm SA. Phase-II study on stereotactic radiotherapy of locally advanced pancreatic carcinoma. Radiother Oncol. 2005;76:48-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 236] [Cited by in RCA: 263] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 9. | Lominska CE, Unger K, Nasr NM, Haddad N, Gagnon G. Stereotactic body radiation therapy for reirradiation of localized adenocarcinoma of the pancreas. Radiat Oncol. 2012;7:74. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 58] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 10. | Macchia G, Morganti AG, Cilla S, Ippolito E, Massaccesi M, Picardi V, Mattiucci GC, Bonomo P, Tambaro R, Pacelli F. Quality of life and toxicity of stereotactic radiotherapy in pancreatic tumors: a case series. Cancer Invest. 2012;30:149-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 11. | Mahadevan A, Jain S, Goldstein M, Miksad R, Pleskow D, Sawhney M, Brennan D, Callery M, Vollmer C. Stereotactic body radiotherapy and gemcitabine for locally advanced pancreatic cancer. Int J Radiat Oncol Biol Phys. 2010;78:735-742. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 172] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 12. | Mahadevan A, Miksad R, Goldstein M, Sullivan R, Bullock A, Buchbinder E, Pleskow D, Sawhney M, Kent T, Vollmer C. Induction gemcitabine and stereotactic body radiotherapy for locally advanced nonmetastatic pancreas cancer. Int J Radiat Oncol Biol Phys. 2011;81:e615-e622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 172] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 13. | Polistina F, Costantin G, Casamassima F, Francescon P, Guglielmi R, Panizzoni G, Febbraro A, Ambrosino G. Unresectable locally advanced pancreatic cancer: a multimodal treatment using neoadjuvant chemoradiotherapy (gemcitabine plus stereotactic radiosurgery) and subsequent surgical exploration. Ann Surg Oncol. 2010;17:2092-2101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 128] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 14. | Rwigema JC, Parikh SD, Heron DE, Howell M, Zeh H, Moser AJ, Bahary N, Quinn A, Burton SA. Stereotactic body radiotherapy in the treatment of advanced adenocarcinoma of the pancreas. Am J Clin Oncol. 2011;34:63-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 123] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 15. | Rwigema JC, Heron DE, Parikh SD, Zeh HJ, Moser JA, Bahary N, Ashby K, Burton SA. Adjuvant stereotactic body radiotherapy for resected pancreatic adenocarcinoma with close or positive margins. J Gastrointest Cancer. 2012;43:70-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 38] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 16. | Shen ZT, Wu XH, Li B, Wang L, Zhu XX. Preliminary efficacy of CyberKnife radiosurgery for locally advanced pancreatic cancer. Chin J Cancer. 2010;29:802-809. [PubMed] |
| 17. | Koong AC, Le QT, Ho A, Fong B, Fisher G, Cho C, Ford J, Poen J, Gibbs IC, Mehta VK. Phase I study of stereotactic radiosurgery in patients with locally advanced pancreatic cancer. Int J Radiat Oncol Biol Phys. 2004;58:1017-1021. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 326] [Cited by in RCA: 316] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 18. | Koong AC, Christofferson E, Le QT, Goodman KA, Ho A, Kuo T, Ford JM, Fisher GA, Greco R, Norton J. Phase II study to assess the efficacy of conventionally fractionated radiotherapy followed by a stereotactic radiosurgery boost in patients with locally advanced pancreatic cancer. Int J Radiat Oncol Biol Phys. 2005;63:320-323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 252] [Cited by in RCA: 246] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 19. | Schellenberg D, Goodman KA, Lee F, Chang S, Kuo T, Ford JM, Fisher GA, Quon A, Desser TS, Norton J. Gemcitabine chemotherapy and single-fraction stereotactic body radiotherapy for locally advanced pancreatic cancer. Int J Radiat Oncol Biol Phys. 2008;72:678-686. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 243] [Cited by in RCA: 253] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 20. | Seo Y, Kim MS, Yoo S, Cho C, Yang K, Yoo H, Choi C, Lee D, Kim J, Kim MS. Stereotactic body radiation therapy boost in locally advanced pancreatic cancer. Int J Radiat Oncol Biol Phys. 2009;75:1456-1461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 31] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 21. | Goyal K, Einstein D, Ibarra RA, Yao M, Kunos C, Ellis R, Brindle J, Singh D, Hardacre J, Zhang Y. Stereotactic body radiation therapy for nonresectable tumors of the pancreas. J Surg Res. 2012;174:319-325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 81] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 22. | Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151:264-269, W64. [PubMed] |
| 23. | Ryan DP, Hong TS, Bardeesy N. Pancreatic adenocarcinoma. N Engl J Med. 2014;371:1039-1049. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1500] [Cited by in RCA: 1711] [Article Influence: 155.5] [Reference Citation Analysis (0)] |
| 24. | Cleary SP, Gryfe R, Guindi M, Greig P, Smith L, Mackenzie R, Strasberg S, Hanna S, Taylor B, Langer B. Prognostic factors in resected pancreatic adenocarcinoma: analysis of actual 5-year survivors. J Am Coll Surg. 2004;198:722-731. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 262] [Cited by in RCA: 257] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 25. | Conlon KC, Klimstra DS, Brennan MF. Long-term survival after curative resection for pancreatic ductal adenocarcinoma. Clinicopathologic analysis of 5-year survivors. Ann Surg. 1996;223:273-279. [PubMed] |
| 26. | Wagner M, Redaelli C, Lietz M, Seiler CA, Friess H, Büchler MW. Curative resection is the single most important factor determining outcome in patients with pancreatic adenocarcinoma. Br J Surg. 2004;91:586-594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 618] [Cited by in RCA: 716] [Article Influence: 34.1] [Reference Citation Analysis (0)] |
| 27. | Huguet F, Mukherjee S, Javle M. Locally advanced pancreatic cancer: the role of definitive chemoradiotherapy. Clin Oncol (R Coll Radiol). 2014;26:560-568. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 41] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 28. | Katz MH, Crane CH, Varadhachary G. Management of borderline resectable pancreatic cancer. Semin Radiat Oncol. 2014;24:105-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 47] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 29. | Laurence JM, Tran PD, Morarji K, Eslick GD, Lam VW, Sandroussi C. A systematic review and meta-analysis of survival and surgical outcomes following neoadjuvant chemoradiotherapy for pancreatic cancer. J Gastrointest Surg. 2011;15:2059-2069. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 99] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 30. | Katz MH, Pisters PW, Evans DB, Sun CC, Lee JE, Fleming JB, Vauthey JN, Abdalla EK, Crane CH, Wolff RA. Borderline resectable pancreatic cancer: the importance of this emerging stage of disease. J Am Coll Surg. 2008;206:833-846; discussion 846-848. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 652] [Cited by in RCA: 605] [Article Influence: 35.6] [Reference Citation Analysis (0)] |
| 31. | Takahashi H, Ohigashi H, Gotoh K, Marubashi S, Yamada T, Murata M, Ioka T, Uehara H, Yano M, Ishikawa O. Preoperative gemcitabine-based chemoradiation therapy for resectable and borderline resectable pancreatic cancer. Ann Surg. 2013;258:1040-1050. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 114] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 32. | Chauffert B, Mornex F, Bonnetain F, Rougier P, Mariette C, Bouché O, Bosset JF, Aparicio T, Mineur L, Azzedine A. Phase III trial comparing intensive induction chemoradiotherapy (60 Gy, infusional 5-FU and intermittent cisplatin) followed by maintenance gemcitabine with gemcitabine alone for locally advanced unresectable pancreatic cancer. Definitive results of the 2000-01 FFCD/SFRO study. Ann Oncol. 2008;19:1592-1599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 494] [Cited by in RCA: 535] [Article Influence: 31.5] [Reference Citation Analysis (0)] |
| 33. | Hammel P, Huguet F, Van Laethem JL, Goldstein D, Glimelius B. Comparison of chemoradiotherapy (CRT) and chemotherapy (CT) in patients with a locally advanced pancreatic cancer (LAPC) controlled after 4 months of gemcitabine with or without erlotinib: final results of the international phase III LAP 07 study. J Clin Oncol. 2013;31 Suppl:LBA4003. |
| 34. | Faris JE, Blaszkowsky LS, McDermott S, Guimaraes AR, Szymonifka J, Huynh MA, Ferrone CR, Wargo JA, Allen JN, Dias LE. FOLFIRINOX in locally advanced pancreatic cancer: the Massachusetts General Hospital Cancer Center experience. Oncologist. 2013;18:543-548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 223] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 35. | Von Hoff DD, Ervin T, Arena FP, Chiorean EG, Infante J, Moore M, Seay T, Tjulandin SA, Ma WW, Saleh MN. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013;369:1691-1703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4035] [Cited by in RCA: 4889] [Article Influence: 407.4] [Reference Citation Analysis (0)] |
| 36. | Yovino S, Poppe M, Jabbour S, David V, Garofalo M, Pandya N, Alexander R, Hanna N, Regine WF. Intensity-modulated radiation therapy significantly improves acute gastrointestinal toxicity in pancreatic and ampullary cancers. Int J Radiat Oncol Biol Phys. 2011;79:158-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 107] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 37. | Ben-Josef E, Schipper M, Francis IR, Hadley S, Ten-Haken R, Lawrence T, Normolle D, Simeone DM, Sonnenday C, Abrams R. A phase I/II trial of intensity modulated radiation (IMRT) dose escalation with concurrent fixed-dose rate gemcitabine (FDR-G) in patients with unresectable pancreatic cancer. Int J Radiat Oncol Biol Phys. 2012;84:1166-1171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 145] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 38. | Chuong MD, Springett GM, Freilich JM, Park CK, Weber JM, Mellon EA, Hodul PJ, Malafa MP, Meredith KL, Hoffe SE. Stereotactic body radiation therapy for locally advanced and borderline resectable pancreatic cancer is effective and well tolerated. Int J Radiat Oncol Biol Phys. 2013;86:516-522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 253] [Cited by in RCA: 263] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 39. | National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: pancreatic adenocarcinoma. Version 2.. 2014; Available from: http://www.nccn.org/professionals/physician_gls/f_guidelines.asp. |
| 40. | Martin JL, Harvey HA, Lipton A, Martin R. Combined chemoradiotherapy for unresectable pancreatic cancer. Am J Clin Oncol. 1999;22:309-314. [PubMed] |
| 41. | Safran H, Cioffi W, Iannitti D, Mega A, Akerman P. Paclitaxel and concurrent radiation for locally advanced pancreatic carcinoma. Front Biosci. 1998;3:E204-E206. [PubMed] |
| 42. | A multi-institutional comparative trial of radiation therapy alone and in combination with 5-fluorouracil for locally unresectable pancreatic carcinoma. The Gastrointestinal Tumor Study Group. Ann Surg. 1979;189:205-208. [PubMed] |
| 43. | Ishii H, Okada S, Tokuuye K, Nose H, Okusaka T, Yoshimori M, Nagahama H, Sumi M, Kagami Y, Ikeda H. Protracted 5-fluorouracil infusion with concurrent radiotherapy as a treatment for locally advanced pancreatic carcinoma. Cancer. 1997;79:1516-1520. [PubMed] |
| 44. | Treatment of locally unresectable carcinoma of the pancreas: comparison of combined-modality therapy (chemotherapy plus radiotherapy) to chemotherapy alone. Gastrointestinal Tumor Study Group. J Natl Cancer Inst. 1988;80:751-755. [PubMed] |
| 45. | Loehrer PJ, Powell ME, Cardenes HR, Wagner L, Brell JM, Ramanathan RK, Crane CH, Alberts SR, Benson AB. A randomized phase III study of gemcitabine in combination with radiation therapy versus gemcitabine alone in patients with localized, unresectable pancreatic cancer. J Clin Oncol. 2008;26:15S. |
| 46. | Aristu J, Cañón R, Pardo F, Martínez-Monge R, Martin-Algarra S, Manuel Ordoñez J, Villafranca E, Moreno M, Cambeiro M, Azinovic I. Surgical resection after preoperative chemoradiotherapy benefits selected patients with unresectable pancreatic cancer. Am J Clin Oncol. 2003;26:30-36. [PubMed] |
| 47. | Adhoute X, Smith D, Vendrely V, Rault A, Sa Cunha A, Legoux JL, Belleannée G, De Lédinghen V, Couzigou P, Masson B. Subsequent resection of locally advanced pancreatic carcinoma after chemoradiotherapy. Gastroenterol Clin Biol. 2006;30:224-230. [PubMed] |
| 48. | Wilkowski R, Thoma M, Schauer R, Wagner A, Heinemann V. Effect of chemoradiotherapy with gemcitabine and cisplatin on locoregional control in patients with primary inoperable pancreatic cancer. World J Surg. 2004;28:1011-1018. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 41] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 49. | Wilkowski R, Thoma M, Bruns C, Wagner A, Heinemann V. Chemoradiotherapy with gemcitabine and continuous 5-FU in patients with primary inoperable pancreatic cancer. JOP. 2006;7:349-360. [PubMed] |
| 50. | Snady H, Bruckner H, Cooperman A, Paradiso J, Kiefer L. Survival advantage of combined chemoradiotherapy compared with resection as the initial treatment of patients with regional pancreatic carcinoma. An outcomes trial. Cancer. 2000;89:314-327. [PubMed] |
| 51. | Boone BA, Steve J, Krasinskas AM, Zureikat AH, Lembersky BC, Gibson MK, Stoller RG, Zeh HJ, Bahary N. Outcomes with FOLFIRINOX for borderline resectable and locally unresectable pancreatic cancer. J Surg Oncol. 2013;108:236-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 146] [Article Influence: 12.2] [Reference Citation Analysis (0)] |