Published online Aug 21, 2015. doi: 10.3748/wjg.v21.i31.9403
Peer-review started: April 8, 2015
First decision: April 23, 2015
Revised: May 1, 2015
Accepted: June 10, 2015
Article in press: June 10, 2015
Published online: August 21, 2015
Processing time: 134 Days and 23.6 Hours
AIM: To determine the relationship between CD11c expression level and prognosis in patients with gastric cancer (GC).
METHODS: This retrospective survival study was performed from July 31, 2008 to June 30, 2014. Our study inclusion criteria included all the patients with GC who underwent surgical resection between January 1998 and December 2009 in the Third Affiliated Hospital of Soochow University. CD11c expression levels in 140 patients with GC at different UICC stages were evaluated using immunohistochemistry, and GC tissues from 16 cases were further verified by qRT-PCR. The χ2 test was used to compare the patient- and disease-related factors between the low CD11c expression group and the high expression group. Univariate probabilities of overall survival (OS) and disease-free survival (DFS) were assessed using the Kaplan-Meier method. The log rank test was used to compare survival curves. Different multivariate COX models were used to estimate the association between CD11c expression and both death and recurrence risk in GC patients.
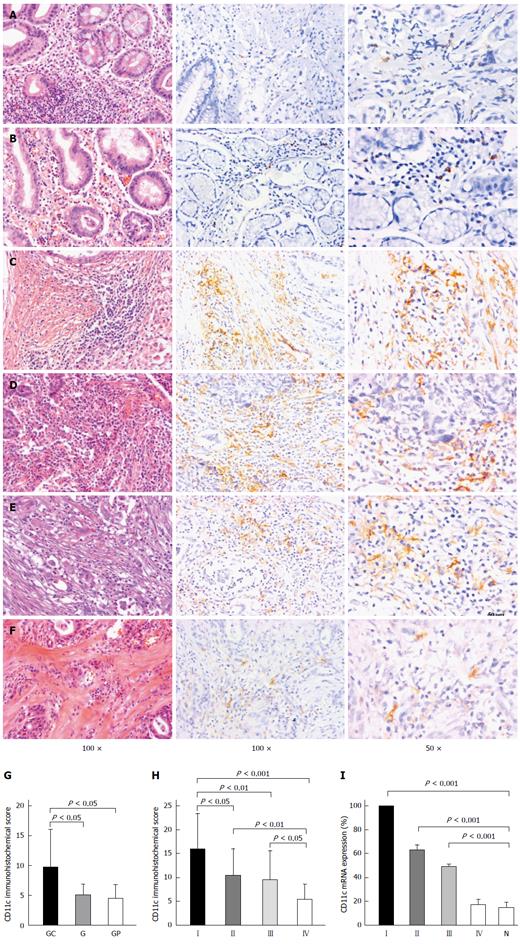
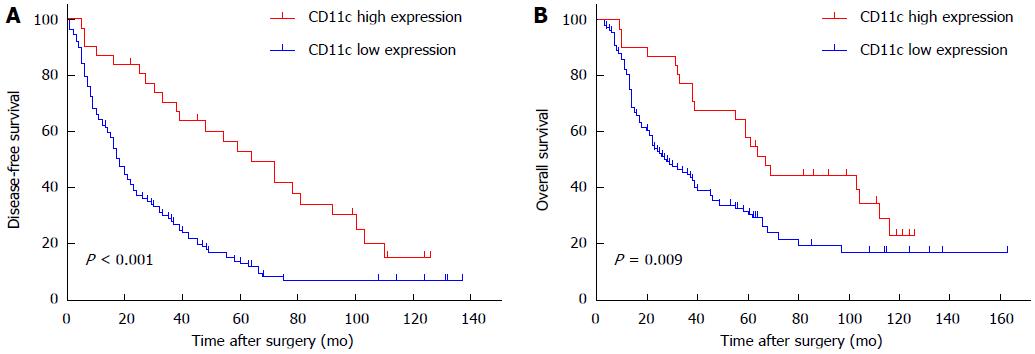
RESULTS: The average CD11c expression level was 5.1 ± 1.8/high power field (HPF) in 10 gastritis samples, 4.5 ± 2.3/HPF in 10 gastric polyp samples and 9.7 ± 6.3/HPF in 140 gastric cancer samples, respectively. The CD11c expression level was significantly decreased from UICC stage I to stage IV (stage I: 16.0 ± 7.4, stage II: 10.4 ± 5.5, stage III: 9.4 ± 6.1, stage IV: 5.3 ± 3.2, P < 0.001). Patients in the high CD11c expression group had a greater 3- and 5-year OS probability and longer median survival time compared with the low CD11c expression group, (67.7% vs 39.2%; 51.4% vs 29.0%; 67.0 mo vs 28.0 mo; χ2 = 6.80, P = 0.009), and had a greater 3- and 5-year DFS probability and longer median DFS time (63.7% vs 24.0%; 49.1% vs 11.9%; 64.0 mo vs 18.0 mo; χ2 = 15.39, P < 0.001). Patients with high CD11c high expression had a reduced risk of death (HR = 0.56, 95%CI: 0.33-0.98, P < 0.05) and relapse (HR = 0.39, 95%CI: 0.23-0.67, P < 0.01) compared with patients with low CD11c expression after adjustment of potential confounders, with the exception of tumor size. However, the protective effect related to death (HR = 0.90, 95%CI: 0.49-1.67, P = 0.749) and relapse (HR = 0.65, 95%CI: 0.36-1.19, P = 0.160) disappeared when tumor size was incorporated into the model.
CONCLUSION: High expression of CD11c decreased the risk of death and relapse, and may be regarded as an alternative indicator of favorable prognosis in patients with GC.
Core tip: The progression of gastric cancer is closely related to the tumor microenvironment. In the present study, we found that CD11c expression level significantly decreased from UICC stage I to stage IV, and patients with high CD11c expression had a reduced risk of death and relapse compared with patients with low CD11c expression after adjustment of potential confounders, with the exception of tumor size. Based on our research, we suggest that high expression of CD11c decreased the risk of death and relapse and may act as an indicator of favorable prognosis in patients with gastric cancer.
- Citation: Wang Y, Xu B, Hu WW, Chen LJ, Wu CP, Lu BF, Shen YP, Jiang JT. High expression of CD11c indicates favorable prognosis in patients with gastric cancer. World J Gastroenterol 2015; 21(31): 9403-9412
- URL: https://www.wjgnet.com/1007-9327/full/v21/i31/9403.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i31.9403
Throughout the history of human civilization, cancer has been a major health problem. As one of the most common malignancies, gastric cancer (GC) remains the second leading cause of cancer-related death worldwide. Over 70% of new cases and deaths are observed in developing countries[1]. In China, GC has the second highest incidence among commonly diagnosed cancers[2]. Currently, the prevalence of GC is high and a large number of patients are first diagnosed in the advanced stages. Despite the availability of several anticancer drugs and treatments, GC cannot be cured, especially in the late stages, without exhibiting side effects, and the prognosis of these patients in the late stages is poor[3,4].
Dendritic cells (DCs) exhibit a strong capacity to receive and integrate innate and adaptive immune signals. In general, DCs are divided into myeloid-, plasmacytoid-, and monocyte-associated DCs[5-7]. Several studies have determined the relationship between immune cell infiltration and tumor progression in various cancers, and tumor-infiltrating mature DCs are associated with a favorable prognosis, but immature DCs are not associated with a favorable prognosis[8,9]. Therefore, they are regarded as key antigen-presenting cells (APCs), which are crucial in the enhancement and regulation of cell-mediated immune responses, and DCs play an important role in most cancers[10,11]. As a member of the adhesion molecule integrin family β2, CD11c is over-expressed in myeloid- and monocyte-associated DCs, and the expression of CD11c has been observed in NK cells, macrophages and even some activated B and T cells[12]. Currently, there are few clinical data available on the impact of CD11c expression level in the tumor microenvironment on the prognosis of patients with GC. In the present study, we aimed to determine the relationship between CD11c expression in the tumor and the progression of GC.
The study inclusion criteria included all the patients with GC who underwent surgical resection between January 1998 and December 2009 in the Third Affiliated Hospital of Soochow University, Changzhou, China. None of the subjects had received chemotherapy or radiotherapy before surgery. A total of 202 patients were enrolled; however, 62 patients were excluded due to lack of pathological specimen, failed assessment of CD11c scores or loss to follow-up at the early stage of the study, resulting in 140 participants in the final study population. The tumor stages were determined using the International Union against Cancer Staging System[13].
Demographics and clinical data were obtained by reviewing the medical records. All the patients were followed up yearly from July 31, 2008 to June 30, 2014.
Primary endpoints were overall survival (OS) and disease-free survival (DFS). OS was defined as the time from registration until death from any cause, and DFS was defined as the time from randomization until recurrence of tumor or death from any cause. Surviving patients were censored on June 30, 2014.
Formalin-fixed and paraffin-embedded tissues were cut into 3-μm-thick sections, de-waxed in xylene and then progressively rehydrated by gradient concentrations of ethanol. Antigens were retrieved by heating the tissue sections at 100 °C for 30 min in citrate solution (10 mmol/L, pH 6.0). The sections were cooled and immersed in methanol in the presence of 0.3% hydrogen peroxide for 15 min to block the endogenous peroxidase activity. The sections were subsequently rinsed in PBS for 5 min and then incubated with primary antibody against CD11c (1:150, Epitomics, Burlingame, CA, United States) at 4 °C overnight. Sections incubated without the primary antibody were used as negative controls. The sections were then incubated with horseradish peroxidase-labeled goat against mouse/rabbit secondary antibody (Maixin Biotechnology, Fuzhou, China). Diaminobenzene was used as the chromogen, and hematoxylin was used as the nuclear counterstain. Finally, the sections were dehydrated, cleared and mounted.
The stained sections were independently reviewed by two pathologists without knowing the clinical diagnosis. The slides were graded according to the staining intensity. CD11c-positive signals located in the cell membrane were counted according to the brown diaminobenzidine precipitate. The CD11c signals from five visual areas enriched with tumor-infiltrating lymphocytes were examined using a low-magnification lens, and then examined using a high-magnification lens (× 200) to determine the average number of CD11c positive cells. Immunohistochemical expression scores of CD11c were obtained using a Leica microscope. The patients were classified into two groups as follows: low expression [≤ 14/high power field (HPF)] and high expression (> 14/HPF) based on expression scores of CD11c per slide.
Total RNA was extracted from different gastric cancer tissues using TRIzol reagent (Invitrogen Company, St. Louis, MO, United States). The RNA quality was evaluated according to the absorbance at a wavelength of 260/280 nm. Subsequently, purified RNA was reversely transcribed into cDNA using a high-capacity cDNA reverse transcription kit (Applied Biosystems, Foster City, CA, United States). Table 1 lists the sequences of all primers used in the present study. The CD11c expression at the mRNA level was evaluated using a 7500 Real-Time PCR System with SYBR® Green PCR Master Mix (Applied Biosystems). GAPDH was selected as a housekeeping gene. The CD11c expression was normalized to the level of the housekeeping gene and analyzed using the comparative CT method (2-ΔΔCT method).
| Gene | Forward primer sequence (5'→3') | Reverse primer sequence (5'→3') |
| CD11c | GGGATGCCGCCAAAATTCTC | ATTGCATAGCGGATGATGCCT |
| GAPDH | GGAAGGTGAAGGTCGGAGTC | CGTTCTCAGCCTTGACGGT |
The χ2 test was used to compare the patient- and disease-related factors between the low CD11c expression group and the high expression group. Univariate probabilities of OS and DFS were assessed using the Kaplan-Meier method. The log rank test was used to compare survival curves. The Cox model, HR with a 95%CI was used to estimate the association between CD11c expression (> 14/HPF = 1; ≤ 14/HPF = 0) and death or relapse of all patients with adjustments of the following potential confounders: age (about 45 = 1; 60 = 2; > 60 = 3), gender (male = 0; female = 1), tumor location: gastric cardia or not (yes = 1; no = 0), gastric body or not (yes = 1; no = 0), gastric antrum or not (yes = 1; no = 0), tumor size (< 5 cm = 0; ≥ 5 cm = 1), histological type (poorly differentiated = 1; differentiated = 0), invasion to muscular layer (yes = 1; no = 0), nodal metastasis (yes = 1; no = 0), recurrence (yes = 1; no = 0), pathological grade (grade 1-2 = 1; grade 3 = 2; grade 4 = 3), and UICC stage (I = 1; II = 2; III = 3; IV = 4).
Immunohistochemistry (IHC) revealed that the staining intensity of CD11c was differently distributed among various tissues, including gastritis (Figure 1A), gastric polyps (Figure 1B) and gastric cancer tissues (Figure 1C-F). As shown in Figure 1, the average expression levels of CD11c were 5.1 ± 1.8/HPF in 10 gastritis samples, 4.5 ± 2.3/HPF in 10 gastric polyp samples and 9.7 ± 6.3/HPF in 140 gastric cancer samples, respectively, which suggests that CD11c is involved in the pathological changes in GC. Furthermore, CD11c expression gradually decreased from UICC stage I to stage IV (stage I: 16.0 ± 7.4, stage II: 10.4 ± 5.5, stage III: 9.4 ± 6.1, stage IV: 5.3 ± 3.2, P < 0.001, Figure 1H). Based on these results, qRT-PCR was subsequently performed to further validate these findings. Moreover, as shown in Figure 1I, low expression of CD11c in normal and para-carcinoma tissues was also observed, which indicated that tumor cells recruited more CD11c+TILs than inflammation and polyps.
Table 2 shows the patient characteristics. The median age of all 140 participants was 60 years, and the age range was 35 to 86 years. More male patients were included in the study population than females (76.43% vs 23.57%). In addition, 73.57% and 85.71% of the 140 patients died or experienced disease progression during the study period, respectively.
| Clinicopathological characteristics | All | CD11c expression | P value | ||||
| Low | High | ||||||
| No. | % | No. | % | No. | % | ||
| Gender | 0.113 | ||||||
| Male | 107 | 76.43 | 80 | 73.39 | 27 | 87.10 | |
| Female | 33 | 23.57 | 29 | 26.61 | 4 | 12.90 | |
| Age (yr) | 0.162 | ||||||
| About 45 | 12 | 8.57 | 9 | 8.26 | 3 | 9.68 | |
| About 60 | 66 | 47.14 | 56 | 51.38 | 10 | 32.26 | |
| > 60 | 62 | 44.29 | 44 | 40.37 | 18 | 58.06 | |
| Tumor location | |||||||
| Gastric cardia | 0.342 | ||||||
| No | 87 | 62.14 | 70 | 64.22 | 17 | 54.84 | |
| Yes | 53 | 37.86 | 39 | 35.78 | 14 | 45.16 | |
| Tumor size (cm)1 | < 0.001 | ||||||
| < 5 | 56 | 47.46 | 36 | 38.71 | 20 | 80.00 | |
| ≥ 5 | 62 | 52.54 | 57 | 61.29 | 5 | 20.00 | |
| Histological type2 | 0.042 | ||||||
| Differentiated | 54 | 40.60 | 37 | 35.92 | 17 | 56.67 | |
| Poorly differentiated | 79 | 59.40 | 66 | 64.08 | 13 | 43.33 | |
| Invasion to muscular layer3 | 0.004 | ||||||
| No | 14 | 11.57 | 7 | 7.29 | 7 | 28.00 | |
| Yes | 107 | 88.43 | 89 | 92.71 | 18 | 72.00 | |
| Nodal metastasis4 | 0.001 | ||||||
| No | 33 | 27.27 | 19 | 20.00 | 14 | 53.85 | |
| Yes | 88 | 72.73 | 76 | 80.00 | 12 | 46.15 | |
| Pathological grade | 0.047 | ||||||
| 1-2 | 17 | 12.14 | 13 | 11.93 | 4 | 12.90 | |
| 3 | 70 | 50.00 | 49 | 44.95 | 21 | 67.74 | |
| 4 | 53 | 37.86 | 47 | 43.12 | 6 | 19.35 | |
| UICC stage | 0.002 | ||||||
| I | 11 | 7.86 | 4 | 3.67 | 7 | 22.58 | |
| II | 27 | 19.29 | 22 | 20.18 | 5 | 16.13 | |
| III | 88 | 62.86 | 69 | 63.30 | 19 | 61.29 | |
| IV | 14 | 10.00 | 14 | 12.84 | 0 | 0.00 | |
| Endpoint: DFS | 0.038 | ||||||
| No recurrence | 20 | 14.29 | 12 | 11.01 | 8 | 25.81 | |
| Recurrence | 120 | 85.71 | 97 | 88.99 | 23 | 74.19 | |
| Endpoint: OS | 0.404 | ||||||
| Survival | 37 | 26.43 | 27 | 24.77 | 10 | 32.26 | |
| Died | 103 | 73.57 | 82 | 75.23 | 21 | 67.74 | |
Significant differences in tumor size, histological type, invasion depth, nodal metastasis status, pathological grade, UICC stage and DFS outcome were detected between the two CD11c groups (all P < 0.05). No significant differences in other clinical variables were observed between the CD11c groups (all P > 0.05) (Table 2).
Table 3 shows that patients in the high CD11c expression group had a greater 3- and 5-year OS probability and longer median survival time compared with the low CD11c expression group, (67.7% vs 39.2%; 51.4% vs 29.0%; 67.0 mo vs 28.0 mo; χ2 = 6.80, P = 0.009), and had a greater 3- and 5-year DFS probability and longer median DFS time (63.7% vs 24.0%; 49.1% vs 11.9%; 64.0 mo vs 18.0 mo; χ2 = 15.39, P < 0.001).
| Expression level of CD11c | 3-yr OS (95%CI) | 5-yr OS (95%CI) | Median survival time (95%CI)1 | 3-yr DFS (95%CI) | 5-yr DFS (95%CI) | Median disease free time (95%CI)2 |
| Low | 39.2 (30.0-48.4) | 29.0 (20.2-37.8) | 28.0 (15.1-40.9) | 24.0 (19.8-28.2) | 11.9 (8.7-15.1) | 18.0 (14.6-21.4) |
| High | 67.7 (59.3-76.1) | 51.4 (42.4-60.4) | 67.0 (53.9-80.1) | 63.7 (54.9-72.5) | 49.1 (39.8-58.4) | 64.0 (42.0-86.0) |
Figure 2 show the OS and DFS curves for the low CD11c expression group and high expression group, respectively. Patients in the high CD11c expression group had a significantly better OS and DFS compared with the low CD11c expression group (log-rank, P = 0.009 for OS and P < 0.001 for DFS).
The above-mentioned statistical results comprehensively revealed that stage IV was related to an increased risk of death and relapse.
Table 4 shows that the risk of death and relapse in patients with high CD11c expression was significantly decreased by 44% (HR = 0.56, 95%CI: 0.33-0.98, P = 0.041) and 61% (HR = 0.39, 95%CI: 0.23-0.67, P = 0.001) compared with the reference group (patients with low CD11c expression) after adjustments for gender, age, tumor location, histological type, pathological grade and UICC stage (Table 5, model 1 and 3). When tumor size was incorporated into the Cox model (Table 5, model 2 and 4), the risk of death and relapse in patients with high CD11c expression was increased to 0.90 and 0.65, with a P value of 0.749 and 0.160, respectively.
| Clinicopathological parameters | Number | OS | DFS | ||||
| HR | 95%CI | P value | HR | 95%CI | P value | ||
| CD11C expression | |||||||
| Low | 109 (77.86) | 1.00 (ref) | 1.00 (ref) | ||||
| High | 31 (22.14) | 0.56 | 0.33-0.98 | 0.041 | 0.39 | 0.23-0.67 | 0.001 |
| Gender | |||||||
| Male | 107 (76.43) | 1.00 (ref) | 1.00 (ref) | ||||
| Female | 33 (23.57) | 1.08 | 0.64-1.83 | 0.769 | 0.77 | 0.46-1.27 | 0.306 |
| Age groups | |||||||
| About 45 | 12 (8.57) | 1.00 (ref) | 1.00 (ref) | ||||
| About 60 | 66 (47.14) | 0.52 | 0.25-1.08 | 0.080 | 0.51 | 0.26-1.03 | 0.060 |
| > 60 | 62 (44.29) | 0.82 | 0.39-1.73 | 0.608 | 0.70 | 0.35-1.41 | 0.316 |
| Gastric cardia | |||||||
| No | 87 (62.14) | 1.00 (ref) | 1.00 (ref) | ||||
| Yes | 53 (37.86) | 1.58 | 1.02-2.43 | 0.039 | 1.42 | 0.95-2.11 | 0.085 |
| Histological type% | |||||||
| Differentiated | 54 (40.60) | 1.00 (ref) | 1.00 (ref) | ||||
| Poorly differentiated | 79 (59.40) | 1.26 | 0.79-2.02 | 0.333 | 1.025 | 0.68-1.56 | 0.907 |
| Pathological grade | |||||||
| 1-2 | 17 (12.14) | 1.00 (ref) | 1.00 (ref) | ||||
| 3 | 70 (50.00) | 0.79 | 0.35-1.76 | 0.564 | 0.85 | 0.41-1.75 | 0.659 |
| 4 | 53 (37.86) | 1.08 | 0.47-2.48 | 0.849 | 1.32 | 0.63-2.79 | 0.466 |
| UICC stage | |||||||
| I | 11 (7.86) | 1.00 (ref) | 1.00 (ref) | ||||
| II | 27 (19.20) | 2.90 | 0.74-11.32 | 0.126 | 1.99 | 0.65-6.10 | 0.230 |
| III | 88 (62.86) | 3.36 | 0.91-12.39 | 0.069 | 2.12 | 0.74-6.10 | 0.162 |
| IV | 14 (10.00) | 9.94 | 2.46-40.19 | 0.001 | 9.20 | 2.75-30.78 | < 0.001 |
In the present study, we demonstrated that the infiltrating intensity of CD11c positive immune cells varied in different gastric tissues, including gastritis, gastric polyps and gastric cancer tissues. CD11c expression level in patients with GC was significantly higher than that in patients with gastritis or gastric polyps. Furthermore, CD11c expression level gradually decreased from UICC stage I to stage IV, as confirmed by the qRT-PCR. We also found that patients with higher infiltrating intensity of CD11c positive cells had a significantly reduced risk of cancer-related death and relapse compared with patients with lower infiltrating intensity of CD11c positive cells after adjustments for potential confounders, with the exception of tumor size. However, the protective effect related to death and relapse disappeared when tumor size was incorporated into the model. Our findings suggested that low CD11c expression was associated with the risk of death and relapse in patients with GC, but was not an independent risk factor.
Interestingly, our previous findings suggested that a decrease in CD11c in GC tissues compared with normal gastric tissue samples may be related to a local imbalance in the tumor immune microenvironment[14]. A previous study suggested that tumor-infiltrating CD11b+ DCs are associated with worse prognosis in patients with GC, and that almost all CD11b+ DCs showed CD11c[15]. In addition, it has been reported that CD11b+ DCs are potent inducers of antigen-specific IL-10, producing type 1 regulatory T cells that induce antigen-specific tolerance[16]. Given these varied results, it is possible that the authors did not completely distinguish CD11b+ cells from macrophages and DCs due to the similar characteristics in inducing immune tolerance between CD11b+ DCs and tumor-associated M2 macrophages[17-19].
APCs, such as macrophages and DCs, can directly activate antigen-specific Th1 or CTLs, which can activate the anti-tumor immune response and are associated with favorable prognosis of patients with many types of cancers[8,9,20,21]. Early studies also demonstrated a significant association between tumor-infiltrating CD1a+ DCs and unfavorable prognosis in patients with colorectal cancer and lung cancer[8,9,20]. In contrast, high expression of CD208 or CD86 in DCs has been found to contribute to a better prognosis in colorectal cancer, gastric cancer, and malignant melanoma[8,22,23]. Based on their polarization, tumor-infiltrating antigen-presenting cells have dual characteristics, which include the capacity to trigger an antitumor or protumor immune response. CD11c is a component of complement receptor 4, which is predominantly expressed on DCs, some macrophages, natural killer (NK) and activated T cells[24-26]. Therefore, the staining scores of CD11c were composed of not only DCs, but also other effector cells in the local tumor microenvironment, such as some macrophages, NK and activated T cells. To the best of our knowledge, a DC-enriched infiltration is necessary for the activation of naive CD8+ T cells in vivo. Fahlén-Yrlid et al[27] showed that CD11c+DCs can activate CD4+ T cells during the process of mucosal immunization. In addition, CD11c is also expressed on some CD8+ T cells, and these CD11c+CD8+ T cells sometimes act as immune regulators by suppressing CD4+ T cells and sometimes as immune effectors[28]. Interestingly, their activities are beneficial to the hosts and boost immune potential in the tumor microenvironment in both cases[29].
Our findings suggested that low CD11c expression was associated with the risk of death and relapse in patients with GC, but was not an independent risk factor. This finding may have been caused by the following reasons: Firstly, univariate survival analysis revealed that low CD11c expression in the tumor was significantly associated with a high risk of death and relapse. Secondly, there was a strong correlation between the CD11c score and tumor size, which may have caused multicolinearity in the Cox model, leading to this result. When CD11c expression and tumor size, respectively, were incorporated into the Cox model (adjustments for other variables in the Cox model remained the same: gender, age, tumor location, histological type, pathological grade and UICC stage), high CD11c expression and small tumor size both showed a significant protective effect. However, when these two variables were simultaneously incorporated into the Cox model, the protective effect disappeared. Previous studies have also shown that lower CD11c expression in the tumor is associated with lower infiltration of effector cells[27-29], indicating low control of tumor progression, leading to rapid tumor growth. Consequently, CD11c may be a potential alternative indicator of tumor size. The causal relationship between CD11c expression and tumor progression (tumor size) has not been clarified.
More and more attention is being paid to tumor size, an important characteristic of the tumor, in order to predict tumor burden, prognosis and UICC staging. Some studies suggested that tumor size was closely correlated with the number of metastatic lymph nodes, which also improved the power of UICC staging in predicting 5-year survival in GC, and the change in tumor size at the first follow-up CT was strongly prognostic for DFS and OS in colorectal cancer[30-32]. However, it is difficult to assess tumor size in some relapsed and metastatic patients, and tumor burden is also difficult to evaluate accurately. Thus, CD11c expression might be useful in predicting tumor burden, prognosis and UICC staging, and in helping clinicians to create rational treatment programs.
In summary, low CD11c expression may be a risk factor for relapse and death of patients with GC. In addition, CD11c could act as an alternative index of tumor size or lymph node metastasis in predicting prognosis in patients with GC in special cases.
CD11c is an antigen receptor predominantly expressed on dendritic cells (DC), to which antigen targeting has been shown to induce robust antigen-specific immune responses. It has been demonstrated that CD11c provided costimulatory signals to activated lymphocytes. The exact roles and regulatory mechanisms of CD11c in the tumor microenvironment have not yet been defined.
Over the decades, more and more studies have shown that the progression of gastric cancer is closely related to the tumor microenvironment. CD11c is a positive regulator present on CD11c+DCs and activates lymphocytes, leading to amelioration of immune status. Therefore, it is necessary to confirm the influence of C11c on the tumor microenvironment in patients with gastric cancer.
Based on the investigation of clinical characteristics and CD11c expression level in 140 patients with gastric cancer, the authors demonstrated that CD11c expression levels significantly decreased from UICC stage I to stage IV, and patients with high CD11c expression had a reduced risk of death and relapse. These findings suggest that high CD11c expression decreased the risk of death and relapse, and may act as an indicator of favorable prognosis in patients with gastric cancer.
The results of this study may provide clinicians with information on the influence of CD11c on the tumor microenvironment in patients with gastric cancer, and provide strategies for the treatment of patients with advanced gastric cancer. CD11c may be regarded as a potential prognostic indicator in patients with gastric cancer.
Tumor microenvironment refers to the unique properties of the tissue microenvironment conferred by abnormal interactions between tumor and host cells. The tumor microenvironment is often characterized by hypoxia, nutrient deprivation, acidosis, and aberrant stroma.
This is an interesting study on evaluating prognosis of patients with GC by means of measuring CD11c expression level.
P- Reviewer: Ali I, Merino G, Syed V, Weyemi U S- Editor: Ma YJ L- Editor: O’Neill M E- Editor: Ma S
| 1. | Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23762] [Cited by in RCA: 25543] [Article Influence: 1824.5] [Reference Citation Analysis (7)] |
| 2. | Chen W, Zheng R, Zhang S, Zhao P, Zeng H, Zou X, He J. Annual report on status of cancer in China, 2010. Chin J Cancer Res. 2014;26:48-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 163] [Reference Citation Analysis (0)] |
| 3. | Kim HJ, Karpeh MS. Surgical approaches and outcomes in the treatment of gastric cancer. Semin Radiat Oncol. 2002;12:162-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 4. | Ali I, Lone MN, Al-Othman ZA, Al-Warthan A, Sanagi MM. Heterocyclic Scaffolds: Centrality in Anticancer Drug Development. Curr Drug Targets. 2015;16:711-734. [PubMed] |
| 5. | Ju X, Clark G, Hart DN. Review of human DC subtypes. Methods Mol Biol. 2010;595:3-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 46] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 6. | Steinman RM, Banchereau J. Taking dendritic cells into medicine. Nature. 2007;449:419-426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1541] [Cited by in RCA: 1579] [Article Influence: 87.7] [Reference Citation Analysis (0)] |
| 7. | Dzionek A, Sohma Y, Nagafune J, Cella M, Colonna M, Facchetti F, Günther G, Johnston I, Lanzavecchia A, Nagasaka T. BDCA-2, a novel plasmacytoid dendritic cell-specific type II C-type lectin, mediates antigen capture and is a potent inhibitor of interferon alpha/beta induction. J Exp Med. 2001;194:1823-1834. [PubMed] |
| 8. | Gulubova MV, Ananiev JR, Vlaykova TI, Yovchev Y, Tsoneva V, Manolova IM. Role of dendritic cells in progression and clinical outcome of colon cancer. Int J Colorectal Dis. 2012;27:159-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 78] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 9. | Sandel MH, Dadabayev AR, Menon AG, Morreau H, Melief CJ, Offringa R, van der Burg SH, Janssen-van Rhijn CM, Ensink NG, Tollenaar RA. Prognostic value of tumor-infiltrating dendritic cells in colorectal cancer: role of maturation status and intratumoral localization. Clin Cancer Res. 2005;11:2576-2582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 132] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 10. | Swartz MA. Immunomodulatory roles of lymphatic vessels in cancer progression. Cancer Immunol Res. 2014;2:701-707. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 72] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 11. | Ramanathapuram LV, Hopkin D, Kurago ZB. Dendritic Cells (DC) Facilitate Detachment of Squamous Carcinoma Cells (SCC), While SCC Promote an Immature CD16(+) DC Phenotype and Control DC Migration. Cancer Microenviron. 2013;6:41-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 12. | Castro FV, Tutt AL, White AL, Teeling JL, James S, French RR, Glennie MJ. CD11c provides an effective immunotarget for the generation of both CD4 and CD8 T cell responses. Eur J Immunol. 2008;38:2263-2273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 89] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 13. | Tanji Y, Mizoguchi K, Yoichi M, Morita M, Hori K, Unno H. Fate of coliphage in a wastewater treatment process. J Biosci Bioeng. 2002;94:172-174. [PubMed] |
| 14. | Chen J, Yang J, Jiang J, Zhuang Y, He W. Function and subsets of dendritic cells and natural killer cells were decreased in gastric cancer. Int J Clin Exp Pathol. 2014;7:8304-8311. [PubMed] |
| 15. | Okita Y, Tanaka H, Ohira M, Muguruma K, Kubo N, Watanabe M, Fukushima W, Hirakawa K. Role of tumor-infiltrating CD11b+ antigen-presenting cells in the progression of gastric cancer. J Surg Res. 2014;186:192-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 34] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 16. | Gregori S, Tomasoni D, Pacciani V, Scirpoli M, Battaglia M, Magnani CF, Hauben E, Roncarolo MG. Differentiation of type 1 T regulatory cells (Tr1) by tolerogenic DC-10 requires the IL-10-dependent ILT4/HLA-G pathway. Blood. 2010;116:935-944. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 441] [Cited by in RCA: 446] [Article Influence: 29.7] [Reference Citation Analysis (0)] |
| 17. | Allavena P, Sica A, Garlanda C, Mantovani A. The Yin-Yang of tumor-associated macrophages in neoplastic progression and immune surveillance. Immunol Rev. 2008;222:155-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 438] [Cited by in RCA: 490] [Article Influence: 30.6] [Reference Citation Analysis (0)] |
| 18. | Liu YJ. Dendritic cell subsets and lineages, and their functions in innate and adaptive immunity. Cell. 2001;106:259-262. [PubMed] |
| 19. | Ratzinger G, Baggers J, de Cos MA, Yuan J, Dao T, Reagan JL, Münz C, Heller G, Young JW. Mature human Langerhans cells derived from CD34+ hematopoietic progenitors stimulate greater cytolytic T lymphocyte activity in the absence of bioactive IL-12p70, by either single peptide presentation or cross-priming, than do dermal-interstitial or monocyte-derived dendritic cells. J Immunol. 2004;173:2780-2791. [PubMed] |
| 20. | Al-Shibli K, Al-Saad S, Donnem T, Persson M, Bremnes RM, Busund LT. The prognostic value of intraepithelial and stromal innate immune system cells in non-small cell lung carcinoma. Histopathology. 2009;55:301-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 81] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 21. | Mahmoud SM, Lee AH, Paish EC, Macmillan RD, Ellis IO, Green AR. Tumour-infiltrating macrophages and clinical outcome in breast cancer. J Clin Pathol. 2012;65:159-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 210] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 22. | Ishigami S, Ueno S, Matsumoto M, Okumura H, Arigami T, Uchikado Y, Setoyama T, Arima H, Sasaki K, Kitazono M. Prognostic value of CD208-positive cell infiltration in gastric cancer. Cancer Immunol Immunother. 2010;59:389-395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 23. | Ladányi A, Kiss J, Somlai B, Gilde K, Fejos Z, Mohos A, Gaudi I, Tímár J. Density of DC-LAMP(+) mature dendritic cells in combination with activated T lymphocytes infiltrating primary cutaneous melanoma is a strong independent prognostic factor. Cancer Immunol Immunother. 2007;56:1459-1469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 217] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 24. | Cooney LA, Gupta M, Thomas S, Mikolajczak S, Choi KY, Gibson C, Jang IK, Danziger S, Aitchison J, Gardner MJ. Short-lived effector CD8 T cells induced by genetically attenuated malaria parasite vaccination express CD11c. Infect Immun. 2013;81:4171-4181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 25. | Chan CW, Crafton E, Fan HN, Flook J, Yoshimura K, Skarica M, Brockstedt D, Dubensky TW, Stins MF, Lanier LL. Interferon-producing killer dendritic cells provide a link between innate and adaptive immunity. Nat Med. 2006;12:207-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 306] [Cited by in RCA: 298] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 26. | Caminschi I, Ahmet F, Heger K, Brady J, Nutt SL, Vremec D, Pietersz S, Lahoud MH, Schofield L, Hansen DS. Putative IKDCs are functionally and developmentally similar to natural killer cells, but not to dendritic cells. J Exp Med. 2007;204:2579-2590. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 89] [Cited by in RCA: 91] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 27. | Fahlén-Yrlid L, Gustafsson T, Westlund J, Holmberg A, Strömbeck A, Blomquist M, MacPherson GG, Holmgren J, Yrlid U. CD11c(high )dendritic cells are essential for activation of CD4+ T cells and generation of specific antibodies following mucosal immunization. J Immunol. 2009;183:5032-5041. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 53] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 28. | Choi BK, Kim YH, Kang WJ, Lee SK, Kim KH, Shin SM, Yokoyama WM, Kim TY, Kwon BS. Mechanisms involved in synergistic anticancer immunity of anti-4-1BB and anti-CD4 therapy. Cancer Res. 2007;67:8891-8899. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 53] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 29. | Vinay DS, Kwon BS. CD11c+CD8+ T cells: two-faced adaptive immune regulators. Cell Immunol. 2010;264:18-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 38] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 30. | Huang CM, Xu M, Wang JB, Zheng CH, Li P, Xie JW, Lin JX, Lu J. Is tumor size a predictor of preoperative N staging in T2-T4a stage advanced gastric cancer? Surg Oncol. 2014;23:5-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 31. | Lu J, Huang CM, Zheng CH, Li P, Xie JW, Wang JB, Lin JX. Consideration of tumor size improves the accuracy of TNM predictions in patients with gastric cancer after curative gastrectomy. Surg Oncol. 2013;22:167-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 45] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 32. | Suzuki C, Blomqvist L, Sundin A, Jacobsson H, Byström P, Berglund Å, Nygren P, Glimelius B. The initial change in tumor size predicts response and survival in patients with metastatic colorectal cancer treated with combination chemotherapy. Ann Oncol. 2012;23:948-954. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 87] [Article Influence: 6.2] [Reference Citation Analysis (0)] |