Published online Aug 21, 2015. doi: 10.3748/wjg.v21.i31.9373
Peer-review started: March 11, 2015
First decision: March 26, 2015
Revised: April 15, 2015
Accepted: June 10, 2015
Article in press: June 10, 2015
Published online: August 21, 2015
Processing time: 162 Days and 22.9 Hours
AIM: To evaluate the long-term effectiveness of colonic stents in colorectal tumors causing large bowel obstruction.
METHODS: We retrospectively analyzed data from 49 patients with colorectal cancer who had undergone colorectal stent placement between January 2008 and January 2013. Patients’ symptoms, characteristics and clinicopathological data were obtained by reviewing medical records. The obstruction was diagnosed clinically and radiologically. Histopathological diagnosis was achieved endoscopically. Technical success rate (TSR) was defined as the ratio of patients with correctly placed SEMS upon stent deployment across the entire stricture length to total number of patients. Clinical success rate (CSR) was defined as the ratio of patients with technical success and successful maintenance of stent function before elective surgery (regardless of number of SEMS deployed) to total number of patients. The surgical success rate (SSR) of colorectal stent as a bridge to surgery was defined as the ratio of patients with successful surgical procedures. Unsuccessful surgical outcomes were defined as being due to insufficient colonic decompression. The technical, clinical, surgical success rates and complications after stenting were assessed.
RESULTS: The median age of patients was 64 (36 to 89). 44.9% of patients were male and 55.1% were female. Eighteen patients had the obstruction located in the rectum, 15 patients in the rectosigmoid region, 10 patients in the sigmoid region, and 6 patients had a tumor causing obstruction in the proximal colon. Each patient was categorized pathologically as stage 2 (32.7%, 16 patients) or stage 3 (42.9%, 21 patients) and 12 patients (24.4%) had metastatic disease. None of the patients received chemotherapy before stenting. Stenting was undertaken in 37 patients as a bridge to surgery, and in 12 patients stents were used for palliation. Median time to surgery after stenting was 30 ± 91.9 d. All surgery was completed in one single operation and thus no colostomy with stoma was needed. The median overall survival rate of patients with stage 2-3 colorectal cancer was 53.1 mo and stage 4 was 37.1 mo (P = 0.04). Metastatic colorectal patients who were treated palliatively with stents had backbone chemotherapy with oxaliplatin and/or irinotecan-based regimens plus antiangiogenic therapies, especially bevacizumab. Resolution of the obstruction and clinical improvement was achieved in all patients. The technical, clinical and surgical success rates were 95.9%, 100% and 94.6%, respectively.
CONCLUSION: The efficacy and safety of colonic stents was demonstrated both as a bridge to surgery and for palliative decompression. In addition, results emphasize the importance of the skills of the endoscopist in colonic stenting.
Core tip: Colorectal stents can be used for two indications in colorectal malignancies; palliative dilatation of advanced disease, and preoperative decompression as a bridge to surgery. In both indications, colonic stents prevent colostomy with stoma. Decompression of the bowel gives time for surgeons to stabilize the patient, stage the disease with imaging techniques, and take a biopsy. Thus, it allows one-stage surgery with primary anastomosis. Palliative colorectal stenting was shown to be as effective and acceptable as palliative surgery. Colonic stents showed long-term efficacy comparable to that of surgery.
- Citation: Bayraktar B, Ozemir IA, Kefeli U, Demiral G, Sagiroğlu J, Bayraktar O, Adali G, Ozcelik A, Tortum OB. Colorectal stenting for palliation and as a bridge to surgery: A 5-year follow-up study. World J Gastroenterol 2015; 21(31): 9373-9379
- URL: https://www.wjgnet.com/1007-9327/full/v21/i31/9373.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i31.9373
Colorectal cancer alone is expected to account for 8.2% (136830) of all new cancer cases and it is estimated that about 50310 (8.3%) deaths from colorectal cancer will occur in the United States in 2014[1]. The rate of colorectal tumors causing large bowel obstruction is between 15% and 20%[2,3]. Obstruction requires immediate treatment and the mortality rate of emergency surgery is high. However, colonic stents are being safely used in malignant colorectal obstruction. Colorectal stents can be successfully placed in the majority of cases with good clinical results[4,5]. They are used for two indications in colorectal malignancies; palliative dilatation of advanced disease, and preoperative decompression as a bridge to surgery[6]. In both indications, colonic stents prevent colostomy with stoma[7]. Colonic stents are well-tolerated and have low rates of morbidity and mortality[4,8]. These stents have therefore attracted wide attention. In the light of these facts, we here report a long-term (5 year) follow-up assessment of the management of malignant colorectal obstructions using colonic stents, both as a bridge to surgery and as palliation.
Forty-nine patients with colorectal cancer who had undergone colorectal stent placement at two hospitals in Istanbul (Istanbul University Cerrahpasa School of Medicine and Medeniyet University Education and Research Hospital) were reviewed retrospectively over a 5-year period from January 2008 to January 2013. The obstruction was diagnosed clinically and radiologically. Histopathological diagnosis was achieved endoscopically. Stages of the disease for each patient were determined with pathological (if the patient underwent surgery) and clinical findings. Patients’ symptoms, characteristics and clinicopathological data were obtained by reviewing medical records. All patients were enrolled after informed consent. All patients were staged according to the American Joint Committee on Cancer (7th edition) tumor node metastasis (TNM) staging manual[9]. Clinical follow-up included history-taking, physical examination, laboratory tests, and radiological imaging tests if indicated for detection of relapse.
Briefly, all self-expanding metallic stent (SEMS) placement procedures were performed by experienced endoscopists using colonoscopes (CV-40; Olympus, Tokyo, Japan and EPX-4400HD; Fujinon, Tokyo, Japan) with fluoroscopic guidance. Before the procedure was carried out, sedation with midazolam 2-5 mg was applied in the presence of an anesthesiologist. Water-soluble contrast material was injected through the catheter to visualize the stricture. The luminal diameters of the Over-the-Wire (OTW) and Through-the-Stent (TTS) stents were 30 mm (body of stent) and 36 mm (both ends) respectively, and the lengths varied from 8 to 12 cm. The length of the stent was at least an additional 3 cm on each side of the stricture preference. Uncovered SEMS (Micro-Tech Europe GmbH, Düsseldorf, Germany) were placed in all patients.
Routine checks with direct radiography were performed after the procedure had been completed. All patients were allowed to take liquid 6 h after the procedure and were discharged after 12 to 24 h. A liquid and semi-solid diet was recommended to patients for the first week. Thereafter, on the seventh post-procedure day, direct control radiography was performed.
Here the technical success rate (TSR) was defined as the ratio of patients with a correctly placed SEMS upon stent deployment across the entire stricture length to the total number of patients. The clinical success rate (CSR) was defined as the ratio of patients with technical success and successful maintenance of stent function before elective surgery (regardless of number of SEMS deployed) to the total number of patients. The surgical success rate (SSR) of colorectal stents as a bridge to surgery was defined as the ratio of patients with successful surgical procedures. Unsuccessful surgical outcomes were defined as being due to insufficient colonic decompression.
Statistical analyses were conducted using SPSS 20.0 (SPSS Inc., Chicago, IL) software. Survival analysis and curves were established according to the Kaplan-Meier method and compared using the log-rank test. Median follow-up time was calculated as the median observation interval for all patients, being the time from diagnosis or colorectal stenting for obstruction to the last follow-up or death. Disease-free survival (DFS) was defined as the time since diagnosis or stenting to the first evidence of relapse in stages 2-3 of the disease. Progression-free survival (PFS) was described as the period following diagnosis or stenting to the first evidence of relapse in stage 4 of the disease. In addition, overall survival (OS) was described as the time from diagnosis to the date of the patient’s death or last known contact. Prognostic factors analyzed by univariate analysis were also evaluated with multivariate analysis using the Cox proportional hazards model to predict the risk factors for relapse. Multivariate P values were used to characterize the independence of these factors. A 95% confidence interval (CI) was used to quantify the relationship between survival time and each independent factor. All P values were 2-sided in tests and P values ≤ 0.05 were considered significant.
A total of 49 colorectal cancer patients were evaluated. The median age of patients was 64, ranging from 36 to 89 years. 44.9% of patients were male and 55.1% were female. Each patient’s disease was categorized pathologically as stage 2 (32.7%, 16 patients) or stage 3 (42.9%, 21 patients). Twelve patients (24.4%) had metastatic disease. Eighteen patients had an obstruction located in the rectum, 15 patients in the rectosigmoid region, 10 patients in the sigmoid region, and 6 patients had a tumor causing obstruction in the proximal colon. None of the patients received chemotherapy before stenting. Clinicopathological characteristics are shown in Table 1.
| Characteristics | Patients (n = 49) |
| Gender | |
| Men | 22 (44.9) |
| Women | 27 (55.1) |
| Age | 64.0 (36-89) |
| Men | 64.1 (34-89) |
| Women | 64.6 (42-87) |
| Stage | |
| Stage 2 | 16 (32.7) |
| Stage 3 | 21 (42.9) |
| Stage 4 | 12 (24.4) |
| Obstruction | |
| Proximal colon | 6 (12.2) |
| Sigmoid colon | 10 (20.4) |
| Rectosigmoid | 15 (30.6) |
| Rectum | 8 (36.8) |
Only in two patients was stenting done at the second attempt. Except for these, in all patients only one colorectal stent was used. In all patients, resolution of the obstruction and clinical improvement was achieved. Thus, the TSR and CSR were 95.9% and 100%, respectively. Stenting was undertaken in 37 patients as a bridge to surgery, and in 12 patients stents were used for palliation. Median duration of the stenting procedure was 18 (± 4.48) min for the entire group.
In the bridge to surgery group, abdominal pain occurred in one patient (2.7%) as an early complication (< 7 d), while one patient (2.7%) experienced tenesmus. Three patients suffered tenesmus (8.1%), in two patients stent migration occurred (5.4%), and one patient (2.7%) had a fecal obstruction as a late complication (> 7 d) that was solved endoscopically. Patients defined with tenesmus who experienced stent migration after the stenting procedure had rectal cancer. Thus, decompression caused complications for 41.6% of patients with rectal cancer, who were in stages 2 and 3 of the disease.
As early complications in the palliative stage 4 group, two patients suffered abdominal pain (16.6%), one patient (8.3%) had tenesmus, and one patient (8.3%) experienced fever. As late complications, stent migration and obstruction occurred in one patient (8.3%) and two patients had an obstruction due to tumor migration (16.6%). Each complication was recorded in a different tumor region.
For the entire group, early complication rates totaled 13.1%; three patients with abdominal pain (6.2%), two patients with tenesmus (4.2%) and one patient with fever (2.1%). Late complications totaled 18.7%; three patients with tenesmus (6.2%), one patient with fecal obstruction (2.1%), three patients with stent migration (6.2%), and two patients with tumor migration and obstruction (4.2%) (Table 2). For the entire group, the median duration of hospitalization was 4 ± 1.17 d. The median time to surgery after stenting was 30 ± 91.9 d. All surgery was done in one single operation; therefore no colostomy with stoma was needed. All patients who had rectal cancer received neoadjuvant chemoradiation and patients with stage 2 and 3 disease received adjuvant chemotherapy. Only two patients with stage 2 and 3 disease failed to undergo surgery due to cardiac problems. Thus, SSR was 94.6%. Metastatic colorectal patients who were managed palliatively with stents had backbone chemotherapy with oxaliplatin and/or irinotecan-based regimens plus antiangiogenic therapies, especially bevacizumab. Colorectal stent and patient characteristics are shown in Table 1.
| Groups | Complications | |
| Early complications | Late complications | |
| Bridge to surgery group | Abdominal pain 1 (2.7) | Stent migration 2 (5.4) |
| Tenesmus 1 (2.7) | Tenesmus 3 (8.1) | |
| Obstruction with feces 1 (2.7) | ||
| Palliation group | Abdominal pain 2 (16.6) | Stent migration 1 (8.3) |
| Tenesmus 1 (8.3) | Tumor migration 2 (16.6) | |
| Fever 1 (8.3) | ||
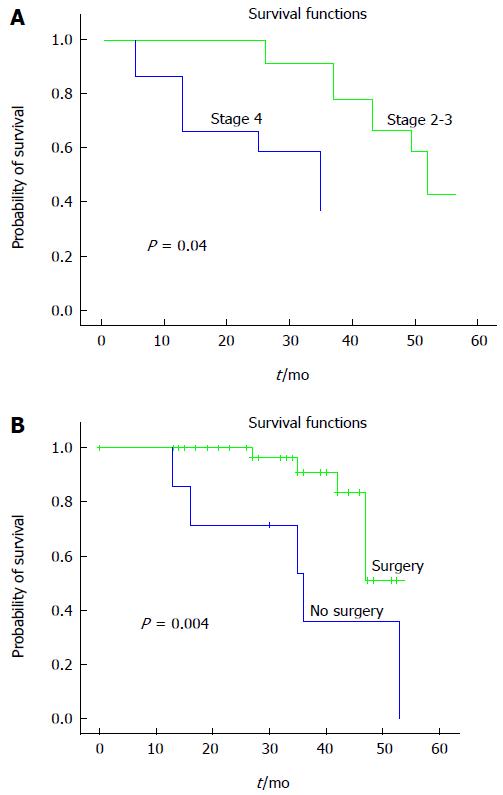
At a median follow-up of 33.0 mo, median OS was 53.0 (95%CI: 46.57-59.42) mo. Median OS of the entire group was 47.0 mo (95%CI: 21.71-72.28). Median OS of stage 2-3 patients was 53.1 mo and stage 4 patients was 37.1 mo (P = 0.04). Patients who underwent surgery had an OS of 49.1 mo, while patients who were unable to undergo surgery had an OS of 37.2 mo (P = 0.004) (Figure 1). There was no statistically significant relationship between OS, age, location of obstruction and gender (P > 0.05). In multivariate analysis, only surgery was an independent prognostic factor for OS (Table 3). Median PFS time was not reached. The two and three-year PFS rate for stage 2 disease was 100%; and the rates were 94.0% and 84.7% for stage 3 disease (P > 0.05), respectively. The two-year DFS rate was 91.3% and three-year DFS rate was 87.4% in stage 4 patients. There was no statistically significant relationship between PFS, age, gender, location of obstruction and surgery (P > 0.05).
| Variables | OS | ||
| HR | 95%CI | P value | |
| Surgery | 0.069 | 0.06-0.96 | 0.044 |
| Stage | 0.082 | 0.59-10.29 | 0.214 |
Colonic obstruction occurs in 15%-20% of colorectal cancers[2,3]. Colonic stenting is recommended only for those patients with both obstructive symptoms and radiological or endoscopic findings suspicious of malignant large-bowel obstruction[6]. Experience with stenting has generally been performed for left-sided lesions and only one randomized trial was carried out in the case of malignant obstruction of rectal cancer. Rectal stenting is often avoided because of presumed association with complications such as pain, tenesmus, incontinence, and stent migration. For this reason, guidelines suggest that stenting only be applied for malignant colonic obstruction[6,10]. In our study, no difficulty was encountered in applying the stenting procedure, except for one patient who had a tumor in the colonic region. However, the stenting process caused complications both in the early and late phases of patients with obstructed rectal cancer. Tenesmus (8.1% overall, 25.0% for rectal cancer) and stent migration (5.4% overall, 16.6% for rectal cancer) occurred in two patients with stage 2 and 3 of the disease. Thus, we found that the stenting procedure for cancers in the rectal region caused relatively more complications compared with proximal tumors.
Our TSR was 95.9% and CSR was 100%. In the literature, TSR varies between 70%-100% and CSR from 85% to 96%[11-14]. In a trial where most of the endoscopists were from a non-university setting, TSR was reported as 70%. Factors associated with technical failure included the severity of obstruction, extra-colonic origin of tumor, proximal colonic obstruction, and presence of carcinomatosis[14]. In our study, a low technical failure rate (only two patients) did not allow us to determine the factors associated with technical failure. Our total early and late complication rates for the entire group were 13.1% and 18.7%, respectively. Early complications were three patients with abdominal pain (6.2%), two patients with tenesmus (4.2%), and one patient with fever (2.1%). Late complications were three patients with tenesmus (6.2%), one patient with fecal obstruction (2.1%), three patients with stent migration (6.2%), and two patients with tumor migration and obstruction (4.2%). These complication rates are much lower than previous studies[15,16]. In this study, all endoscopic stent placements were handled by the same general surgeon in a university setting. Therefore, higher success rates and lower complication rates with no perforation can be attributable to the circumstances in the particular treatment facility and experience of well-trained endoscopists.
The use of stents for acute colorectal obstruction due to malignancy is controversial. van Hooft et al[6] found high perforation rates related to the use of colorectal stenting in malignant obstructions[2,3]. A decision analysis concluded that colonic stent insertion followed by elective surgery was more effective and less costly than emergency surgery, but results of randomized trials and meta-analyses are contradictory[17-19]. Decompression of the bowel gives time for surgeons to stabilize the patient, stage the disease with imaging techniques, and take a biopsy. Thus, it allows one-stage surgery with primary anastomosis. In our study, except for two patients who had cardiac problems, all patients with stage 2 and 3 disease were able to undergo one-stage surgery without stoma. In the group overall, patients who underwent surgery had longer OS. Based on our results, we oppose the belief in favor of emergency surgery rather than stenting in situations of malignant colonic obstruction, based on high perforation rates[14].
Palliative colorectal stenting was shown to be as effective and acceptable as palliative surgery in one retrospective study, where colonic stents showed long-term efficacy comparable to that of surgery[20]. Published follow-up data are limited because of poor survival rates of the patient population in the palliative group. Stefanidis et al[21] reported one-year effectiveness and the patency of colonic stents used for palliation. Bevacizumab is an antiangiogenic agent used in treating metastatic colorectal cancer[22]. Bevacizumab was found to be associated with high complication rates in patients who had palliative stents for malignant obstruction[23]. In our study, at a median follow-up of 33 mo, we found no significant complications due to the addition of bevacizumab.
In the bridge to surgery group, the surgical success rate, i.e., the ratio of patients that had stent placement who underwent elective primary anastomosis surgery, was 95.6%. This ratio is very high compared to other studies, which were between 55.3%-77.9%[24-26]. This might be explained by low TSR and CSR in these studies vs high rates in our study. Previous studies have reported that the factors associated with technical failure included severity of obstruction, extra-colonic origin of tumor, proximal colonic obstruction, and presence of carcinomatosis.
In conclusion, we demonstrated in this study the efficacy and safety of colonic stents both as a bridge to surgery and for palliative decompression. Surgery was found to be an independent prognostic factor in patients with malignant colorectal obstruction. Although technical and clinical success rates were high, rectal stents had greater complication rates, consistent with the literature. Palliative stenting with a median follow-up of 33 mo did not add any severe extra complications in the era of bevacizumab. In addition, results highlight the importance of the skill of the endoscopist when carrying out colonic stenting.
Colorectal tumors may cause large bowel obstruction and in such cases they require immediate treatment. The mortality rate of emergency surgery is high. However, colonic stents are being safely used in malignant colorectal obstruction in the majority of cases with good clinical results. They are used for two indications in colorectal malignancies; palliative dilatation of advanced disease, and preoperative decompression as a bridge to surgery. In both indications, colonic stents prevent colostomy with stoma.
Colorectal stents can be used for two indications in colorectal malignancies; palliative dilatation of advanced disease, and preoperative decompression as a bridge to surgery. In both indications, colonic stents prevent colostomy with stoma.
The authors proved the efficacy and safety of colonic stents in both bridge to surgery and for palliative decompression. In addition, the importance of the skills of the endoscopist in colonic stenting was also exposed. Decompression of the bowel gives time for surgeons to stabilize and prepare the patient for further treatment approaches.
Colorectal stents are used increasingly as a nonsurgical alternative for the palliation of luminal gastrointestinal neoplasms. They also have an emerging role in the treatment of obstruction in gastrointestinal segments.
A colostomy is a surgically created opening on the abdomen which allows stools to exit the body.
The manuscript describes the evaluation of colonic stents. The manuscript is well presented and tackles an important aspect in clinical colon cancer biology. A significant group of nearly 50 patients have been studied. Statistical analysis is well carried out.
P- Reviewer: Braet F, Lakatos PL S- Editor: Ma YJ L- Editor: O’Neill M E- Editor: Liu XM
| 1. | Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8789] [Cited by in RCA: 9568] [Article Influence: 869.8] [Reference Citation Analysis (0)] |
| 2. | Phillips RK, Hittinger R, Fry JS, Fielding LP. Malignant large bowel obstruction. Br J Surg. 1985;72:296-302. [PubMed] |
| 3. | Mella J, Biffin A, Radcliffe AG, Stamatakis JD, Steele RJ. Population-based audit of colorectal cancer management in two UK health regions. Colorectal Cancer Working Group, Royal College of Surgeons of England Clinical Epidemiology and Audit Unit. Br J Surg. 1997;84:1731-1736. [PubMed] |
| 4. | Khot UP, Lang AW, Murali K, Parker MC. Systematic review of the efficacy and safety of colorectal stents. Br J Surg. 2002;89:1096-1102. [PubMed] |
| 5. | Sebastian S, Johnston S, Geoghegan T, Torreggiani W, Buckley M. Pooled analysis of the efficacy and safety of self-expanding metal stenting in malignant colorectal obstruction. Am J Gastroenterol. 2004;99:2051-2057. [PubMed] |
| 6. | van Hooft JE, van Halsema EE, Vanbiervliet G, Beets-Tan RG, DeWitt JM, Donnellan F, Dumonceau JM, Glynne-Jones RG, Hassan C, Jiménez-Perez J. Self-expandable metal stents for obstructing colonic and extracolonic cancer: European Society of Gastrointestinal Endoscopy (ESGE) Clinical Guideline. Endoscopy. 2014;46:990-1053. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 262] [Cited by in RCA: 218] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 7. | Karoui M, Charachon A, Delbaldo C, Loriau J, Laurent A, Sobhani I, Tran Van Nhieu J, Delchier JC, Fagniez PL, Piedbois P. Stents for palliation of obstructive metastatic colon cancer: impact on management and chemotherapy administration. Arch Surg. 2007;142:619-623; discussion 623. [PubMed] |
| 8. | Faragher IG, Chaitowitz IM, Stupart DA. Long-term results of palliative stenting or surgery for incurable obstructing colon cancer. Colorectal Dis. 2008;10:668-672. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 59] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 9. | Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A. AJCC Cancer Staging Manual. 7th ed. New York, NY: Springer 2010; 347-376. |
| 10. | Fiori E, Lamazza A, De Cesare A, Bononi M, Volpino P, Schillaci A, Cavallaro A, Cangemi V. Palliative management of malignant rectosigmoidal obstruction. Colostomy vs. endoscopic stenting. A randomized prospective trial. Anticancer Res. 2004;24:265-268. [PubMed] |
| 11. | Baron TH, Dean PA, Yates MR, Canon C, Koehler RE. Expandable metal stents for the treatment of colonic obstruction: techniques and outcomes. Gastrointest Endosc. 1998;47:277-286. [PubMed] |
| 12. | Mainar A, De Gregorio Ariza MA, Tejero E, Tobío R, Alfonso E, Pinto I, Herrera M, Fernández JA. Acute colorectal obstruction: treatment with self-expandable metallic stents before scheduled surgery--results of a multicenter study. Radiology. 1999;210:65-69. [PubMed] |
| 13. | de Gregorio MA, Mainar A, Tejero E, Tobío R, Alfonso E, Pinto I, Fernández R, Herrera M, Fernández JA. Acute colorectal obstruction: stent placement for palliative treatment--results of a multicenter study. Radiology. 1998;209:117-120. [PubMed] |
| 14. | van Hooft JE, Bemelman WA, Oldenburg B, Marinelli AW, Lutke Holzik MF, Grubben MJ, Sprangers MA, Dijkgraaf MG, Fockens P. Colonic stenting versus emergency surgery for acute left-sided malignant colonic obstruction: a multicentre randomised trial. Lancet Oncol. 2011;12:344-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 303] [Cited by in RCA: 313] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 15. | Jost RS, Jost R, Schoch E, Brunner B, Decurtins M, Zollikofer CL. Colorectal stenting: an effective therapy for preoperative and palliative treatment. Cardiovasc Intervent Radiol. 2007;30:433-440. [PubMed] |
| 16. | Watt AM, Faragher IG, Griffin TT, Rieger NA, Maddern GJ. Self-expanding metallic stents for relieving malignant colorectal obstruction: a systematic review. Ann Surg. 2007;246:24-30. [PubMed] |
| 17. | Targownik LE, Spiegel BM, Sack J, Hines OJ, Dulai GS, Gralnek IM, Farrell JJ. Colonic stent vs. emergency surgery for management of acute left-sided malignant colonic obstruction: a decision analysis. Gastrointest Endosc. 2004;60:865-874. [PubMed] |
| 18. | Sagar J. Colorectal stents for the management of malignant colonic obstructions. Cochrane Database Syst Rev. 2011;CD007378. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3573] [Cited by in RCA: 2754] [Article Influence: 1377.0] [Reference Citation Analysis (0)] |
| 19. | Zhang Y, Shi J, Shi B, Song CY, Xie WF, Chen YX. Self-expanding metallic stent as a bridge to surgery versus emergency surgery for obstructive colorectal cancer: a meta-analysis. Surg Endosc. 2012;26:110-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 135] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 20. | Lee HJ, Hong SP, Cheon JH, Kim TI, Min BS, Kim NK, Kim WH. Long-term outcome of palliative therapy for malignant colorectal obstruction in patients with unresectable metastatic colorectal cancers: endoscopic stenting versus surgery. Gastrointest Endosc. 2011;73:535-542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 94] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 21. | Stefanidis D, Brown K, Nazario H, Trevino HH, Ferral H, Brady CE, Gross GW, Postoak DW, Chadhury R, Rousseau DL. Safety and efficacy of metallic stents in the management of colorectal obstruction. JSLS. 2005;9:454-459. [PubMed] |
| 22. | Bendell JC, Bekaii-Saab TS, Cohn AL, Hurwitz HI, Kozloff M, Tezcan H, Roach N, Mun Y, Fish S, Flick ED. Treatment patterns and clinical outcomes in patients with metastatic colorectal cancer initially treated with FOLFOX-bevacizumab or FOLFIRI-bevacizumab: results from ARIES, a bevacizumab observational cohort study. Oncologist. 2012;17:1486-1495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 78] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 23. | Small AJ, Coelho-Prabhu N, Baron TH. Endoscopic placement of self-expandable metal stents for malignant colonic obstruction: long-term outcomes and complication factors. Gastrointest Endosc. 2010;71:560-572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 227] [Cited by in RCA: 202] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 24. | Ye GY, Cui Z, Chen L, Zhong M. Colonic stenting vs emergent surgery for acute left-sided malignant colonic obstruction: a systematic review and meta-analysis. World J Gastroenterol. 2012;18:5608-5615. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 39] [Cited by in RCA: 37] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 25. | Cirocchi R, Farinella E, Trastulli S, Desiderio J, Listorti C, Boselli C, Parisi A, Noya G, Sagar J. Safety and efficacy of endoscopic colonic stenting as a bridge to surgery in the management of intestinal obstruction due to left colon and rectal cancer: a systematic review and meta-analysis. Surg Oncol. 2013;22:14-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 142] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 26. | Kim JH, Kwon KA, Lee JJ, Lee WS, Baek JH, Kim YJ, Chung JW, Kim KO, Park DK, Kim JH. Surgical failure after colonic stenting as a bridge to surgery. World J Gastroenterol. 2014;20:11826-11834. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 12] [Cited by in RCA: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |