Published online Aug 21, 2015. doi: 10.3748/wjg.v21.i31.9286
Peer-review started: March 16, 2015
First decision: April 23, 2015
Revised: May 10, 2015
Accepted: July 3, 2015
Article in press: July 3, 2015
Published online: August 21, 2015
Processing time: 159 Days and 17.4 Hours
Transanal endoscopic surgery (TES) consists of a series of anorectal surgical procedures using different devices that are introduced into the anal canal. TES has been developed significantly since it was first used in the 1980s. The key point for the success of these techniques is how accurately patients are selected. The main indication was the resection of endoscopically unresectable adenomas. In recent years, these techniques have become more widespread which has allowed them to be applied in conservative rectal procedures for both benign diseases and selected cases of rectal cancer. For more advanced rectal cancers it should be considered palliative or, in some controlled trials, experimental. The role of newer endoscopic techniques available has not yet been defined. TES may allow for new strategies in the treatment of rectal pathology, like transanal natural orifice transluminal endoscopic surgery or total mesorectal excision.
Core tip: In recent years, the diffusion of transanal endoscopic surgery techniques has allowed the application of conservative rectal procedures in both benign diseases and selected cases of early rectal cancer. For more advanced rectal cancers it should be considered palliative or, in some controlled trials, experimental and may allow for new strategies in the treatment of rectal pathologies.
- Citation: García-Flórez LJ, Otero-Díez JL. Local excision by transanal endoscopic surgery. World J Gastroenterol 2015; 21(31): 9286-9296
- URL: https://www.wjgnet.com/1007-9327/full/v21/i31/9286.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i31.9286
Transanal endoscopic surgery (TES) consists of a series of anorectal surgical procedures using different devices that are introduced into the anal canal. These devices allow the creation of a stable pneumorectum and offer a number of working channels that enable the introduction of optics and instruments for maneuvers of dissection, cutting, coagulation and suture. In recent years, the greater spread of these techniques has allowed the application of conservative rectal procedures in both benign diseases and selected cases of rectal cancer. The purpose of this article is to review the current indications of local excision, technical options available, and outcomes.
The key point for the success of these techniques lies in how accurately patients are selected. Preoperative workup includes physical exploration with digital rectal examination, fecal incontinence score, endoscopy, endoscopic rectal ultrasound (ERUS), pelvic magnetic resonance imaging (MRI) and abdominal CT in case of malignancies. Digital examination plays a key role. Magnification chromoendoscopy, ERUS and MRI are complementary staging modalities. TES has developed significantly since it was first used in the 1980s. The main indication was the resection of endoscopically unresectable adenomas. Local excision with TES has also shown benefits treating selected early rectal cancers. For more advanced rectal cancers it should be considered palliative or, in some controlled trials, experimental. With the development of the technique and the experience of surgeons, the indications have been increased. There are many applications beyond local excision, the most important in recent years is the development of transanal total mesorectal excision (TME)[1-3] (Table 1). Nowadays, we can consider these platforms as an important part of the colorectal surgeon’s armamentarium, available for solving complex proctologic diseases, and may offer new strategies in the treatment of rectal cancer.
| Pelvic abscess |
| Benign rectal stenosis |
| Rectal Dieulafoy's lesion |
| Rectourethral fistula |
| Gastrointestinal stromal tumour |
| Rectal condylomata acuminata |
| Rectal prolapse |
| Impacted fecaloma |
| Rectal perforations |
| Presacral tumor |
| Foreign body |
| Neuroendocrine tumour |
| Transanal total mesorectal excision |
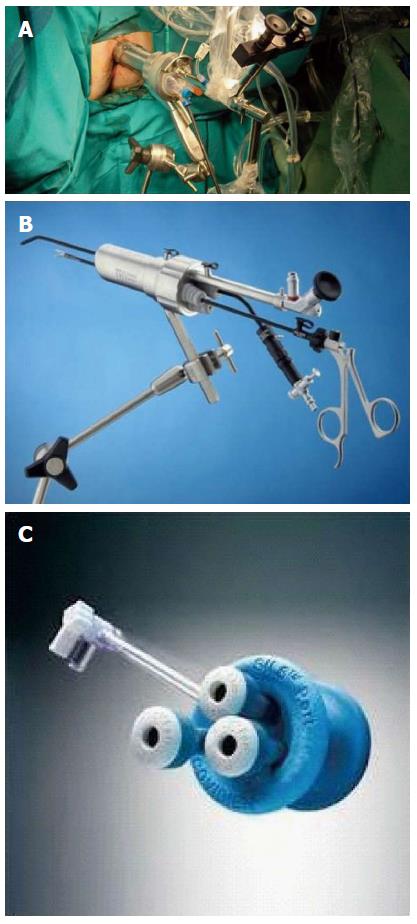
The first specifically designed device for these procedures was developed by Buess et al[4] in the mid 1980s. (Transanal Endoscopic Microsurgery, TEM, Richard Wolf, Germany). TEM instruments consist of a 3D optic viewing system with specific operating instruments and an endosurgical unit. The operating instruments include the operating rectoscope (4 cm in diameter, 12 or 20 cm in length), the stereoscope and instruments for dissection, excision and suturing. The endosurgical unit provides insufflation, suction, irrigation and continuous monitoring of intrarectal pressure. The rectoscope and its attachments are secured to the operating room table using a multijointed clamp, Martin’s arm (Figure 1A). It was costly equipment and required specific training, so its spread, mainly in high-volume colorectal surgery units, was slow.
A few years later a cheaper alternative was introduced. The transanal endoscopic operation (TEO, Karl Storz, Germany), which was a newer and simpler system, has become widely implemented. It does not use the 3D optic system and has a shorter rectoscope (4 cm in diameter, 7.5 or 15 cm in length). Standard laparoscopic instruments, equipment and set up costs are lower, potentially opening the technique to any surgeon with previous laparoscopic experience. The main difference is a lack of binocular vision (Figure 1B). Several studies have compared TEO with TEM for benign and malignant lesions and have shown satisfactory outcomes[5,6].
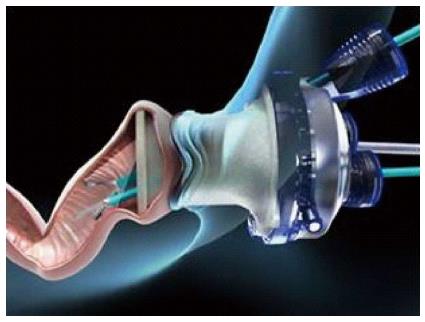
In recent years, the procedure known under the name of Transanal Minimally Invasive Surgery (TAMIS) has become increasingly more popular. Reported by Atallah et al[7] in 2010, the technique stems from the use of a single port initially designed for abdominal surgery. TAMIS is defined as the use of any multichannel port combined with ordinary laparoscopic instruments, a laparoscopic camera lens (preferably 5 mm and 30º), and a standard laparoscopic insufflator. Currently in the United States there are two ports with Food and Drug Administration (FDA) approval for TAMIS. They are the single-incision assisted laparoscopic surgery (SILS) Port (Covidien, United States) and the GelPOINT Path Transanal Access Platform (Applied Medical, United States). The latter is the only multichannel port specifically designed for TAMIS procedures (Figures 1C and 2). Standard laparoscopic instruments available at any operating theatre allow any experienced colorectal laparoscopic surgeon to perform the procedure without additional investment. It is also possible to use a flexible endoscope as a camera and offers an additional way to grasp or retract the bowel with an endoscopic grasper (eTAMIS)[8].
A variety of multichannel ports can be applied transanally[9-14] (Table 2). Since the inception of TAMIS, at least 390 procedures were reported worldwide from 2010 to 2013[15]. Robotic-TAMIS have also been reported, but with limited data. Success with robotic-TAMIS has been demonstrated with various patient positions and using a glove port[16-18]. Regardless of which platform is used, the basic principles of the procedure remain the same. Although alternate synonyms for TAMIS exist, it could be a valid generic term for all procedures using multichannel disposable ports regarding transanal minimal access surgery.
| SILS port (Covidien, United States) |
| Gel POINT Path (Applied Medical, United States) |
| SSL port (Ethicon, United States) |
| Triport (Olympus, Japan) |
| Glove port |
| Long Gel POINT Path (Applied Medical, United States) |
| Endorec (Aspide, France) |
| Single ballon trocar (pediatric use) |
Mechanical bowel preparation, antibiotic and antithrombotic prophylaxis are usually recommended. Anesthesia may be general or spinal. Lee et al[19] have reported a series of 25 TAMIS using spinal anesthesia. In TEM the surgeon works with the tumour visible in the lower part of the rectoscope at all times, so the positioning of the patient depends on the location of the rectal tumour. In TAMIS the majority of lesions can be excised in a lithotomy position. However, we still recommend turning the patient for large anterior lesions, especially if the distance from the anal verge is in a range where there might be a risk of opening the peritoneum.
The pneumorectum is maintained at a constant pressure. Rectal distension created in this way exposes the tumour and the rectal wall. Some groups are now using the AirSeal system insufflator (SurgiQuest, United States) to maintain a stable pneumorectum during TAMIS[20]. Right-angle camera cords can improve ergonomics and decrease instrument collision[21]. Care must be taken to avoid entering the abdominal cavity whenever it is possible. As usual, we recommend beginning the dissection making a dotted line with the monopolar scalpel about 10 mm from the tumour. We then open the mucosa over the dotted line and begin the full-thickness excision of the rectal wall reaching the mesorectal fat using an ultrasound scalpel (Ultracision, Ethicon Endo-Surgery, United States) allowing a good hemostasis. Conventional laparoscopic instruments are suitable for TAMIS, but advanced laparoscopic instruments can be employed, like linear staplers, vessel-sealing systems or articulating instruments[7,9]. We usually wash the rectal defect with diluted povidone, and we recommend exsufflating the rectum after complete resection, then wait 3-5 min and reinsufflate in order to assure good hemostasis. The defect is sutured transversally to avoid stenosis of the rectal lumen and postoperative bleeding. Suturing in this area is sometimes difficult for technical reasons as the working space is limited. For defect closure different techniques are frequently used, such as clip-fixated sutures. If the defect cannot be completely closed, it should be reduced to the maximum, especially in the upper rectum, due to the risk of perforation. We are now using barbed sutures, that display the same bursting pressure as monofilament sutures, and their use for rectal wall closure seems feasible[22]. Suture line dehiscence is described in up to one-third of patients but mainly remains clinically unrecognized[23]. The dehiscence is presumably related to the wider size of the residual cavity. Obliteration of the residual perirectal space with a hemostatic agent and by the introduction of gauzes into the rectal ampulla, may reduce the risk of postoperative perirectal abscess, and thus reduce the suture line dehiscence rate[24].
In centres with TEM experience, the average duration of surgery ranges from 45 to 120 min. Also, a number of studies have reported significantly decreased duration of surgery for TEM compared with radical surgery[25]. Several large studies have reported hospital stays of 4-5 d with low readmission rates. Some articles have also shown that 23-h discharge is safe, although the number of patients in these studies is small[26].
Most experience with TES is derived from TEM and TEO. In a recent prospective randomized clinical trial, no technical or clinical differences were observed between the results obtained with the two systems except lower cost with TEO[6]. For some authors, the introduction of the TAMIS port into the anal canal is more complex than in TEM or TEO[9]. A SILS port can be used in patients with narrow or fibrotic anal canals which do not allow the GelPOINT Path transanal access device to be introduced. A further disadvantage of TAMIS is that the rectoscope cannot be mobilized at the site of the lesion; rectal lesions located behind a rectal haustral valve may be more difficult to access and remove. The longer channels associated with TEM and TEO equipments facilitate intraluminal rectal retraction. A new disposable port (GelPOINT Path Long Channel) can reach lesions up to 15 cm from the anal verge[27]. In addition, an assistant is required to hold and manipulate the laparoscope during the TAMIS procedure.
Traditionally, the upper limit of dissection is 10 cm for anterior tumours, 12 for lateral and 15 cm for posterior tumours. The limit for low lesions is the anal verge itself, but air-tightness of the insufflation system may be compromised for tumours less than 4 cm from the anal verge and therefore traditional transanal local excision (TAE) is sometimes better for these lower tumours. Nonetheless, the operation can begin by TAE and then be converted to TES to finish. In experienced hands, TES is capable of providing high quality local excision with a reach triple to that of Park’s TAE. TEM offers excellent magnified exposure of the operative site, especially for the upper and deep limits of the tumour, enhancing monobloc excision. TAE was associated with a significantly increased risk of fragmentation and R1 resection, leading to a higher risk of local recurrence (LR)[28,29]. A meta-analysis has confirmed a higher rate of R0 resection and disease-free survival after TEM[30]. Recently reported by Elmessiry et al[31], when TAE and TEM were compared, the latter resulted in a greater number of tumour-free excision margins, especially at the deep margin, and enabled a full-thickness whole specimen rather than a fragmented one. There was, however, no significant difference in LR or survival between the two techniques.
The limits of these techniques lie chiefly in the size and the circumferential extension of the lesions. Classically, the technique is recommended for superficial rectal tumours up to 3 cm in diameter and involving up to 40% of the rectal circumference. Nowadays we can consider that there are very few limits in terms of the location (anterior, lateral) of the lesion. In fact all four quadrants can sometimes be reached if the lesions are not particularly wide and if the size does not exceed the height permitted. It is possible to excise adenomatous lesions that cover even more than three quadrants of the circumference.
Whichever technique is used, morbidity and mortality are lower than for radical surgery. Operative mortality is less than 0.5% and morbidity ranges from 4% to 30% in large series, depending on the inclusion of minor complications. Bignell et al[32] found that the use of the harmonic scalpel reduces the complication rate. The most frequent complications include acute urinary retention (0%-11%), bleeding requiring re-operation (0.7%-9%), entry into the peritoneum (6%-20%) and recto-vaginal fistula (0.3%-1.4%)[33]. Kumar et al[34] found that the size of the tumour was associated with a risk of bleeding and anterior and lateral location was associated with a risk of peritoneal violation and acute urinary retention. Kreissler-Haag et al[35] assessed the anatomical variables of rectal neoplasia as well as surgeon experience on postoperative complications in patients undergoing TEM, they found 0.3% mortality and a 9% overall complication rate, including bleeding, fecal incontinence, dysuria, pneumonia, myocardial infarction and pulmonary emboli. Complications correlate with tumours located laterally and more than 8 cm from the anal verge. Overall surgical complications did not correlate with the number of TEM procedures performed, suggesting a short learning curve for the procedure in surgeons with previous experience in minimally invasive surgery.
Pelvic sepsis, which occurs in about 3% of cases, is more common in lesions within 2 cm of the dentate line. Regarding peritoneal perforation, although it was once thought to represent a complication requiring conversion to laparotomy or even a stoma, in experienced hands this can usually be salvaged by TES[36,37]. A multicenter study performed from a database of 888 TEM procedures, found 22 perforations into the peritoneal cavity. They reported no association with major short-term complications or adverse long-term oncological outcomes[38]. Postoperative complications may be greater after neoadjuvant chemoradiation and include pain and wound dehiscence, but the majority seems to be minor and can be treated conservatively[25,39-43].
Anorectal function after TEM has been addressed in several studies[26,43,44]. The evidence available suggests that the TEM procedure seems to have no permanent deleterious effect on fecal continence. Although TEM can cause manometric alterations, it does not affect clinical continence scores[45]. Short-term functional results of TAMIS are also excellent and comparable to functional results using the TEM equipment[46]. In many patients with pre-existent impairment of anorectal function, their functional outcome after TES is significantly improved, probably secondary to excision of a mucous producing lesion[47]. Some patients will develop anorectal dysfunction but this is associated with excision of large lesions with changes in rectal capacity and compliance[48].
Sexual or urinary disorders are very rare. When circumferential lesions are resected, particularly carpet adenomas, there can be a higher rate of rectal stenosis. Stenosis will normally respond to surgical or endoscopic balloon dilatations[49,50].
TEM has been used primarily for resection of large adenomas of the rectum[25,51,52]. The evidence supports TEM as the preferred approach to rectal adenoma resection when endoscopic removal is not possible with safety or without fragmentation, with excellent results, low recurrence rates and a favorable complication profile compared with TAE or radical resection[25,53,54]. It would be of interest to report and evaluate the results with more TAMIS series including margin status, specimen fragmentation, and complications associated with the technique in a similar way to the TEM-TEO series. Since a LR rate is higher after excision of adenomas larger than 5 cm, a strict follow-up is recommended. In cases of recurrent adenoma, TEM has been shown to be an important therapeutic option with no increased morbidity[55].
There are still some limitations in the pre-operative diagnosis of large rectal adenomas. Even though ERUS appears to be the most accurate pre-operative diagnostic tool for investigating tumour invasion, the rate of incidental carcinoma in lesions with benign appearance is significant even with multimodal pre-operative assessment. Serra-Aracil et al[56], found 52 out of 277 lesions (18.8%) with preoperative diagnosis of adenoma to be invasive carcinomas. Dash et al[57] found that 13% of 167 benign lesions (with non full-thickness excision) were unexpected cancers. This is not related to the type of lesion, although exophytic lesions may be harder to assess and classify by ERUS[57]. The rate of occult carcinoma may be as high as 40%, depending on pre-operative imaging assessment[43]. Higher frequency scanning probes and coupling gels seem to have shown better accuracy for early stage cancer[58]. Real-time elastography has been used to assess adenomas and early cancers, and has shown promising discrimination between them[59]. Some recent studies have investigated the role of ERUS, compared with MRI, for the staging of large rectal adenomas, reporting similar rates of over-staging, but MRI might be more appropriate in case of proximal tumours that cannot be reached by the ERUS probe[43].
Radical surgery with TME is still the cornerstone for the treatment of rectal cancer, offering patients the best results in terms of LR and disease-free survival[60]; however it is associated with significant mortality and morbidity rates[61]. According to the accumulated experience over the last years, TEM-TEO procedures have been accepted as effective treatments in selected patients with early rectal cancer, with similar oncologic outcomes as radical surgery and better functional results[62,63]. Recently, TAMIS has been proposed as an alternative with the same indications, but there is still limited experience in rectal cancer using this approach because of its short follow-up[27,15,31,43,64-67].
Appropriate patient and tumour selection is the main challenge and preoperative staging is of paramount importance for decision-making. Tumour biopsy offers a low accuracy, with a histological discrepancy of up to 20% or even higher[68]. Despite improvement in imaging techniques, there are patients who are not accurately staged[43]. ERUS appears to be the most accurate diagnostic tool for assessing tumour invasion of the rectal wall, especially for small lesions. MRI is more accurate detecting mesorectal invasion and the relation of the tumour with puborectalis muscle and anal sphincter in low cancers, and is preferred for N-staging because it allows for the evaluation of the whole mesorectum[25,33,40,61,63,69-71].
Unfavourable histological characteristics related to a high incidence of positive lymph nodes (N+) and recurrences are: Tumours larger than 3 cm or involving more than one third of the rectal lumen, rectal wall invasion more than T1 sm1, positive resection margins (< 1 mm), poorly differentiated adenocarcinomas, presence of lymphatic, venous or perineural invasion, mucinous component and tumour budding[25,31,40,63,72-76]. High-risk T1 tumours are more likely to be N+ compared to low-risk T2 tumours. Due to a lack of accuracy in the preoperative staging, full-thickness resection with a macroscopic margin of 10 mm is generally recommended[33,40,54,61,62,77]. Some authors[78] remove the perirectal fat to reach the mesorectal fascia, but there are some concerns about the possible major interference in case of completion surgery.
As mentioned above, TEM allows local excision to be performed with a lower positive margin rate compared to conventional TAE, less fragmented specimens and better oncologic outcomes[28,29]. In a multivariate analysis, TAE was an independent predictor of LR when it was compared to TEM[31]. The rate of reported involved margin in the surgical specimen in TAMIS procedures is 4.4%-6%[27,15,66], figures similar to those obtained with TEM, and seems to be related with the T stage[25,54,79]. Some studies have compared TEM with radical TME resection in early rectal cancer, finding similar results in terms of LR and survival[77,80]. In the meta-analysis performed by Winde et al[81], the rate of LR was higher with TEM (12% vs 0.5%) but no difference in survival was found. Similar conclusions were reported by others[25,64,80,82]. We have to take into account that TES is more frequently used in distal tumours, which have poorer prognosis when they are compared with upper rectal lesions.
TES seems to be a reasonable alternative to radical resection in patients with low-risk T1N0 rectal cancer[25,33,40,61,62,64,80,83] with LR rates ranging from 0%-39%. These wide differences can be explained by the heterogeneity of cases, different selection criteria, risk characteristics, and surgical techniques, but the majority are under 10%[25,30,68,77,78,81,84-87]. The level of submucosal invasion (sm level) has been demonstrated to be a strong predictor of recurrence, with sm1 lesions showing lowest levels of LR and sm2-3 lesions with LR rates similar to T2[66,72]. 5-year survival is consistently high in pT1, ranging 80%-100%, depending on the number of patients with high-risk tumours[25,68,78,81,83,85,87,88].
TES alone is not suitable treatment for fit patients with staged T2 or worse tumours, considering that the risk of LR varies between 9.5% and 47%[25,54,61,64,77,79,80,83-89]. But even in these cases there are considerable differences between low and high-risk cancers[90]. TES might be offered to patients with high-risk T1 or T2-3 tumours with poor life expectancy and multiple co-morbidity, unfit for major surgery, offering a reasonable chance of success, or simply as palliative treatment in case of disseminated disease[40,61,80].
When pathological evaluation of the TES specimen reveals tumour invasion beyond pT1 sm1 or high-risk features, immediate radical surgery with TME is recommended[25,27,40,43,54,65,85,87,91]. As a matter for concern, Hompes et al[92] reported that they had found the completion surgery procedure difficult in 53% of cases. The quality of mesorectum was moderate or poor in 36% of cases in the pathological exam; all of them were in the difficult group, associated with previous full-thickness resection and low tumours. The resected tumours with a good TME specimen had a significantly better 5-year disease-free survival compared to inferior specimens (100% vs 51%).
Salvage surgery for recurrence after TES offers disappointing oncologic outcomes, the stage is usually more advanced than in primary tumours and may require multivisceral resection and an ostomy in up to 43% of cases. Survival is seriously compromised, with a 5-year survival ranging 43%-68%, dropping to 29% in patients with unfavourable histology[40,84,93].
In patients with high-risk pT1 or pT2 after local resection, adjuvant radiotherapy could be an option for selected patients who decline completion surgery or are too frail for radical surgery. Even though radiotherapy appears to have some benefits added to TES, it is not equivalent to radical surgery[62,64]. There are some promising studies, such as Duek et al[94], with no LR at 3-year follow-up in 12 patients with T2 tumours treated with local resection and adjuvant radiotherapy. Ramirez et al[95] reported 28 pT1 high-risk and pT2 low-risk patients treated with local excision and radiotherapy, with a LR rate of 10.7% and a 5-year cancer-specific survival rate of 93%. Borschitz et al[90] had a LR rate 16% in low-risk pT2. But other groups showed worse results, with LR rates over 30%[79,86].
Tumour response to chemoradiation therapy (CRT) may define a subset of patients with a particularly good prognosis, who could benefit from a rectal sparing approach. Patients with early stage rectal cancer seem to respond better. Complete pathological response can be achieved usually in 10%-25%, but can even reach 45%[42,96]. Reliable assessment of the rectal wall and nodal status of mesorectum after CRT remains challenging, because of the induced edema, inflammation and fibrosis. Furthermore, endoscopic biopsies are unreliable to rule out residual cancer cells in rectal wall[42,96]. TES may play a role as a diagnostic procedure in selected patients with complete clinical response to rule out tumour persistence[85,97].
There are several studies which have shown similar oncological outcomes for T2 rectal cancers comparing neoadjuvant therapy and TEM with radical resection[40,79,98]. Other authors confirmed these good results in T2, with no LR in patients who had a significant response to CRT[54,78]. The best candidates for TES following CRT are those with complete pathological response[41]. Partial tumour response to CRT has increased risk of recurrence after TES, being ypT stage the strongest prognostic factor[99-101]. CRT followed by TES may be a promising way to treat the best responding patients with distal rectal cancer who may require an abdomino-perineal resection or a coloanal anastomosis, but only in well selected cases and in the setting of a controlled clinical trial. It may also be an option as palliative treatment, for patients who refuse a permanent ostomy or are unfit for major surgery.
Conventional endoscopic mucosal resection (EMR) cannot provide en bloc resection in cases of large lesions. Barendse et al[102] have published a systematic review on safety and effectiveness of EMR vs TEM for large rectal adenomas. The study has shown the safety of EMR with a lower rate of morbidity but a higher recurrence rate. We are waiting for the results of an ongoing prospective randomized trial by a multicenter collaboration group of Dutch endoscopists and surgeons (TREND study) that compares the cost-effectiveness of EMR and TEM for the resection of large (> 3 cm) rectal adenomas[103].
In recent years, the endoscopic submucosal dissection (ESD) technique was introduced to allow more en-bloc resections, especially in lesions larger than 20 mm. However, ESD has not gained wide acceptance in western countries because it is technically challenging and time consuming, requiring a steep learning curve, while it is affected by a consistent rate of complications (29.2%) and allows a rate of R0 resections of no more than 72.9% of cases[104].
The standard care of rectal cancer is changing. In recent years, the impact of screening is increasing the diagnosis of early cancer and its management is becoming bespoke and has not yet been defined. Molecular markers associated with tumour progression or response to neoadjuvant therapies may help in stratifying patients at high or low risk for local therapies[105,106]. The role of organ-sparing approaches including neoadjuvant therapies followed by TES should be formally assessed by randomized controlled trials. Improvements in preoperative discrimination of benign and malignant rectal lesions are also needed. The management of early rectal cancer should always be based on a multidisciplinary approach without jeopardizing survival[65]. Currently, two controlled trials are examining this. The CARTS study (CRT for rectal cancer in the distal rectum followed by organ-sparing TEM) has been designed to assess the adequacy of TEM following neoadjuvant radiotherapy. Patients with a clinical T1-3N0M0 rectal cancer below 10 cm from the anal verge will receive CRT followed by TEM 8-10 wk later. The UK TREC trial (TEM and Radiotherapy in Early Rectal Cancer) is offered for patients with early rectal cancer (T1-2N0). Patients are randomized between radical TME surgery and short-course neoadjuvant radiotherapy with delayed local excision at 8-10 wk[43].
There are several new techniques and approaches under investigation, which are still preclinical or experimental, such as transanal natural orifice transluminal endoscopic surgery (NOTES), transanal TME, and robotic-TES[25,43,107]. TES platforms seem to be safe for both transanal NOTES and TME procedures[108,109]. Robotic technology can lower the difficulty inherent in the TES platforms for performing such procedures[110]. Clinical trials are necessary for full evaluation of these techniques.
TES has improved significantly since its introduction in the 1980s. In recent years, the spread of these techniques has allowed the application of conservative rectal procedures in both benign diseases and selected cases of early rectal cancer. For more advanced rectal cancers it should be considered palliative or, in some controlled trials, experimental. The role of newer endoscopic techniques available has not yet been defined. TES may offer new strategies in the treatment of rectal pathology, like transanal NOTES or TME.
P- Reviewer: Coffey JC, Furka A S- Editor: Ma YJ L- Editor: A E- Editor: Liu XM
| 1. | Serra-Aracil X, Mora-Lopez L, Alcantara-Moral M, Corredera-Cantarin C, Gomez-Diaz C, Navarro-Soto S. Atypical indications for transanal endoscopic microsurgery to avoid major surgery. Tech Coloproctol. 2014;18:157-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 33] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 2. | Atallah S, Albert M, Debeche-Adams T, Larach S. Transanal minimally invasive surgery (TAMIS): applications beyond local excision. Tech Coloproctol. 2013;17:239-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 49] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 3. | Sylla P, Rattner DW, Delgado S, Lacy AM. NOTES transanal rectal cancer resection using transanal endoscopic microsurgery and laparoscopic assistance. Surg Endosc. 2010;24:1205-1210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 507] [Cited by in RCA: 534] [Article Influence: 35.6] [Reference Citation Analysis (0)] |
| 4. | Buess G, Theiss R, Günther M, Hutterer F, Pichlmaier H. Endoscopic surgery in the rectum. Endoscopy. 1985;17:31-35. [PubMed] |
| 5. | Nieuwenhuis DH, Draaisma WA, Verberne GH, van Overbeeke AJ, Consten EC. Transanal endoscopic operation for rectal lesions using two-dimensional visualization and standard endoscopic instruments: a prospective cohort study and comparison with the literature. Surg Endosc. 2009;23:80-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 6. | Serra-Aracil X, Mora-Lopez L, Alcantara-Moral M, Caro-Tarrago A, Navarro-Soto S. Transanal endoscopic microsurgery with 3-D (TEM) or high-definition 2-D transanal endoscopic operation (TEO) for rectal tumors. A prospective, randomized clinical trial. Int J Colorectal Dis. 2014;29:605-610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 31] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 7. | Atallah S, Albert M, Larach S. Transanal minimally invasive surgery: a giant leap forward. Surg Endosc. 2010;24:2200-2205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 387] [Cited by in RCA: 391] [Article Influence: 26.1] [Reference Citation Analysis (0)] |
| 8. | McLemore EC, Coker A, Jacobsen G, Talamini MA, Horgan S. eTAMIS: endoscopic visualization for transanal minimally invasive surgery. Surg Endosc. 2013;27:1842-1845. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 9. | Barendse RM, Doornebosch PG, Bemelman WA, Fockens P, Dekker E, de Graaf EJ. Transanal employment of single access ports is feasible for rectal surgery. Ann Surg. 2012;256:1030-1033. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 68] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 10. | Michalik M, Bobowicz M, Orlowski M. Transanal endoscopic microsurgery via TriPort Access System with no general anesthesia and without sphincter damage. Surg Laparosc Endosc Percutan Tech. 2011;21:e308-e310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 11. | Alessandro C, Daniela M, Michele M, Andrea T, Gianmarco G, Massimo S, Orazio Z, Fabio G, Giuseppe T. Glove port technique for transanal endoscopic microsurgery. Int J Surg Oncol. 2012;2012:383025. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 13] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 12. | Mclemore EC, Coker A, Leland H, Yu PT, Devaraj B, Jacobsen G, Talamini MA, Horgan S, Ramamoorthy S. New disposable transanal endoscopic surgery platform: longer channel, longer reach. Glob J Gastroenterol Hepatol. 2013;1:46-50. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 13. | Esposito C, Escolino M, Ascione G, Settimi A. Single trocar transanal endoscopy in a child. APSP J Case Rep. 2013;4:22. [PubMed] |
| 14. | Bridoux V, Schwarz L, Suaud L, Dazza M, Michot F, Tuech JJ. Transanal minimal invasive surgery with the Endorec(TM) trocar: a low cost but effective technique. Int J Colorectal Dis. 2014;29:177-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 15. | Martin-Perez B, Andrade-Ribeiro GD, Hunter L, Atallah S. A systematic review of transanal minimally invasive surgery (TAMIS) from 2010 to 2013. Tech Coloproctol. 2014;18:775-788. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 129] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 16. | Atallah S, Parra-Davila E, DeBeche-Adams T, Albert M, Larach S. Excision of a rectal neoplasm using robotic transanal surgery (RTS): a description of the technique. Tech Coloproctol. 2012;16:389-392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 53] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 17. | Bardakcioglu O. Robotic transanal access surgery. Surg Endosc. 2013;27:1407-1409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 29] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 18. | Hompes R, Rauh SM, Ris F, Tuynman JB, Mortensen NJ. Robotic transanal minimally invasive surgery for local excision of rectal neoplasms. Br J Surg. 2014;101:578-581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 58] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 19. | Lee TG, Lee SJ. Transanal single-port microsurgery for rectal tumors: minimal invasive surgery under spinal anesthesia. Surg Endosc. 2014;28:271-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 41] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 20. | Bislenghi G, Wolthuis AM, de Buck van Overstraeten A, D’Hoore A. AirSeal system insufflator to maintain a stable pneumorectum during TAMIS. Tech Coloproctol. 2015;19:43-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 30] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 21. | Ragupathi M, Vande Maele D, Nieto J, Pickron TB, Haas EM. Transanal endoscopic video-assisted (TEVA) excision. Surg Endosc. 2012;26:3528-3535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 20] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 22. | Wilhelm P, Storz P, Axt S, Falch C, Kirschniak A, Muller S. Use of self-retaining barbed suture for rectal wall closure in transanal endoscopic microsurgery. Tech Coloproctol. 2014;18:813-816. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 23. | Ramirez JM, Aguilella V, Arribas D, Martinez M. Transanal full-thickness excision of rectal tumours: should the defect be sutured? a randomized controlled trial. Colorectal Dis. 2002;4:51-55. [PubMed] |
| 24. | Paganini AM, Balla A, Quaresima S, D’Ambrosio G, Bruzzone P, Lezoche E. Tricks to decrease the suture line dehiscence rate during endoluminal loco-regional resection (ELRR) by transanal endoscopic microsurgery (TEM). Surg Endosc. 2015;29:1045-1050. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 25. | Heidary B, Phang TP, Raval MJ, Brown CJ. Transanal endoscopic microsurgery: a review. Can J Surg. 2014;57:127-138. [PubMed] |
| 26. | Wright CJ, Tutton M. Early discharge following transanal endoscopic microsurgery is safe. J Laparoendosc Adv Surg Tech A. 2014;24:399-402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 27. | McLemore EC, Weston LA, Coker AM, Jacobsen GR, Talamini MA, Horgan S, Ramamoorthy SL. Transanal minimally invasive surgery for benign and malignant rectal neoplasia. Am J Surg. 2014;208:372-381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 49] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 28. | Moore JS, Cataldo PA, Osler T, Hyman NH. Transanal endoscopic microsurgery is more effective than traditional transanal excision for resection of rectal masses. Dis Colon Rectum. 2008;51:1026-1030; discussion 1030-1031. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 272] [Cited by in RCA: 253] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 29. | Clancy C, Burke JP, Albert MR, O’Connell PR, Winter DC. Transanal endoscopic microsurgery versus standard transanal excision for the removal of rectal neoplasms: a systematic review and meta-analysis. Dis Colon Rectum. 2015;58:254-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 133] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 30. | Sgourakis G, Lanitis S, Gockel I, Kontovounisios C, Karaliotas C, Tsiftsi K, Tsiamis A, Karaliotas CC. Transanal endoscopic microsurgery for T1 and T2 rectal cancers: a meta-analysis and meta-regression analysis of outcomes. Am Surg. 2011;77:761-772. [PubMed] |
| 31. | Elmessiry MM, Van Koughnett JA, Maya A, DaSilva G, Wexner SD, Bejarano P, Berho M. Local excision of T1 and T2 rectal cancer: proceed with caution. Colorectal Dis. 2014;16:703-709. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 32. | Bignell MB, Ramwell A, Evans JR, Dastur N, Simson JN. Complications of transanal endoscopic microsurgery (TEMS): a prospective audit. Colorectal Dis. 2010;12:e99-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 28] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 33. | Lartigau C, Lebreton G, Alves A. Local resection for small rectal cancer. J Visc Surg. 2013;150:325-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 34. | Kumar AS, Coralic J, Kelleher DC, Sidani S, Kolli K, Smith LE. Complications of transanal endoscopic microsurgery are rare and minor: a single institution’s analysis and comparison to existing data. Dis Colon Rectum. 2013;56:295-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 49] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 35. | Kreissler-Haag D, Schuld J, Lindemann W, König J, Hildebrandt U, Schilling M. Complications after transanal endoscopic microsurgical resection correlate with location of rectal neoplasms. Surg Endosc. 2008;22:612-616. [PubMed] |
| 36. | Marks JH, Frenkel JL, Greenleaf CE, D’Andrea AP. Transanal endoscopic microsurgery with entrance into the peritoneal cavity: is it safe? Dis Colon Rectum. 2014;57:1176-1182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 40] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 37. | Morino M, Allaix ME, Famiglietti F, Caldart M, Arezzo A. Does peritoneal perforation affect short- and long-term outcomes after transanal endoscopic microsurgery? Surg Endosc. 2013;27:181-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 66] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 38. | Baatrup G, Borschitz T, Cunningham C, Qvist N. Perforation into the peritoneal cavity during transanal endoscopic microsurgery for rectal cancer is not associated with major complications or oncological compromise. Surg Endosc. 2009;23:2680-2683. [PubMed] |
| 39. | Marks JH, Valsdottir EB, DeNittis A, Yarandi SS, Newman DA, Nweze I, Mohiuddin M, Marks GJ. Transanal endoscopic microsurgery for the treatment of rectal cancer: comparison of wound complication rates with and without neoadjuvant radiation therapy. Surg Endosc. 2009;23:1081-1087. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 84] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 40. | Heafner TA, Glasgow SC. A critical review of the role of local excision in the treatment of early (T1 and T2) rectal tumors. J Gastrointest Oncol. 2014;5:345-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 41. | Stipa F, Picchio M, Burza A, Soricelli E, Vitelli CE. Long-term outcome of local excision after preoperative chemoradiation for ypT0 rectal cancer. Dis Colon Rectum. 2014;57:1245-1252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 42. | Garcia-Aguilar J, Shi Q, Thomas CR, Chan E, Cataldo P, Marcet J, Medich D, Pigazzi A, Oommen S, Posner MC. A phase II trial of neoadjuvant chemoradiation and local excision for T2N0 rectal cancer: preliminary results of the ACOSOG Z6041 trial. Ann Surg Oncol. 2012;19:384-391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 231] [Cited by in RCA: 216] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 43. | Smart CJ, Cunningham C, Bach SP. Transanal endoscopic microsurgery. Best Pract Res Clin Gastroenterol. 2014;28:143-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 44. | Planting A, Phang PT, Raval MJ, Brown CJ. Transanal endoscopic microsurgery: impact on fecal incontinence and quality of life. Can J Surg. 2013;56:243-248. [PubMed] |
| 45. | Mora López L, Serra Aracil J, Rebasa Cladera P, Puig Divi V, Hermoso Bosch J, Bombardo Junca J, Alcántara Moral M, Hernando Tavira R, Ayguavives Garnica I, Navarro Soto S. [Anorectal disorders in the immediate and late postoperative period after transanal endoscopic microsurgery]. Cir Esp. 2007;82:285-289. [PubMed] |
| 46. | Schiphorst AH, Langenhoff BS, Maring J, Pronk A, Zimmerman DD. Transanal minimally invasive surgery: initial experience and short-term functional results. Dis Colon Rectum. 2014;57:927-932. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 34] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 47. | Doornebosch PG, Gosselink MP, Neijenhuis PA, Schouten WR, Tollenaar RA, de Graaf EJ. Impact of transanal endoscopic microsurgery on functional outcome and quality of life. Int J Colorectal Dis. 2008;23:709-713. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 66] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 48. | Herman RM, Richter P, Walega P, Popiela T. Anorectal sphincter function and rectal barostat study in patients following transanal endoscopic microsurgery. Int J Colorectal Dis. 2001;16:370-376. [PubMed] |
| 49. | Barker JA, Hill J. Incidence, treatment and outcome of rectal stenosis following transanal endoscopic microsurgery. Tech Coloproctol. 2011;15:281-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 50. | Flexer SM, Durham-Hall AC, Steward MA, Robinson JM. TEMS: results of a specialist centre. Surg Endosc. 2014;28:1874-1878. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 51. | Steele RJ, Hershman MJ, Mortensen NJ, Armitage NC, Scholefield JH. Transanal endoscopic microsurgery--initial experience from three centres in the United Kingdom. Br J Surg. 1996;83:207-210. [PubMed] |
| 52. | Cocilovo C, Smith LE, Stahl T, Douglas J. Transanal endoscopic excision of rectal adenomas. Surg Endosc. 2003;17:1461-1463. [PubMed] |
| 53. | Langer C, Liersch T, Süss M, Siemer A, Markus P, Ghadimi BM, Füzesi L, Becker H. Surgical cure for early rectal carcinoma and large adenoma: transanal endoscopic microsurgery (using ultrasound or electrosurgery) compared to conventional local and radical resection. Int J Colorectal Dis. 2003;18:222-229. [PubMed] |
| 54. | Morino M, Allaix ME. Transanal endoscopic microsurgery: what indications in 2013? Gastroenterol Rep (Oxf). 2013;1:75-84. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 55. | Allaix ME, Arezzo A, Cassoni P, Famiglietti F, Morino M. Recurrence after transanal endoscopic microsurgery for large rectal adenomas. Surg Endosc. 2012;26:2594-2600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 47] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 56. | Serra-Aracil X, Caro-Tarrago A, Mora-López L, Casalots A, Rebasa P, Navarro-Soto S. Transanal endoscopic surgery with total wall excision is required with rectal adenomas due to the high frequency of adenocarcinoma. Dis Colon Rectum. 2014;57:823-829. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 57. | Dash I, Walter CJ, Wheeler JM, Borley NR. Does the incidence of unexpected malignancy in ‘benign’ rectal neoplasms undergoing trans-anal endoscopic microsurgery vary according to lesion morphology? Colorectal Dis. 2013;15:183-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 58. | Wang Y, Zhou CW, Hao YZ, Li L, Liu SM, Feng XL, Zhou ZX, Leung VY. Improvement in T-staging of rectal carcinoma: using a novel endorectal ultrasonography technique with sterile coupling gel filling the rectum. Ultrasound Med Biol. 2012;38:574-579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 59. | Waage JE, Havre RF, Odegaard S, Leh S, Eide GE, Baatrup G. Endorectal elastography in the evaluation of rectal tumours. Colorectal Dis. 2011;13:1130-1137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 35] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 60. | Peeters KC, Marijnen CA, Nagtegaal ID, Kranenbarg EK, Putter H, Wiggers T, Rutten H, Pahlman L, Glimelius B, Leer JW. The TME trial after a median follow-up of 6 years: increased local control but no survival benefit in irradiated patients with resectable rectal carcinoma. Ann Surg. 2007;246:693-701. [PubMed] |
| 61. | Lezoche G, Paganini AM, Campagnacci R, Ghiselli R, Pelloni M, Rombini A, Guerrieri M. Treatment of rectal cancer by transanal endoscopic microsurgery: review of the literature. Minerva Chir. 2013;68:1-9. [PubMed] |
| 62. | Monson JR, Weiser MR, Buie WD, Chang GJ, Rafferty JF, Buie WD, Rafferty J. Practice parameters for the management of rectal cancer (revised). Dis Colon Rectum. 2013;56:535-550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 304] [Cited by in RCA: 301] [Article Influence: 25.1] [Reference Citation Analysis (0)] |
| 63. | Morino M, Risio M, Bach S, Beets-Tan R, Bujko K, Panis Y, Quirke P, Rembacken B, Rullier E, Saito Y. Early rectal cancer: the European Association for Endoscopic Surgery (EAES) clinical consensus conference. Surg Endosc. 2015;29:755-773. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 93] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 64. | Kidane B, Chadi SA, Kanters S, Colquhoun PH, Ott MC. Local resection compared with radical resection in the treatment of T1N0M0 rectal adenocarcinoma: a systematic review and meta-analysis. Dis Colon Rectum. 2015;58:122-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 90] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 65. | Morino M, Arezzo A, Allaix ME. Transanal endoscopic microsurgery. Tech Coloproctol. 2013;17 Suppl 1:S55-S61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 66. | Albert MR, Atallah SB, deBeche-Adams TC, Izfar S, Larach SW. Transanal minimally invasive surgery (TAMIS) for local excision of benign neoplasms and early-stage rectal cancer: efficacy and outcomes in the first 50 patients. Dis Colon Rectum. 2013;56:301-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 161] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 67. | Lim SB, Seo SI, Lee JL, Kwak JY, Jang TY, Kim CW, Yoon YS, Yu CS, Kim JC. Feasibility of transanal minimally invasive surgery for mid-rectal lesions. Surg Endosc. 2012;26:3127-3132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 73] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 68. | Morino M, Allaix ME, Caldart M, Scozzari G, Arezzo A. Risk factors for recurrence after transanal endoscopic microsurgery for rectal malignant neoplasm. Surg Endosc. 2011;25:3683-3690. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 68] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 69. | Bipat S, Glas AS, Slors FJ, Zwinderman AH, Bossuyt PM, Stoker J. Rectal cancer: local staging and assessment of lymph node involvement with endoluminal US, CT, and MR imaging--a meta-analysis. Radiology. 2004;232:773-783. [PubMed] |
| 70. | Puli SR, Reddy JB, Bechtold ML, Choudhary A, Antillon MR, Brugge WR. Accuracy of endoscopic ultrasound to diagnose nodal invasion by rectal cancers: a meta-analysis and systematic review. Ann Surg Oncol. 2009;16:1255-1265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 91] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 71. | Ashraf S, Hompes R, Slater A, Lindsey I, Bach S, Mortensen NJ, Cunningham C. A critical appraisal of endorectal ultrasound and transanal endoscopic microsurgery and decision-making in early rectal cancer. Colorectal Dis. 2012;14:821-826. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 76] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 72. | Bach SP, Hill J, Monson JR, Simson JN, Lane L, Merrie A, Warren B, Mortensen NJ. A predictive model for local recurrence after transanal endoscopic microsurgery for rectal cancer. Br J Surg. 2009;96:280-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 277] [Cited by in RCA: 261] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 73. | Saraste D, Gunnarsson U, Janson M. Predicting lymph node metastases in early rectal cancer. Eur J Cancer. 2013;49:1104-1108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 58] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 74. | Beaton C, Twine CP, Williams GL, Radcliffe AG. Systematic review and meta-analysis of histopathological factors influencing the risk of lymph node metastasis in early colorectal cancer. Colorectal Dis. 2013;15:788-797. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 189] [Article Influence: 15.8] [Reference Citation Analysis (1)] |
| 75. | Yamamoto S, Watanabe M, Hasegawa H, Baba H, Yoshinare K, Shiraishi J, Kitajima M. The risk of lymph node metastasis in T1 colorectal carcinoma. Hepatogastroenterology. 2004;51:998-1000. [PubMed] |
| 76. | Bosch SL, Teerenstra S, de Wilt JH, Cunningham C, Nagtegaal ID. Predicting lymph node metastasis in pT1 colorectal cancer: a systematic review of risk factors providing rationale for therapy decisions. Endoscopy. 2013;45:827-834. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 256] [Cited by in RCA: 297] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 77. | Hershman MJ, Mohammad H, Hussain A, Ahmed A. Local excision of rectal tumours by minimally invasive transanal surgery. Br J Hosp Med (Lond). 2013;74:387-390. [PubMed] |
| 78. | Guerrieri M, Gesuita R, Ghiselli R, Lezoche G, Budassi A, Baldarelli M. Treatment of rectal cancer by transanal endoscopic microsurgery: experience with 425 patients. World J Gastroenterol. 2014;20:9556-9563. [PubMed] [DOI] [Full Text] |
| 79. | Allaix ME, Arezzo A, Giraudo G, Morino M. Transanal endoscopic microsurgery vs. laparoscopic total mesorectal excision for T2N0 rectal cancer. J Gastrointest Surg. 2012;16:2280-2287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 36] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 80. | Sajid MS, Farag S, Leung P, Sains P, Miles WF, Baig MK. Systematic review and meta-analysis of published trials comparing the effectiveness of transanal endoscopic microsurgery and radical resection in the management of early rectal cancer. Colorectal Dis. 2014;16:2-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 58] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 81. | Winde G, Nottberg H, Keller R, Schmid KW, Bünte H. Surgical cure for early rectal carcinomas (T1). Transanal endoscopic microsurgery vs. anterior resection. Dis Colon Rectum. 1996;39:969-976. [PubMed] |
| 82. | Heintz A, Mörschel M, Junginger T. Comparison of results after transanal endoscopic microsurgery and radical resection for T1 carcinoma of the rectum. Surg Endosc. 1998;12:1145-1148. [PubMed] |
| 83. | Damin DC, Lazzaron AR. Evolving treatment strategies for colorectal cancer: a critical review of current therapeutic options. World J Gastroenterol. 2014;20:877-887. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 27] [Cited by in RCA: 35] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 84. | Doornebosch PG, Ferenschild FT, de Wilt JH, Dawson I, Tetteroo GW, de Graaf EJ. Treatment of recurrence after transanal endoscopic microsurgery (TEM) for T1 rectal cancer. Dis Colon Rectum. 2010;53:1234-1239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 87] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 85. | Dias AR, Nahas CS, Marques CF, Nahas SC, Cecconello I. Transanal endoscopic microsurgery: indications, results and controversies. Tech Coloproctol. 2009;13:105-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 40] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 86. | Serra-Aracil X, Mora-Lopez L, Alcantara-Moral M, Caro-Tarrago A, Gomez-Diaz CJ, Navarro-Soto S. Transanal endoscopic surgery in rectal cancer. World J Gastroenterol. 2014;20:11538-11545. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 48] [Cited by in RCA: 55] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 87. | Borschitz T, Heintz A, Junginger T. The influence of histopathologic criteria on the long-term prognosis of locally excised pT1 rectal carcinomas: results of local excision (transanal endoscopic microsurgery) and immediate reoperation. Dis Colon Rectum. 2006;49:1492-1506; discussion 1500-1505. [PubMed] |
| 88. | Stipa F, Burza A, Lucandri G, Ferri M, Pigazzi A, Ziparo V, Casula G, Stipa S. Outcomes for early rectal cancer managed with transanal endoscopic microsurgery: a 5-year follow-up study. Surg Endosc. 2006;20:541-545. [PubMed] |
| 89. | Bhangu A, Brown G, Nicholls RJ, Wong J, Darzi A, Tekkis P. Survival outcome of local excision versus radical resection of colon or rectal carcinoma: a Surveillance, Epidemiology, and End Results (SEER) population-based study. Ann Surg. 2013;258:563-569; discussion 569-571. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 79] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 90. | Borschitz T, Kneist W, Gockel I, Junginger T. Local excision for more advanced rectal tumors. Acta Oncol. 2008;47:1140-1147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 91. | Levic K, Bulut O, Hesselfeldt P, Bülow S. The outcome of rectal cancer after early salvage TME following TEM compared with primary TME: a case-matched study. Tech Coloproctol. 2013;17:397-403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 65] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 92. | Hompes R, McDonald R, Buskens C, Lindsey I, Armitage N, Hill J, Scott A, Mortensen NJ, Cunningham C. Completion surgery following transanal endoscopic microsurgery: assessment of quality and short- and long-term outcome. Colorectal Dis. 2013;15:e576-e581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 69] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 93. | Bikhchandani J, Ong GK, Dozois EJ, Mathis KL. Outcomes of salvage surgery for cure in patients with locally recurrent disease after local excision of rectal cancer. Dis Colon Rectum. 2015;58:283-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 34] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 94. | Duek SD, Issa N, Hershko DD, Krausz MM. Outcome of transanal endoscopic microsurgery and adjuvant radiotherapy in patients with T2 rectal cancer. Dis Colon Rectum. 2008;51:379-384; discussion 384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 48] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 95. | Ramirez JM, Aguilella V, Valencia J, Ortego J, Gracia JA, Escudero P, Esco R, Martinez M. Transanal endoscopic microsurgery for rectal cancer. Long-term oncologic results. Int J Colorectal Dis. 2011;26:437-443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 44] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 96. | Smith FM, Chang KH, Sheahan K, Hyland J, O’Connell PR, Winter DC. The surgical significance of residual mucosal abnormalities in rectal cancer following neoadjuvant chemoradiotherapy. Br J Surg. 2012;99:993-1001. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 102] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 97. | Garcia-Aguilar J. Transanal endoscopic microsurgery following neoadjuvant chemoradiation therapy in rectal cancer: a word of caution about patient selection? Dis Colon Rectum. 2013;56:1-3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 98. | Lezoche E, Baldarelli M, Lezoche G, Paganini AM, Gesuita R, Guerrieri M. Randomized clinical trial of endoluminal locoregional resection versus laparoscopic total mesorectal excision for T2 rectal cancer after neoadjuvant therapy. Br J Surg. 2012;99:1211-1218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 230] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 99. | Borschitz T, Wachtlin D, Möhler M, Schmidberger H, Junginger T. Neoadjuvant chemoradiation and local excision for T2-3 rectal cancer. Ann Surg Oncol. 2008;15:712-720. [PubMed] |
| 100. | Pucciarelli S, De Paoli A, Guerrieri M, La Torre G, Maretto I, De Marchi F, Mantello G, Gambacorta MA, Canzonieri V, Nitti D. Local excision after preoperative chemoradiotherapy for rectal cancer: results of a multicenter phase II clinical trial. Dis Colon Rectum. 2013;56:1349-1356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 144] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 101. | Perez RO, Habr-Gama A, São Julião GP, Proscurshim I, Scanavini Neto A, Gama-Rodrigues J. Transanal endoscopic microsurgery for residual rectal cancer after neoadjuvant chemoradiation therapy is associated with significant immediate pain and hospital readmission rates. Dis Colon Rectum. 2011;54:545-551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 115] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 102. | Barendse RM, van den Broek FJ, Dekker E, Bemelman WA, de Graaf EJ, Fockens P, Reitsma JB. Systematic review of endoscopic mucosal resection versus transanal endoscopic microsurgery for large rectal adenomas. Endoscopy. 2011;43:941-949. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 68] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 103. | van den Broek FJ, de Graaf EJ, Dijkgraaf MG, Reitsma JB, Haringsma J, Timmer R, Weusten BL, Gerhards MF, Consten EC, Schwartz MP. Transanal endoscopic microsurgery versus endoscopic mucosal resection for large rectal adenomas (TREND-study). BMC Surg. 2009;9:4. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 33] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 104. | Farhat S, Chaussade S, Ponchon T, Coumaros D, Charachon A, Barrioz T, Koch S, Houcke P, Cellier C, Heresbach D. Endoscopic submucosal dissection in a European setting. A multi-institutional report of a technique in development. Endoscopy. 2011;43:664-670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 115] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 105. | Leong KJ, Wei W, Tannahill LA, Caldwell GM, Jones CE, Morton DG, Matthews GM, Bach SP. Methylation profiling of rectal cancer identifies novel markers of early-stage disease. Br J Surg. 2011;98:724-734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 39] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 106. | García-Flórez LJ, Gómez-Álvarez G, Frunza AM, Barneo-Serra L, Martínez-Alonso C, Fresno-Forcelledo MF. Predictive markers of response to neoadjuvant therapy in rectal cancer. J Surg Res. 2015;194:120-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 32] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 107. | Aly EH. SILS TEM: The new armamentarium in transanal endoscopic surgery. J Minim Access Surg. 2014;10:102-103. [PubMed] |
| 108. | Wolthuis AM, de Buck van Overstraeten A, D’Hoore A. Laparoscopic natural orifice specimen extraction-colectomy: a systematic review. World J Gastroenterol. 2014;20:12981-12992. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 100] [Cited by in RCA: 87] [Article Influence: 7.9] [Reference Citation Analysis (1)] |
| 109. | Araujo SE, Crawshaw B, Mendes CR, Delaney CP. Transanal total mesorectal excision: a systematic review of the experimental and clinical evidence. Tech Coloproctol. 2015;19:69-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 51] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 110. | Gómez Ruiz M, Palazuelos CM, Martín Parra JI, Alonso Martín J, Cagigas Fernández C, del Castillo Diego J, Gómez Fleitas M. New technique of transanal proctectomy with completely robotic total mesorrectal excision for rectal cancer. Cir Esp. 2014;92:356-361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |