Published online Aug 14, 2015. doi: 10.3748/wjg.v21.i30.9189
Peer-review started: March 13, 2015
First decision: March 26, 2015
Revised: April 15, 2015
Accepted: June 15, 2015
Article in press: June 16, 2015
Published online: August 14, 2015
Processing time: 157 Days and 19.8 Hours
AIM: To investigate the efficacy and adverse effects of antioxidant therapy in acute pancreatitis (AP), chronic pancreatitis (CP) and post-endoscopic retrograde cholangiopancreatography pancreatitis (PEP).
METHODS: PubMed, Scopus, Google Scholar, Cochrane library database, and Evidence-based medicine/clinical trials published before August 2014 were searched. Clinical and laboratory outcomes of randomized trials of antioxidant therapy in patients with AP, CP and PEP were included. The methodological quality of the trials was assessed by the Jadad score based on the description of randomization, blinding, and dropouts (withdrawals). The results of the studies were pooled and meta-analyzed to provide estimates of the efficacy of antioxidant therapy.
RESULTS: Thirty four trials out of 1069 potentially relevant studies with data for 4898 patients were eligible for inclusion. Antioxidant therapy significantly reduced the length of hospital stay in AP patients {mean difference -2.59 d (95%CI: -4.25-(-0.93)], P = 0.002}. Although, antioxidant therapy had no significant effect on serum C reactive protein (CRP) after 5-7 d in AP patients [mean difference -9.57 (95%CI: -40.61-21.48, P = 0.55], it significantly reduced serum CRP after 10 d {mean difference -45.16 [95%CI: -89.99-(-0.33)], P = 0.048}. In addition, antioxidant therapy had no significant effect on CP-induced pain [mean difference -2.13 (95%CI: -5.87-1.6), P = 0.26]. Antioxidant therapy had no significant effects on the incidence of all types of PEP [mean difference 1.05 (95%CI: 0.74-1.5), P = 0.78], severe PEP [mean difference 0.92 (95%CI: 0.43-1.97), P = 0.83], moderate PEP [mean difference 0.82 (95%CI: 0.54-1.23), P = 0.33], and mild PEP [mean difference 1.33 (95%CI: 0.99-1.78), P = 0.06]. Furthermore, while antioxidant therapy had no significant effect on serum amylase after less than 8 h sampling [mean difference -20.61 (95%CI: -143.61-102.39), P = 0.74], it significantly reduced serum amylase close to 24-h sampling {mean difference -16.13 [95%CI: -22.98-(-9.28)], P < 0.0001}.
CONCLUSION: While there is some evidence to support antioxidant therapy in AP, its effect on CP and PEP is still controversial.
Core tip: Antioxidant therapy reduces the length of hospital stay in acute pancreatitis patients. Although antioxidant therapy shows no significant effect on serum amylase after less than 8 h sampling, it significantly reduces serum amylase after 24 h sampling. Antioxidant therapy has no significant effect on serum C reactive protein (CRP) after 5-7 d sampling, but significantly reduces serum CRP after 10 d sampling. Evidence to support the efficacy of antioxidant therapy in the management of chronic pancreatitis and post-endoscopic retrograde cholangiopancreatography pancreatitis is limited. Further trials should be based on etiology-differentiated designs.
- Citation: Gooshe M, Abdolghaffari AH, Nikfar S, Mahdaviani P, Abdollahi M. Antioxidant therapy in acute, chronic and post-endoscopic retrograde cholangiopancreatography pancreatitis: An updated systematic review and meta-analysis. World J Gastroenterol 2015; 21(30): 9189-9208
- URL: https://www.wjgnet.com/1007-9327/full/v21/i30/9189.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i30.9189
Pancreatitis is an inflammatory metabolic disorder, which is a major cause of physical and socioeconomic loss worldwide[1-3]. Generally, pancreatitis is categorized into two different entities of acute and chronic[4].
Acute pancreatitis (AP) is sudden painful inflammation of the pancreas, basically caused by tissue destruction as a consequence of innate immune-induced epithelial stress pathways[5]. The most common cause of gut-related hospitalization in the United States is AP[6]. Several complicated factors are associated with the development of AP, however, alcohol abuse and ductal obstruction caused by gallstones or bacterial infection are the main factors[5].
Furthermore, pancreatitis remains the most common adverse event of endoscopic retrograde cholangiopancreatography (ERCP). The incidence of post-ERCP pancreatitis (PEP) varies widely, ranging from 1% to 40% in the normal population, to as high as 67% in high-risk patients[7]. While investigations toward preventing or limiting the complications of PEP with pharmacological agents have drawn much attention, these have so far had limited success.
Chronic pancreatitis (CP) is a progressive fibro-inflammatory disorder, representing a continuum from a first inflammatory episode to parenchymal fibrosis and functional insufficiency[8]. While alcohol is the most frequent causative factor in the development of chronic pancreatitis, idiopathic, genetic, and autoimmune factors are considered less frequent causes[8]. CP can eventually give rise to several complications that should be treated accordingly. Principally, the only observable symptom in chronic pancreatitis is pain[9].
Reactive oxidative species (ROS) are inevitable epiphenomenon or the cause of vital processes, particularly aerobic metabolism. When production of ROS exceeds their catabolism in any physiologic and pathologic conditions, oxidant-derived cellular injury can occur which is known as oxidative stress[10,11].
Interestingly, there is ample evidence suggesting that oxidative stress is a common pivotal factor in the pathogenesis of AP, CP and PEP[12]. While an extensive and multilayered antioxidant defense system is present in the human body, dietary intake can play a crucial role in strengthening antioxidant capacity within the blood[13,14]. Thus, it is not surprising that the use of antioxidants have positive effects in pancreatitis.
The question of whether antioxidant supplements might protect against pancreatitis has drawn much attention in recent years, and a meta-analysis has shown some positive effects[15], although the results of randomized trials have been contradictory. The present systematic review with meta-analyses was conducted to critically update the knowledge on the beneficial or harmful effects of antioxidant supplementation in the management of AP, CP and PEP.
All randomized clinical trials (RCTs) evaluating antioxidants for the treatment of pain, hospitalization, C reactive protein (CRP) and serum amylase in CP, AP and the severity and rate of PEP were included. Data were searched from PubMed, Scopus, Google Scholar, Cochrane library database, and Evidence-based medicine/clinical trials published before August 2014 were searched.
The search terms were as follows: AP, CP, PEP, pancreatic inflammation, antioxidant, vitamin, superoxide dismutase, manganese, glutamine, butylated hydroxyanisole, taurine, glutathione, curcumin, catalase, peroxidase, lutein, xanthophylls, zeaxanthin, selenium, riboflavin, zinc, carotenoid, cobalamin, retinol, alpha-tocopherol, ascorbic acid, beta-carotene, carotene and all MeSH terms of pharmacologically active antioxidants. The studies were limited to clinical trials and those written in the English language.
The Jadad score, which indicates the quality of the studies based on their description of randomization, blinding, and dropouts (withdrawals) was used to assess the methodological quality of trials[16]. The quality scale ranges from 0 to 5 points with a score of 2 or less for a low quality report and a score of at least 3 for a high quality report. The description of this score is as follows: (1) whether randomized (yes = 1 point, no = 0); (2) whether randomization was described appropriately (yes = 1 point, no = 0); (3) double-blind (yes = 1 point, no = 0); (4) was the double-blinding described appropriately (yes = 1 point, no = 0); and (5) whether withdrawals and dropouts were described (yes = 1 point, no= 0). The quality score ranges from 0 to 5 points; a low-quality report score is ≤ 2 and a high-quality report score is at least 3.
Data synthesis was conducted by three reviewers who read the title and abstract of the search results separately to eliminate duplicates, reviews, case studies, and uncontrolled trials. The inclusion criteria were that the studies should be clinical trials on the use of an antioxidant for the treatment or prevention of pancreatitis. Outcomes of the studies were not the point of selection and all studies that analyzed the effects of an antioxidant on pancreatitis, from pain reduction to changes in plasma cytokines, were included.
Data from selected studies were extracted in the form of 2 × 2 tables by study characteristics. Included studies were weighted by effect size and pooled. Data were analyzed using Statsdirect software version 3.0.146. Relative risk (RR) and 95% confidence intervals (95%CI) were calculated using Mantel-Haenszel, Rothman-Boice (for fixed effects) and DerSimonian-Laird (for random effects) methods. Standardized effect size and 95%CI were calculated using Mulrow-Oxman (for fixed effects) and Der Simonian-Laird (for random effects) methods. The Cochran Q test was used to test heterogeneity and P < 0.05 was considered significant. In the case of heterogeneity or few included studies, the random effects model was used. Egger and Begg-Mazumdar tests were used to evaluate publication bias indicators in funnel plots.
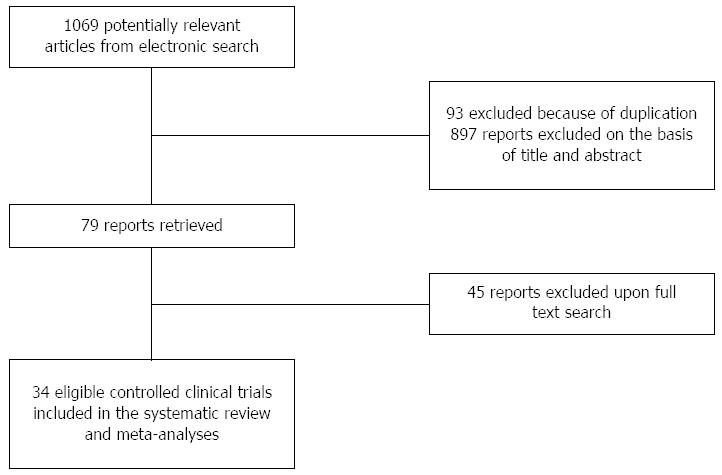
From the 1069 studies identified through the literature search, 34 randomized controlled trials were identified as eligible (4898 patients; 551 AP, 673 CP and 3674 PEP) (Figure 1). Of these, 12 trials used antioxidant therapy in AP (Table 1)[17-28], 12 trials in CP (Table 2)[28-39] and 11 trials in PEP (Table 3)[40-50].
| Ref. | Drug/supplements | Study design | Jadad score | Participants | Treatment (intervention) | Outcome (results) | Adverse effects/events | ||
| Case | Control | Clinical | Laboratory | ||||||
| Bansal et al[18], 2011 | Combined antioxidant (vitamin A, vitamin C, vitamin E) | Single-center, prospective randomized, open-label with blinded endpoint | 4 | 39 patients with severe AP | 19 patients; combined antioxidants: 1000 mg vitamin C in 100 mL normal saline, 200 mg vitamin E oral, and 10000 IU vitamin A intramuscularly; per day; for 14 d | 20 patients; placebo | Multi-organ dysfunction1Length of hospital stay1 | Serum GSH1Serum SOD1 | |
| Sateesh et al[17], 2009 | Combined antioxidant (vitamin C, N-acetyl cysteine, antoxyl forte) | Randomized; placebo-controlled | 3 | 53 patients with AP | 23 patients; combined antioxidants: 500 mg vitamin C, 200 mg 8 hourly N-acetyl cysteine and 1 capsule hourly antoxyl forte); per day; for 7 d | 30 patients; placebo | Length of hospital stay and complications ↓ | Serum MDA1TBARS ↓SOD ↓ | |
| Xue et al[19], 2008 | Glutamine | Randomized; | 1 | 80 patients with severe AP | 38 patients; 100 mL/d of 20% AGD intravenous infusion; for 10 d; starting on the day 1 (Early treatment) | 38 patients; 100 mL/d of 20% AGD intravenous infusion/for 10 d starting on the day 5 (late treatment) | Infection rate ↓Operation rate ↓Mortality ↓Hospitalization ↓Duration of ARDS ↓Renal failure ↓Acute hepatitis ↓Encephalopathy ↓Enteroparalysis ↓ | TAC ↓Vitamin C ↑- | - |
| Fuentes-Orozco et al[20], 2008 | Glutamine | Randomized; double blind; controlled | 4 | 44 patients with AP | 22 patients; 0.4 g/kg per day of L-alanyl-L-Glutamine in standard TPN; 10 d | 22 patients; standard TPN; 10 d | Duration of shock ↓15 d APACHE II core ↓Infectious morbidity ↓Hospital stay day1Mortality1 | Serum IL10 ↑Serum IL-6 ↓CRP ↓Ig A ↑Protein ↑Albumin ↑Leucocyte ↓ | - |
| Sahin et al[21], 2007 | Glutamine enriched total parenteral nutrition (TPN) | Randomized; double blind; placebo- controlled | 3 | 40 patients with AP | 20 patients; 0.3 g/kg per day glutamine; for 7-15 d | 20 patients; placebo | Complication rates ↓ | Total lymphocyte ↑Nitrogen balance was (+) in treated group vs (-) in control groupTransferrin level ↑Fasting blood sugar, albumin1BUN1Creatinine1Total cholesterol concentrations1AST1ALT1LDH activities1Leukocytes, CD4, CD81Serum Zn, Ca and P | |
| Siriwardena et al[22], 2008 | Combined antioxidant (N-acetylcysteine, selenium, vitamin C) | Randomized; double blind; placebo- controlled | 5 | 43 patients with severe AP | 22 patients; N-acetylcysteine, selenium and vitamin C; for 7d | 21 patients; placebo | Organ dysfunction1APACHE- II1Hospitalization1 All case mortality1 | Serum lipase ↓Amylase activities↓CRP ↓Serum vitamin C1Serum selenium1GSH/GSSG ratio1CRP1 | - |
| Pearce CB et al[23], 2006 | Glutamine, arginine, tributyrin and antioxidants | Randomized; double blind; placebo- controlled | 5 | 31 patients with severe AP | 15 patients; glutamine, arginine, tributyrin and antioxidants; for 3 d; If patients required further feeding the study was continued up to 15 d | 16 patients; placebo isocaloric isonitrogenous control feed was undertaken | CRP ↑CAPAP↓ | Diarrhea (1 patient)Vomiting (2 patients) | |
| Du et al[24], 2003 | Vitamin C | Randomized; controlled | 3 | 84 patients with AP | 40 patients; IV vitamin C; 10 g/d; for 5 d | 44 patients; IV vitamin C; 1 g/d; for 5 d | Hospitalization ↓Deterioration of disease ↓ Improvement of disease ↑ Cure rate ↑ | TNF-α↓IL-1 ↓IL-8 ↓CRP ↓Serum interleukin-2 receptor ↓Plasma vitamin C ↑Plasma lipideroxide ↑Plasma vitamin E ↑Plasma β-carotene ↑Whole blood glutathione ↑Activity of erythrocyte surperoxide dismutase ↑Erythrocyte catalase ↑ | Hypernatremia (2 patients)- |
| Ockenga et al[25], 2002 | Glutamine | Randomized, double blind; controlled | 4 | 28 patients with AP | Standard TPN which contains 0.3 g/kg per day L-alanine-L-glutamine; at least 1 wk | Standard TPN | Hospitalization ↓Duration of TPN ↓Cost of TPN1 | Cholinesterase ↑Albumin ↑ | - |
| de Beaux et al[26], 1998 | Glutamine | Randomized; double-blind; controlled | 5 | 14 patients with AP | 6 patients; 0.22 g/kg per day of glycyl-glutamine in standard TPN; for 7 d | 7 patients; standard TPN | Lymphocyte count ↑CRP ↓Lymphocytic proliferation (by DNA synthesis) ↑TNF1 IL61IL8↓ | - | |
| Sharer et al[27], 1995 | Glutathione precursors (S-adenosyl methionine and N-acetylcysteine) | Randomized | 2 | 79 patients with AP | SAMe 43 mg/kg and N-acetylcysteine 300 mg/kg | - | APACHE II score reduction1Complication rate1 | - | - |
| Bilton et al[28], 1994 | S- adenosyl methionine (SAMe)Selenium and β-carotene + SAMe | Randomized; double-blind; crossover; placebo- controlled | 5 | 20 patients with AP or CP | 20 patients; SAMe 2.4 g/d; 10 wk20 patients; SAMe 2.4 g/d, Selenium 600 μg and β-carotene 9000 IU; 10 wk | Placebo | Days in hospital1Mortality1Attack rate and background pain1 | Free radical activity ↓Serum Selenium ↓Serum β-carotene ↓Serum vitamin E ↓Serum vitamin C ↓Serum SAMe ↑Free radical activity ↓Serum selenium ↓Serum β-carotene ↑Serum vitamin E ↑1Serum vitamin C ↓Serum SAMe ↑ | - |
| Ref. | Drug/supplements | Study design | Jadad score | Participants | Treatment (intervention) | Outcome (results) | Adverse effects/events | ||
| Case | Control | Clinical | Laboratory | ||||||
| Dhingra et al[29], 2013 | Combined antioxidant (organic selenium, vitamin C, β carotene, vitamin E, methionine) | Randomized; placebo-controlled | 3 | 61 patients with CP | 31 patients; 600 Hg of organic selenium, 0.54 g of vitamin C, 9000 IU of β carotene, 270 IU of vitamin E, and 2 g of methionine | 30 patients; placebo | Number of painful days per month ↓Number of analgesic tablets per month ↓ | Platelet-derived growth factor (PDGF) AA ↓Transforming growth factor β 11Thiobarbituric acid-reactive substances1Ferric-reducing ability of plasma ↑TBARS ↓FRAP ↑ | |
| Shah et al[30], 2013 | Combined antioxidant (vitamin C, vitamin E, β carotene, selenium, methionine) | Randomized; double blind; placebo-controlled | 5 | 14 patients with CP | 7 patients; Antox tablet: vitamin C, vitamin E, β carotene, selenium, methionine (Pharma Nord, Morpeth, United Kingdom); 6 m | 7 patients; placebo | Opiate usage1 | Serum vitamin C ↑ Serum vitamin E ↑Serum b caroteneSerum vitamin A ↑WCC1Hb1CRP1Serum selenium1IL 1b, 4, 6, and 101TNF-α1 | |
| Siriwardena et al[31], 2012 | Combined antioxidant (selenium, d-a-tocopherol acetate, ascorbic acid, l-methionine) | Randomized; double blind; placebo-controlled | 5 | 70 patients with CP | 33 patients; Antox tablet: 38.5 mg seleniumYeast, 113.4 mg d-a-tocopherol acetate, 126.3 mg ascorbic acid, 480 mg l-methionine; per d; for 6 m | 37 patients; placebo | Quality of life1Average daily pain scores1Opiate use1Number of hospital admissions1Outpatient visits1 | Serum vitamin C ↑Serum vitamin E ↑Serum beta carotene ↑Serum selenium ↑ | Increased frequency of stool, occasional diarrhea, bad taste, and heartburn with nausea |
| Shah et al[32], 2010 | Combined antioxidant (vitamin C, vitamin E, β carotene, selenium, methionine) | Randomized; placebo-controlled | 2 | 137 patients with CP | 68 patients; Antox tablet: vitamin C, vitamin E, β carotene, selenium, methionine (Pharma Nord, Morpeth, United Kingdom); at least 6 m | 69 patients; placebo | Median visual analogue pain score ↓Cognitive, emotional, social, physical and role function ↑Analgesics and opiate usage ↓ | - | - |
| Bhardwaj et al[33], 2009 | Combined antioxidant (organic selenium, vitamin C, β- carotene, α-tocopherol and methionine) | Randomized; double blind; placebo-controlled | 5 | 147 patients with CP | 71 patients; combined antioxidants: 600 μg organic selenium,0.54 g ascorbic acid, 9000 IU β- carotene, 270 IU α-tocopherol and 2 g methionine (Betamore G, Osper Pharmanautics, India); per d; for 6 m | 76 patients; placebo | Number of painful days per month ↓Numbers of oral analgesic tablets and parenteral analgesic injections per month ↓Hospitalization ↓Percentage of patients become pain-free ↓Number of man-days lost per month ↓ | Lipid peroxidation (TBARS) ↓Serum SOD ↓Total antioxidant capacity (FRAP) ↑Serum vitamin A↑Serum vitamin C ↑Serum vitamin E ↑Erythrocyte superoxide dismutase ↓ | Headache & Constipation (all during the first month of treatment) |
| Kirk et al[34], 2006 | Combined antioxidant (selenium, β- carotene, L-methionine, vitamins C and E) | Randomized; double-blind; placebo-controlled; crossover | 4 | 72 patients with CP | 36 patients; Antox tablet: 75 mg of selenium, 3 mg β- carotene, 47 mg vitamin E, 150 mg vitamin C, and 400 mg methionone; 4 times per day; for 10 wk | 36 patients; placebo; 4 times per d; for 10 wk | Quality of life ↑Pain ↓Physical and social functioning ↑Health perception ↑Emotional functioning, energy, mental health:1 | Plasma selenium ↑Plasma vitamin C ↑Plasma vitamin E ↑Plasma β-carotene ↑ | Two patients complained of nausea and one of an unpleasant taste during treatment with Antox |
| Durgaprasad et al[35], 2005 | Curcumin | Randomized; single blind; placebo-controlled | 3 | 20 patients with tropical pancreatitis (CP) | 8 patients; capsule: 500 mg curcumin (95%) with 5 mg of piperine; 3 times per day; for 6 wk | 7 patients; placebo (lactose) | Median visual analogue pain score1Severity of Pain1 | Erythrocyte MDA ↓GSH level1 | - |
| Banks et al[36], 1997 | Allopurinol | Randomized, double-blind, two-period crossover clinical trial | 4 | 26 patients with CP | 13 patients; 300 mg/d All opurinol; 4 wk | 13 patients, placebo | Pain1 | Uric acid level ↓ | - |
| Bilton et al[28], 1994 | S- adenosyl methionine (SAMe)Selenium and β-carotene + SAMe | Randomized; double-blind; crossover; placebo- controlled | 5 | 20 patients with AP or CP | 20 patients; SAMe 2.4 g/d; 10 wk20 patients; SAMe 2.4 g/d, Selenium 600 μg and β-carotene 9000 IU; 10 wk | Placebo | Attack rate and background pain1 | Free radical activity ↓Serum selenium ↓Serum β-carotene ↓Serum vitamin E ↓1Serum vitamin C ↓Serum SAMe ↑Free radical activity ↓Serum selenium ↓Serum β-carotene ↑Serum vitamin E ↑1Serum vitamin C ↓Serum SAMe ↑ | - |
| Salim et al[39], 1991 | Allopurinol;dimethyl sulfoxide | Randomized; double-blind; placebo- controlled | 4 | 78 patients with CP | 25 patients; allopurinol; 50 mg 4 times per day, with analgesic regimen (IM pethidine hydrochloride; 50 mg every 4 hours, and IM metoclopramide hydrochloride; 10 mg every 8 h) | 27 patients; placebo with analgesic regimen | Pain free patients ↑Hospitalization ↓Epigastric tenderness ↓ | WBC count ↓Serum amylase ↓ Serum LDH ↓ | AllergiesGeneral malaiseHeadacheNauseaVomitingDyspepsiaAbdominal pain |
| Uden et al[37,38], 1990, 1992 | Combined antioxidant (selenium , β-carotene, vitamin C, vitamin E, methionine) | Randomized; double-blind; crossover; placebo- controlled | 5 | 28 patients with CP | 26 patients; dimethyl sulfoxide; 500 mg 4 times per day; with analgesic regimen23 patients; daily doses of 600 mg organic selenium, 9000 IU β-carotene, 0.54 g vitamin C, 270 IU vitamin E and 2 g methionine; 10 wk | 23 patients; placebo | Pain (Mc Gill) ↓ | Free radical activity ↓ Serum selenium ↑Serum β-carotene ↑Serum vitamin E ↑Serum SAMe ↓ | - |
| Ref. | Drug/supplements | Study design | Jadad score | n | Treatment (intervention) | Outcome (results) | Adverse effects/events | Other comments | ||
| Case | Control | Primary | Other | |||||||
| Abbasinazari et al[40], 2011 | Allopurinol | Randomized double blind clinical trial | 3 | 74 | 29 patients; | 45 patients; no medication | Rate of PEP1(11.5% vs 12.5%) | Serum amylase activity1 | - | - |
| Martinez-Torres et al[41], 2009 | Allopurinol | Randomized; double-blind; placebo-controlled | 5 | 170 | 85 patients; 300 mg oral allopurinol 15 h and 3 h before ERCP | 85 patients; placebo | Rate of PEP ↓ (2.3% vs 9.4%) | Serum amylase activity ↓ | - | 21.7% absolute benefit in patients with high-risk procedures favoring allopurinol, no difference in low-risk procedures |
| Kapetanos et al[42], 2009 | Pentoxifylline | Randomized; | 2 | 590 | 205 patients; 400 mg oral Pentoxifylline, 40 h, 32 h, 24 h, 16 h and 8 h before ERCP (total dose 2 g) | 205 patients; no medication | Rate of PEP1(7.3% vs 2.9%) | TNF-α1IL-61 | - | - |
| Octreotide | 180 patients; 0.5 mg subcutaneous octreotide, 64 h, 56 h, 48 h, 40 h, 32 h, 24 h, 16 h and 8 h before ERCP (total dose 4 mg) | 205 patients; no medication | Rate of PEP1(5% vs 2.9%) | TNF-α↓IL-61 | ||||||
| Romagnuolo et al[43], 2008 | Allopurinol | Randomized; double blind; placebo- controlled | 4 | 586 | 293 patients; 300 mg oral allopurinol 60 min before ERCP | 293 patients; placebo | Rate of PEP1(5.5% vs 4.1%) | Disease-related adverse events1Procedure-related complications1Hospitalization1 | - | In the non–high-risk group (n = 520), the crude PEP rates were 5.4% for allopurinol and 1.5% for placebo (P = 0.017), favoring placebo, indicating harm associated with allopurinol, whereas in the high-risk group (n = 66), the PEP rates were 6.3% for allopurinol and 23.5% for placebo (P = 0.050), favoring allopurinol |
| Kapetanos et al[44], 2007 | Pentoxifylline | Randomized; | 2 | 320 | 158 patients; 400 mg oral pentoxifylline, 40 h, 32 h, 24 h, 16 h and 8 h before ERCP (total dose 2 g) | 162 patients; no medication | Rate of PEP1(5.6% vs 3%) | Hemorrhage1Serum amylase activity1 | Nausea and vomiting in 10% of the patients who received the drug | - |
| Milewski et al[45], 2006 | N-acetylcysteine | Randomized; placebo-controlled | 2 | 106 | 55 patients; 600 mg oral N-acetylcysteine 24 h and 12 h before ERCP and 1200 mg IV for 2 d after the ERCP | 51 patients; isotonic IV saline b.d for 2 d after the ERCP | Rate of PEP1(7.3% vs 11.8%) | Urine amylase activity1Serum amylase activity1 | - | - |
| Katsinelos et al[46], 2005 | Allopurinol | Randomized; double blind; placebo-controlled | 4 | 250 | 125 patients; 600 mg oral allopurinol 15 and 3 h before ERCP | 118 patients; placebo | Rate of PEP ↓(3.2% vs 17.8%) | Hospitalization ↓Severity of Pancreatitis ↓ | - | - |
| Katsinelos et al[47], 2005 | N-acetylcysteine | Randomized; double-blind; placebo-controlled | 3 | 256 | 124 patients; 70 mg/kg 2 h before and 35 mg/kg at 4 h intervals for a total of 24 h after the procedure | 125 patients; placebo (normal saline solution) | Rate of PEP1Hospitalization1 | - | Nausea Skin rash Diarrhea Vomiting | 2 patients with suspected SOD |
| Mosler et al[48], 2005 | Allopurinol | Randomized; double blind; placebo- controlled | 4 | 701 | 355 patients; 600 mg 4 h and 300 mg 1 h oral allopurinol before ERCP | 346 patients; placebo | Rate of PEP1(13.0% vs 12.1%) | Severity of pancreatitis1 | - | 4% absolute benefit in high-risk patients; 4% absolute harm in average risk |
| Lavy et al[49], 2004 | Natural β-carotene | Randomized; double-blind; placebo-controlled | 5 | 321 | 141 patients; 2 g oral β-carotene 12 h before ERCP | 180 patients; placebo | Rate of PEP1(10% vs 9.4%) | Severe pancreatitis ↓ | - | - |
| Budzyńska et al[50], 2001 | Allopurinol | Randomized; placebo-controlled | 3 | 300 | 99 patients; 200 mg oral Allopurinol 15 h and 3 h before ERCP | 101 patients; placebo | Rate of PEP1(12.1% vs 7.9%) | Severity of pancreatitis1 | - | 3-arm study, with third arm (n = 100) given prednisone |
In these 35 papers, the Jadad score was 5 in 12 papers (34%), 4 in 9 (25%), 3 in 8 (22%), 2 in 5 (14%) and only one study scored 1 (Tables 1-3).
Furthermore, the effects of early discontinuation were minimized by the collection of updates, follow-up and investigated in the analyses.
In each study, patients used antioxidant therapy in order to treat or prevent pancreatitis, although various methods of quantifying outcomes were employed. Tables 1, 2, and 3 detail the characteristics of the trials. In these cases, only the results for length of hospital stay in AP patients, serum CRP in AP patients, pain reduction in CP patients, the incidence and severity of all types of PEP in patients undergoing ERCP, and serum amylase in patients undergoing ERCP were included in the meta-analysis.
In the context of AP, ten of twelve studies assessed clinical presentations, as outcomes of antioxidant therapy[17-22,24,25,27,28]. One of four studies reported that the mortality rate was reduced following antioxidant therapy[19]. Four of eight studies showed a significantly shorter hospital stay in the treatment groups[17,19,24,25]. In addition, four of eight trials reported a reduction in complications and organ dysfunction[17,19,21,24]. However, one study showed that antioxidant therapy did not alleviate pain in AP[28].
On the other hand, ten of twelve studies assessed laboratory outcomes, as outcomes of antioxidant therapy[17,18,20-26,28]. Three of five studies showed a significant increase in serum free radical activity and a significant increase in serum antioxidant levels[17,24,28]. While, three of seven trials reported a decrease in inflammatory biomarkers[20,24,28], one trial reported an increase in inflammatory biomarkers[25]. Indeed, three of the five studies demonstrated a significant decrease in CRP levels[20,21,24,25]. In addition, one study reported a reduction in the levels of serum amylase and lipase[21]. It is noteworthy that one of twelve studies assessing the antioxidant therapies reported diarrhea, vomiting and hypernatremia in 5 patients[23].
In the context of CP, all of the studies (twelve studies) assessed clinical presentations[28-39]. Three of four studies reported that antioxidant therapy improved the quality of life as well as cognitive, emotional, social, physical and role function[32-34]. Two of three studies showed a significantly shorter hospital stay in the treatment groups[33,39]. In addition, six of eleven trials reported a reduction of pain[29,32-34,37-39].
On the other hand, eleven of twelve studies assessed laboratory outcomes, as outcomes of antioxidant therapy[28-39]. Eight of nine studies showed a significant decrease in serum free radical activity and a significant increase in serum antioxidant levels[28-31,33,34,37,38]. Furthermore, one of two trials reported a decrease in inflammatory biomarkers[39]. In addition, one study reported a decrease in the levels of serum amylase[39]. However, three of twelve studies assessing the antioxidant therapies reported adverse effects such as GI complications (nausea, vomiting, dyspepsia, diarrhea, and constipation), unpleasant taste, allergies, heartburn, headaches, general malaise, and abdominal pain[33,34,39].
In the context of PEP, two of eleven studies showed a significant drop in the rate of PEP[41-46]. In addition, one of two studies reported a significant decrease in the rate of hospitalization in the treatment group[46]. On the other hand, two studies showed that antioxidant therapy did not affect disease-related complications[43,44].
One of four studies assessing laboratory outcomes, reported a significant decrease in serum amylase activity[41]. Moreover, one trial reported a non-significant alteration in urine amylase levels[45]. Also, one of two studies demonstrated a significant decrease in serum TNF[42]. Two of eleven trials reported adverse events such as nausea, diarrhea, vomiting and skin rash[44,47].
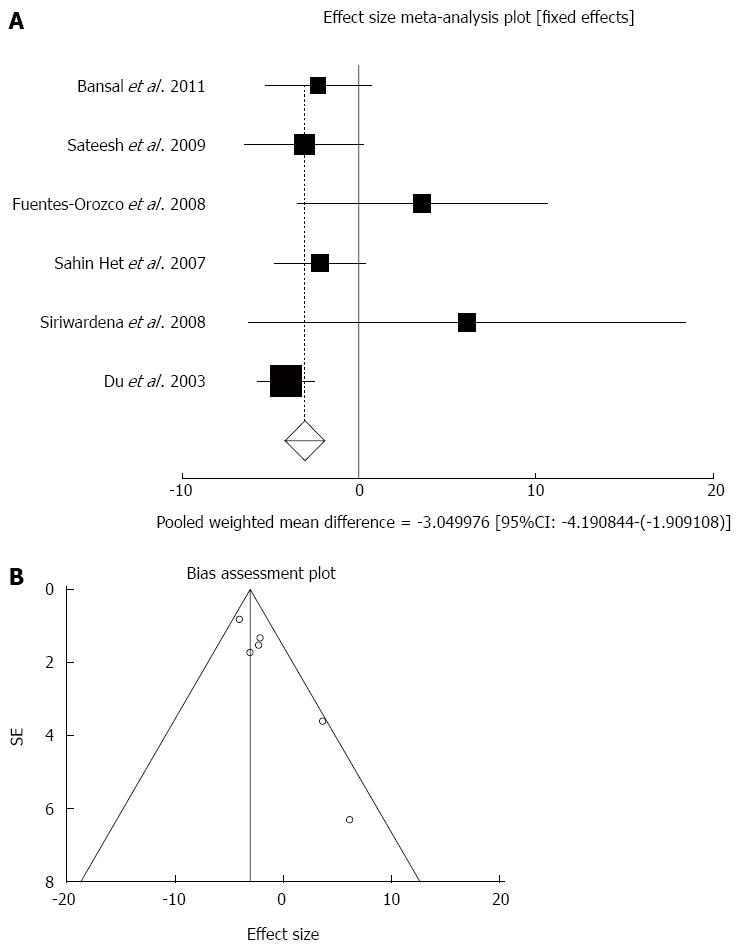
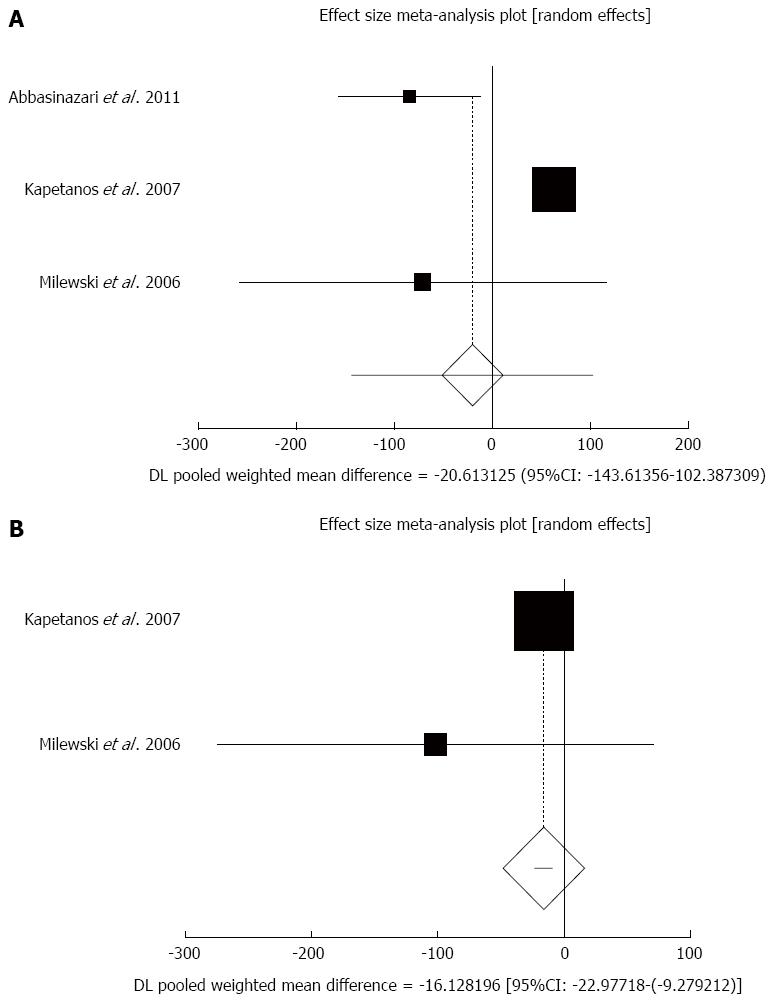
Effect of antioxidants compared with placebo on length of hospital stay (d) in acute pancreatitis patients: The summary for standardized effect size of mean differences in length of hospital stay in 303 AP patients for antioxidants therapy for six included trials compared to placebo[17,18,20-22,24] was -2.59 with 95%CI: -4.25-(-0.93) (P = 0.002, Figure 2A). The Cochrane Q test for heterogeneity indicated that the studies were not heterogeneous (P = 0.16) and could be combined, but due to publication bias the random effects for individual and summary of effect size for standardized mean was applied. For evaluation of publication bias, Egger regression of normalized effect vs precision for all included studies on length of hospital stay in AP patients treated with antioxidants vs placebo therapy was 2.17 (95%CI: 1.04-3.31, P = 0.006) and Begg-Mazumdar Kendall’s test on standardized effect vs variance indicated tau= 0.47, P = 0.27 (Figure 2B).
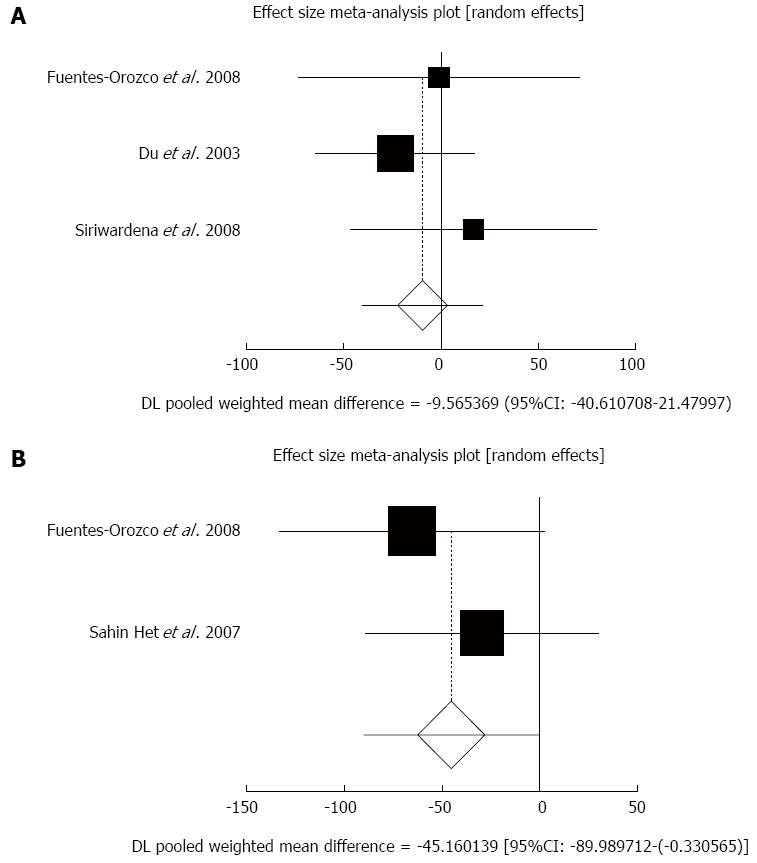
Effect of antioxidants compared with placebo on serum CRP in acute pancreatitis patients after 5-7 d: The summary for standardized effect size of mean differences in serum CRP in 171 AP patients after 5-7 d for antioxidants therapy for three included trials compared to placebo[20,22,24] was -9.57 with 95%CI: -40.61-21.48 (P = 0.55, Figure 3A). The Cochrane Q test for heterogeneity indicated that the studies were not heterogeneous (P = 0.56) and could be combined, but due to few included trials, the random effects for individual and summary of effect size for standardized mean was applied. Publication bias for included studies for serum CRP in AP patients treated with antioxidants vs placebo therapy could not be evaluated because of too few strata.
Effect of antioxidants compared with placebo on serum CRP in acute pancreatitis patients after 10 d: The summary for standardized effect size of mean differences of serum CRP in 84 AP patients after 10 d for antioxidants therapy for two included trials compared to placebo[20,21] was -45.16 with 95%CI: -89.99-(-0.33) (P = 0.048, Figure 3B). The Cochrane Q test for heterogeneity indicated that the studies were not heterogeneous (P = 0.44) and could be combined, but due to few included trials, the random effects for individual and summary of effect size for standardized mean was applied. Publication bias for included studies for serum CRP in AP patients treated with antioxidants vs placebo therapy could not be evaluated because of too few strata.
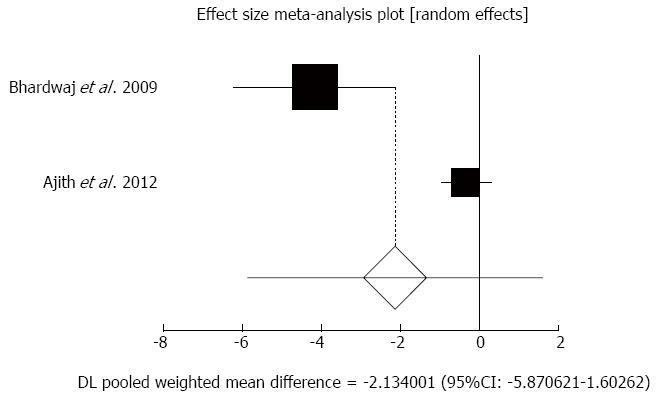
Effect of antioxidants compared with placebo on pain reduction in chronic pancreatitis patients: The summary for standardized effect size of mean differences of pain reduction in 189 CP patients for antioxidants therapy for two included trials compared to placebo[31,33] was -2.13 with 95%CI: -5.87-1.6 (P = 0.26, Figure 4). The Cochrane Q test for heterogeneity indicated that the studies were heterogeneous (P = 0.0003) and could not be combined, thus the random effects for individual and summary of effect size for standardized mean was applied. Publication bias for included studies of pain reduction in CP patients treated with antioxidants vs placebo therapy could not be evaluated because of too few strata.
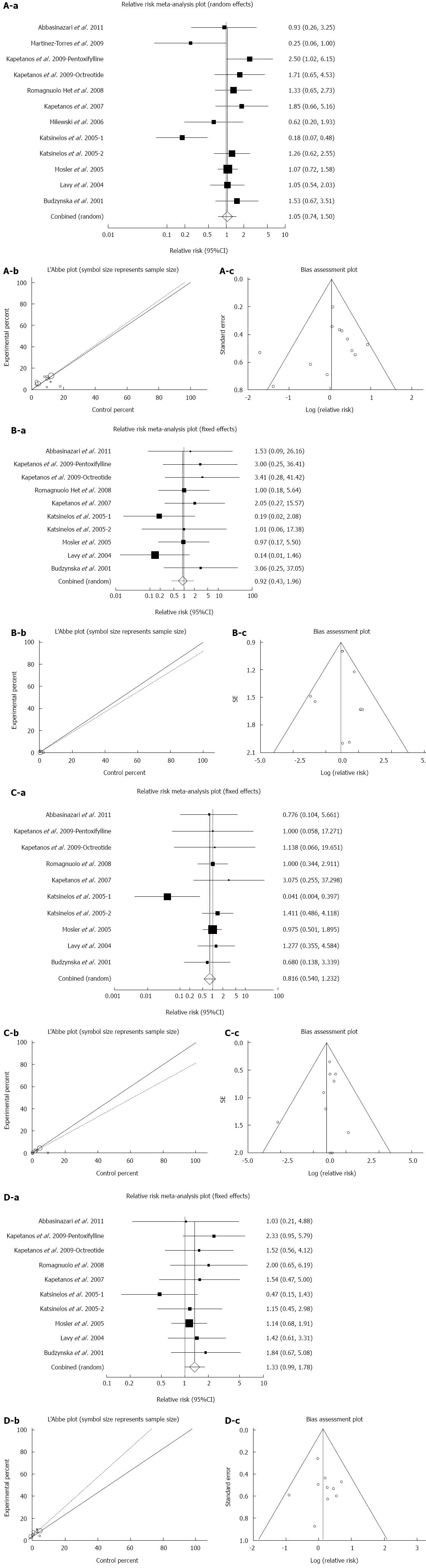
Effect of antioxidants compared with placebo on the incidence of all types of PEP in patients undergoing ERCP: The summary for RR of all types of PEP in patients undergoing ERCP for twelve included trials in eleven studies[40-50] comparing antioxidants to placebo was 1.05 with 95%CI: 0.74-1.5 (P = 0.78, Figure 5A-a). The Cochrane Q test for heterogeneity indicated that the studies were heterogeneous (P = 0.02, Figure 5A-b) and could be not combined, thus the random effects for individual and summary for RR was applied. For evaluation of publication bias Egger regression of normalized effect vs precision for all included studies for “all types of PEP” in 1849 patients treated with antioxidants vs placebo therapy was -0.78 (95%CI: -3.22-1.67, P = 0.5) and Begg-Mazumdar Kendall’s test on standardized effect vs variance indicated tau= -0.06, P = 0.73 (Figure 5A-c).
Effect of antioxidants compared with placebo on the incidence of severe PEP in patients undergoing ERCP: The summary for RR of severe PEP in patients undergoing ERCP for ten included trials in nine studies[40,42-44,46-50] comparing antioxidants to placebo was 0.92 with 95%CI: 0.43-1.97 (P = 0.83, Figure 5B-a). The Cochrane Q test for heterogeneity indicated that the studies were not heterogeneous (P = 0.85, Figure 5B-b) and could be combined, thus the fixed effects for individual and summary for RR was applied. For evaluation of publication bias, Egger regression of normalized effect vs precision for all included studies for “severe PEP” in 1709 patients treated with antioxidants vs placebo therapy was 0.21 (95%CI: -2.12-2.54, P = 0.84) and Begg-Mazumdar Kendall’s test on standardized effect vs variance indicated tau= 0.2, P = 0.48 (Figure 5B-c).
Effect of antioxidants compared with placebo on the incidence of moderate PEP in patients undergoing ERCP: The summary for RR of moderate PEP in patients undergoing ERCP for ten included trials in nine studies[40,42-44,46-50] comparing antioxidants to placebo was 0.82 with 95%CI: 0.54-1.23 (P = 0.33, Figure 5C-a). The Cochrane Q test for heterogeneity indicated that the studies were not heterogeneous (P = 0.66, Figure 5C-b) and could be combined, thus the fixed effects for individual and summary for RR was applied. For evaluation of publication bias, Egger regression of normalized effect vs precision for all included studies for “moderate PEP” in 1709 patients treated with antioxidants vs placebo therapy was -0.37 (95%CI: -1.57-0.83, P = 0.5) and Begg-Mazumdar Kendall’s test on standardized effect vs variance indicated tau= -0.02, P = 0.86 (Figure 5C-c).
Effect of antioxidants compared with placebo on the incidence of mild PEP in patients undergoing ERCP: The summary for RR of mild PEP in patients undergoing ERCP for ten included trials in nine studies[40,42-44,46-50] comparing antioxidants to placebo was 1.33 with 95%CI: 0.99-1.78 (P = 0.06, Figure 5D-a). The Cochrane Q test for heterogeneity indicated that the studies were not heterogeneous (P = 0.76, Figure 5D-b) and could be combined, thus the fixed effects for individual and summary for RR was applied. For evaluation of publication bias, Egger regression of normalized effect vs precision for all included studies for “mild PEP” in 1709 patients treated with antioxidants vs placebo therapy was 0.25 (95%CI: -1.73-2.23, P = 0.78) and Begg-Mazumdar Kendall’s test on standardized effect vs variance indicated tau= 0.07, P = 0.86 (Figure 5D-c).
Effect of antioxidants compared with placebo on serum amylase in patients undergoing ERCP after less than 8 h sampling: The summary for standardized effect size of mean differences in serum amylase in 500 patients undergoing ERCP after less than 8 h sampling for antioxidants therapy for three included trials compared to placebo[40,44,45] was -20.61 with 95%CI: -143.61-102.39 (P = 0.74, Figure 6A). The Cochrane Q test for heterogeneity indicated that the studies were heterogeneous (P < 0.0001) and could not be combined, thus the random effects for individual and summary of effect size for standardized mean was applied. Publication bias for included studies for serum amylase in patients undergoing ERCP treated with antioxidants vs placebo therapy could not be evaluated because of too few strata.
Effect of antioxidants compared with placebo on serum amylase in patients undergoing ERCP after less than 24-h sampling: The summary for standardized effect size of mean differences in serum amylase in 426 patients undergoing ERCP after less than 24-h sampling for antioxidants therapy for two included trials comparing to placebo[44,45] was -16.13 with 95%CI: -22.98-(-9.28) (P < 0.0001, Figure 6B). The Cochrane Q test for heterogeneity indicated that the studies were not heterogeneous (P = 0.34) and could be combined, but because of few included trials, the random effects for individual and summary of effect size for standardized mean was applied. Publication bias for included studies for serum amylase in patients undergoing ERCP treated with antioxidants vs placebo therapy could not be evaluated because of too few strata.
We established that antioxidant therapy significantly shortens hospital stay in AP patients, however, time is needed for the best effects. In addition, we found no significant decrease in serum CRP (as a marker of inflammation) following antioxidant therapy after 5-7 d, while the CRP decreased after 10 d. In addition, our results do not support an ameliorative role of antioxidant supplements in the reduction of pain in CP. Although in this meta-analysis, we aimed to include as many patients as possible, only two trials were eligible and eleven trials (456 patients) were excluded. Therefore, further trials are required to provide more solid evidence. The findings from another study[51] were not consistent with ours.
For interventions focused on PEP, the use of antioxidant supplements resulted in no major clinical evidence (rate and severity of PEP) of efficacy, although a tendency to decrease the rate and severity of PEP was observed. These findings are supported by the results of previous meta-analyses[15,52,53]. Controversially, although we found no significant effect of antioxidant therapy in decreasing serum amylase in PEP patients after less than 8 h sampling, serum amylase after less than 24 h sampling was significantly reduced.
To best of our knowledge, this is the most comprehensive systematic review with meta-analysis on the effect of antioxidant therapy in the management of acute, chronic and post-ERCP pancreatitis. In order to avoid bias, a comprehensive search and data extraction were conducted, however, we reached the conclusion that existing trials have inevitable differences in the use of antioxidants or the study design. Furthermore, excluding languages other than English may lead to language bias.
This meta-analysis suggests that antioxidant supplements are safe and effective in the treatment of AP, while their efficacy in CP and PEP was not confirmed. Although there are several safe and efficacious compounds that can control oxidative stress, yet antioxidant therapy has shown little success in inflammatory disorders such as pancreatitis. Lack of proper understanding of the pathological processes underlying pancreatitis may be the reason behind this failure. Evolving evidence suggests that, depending on the etiology of AP, CP or PEP, different underlying pathological processes might take part in these conditions. Most of these trials targeted AP or CP regardless of their etiology. Indeed, this meta-analysis indicated that antioxidant therapy exerts alleviating effects in the management of AP, but there is limited evidence supporting the efficacy of antioxidant therapy in PEP (as a particular type of AP). Thus, in order to progress in making antioxidant therapy a realistic goal, outcomes should be differentiated, based on their etiology.
Antioxidants, as with all drugs, have adverse events. Therefore, the complications of such compounds are yet to be specified, although they seem less theoretical than supposed.
Current advances in the field of antioxidant therapy should provide the impetus for more clinical trials. However, there is still a long way before such therapies are used in routine clinical use.
We gratefully and sincerely thank Dr. Alireza Aleyasin for his valuable comments. This invited paper (Number ID: 00040588) is the outcome of an in-house financially non-supported study.
Pancreatitis is an inflammatory, metabolic disorder, which is the major cause of physical and socioeconomic loss worldwide. Generally, pancreatitis is categorized into two different entities of acute and chronic. Antioxidant therapy has the potential to ameliorate clinical and laboratory outcomes of acute pancreatitis (AP), chronic pancreatitis (CP) and post-endoscopic retrograde cholangiopancreatography pancreatitis (PEP). Therefore, it is necessary to systematically evaluate the efficacy and adverse effects of antioxidant therapy in the management of different types of pancreatitis.
This systematic review with meta-analyses seeks to critically appraise the beneficial and harmful effects of antioxidant supplements in the management of AP, CP and PEP. The study is focused on the key outcomes of pain, hospitalization, C reactive protein (CRP) and serum amylase in CP or AP, and severity and rate of PEP.
Antioxidant therapy reduces the length of hospital stay in AP patients. Although antioxidant therapy has no significant effect on serum amylase after less than 8-h sampling, it significantly reduces serum amylase after 24-h sampling. Antioxidant therapy has no significant effect on serum CRP after 5-7 d sampling, but significantly reduces serum CRP after 10-d sampling. Future studies should focus on key outcomes of the disease dependent on the type of antioxidant.
This meta-analysis confirmed the efficacy of antioxidant therapy in the management of AP.
This is an interesting meta-analysis on the role of antioxidant therapy in the management of AP, PEP and CP. The manuscript is well-written and the conclusions of the study are acceptable.
P- Reviewer: Cosen-Binker L, Du YQ, Sperti C, Zhang ZM S- Editor: Ma YJ L- Editor: Webster JR E- Editor: Liu XM
| 1. | Teshima CW, Bridges RJ, Fedorak RN. Canadian Digestive Health Foundation Public Impact Series 5: Pancreatitis in Canada. Incidence, prevalence, and direct and indirect economic impact. Can J Gastroenterol. 2012;26:544-545. [PubMed] |
| 2. | Fagenholz PJ, Fernández-del Castillo C, Harris NS, Pelletier AJ, Camargo CA. Direct medical costs of acute pancreatitis hospitalizations in the United States. Pancreas. 2007;35:302-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 218] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 3. | Everhart JE, Ruhl CE. Burden of digestive diseases in the United States part I: overall and upper gastrointestinal diseases. Gastroenterology. 2009;136:376-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 386] [Cited by in RCA: 418] [Article Influence: 26.1] [Reference Citation Analysis (0)] |
| 4. | Mitchell RM, Byrne MF, Baillie J. Pancreatitis. Lancet. 2003;361:1447-1455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 202] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 5. | Frossard JL, Steer ML, Pastor CM. Acute pancreatitis. Lancet. 2008;371:143-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 524] [Cited by in RCA: 511] [Article Influence: 30.1] [Reference Citation Analysis (1)] |
| 6. | Peery AF, Dellon ES, Lund J, Crockett SD, McGowan CE, Bulsiewicz WJ, Gangarosa LM, Thiny MT, Stizenberg K, Morgan DR. Burden of gastrointestinal disease in the United States: 2012 update. Gastroenterology. 2012;143:1179-1187.e1-e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1355] [Cited by in RCA: 1465] [Article Influence: 112.7] [Reference Citation Analysis (1)] |
| 7. | Freeman ML, Guda NM. Prevention of post-ERCP pancreatitis: a comprehensive review. Gastrointest Endosc. 2004;59:845-864. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 321] [Cited by in RCA: 313] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 8. | Braganza JM, Lee SH, McCloy RF, McMahon MJ. Chronic pancreatitis. Lancet. 2011;377:1184-1197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 373] [Cited by in RCA: 343] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 9. | Issa Y, Bruno MJ, Bakker OJ, Besselink MG, Schepers NJ, van Santvoort HC, Gooszen HG, Boermeester MA. Treatment options for chronic pancreatitis. Nat Rev Gastroenterol Hepatol. 2014;11:556-564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 10. | Rezvanfar MA, Shojaei Saadi HA, Gooshe M, Abdolghaffari AH, Baeeri M, Abdollahi M. Ovarian aging-like phenotype in the hyperandrogenism-induced murine model of polycystic ovary. Oxid Med Cell Longev. 2014;2014:948951. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 34] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 11. | Nathan C, Cunningham-Bussel A. Beyond oxidative stress: an immunologist’s guide to reactive oxygen species. Nat Rev Immunol. 2013;13:349-361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 910] [Cited by in RCA: 1055] [Article Influence: 87.9] [Reference Citation Analysis (0)] |
| 12. | Robles L, Vaziri ND, Ichii H. Role of Oxidative Stress in the Pathogenesis of Pancreatitis: Effect of Antioxidant Therapy. Pancreat Disord Ther. 2013;3:112. [PubMed] |
| 13. | Willett WC, Stampfer MJ, Underwood BA, Speizer FE, Rosner B, Hennekens CH. Validation of a dietary questionnaire with plasma carotenoid and alpha-tocopherol levels. Am J Clin Nutr. 1983;38:631-639. [PubMed] |
| 14. | Garry PJ, Vanderjagt DJ, Hunt WC. Ascorbic acid intakes and plasma levels in healthy elderly. Ann N Y Acad Sci. 1987;498:90-99. [PubMed] |
| 15. | Mohseni Salehi Monfared SS, Vahidi H, Abdolghaffari AH, Nikfar S, Abdollahi M. Antioxidant therapy in the management of acute, chronic and post-ERCP pancreatitis: a systematic review. World J Gastroenterol. 2009;15:4481-4490. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 40] [Cited by in RCA: 36] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 16. | Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, McQuay HJ. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17:1-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12275] [Cited by in RCA: 12878] [Article Influence: 444.1] [Reference Citation Analysis (1)] |
| 17. | Sateesh J, Bhardwaj P, Singh N, Saraya A. Effect of antioxidant therapy on hospital stay and complications in patients with early acute pancreatitis: a randomised controlled trial. Trop Gastroenterol. 2009;30:201-206. [PubMed] |
| 18. | Bansal D, Bhalla A, Bhasin DK, Pandhi P, Sharma N, Rana S, Malhotra S. Safety and efficacy of vitamin-based antioxidant therapy in patients with severe acute pancreatitis: a randomized controlled trial. Saudi J Gastroenterol. 2011;17:174-179. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 35] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 19. | Xue P, Deng LH, Xia Q, Zhang ZD, Hu WM, Yang XN, Song B, Huang ZW. Impact of alanyl-glutamine dipeptide on severe acute pancreatitis in early stage. World J Gastroenterol. 2008;14:474-478. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 36] [Cited by in RCA: 36] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 20. | Fuentes-Orozco C, Cervantes-Guevara G, Muciño-Hernández I, López-Ortega A, Ambriz-González G, Gutiérrez-de-la-Rosa JL, Gómez-Herrera E, Hermosillo-Sandoval JM, González-Ojeda A. L-alanyl-L-glutamine-supplemented parenteral nutrition decreases infectious morbidity rate in patients with severe acute pancreatitis. JPEN J Parenter Enteral Nutr. 2008;32:403-411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 56] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 21. | Sahin H, Mercanligil SM, Inanç N, Ok E. Effects of glutamine-enriched total parenteral nutrition on acute pancreatitis. Eur J Clin Nutr. 2007;61:1429-1434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 39] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 22. | Siriwardena AK, Mason JM, Balachandra S, Bagul A, Galloway S, Formela L, Hardman JG, Jamdar S. Randomised, double blind, placebo controlled trial of intravenous antioxidant (n-acetylcysteine, selenium, vitamin C) therapy in severe acute pancreatitis. Gut. 2007;56:1439-1444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 125] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 23. | Pearce CB, Sadek SA, Walters AM, Goggin PM, Somers SS, Toh SK, Johns T, Duncan HD. A double-blind, randomised, controlled trial to study the effects of an enteral feed supplemented with glutamine, arginine, and omega-3 fatty acid in predicted acute severe pancreatitis. JOP. 2006;7:361-371. [PubMed] |
| 24. | Du WD, Yuan ZR, Sun J, Tang JX, Cheng AQ, Shen DM, Huang CJ, Song XH, Yu XF, Zheng SB. Therapeutic efficacy of high-dose vitamin C on acute pancreatitis and its potential mechanisms. World J Gastroenterol. 2003;9:2565-2569. [PubMed] |
| 25. | Ockenga J, Borchert K, Rifai K, Manns MP, Bischoff SC. Effect of glutamine-enriched total parenteral nutrition in patients with acute pancreatitis. Clin Nutr. 2002;21:409-416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 67] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 26. | de Beaux AC, O’Riordain MG, Ross JA, Jodozi L, Carter DC, Fearon KC. Glutamine-supplemented total parenteral nutrition reduces blood mononuclear cell interleukin-8 release in severe acute pancreatitis. Nutrition. 1998;14:261-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 95] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 27. | Sharer N, Scott P, Deardon D, Lee S, Taylor P, Braganza J. Clinical trial of 24 hours’ treatment with glutathione precursors in acute pancreatitis. Clinical Drug Investigation. 1995;10:147-157. [RCA] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 28. | Bilton D, Schofield D, Mei G, Kay P, Bottiglieri T, Braganza J. Placebo-controlled trials of antioxidant therapy including S-adenosulmethionine in patients with recurrent non-gallstone pancreatitis. Drug Invest. 1994;8:10-20. [RCA] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 21] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 29. | Dhingra R, Singh N, Sachdev V, Upadhyay AD, Saraya A. Effect of antioxidant supplementation on surrogate markers of fibrosis in chronic pancreatitis: a randomized, placebo-controlled trial. Pancreas. 2013;42:589-595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 30. | Shah N, Siriwardena AK. Cytokine profiles in patients receiving antioxidant therapy within the ANTICIPATE trial. World J Gastroenterol. 2013;19:4001-4006. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 31. | Siriwardena AK, Mason JM, Sheen AJ, Makin AJ, Shah NS. Antioxidant therapy does not reduce pain in patients with chronic pancreatitis: the ANTICIPATE study. Gastroenterology. 2012;143:655-663.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 76] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 32. | Shah NS, Makin AJ, Sheen AJ, Siriwardena AK. Quality of life assessment in patients with chronic pancreatitis receiving antioxidant therapy. World J Gastroenterol. 2010;16:4066-4071. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 26] [Cited by in RCA: 24] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 33. | Bhardwaj P, Garg PK, Maulik SK, Saraya A, Tandon RK, Acharya SK. A randomized controlled trial of antioxidant supplementation for pain relief in patients with chronic pancreatitis. Gastroenterology. 2009;136:149-159.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 158] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 34. | Kirk GR, White JS, McKie L, Stevenson M, Young I, Clements WD, Rowlands BJ. Combined antioxidant therapy reduces pain and improves quality of life in chronic pancreatitis. J Gastrointest Surg. 2006;10:499-503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 89] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 35. | Durgaprasad S, Pai CG, Vasanthkumar JF, Namitha S. A pilot study of the antioxidant effect of curcumin in tropical pancreatitis. Indian J Med Res. 2005;122:315-318. [PubMed] |
| 36. | Banks PA, Hughes M, Ferrante M, Noordhoek EC, Ramagopal V, Slivka A. Does allopurinol reduce pain of chronic pancreatitis? Int J Pancreatol. 1997;22:171-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 29] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 37. | Uden S, Bilton D, Nathan L, Hunt LP, Main C, Braganza JM. Antioxidant therapy for recurrent pancreatitis: placebo-controlled trial. Aliment Pharmacol Ther. 1990;4:357-371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 113] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 38. | Uden S, Schofield D, Miller PF, Day JP, Bottiglier T, Braganza JM. Antioxidant therapy for recurrent pancreatitis: biochemical profiles in a placebo-controlled trial. Aliment Pharmacol Ther. 1992;6:229-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 61] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 39. | Salim AS. Role of oxygen-derived free radical scavengers in the treatment of recurrent pain produced by chronic pancreatitis. A new approach. Arch Surg. 1991;126:1109-1114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 29] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 40. | Abbasinazari M, Mohammad Alizadeh AH, Moshiri K, Pourhoseingholi MA, Zali MR. Does allopurinol prevent post endoscopic retrograde cholangio- pancreatography pancreatitis? A randomized double blind trial. Acta Med Iran. 2011;49:579-583. [PubMed] |
| 41. | Martinez-Torres H, Rodriguez-Lomeli X, Davalos-Cobian C, Garcia-Correa J, Maldonado-Martinez JM, Medrano-Muñoz F, Fuentes-Orozco C, Gonzalez-Ojeda A. Oral allopurinol to prevent hyperamylasemia and acute pancreatitis after endoscopic retrograde cholangiopancreatography. World J Gastroenterol. 2009;15:1600-1606. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 19] [Cited by in RCA: 22] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 42. | Kapetanos D, Christodoulou D, Chatzizisi O, Sigounas D, Vasiliou K, Stavropoulou E, Katodritou E, Kokozidis G, Kiriazis G, Kitis G. Randomized study of the effect of pentoxifylline or octreotide on serum levels of TNF-alpha and IL-6 after endoscopic retrograde cholangiopancreatography. Eur J Gastroenterol Hepatol. 2009;21:529-533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 43. | Romagnuolo J, Hilsden R, Sandha GS, Cole M, Bass S, May G, Love J, Bain VG, McKaigney J, Fedorak RN. Allopurinol to prevent pancreatitis after endoscopic retrograde cholangiopancreatography: a randomized placebo-controlled trial. Clin Gastroenterol Hepatol. 2008;6:465-471; quiz 371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 44. | Kapetanos D, Kokozidis G, Christodoulou D, Mistakidis K, Sigounas D, Dimakopoulos K, Kitis G, Tsianos EV. A randomized controlled trial of pentoxifylline for the prevention of post-ERCP pancreatitis. Gastrointest Endosc. 2007;66:513-518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 45. | Milewski J, Rydzewska G, Degowska M, Kierzkiewicz M, Rydzewski A. N-acetylcysteine does not prevent post-endoscopic retrograde cholangiopancreatography hyperamylasemia and acute pancreatitis. World J Gastroenterol. 2006;12:3751-3755. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 28] [Cited by in RCA: 31] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 46. | Katsinelos P, Kountouras J, Chatzis J, Christodoulou K, Paroutoglou G, Mimidis K, Beltsis A, Zavos C. High-dose allopurinol for prevention of post-ERCP pancreatitis: a prospective randomized double-blind controlled trial. Gastrointest Endosc. 2005;61:407-415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 53] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 47. | Katsinelos P, Kountouras J, Paroutoglou G, Beltsis A, Mimidis K, Zavos C. Intravenous N-acetylcysteine does not prevent post-ERCP pancreatitis. Gastrointest Endosc. 2005;62:105-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 43] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 48. | Mosler P, Sherman S, Marks J, Watkins JL, Geenen JE, Jamidar P, Fogel EL, Lazzell-Pannell L, Temkit M, Tarnasky P. Oral allopurinol does not prevent the frequency or the severity of post-ERCP pancreatitis. Gastrointest Endosc. 2005;62:245-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 40] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 49. | Lavy A, Karban A, Suissa A, Yassin K, Hermesh I, Ben-Amotz A. Natural beta-carotene for the prevention of post-ERCP pancreatitis. Pancreas. 2004;29:e45-e50. [PubMed] |
| 50. | Budzyńska A, Marek T, Nowak A, Kaczor R, Nowakowska-Dulawa E. A prospective, randomized, placebo-controlled trial of prednisone and allopurinol in the prevention of ERCP-induced pancreatitis. Endoscopy. 2001;33:766-772. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 75] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 51. | Ahmed Ali U, Jens S, Busch OR, Keus F, van Goor H, Gooszen HG, Boermeester MA. Antioxidants for pain in chronic pancreatitis. Cochrane Database Syst Rev. 2014;8:CD008945. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 52. | Gu WJ, Wei CY, Yin RX. Antioxidant supplementation for the prevention of post-endoscopic retrograde cholangiopancreatography pancreatitis: a meta-analysis of randomized controlled trials. Nutr J. 2013;12:23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 53. | Zheng M, Chen Y, Bai J, Xin Y, Pan X, Zhao L. Meta-analysis of prophylactic allopurinol use in post-endoscopic retrograde cholangiopancreatography pancreatitis. Pancreas. 2008;37:247-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 1.4] [Reference Citation Analysis (0)] |