Published online Aug 14, 2015. doi: 10.3748/wjg.v21.i30.9163
Peer-review started: March 5, 2015
First decision: April 13, 2015
Revised: April 29, 2015
Accepted: June 26, 2015
Article in press: June 26, 2015
Published online: August 14, 2015
AIM: To evaluates the effectiveness and safety of the first generation, NS3/4A protease inhibitors (PIs) in clinical practice against chronic C virus, especially in patients with advanced fibrosis.
METHODS: Prospective study and non-experimental analysis of a multicentre cohort of 38 Spanish hospitals that includes patients with chronic hepatitis C genotype 1, treatment-naïve (TN) or treatment-experienced (TE), who underwent triple therapy with the first generation NS3/4A protease inhibitors, boceprevir (BOC) and telaprevir (TVR), in combination with pegylated interferon and ribavirin. The patients were treatment in routine practice settings. Data on the study population and on adverse clinical and virologic effects were compiled during the treatment period and during follow up.
RESULTS: One thousand and fifty seven patients were included, 405 (38%) were treated with BOC and 652 (62%) with TVR. Of this total, 30% (n = 319) were TN and the remaining were TE: 28% (n = 298) relapsers, 12% (n = 123) partial responders (PR), 25% (n = 260) null-responders (NR) and for 5% (n = 57) with prior response unknown. The rate of sustained virologic response (SVR) by intention-to-treatment (ITT) was greater in those treated with TVR (65%) than in those treated with BOC (52%) (P < 0.0001), whereas by modified intention-to-treatment (mITT) no were found significant differences. By degree of fibrosis, 56% of patients were F4 and the highest SVR rates were recorded in the non-F4 patients, both TN and TE. In the analysis by groups, the TN patients treated with TVR by ITT showed a higher SVR (P = 0.005). However, by mITT there were no significant differences between BOC and TVR. In the multivariate analysis by mITT, the significant SVR factors were relapsers, IL28B CC and non-F4; the type of treatment (BOC or TVR) was not significant. The lowest SVR values were presented by the F4-NR patients, treated with BOC (46%) or with TVR (45%). 28% of the patients interrupted the treatment, mainly by non-viral response (51%): this outcome was more frequent in the TE than in the TN patients (57% vs 40%, P = 0.01). With respect to severe haematological disorders, neutropaenia was more likely to affect the patients treated with BOC (33% vs 20%, P≤ 0.0001), and thrombocytopaenia and anaemia, the F4 patients (P = 0.000, P = 0.025, respectively).
CONCLUSION: In a real clinical practice setting with a high proportion of patients with advanced fibrosis, effectiveness of first-generation PIs was high except for NR patients, with similar SVR rates being achieved by BOC and TVR.
Core tip: To the best of our knowledge, this study objectively evaluates the effectiveness and safety of first-generation protease inhibitors in routine clinical practice against chronic C virus infection. A total of 1057 patients with chronic hepatitis C genotype 1, treatment with triple therapy (boceprevir or telaprevir in combination with peginterferon and ribavirin) were included: 30% (n = 319) were treatment-naïve and the remaining 738 (70%) were treatment-experienced: 28% were relapsers, 12% were partial responders, 25% were null-responders and for 5% the prior response was unknown. At present not all patients can be treated with new molecules as simeprevir or sofosbuvir.
- Citation: Salmerón J, Vinaixa C, Berenguer R, Pascasio JM, Sánchez Ruano JJ, Serra M&, Gila A, Diago M, Romero-Gómez M, Navarro JM, Testillano M, Fernández C, Espinosa D, Carmona I, Pons JA, Jorquera F, Rodriguez FJ, Pérez R, Montero JL, Granados R, Fernández M, Martín AB, Muñoz de Rueda P, Quiles R, Alhambra Spanish Study Group. Effectiveness and safety of first-generation protease inhibitors in clinical practice: Hepatitis C virus patients with advanced fibrosis. World J Gastroenterol 2015; 21(30): 9163-9174
- URL: https://www.wjgnet.com/1007-9327/full/v21/i30/9163.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i30.9163
The hepatitis C virus (HCV) is a global health problem. Estimates suggest that in 2005 about 185 million people worldwide were infected with HCV and that the prevalence had increased by 2.3% with respect to 1990[1]. Moreover, about 80% of patients progress to chronicity, and between 5%-30% to liver cirrhosis and hepatocellular carcinoma[2], which provoke 366000 deaths annually[3].
For decades, the standard treatment for chronic hepatitis C (CHC) has been based on a combination of pegylated interferon (PEG-IFN) and ribavirin (RBV), which achieve a rate of sustained virologic response (SVR) of 41%[4,5]. However, a few years ago, direct-acting antiviral agents (DAAs) such as boceprevir (BOC) and telaprevir (TVR), which are first-generation NS3/4A protease inhibitors (PIs), were incorporated into treatment protocols. With the use of these PIs, the rate of SVR has increased in clinical phase III trials by up to 75% in HCV genotype 1 patients, although the treatment is complicated by problems of tolerability and other adverse effects. However, to date most clinical trials have not accurately reflected the patients being treated in clinical practice, because the study population tends to be composed of carefully selected subjects, with very few cirrhotic patients being included[6-9]. For this reason, some studies conducted to examine the efficacy and safety of triple therapy in patients with advanced liver disease, such as those examining the CUPIC cohort in the French Early Access programme and the American Veterans cohort, have concluded that despite achieving high levels of SVR, PIs are associated with a higher rate of adverse events, including deaths, cirrhotic decompensation, dermatologic disorders and severe anaemias, with 51%-54% of the patients treated presenting at least one serious adverse effect[10-12].
In Spain, this approach has mainly been used to treat patients presenting a high degree of liver fibrosis, in accordance with Ministry of Health recommendations[13], in the view that this population is a more complex one, with more comorbidities than HCV patients detected at earlier stages of the disease[7,8,14,15]. The aim of the present study is to evaluate the efficacy and safety of triple therapy with first-generation PIs in clinical practice, both in treatment-naïve patients (TN) and in treatment-experienced ones (TE).
A total of 1057 Spanish patients have been included in the national Register of Patients with Chronic Hepatitis C treated with PIs, administered by the Andalusian Public Health and Progress Foundation (protocol code FSE-TEL-2013-01), designed in compliance with applicable national legislation (Act 15/1999 LOPD and the Biomedical Research Act 14/2007). All patients involved in the study were informed verbally and in writing of the characteristics of the study obtaining their consent to participate by signing the informed consent (in accordance with the Declaration of Helsinki). This study was approved of the Andalusian Coordinating Committee for Biomedical Research Ethics, the Ethics Committees of each of the 38 hospitals that have contributed to the Register and Spanish Agency of Medicines and Health Product.
The present study is an open, prospective, non-experimental analysis of a multicentre cohort including patients with HCV genotype 1, both TN and TE, who meet the requirements, according to rules set out by the Health Ministry and by each Autonomous Community, to be treated with first-generation PIs, BOC or TVR, i.e., these patients all present moderate or advanced liver fibrosis (Metavir fibrosis score of F3 or F4). The diagnosis of liver fibrosis was established according to local clinical practice. For most patients, Fibroscan was used according to the manufacturer’s instructions (Fibroscan, Echosens). The results were expressed in kilopascals (kPa) applying the following cut-off values to determine the degree of fibrosis[16]: < 7.5 kPa, mild or no fibrosis (F0-F1); ≥ 7.5 kPa and < 9.5 kPa, significant fibrosis (F2); ≥ 9.5 kPa and < 12.5 kPa, severe fibrosis (F3); ≥ 12.5 kPa, liver cirrhosis (F4). All patients in the study, and in accordance with applicable national legislation, gave their informed consent to be included in the Register. The following exclusion criteria were applied: simultaneous participation in another research study, non-availability to follow up, contraindications for triple therapy, coinfection with HBV and/or HIV, advanced liver cirrhosis and liver transplantation.
Treatment was prescribed according to the recommendations of product characteristics for each of the drugs used. Data were compiled by the specialist in question during the treatment and follow-up. The minimum set of common data was obtained using data collection protocols, taking into account sociodemographic, diagnostic, clinical, treatment and analytical variables.
The viral load, or level of HCV RNA, was determined, following the recommendations of standard clinical practice, at the following times: baseline, at weeks 4, 12, 24 and 48 wk of treatment and at 12 wk post-treatment. HCV-RNA was determined in serum with the Amplicor HCV Kit (Roche Diagnostics System). HCV-RNA serum levels were validated real-time PCR-based assays COBAS AmpliPrep/COBAS TaqMan (Roche Molecular Systems, Pleasanton, CA, United States) with a lower limit of detection of 15 IU/mL. Testing was performed at each site, following local practice.
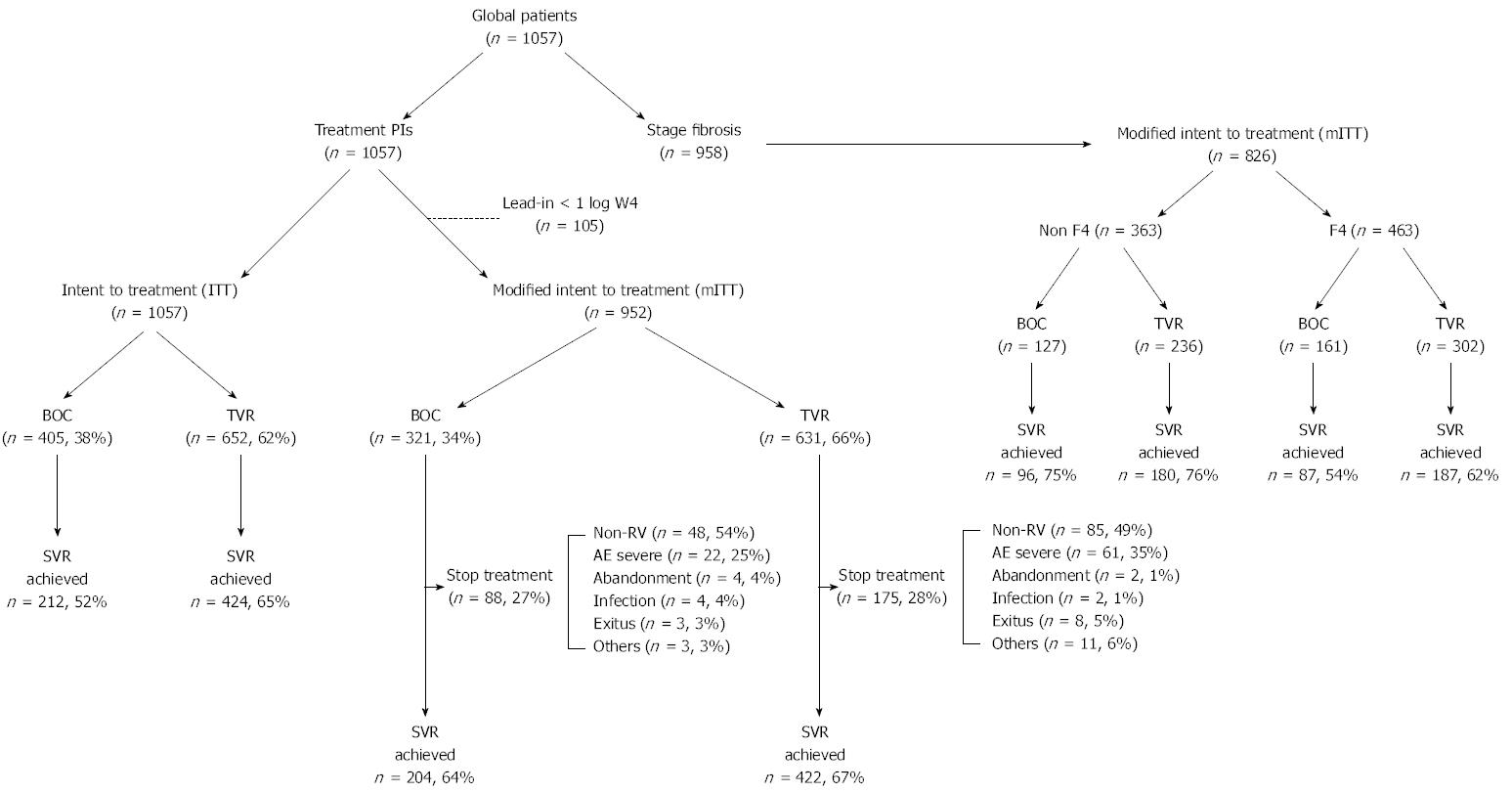
Treatment effectiveness, i.e., the likelihood of achieving SVR at 12 wk post-treatment, was assessed by intention to treatment (ITT), including all patients (n = 1057), and by modified intention to treatment (mITT; n = 952), excluding patients who had a decrease of less than 1log10 IU per millilitre in the HCV RNA level during the 4-wk lead-in period and had not received any dose of BOC or TVR (Figure 1). The adverse effects reported by patients were compiled prospectively during the treatment period and during follow-up (12 wk post-treatment). Anaemia events were managed according to the usual practice at each hospital. Anaemia was defined as mild or grade 0-1-2 for haemoglobin values > 8 gr/dL, and as severe or grade 3-4 for Hb < 8 gr/dL. Other cytopaenias were also classed as mild (grade 0-1-2) or severe (grade 3-4) according to the values of neutrophils (neutropaenia grade 0-1-2, > 750 μL; grade 3-4 neutropaenia, < 750 μL) and of platelets (thrombocytopaenia grade 0-1-2, > 50000 μL; grade 3-4 thrombocytopaenia, < 50000 μL). The use of erythropoietin or other growth factors, and/or of blood transfusion, was also noted.
The baseline and demographic characteristics of the study population were analysed descriptively by treatment arm (BOC and TVR) and clinical situation (TN and TE). The quantitative variables are expressed as mean ± SD and the qualitative variables as absolute values with percentages. Inter-group comparisons for qualitative variables were made using the χ2 test or Fisher’s exact test, and the quantitative variables, using Student’s t-test; non-normal distribution variables were tested using the Mann-Whitney U nonparametric test. The response to antiviral therapy was tested using binary logistic regression with bivariate and multivariate analysis, together with the propensity score. In nonrandomized studies treatment selection is often influenced by subject characteristics. Thus, in our case, the baseline characteristics of the subjects may differ depending on the treatment used. Therefore, one must account for systematic differences in baseline characteristics between different treatments when estimating the effect of treatment on outcomes. Therefore, one must account for systematic differences in baseline characteristics between different treatments when estimating the effect of treatment on outcomes. For this reason, we used methods based on the propensity score to reduce or eliminate confounding effects when using observational data[17,18]. Thus, in a set of subjects, all of whom have the same propensity score, the distribution of the observed baseline covariates will be the same between the two treatment groups. To adjust the regression, we incorporated a large set of background covariates to estimate the propensity score and then used a subset of these covariates (previous response, IL28B, fibrosis, treatment, logarithm GGT and logarithm viral load) and the propensity score in the regression adjustment. The criterion for statistical significance was P≤ 0.05. Data management and analysis were performed using SPSS 15.0 for Windows.
The statistical methods of this study were reviewed by Paloma Muñoz de Rueda from San Cecilio University Hospital
A total of 1057 patients with CHC participated in this study. 405 (38%) were treated with BOC and 652 (62%) with TVR (Table 1). Of the 1057 patients, 30% (n = 319) were TN and the rest were TE. 34% of the patients treated with TVR were relapsers (R), vs 19% of those treated with BOC (P≤ 0.0001). 958 patients were tested for liver fibrosis (Table 1), and 33% of the non-F4 patients were found to be R (compared to 24% of the F4 patients), while 28% of the F4 patients were null responders (NR) (vs 20% of the non-F4 patients, P = 0.01) (Table 1).
| All patients | BOC | TVR | P value | Fibrosis | Non-F4 | F4 | P value | |
| (n = 1057) (100) | (n = 405) (38) | (n = 652) (62) | All patients | (n = 423) (44) | (n = 545) (56) | |||
| (n = 958) (100) | ||||||||
| TN | 319 (30) | 130 (32) | 189 (29) | 0.000 | 294 (30) | 129 (31) | 165 (30) | 0.010 |
| TE | 738 (70) | 275 (68) | 463 (71) | 664 (70) | 294 (67) | 380 (70) | ||
| R | 298 (28) | 77 (19) | 221 (34) | 270 (28) | 138 (33) | 132 (24) | ||
| PR | 123 (12) | 55 (14) | 68 (10) | 113 (12) | 44 (10) | 69 (13) | ||
| NR | 260 (25) | 119 (29) | 141 (22) | 238 (25) | 86 (20) | 152 (28) | ||
| Unknown responders | 57 (5) | 24 (6) | 33 (5) | 53 (6) | 26 (6) | 27 (5) |
The baseline characteristics of all patients and those according to prior response are shown in Table 2. In the total patients, there were significant differences between BOC and TVR in liver fibrosis (P = 0.002), GGT (P = 0.05) and albumin (P = 0.04); in the TN patients, in liver fibrosis (P = 0.000); in the R patients, in albumin (P = 0.03); in the partial responders (PR) patients, in liver fibrosis (P = 0.03) and in albumin (P = 0.02) and, finally, in the NR patients, in GGT (P = 0.04).
| All patients (n = 1057) | TN (n = 319) | R (n = 298) | PR (n = 123) | NR (n = 260) | ||||||||||||
| All (n = 1057) | BOC (n = 405) | TVR (n = 652) | P value | BOC (n = 130) | TVR (n = 189) | P value | BOC (n = 77) | TVR (n = 221) | P value | BOC (n = 55) | TVR (n = 68) | P value | BOC (n = 119) | TVR (n = 141) | P value | |
| Age (yr), mean ± SD | 54 ± 8 | 54 ± 9 | 53 ± 8 | 52 ± 10 | 53 ± 9 | 54.6 ± 8 | 53 ± 8 | 54.6 ± 8 | 53 ± 8 | 54 ± 9 | 54 ± 7 | |||||
| Male sex | 724 (69) | 264 (65) | 460 (71) | NS | 85 (65) | 124 (66) | NS | 46 (60) | 157 (71) | NS | 35 (64) | 55 (81) | 0.03 | 85 (71) | 101 (72) | NS |
| HCV genotype 1 subtype | ||||||||||||||||
| 1a | 239 (26) | 102 (28) | 137 (24) | NS | 32 (27) | 47 (29) | NS | 18 (25) | 38 (20) | NS | 15 (31) | 13 (23) | NS | 34 (32) | 31 (24) | NS |
| 1b | 692 (74) | 263 (72) | 429 (76) | 85 (73) | 116 (71) | 54 (75) | 151 (80) | 34 (69) | 44 (77) | 72 (68) | 99 (76) | |||||
| IL28b genotype | ||||||||||||||||
| CC | 157 (20) | 55 (18) | 102 (20) | NS | 27 (26) | 35 (23) | NS | 8 (16) | 46 (29) | NS | 5 (13) | 5 (9) | NS | 9 (10) | 9 (8) | NS |
| CT | 510 (63) | 191 (64) | 319 (63) | 57 (54) | 94 (61) | 36 (74) | 97 (61) | 24 (63) | 38 (72) | 61 (70) | 77 (68) | |||||
| TT | 138 (17) | 54 (18) | 84 (17) | 21 (20) | 24 (16) | 5 (10) | 16 (10) | 9 (24) | 10 (19) | 17 (20) | 27 (24) | |||||
| Stage of fibrosis | ||||||||||||||||
| F0F1 | 81 (8) | 46 (12) | 35 (6) | 0.002 | 19 (15) | 4 (2) | 0 | 11 (15) | 27 (14) | NS | 8 (16) | 2 (3) | 0.03 | 3 (3) | 1 (1) | NS |
| F2 | 138 (14) | 42 (11) | 96 (16) | 17 (14) | 28 (16) | 7 (10) | 31 (16) | 3 (6) | 9 (14) | 14 (12) | 20 (16) | |||||
| F3 | 204 (21) | 76 (20) | 128 (22) | 27 (22) | 34 (20) | 21 (29) | 41 (21) | 7 (14) | 15 (24) | 16 (14) | 32 (25) | |||||
| F4 | 545 (56) | 217 (57) | 328 (56) | 60 (49) | 105 (62) | 34 (46) | 98 (49) | 32 (64) | 37 (59) | 80 (71) | 72 (58) | |||||
| Hb (g/dL) | 15 ± 1 | 15 ± 1.5 | 15.1 ± 1.5 | NS | 15 ± 1.6 | 14.8 ± 1.5 | NS | 15 ± 1.6 | 15.3 ± 1.3 | NS | 14.9 ± 1.5 | 15.2 ± 1.5 | NS | 15.2 ± 1.5 | 15.3 ± 1.6 | NS |
| PMN (mL) | 3228 ± 1305 | 3241.4 ± 1329.6 | 3220.4 ± 1290.3 | NS | 3317.8 ± 1250.4 | 3155.1 ± 1276.1 | NS | 3499 ± 1405 | 3366 ± 1392 | NS | 3002 ± 1364.5 | 3168 ± 1182.5 | NS | 3156 ± 1385.5 | 3176.0 ± 1208.1 | NS |
| Platelets (%) | 161358 ± 66199 | 162194.7 ± 65144.4 | 160841.2 ± 66889.7 | NS | 169727.3 ± 68082 | 159038.1 ± 72104.9 | NS | 172671 ± 69822 | 178393 ± 66412 | NS | 156907 ± 57085 | 147308 ± 61828 | NS | 154046 ± 62558 | 144128.6 ± 55066 | NS |
| Prothrombin (%) | 94 ± 14 | 94.7 ± 12.6 | 92.7 ± 14.8 | NS | 93.7 ± 11 | 95.6 ± 15.3 | NS | 94.2 ± 13.0 | 90 ± 15 | NS | 95.8 ± 16.0 | 93.4 ± 11.0 | NS | 95.6 ± 0.7 | 92.6 ± 15.5 | NS |
| ALT (IU/mL) | 89 ± 65 | 88.9 ± 70.1 | 89.8 ± 61.6 | NS | 80.6 ± 55 | 92.4 ± 62.0 | NS | 80 ± 64 | 76.0 ± 51.2 | NS | 90 ± 53 | 87 ± 46 | NS | 93.6 ± 48.8 | 106.3 ± 77.0 | NS |
| GGT (IU/mL) | 107 ± 103 | 115.3 ± 106.1 | 101.8 ± 101.3 | 0.050 | 90.6 ± 86.5 | 107.0 ± 133.8 | NS | 110 ± 144 | 81.2 ± 70.0 | NS | 116.8 ± 79.0 | 113.0 ± 87.5 | NS | 143.1 ± 107.0 | 117.7 ± 93.0 | 0.040 |
| Alkaline phosphatase (mL) | 95 ± 46 | 93.6 ± 40.8 | 95.2 ± 49.6 | NS | 88.4 ± 31 | 99 ± 57 | NS | 89.4 ± 30.0 | 89.3 ± 43.0 | NS | 99.6 ± 50.6 | 101.8 ± 48.1 | NS | 96.7 ± 46.6 | 95.1 ± 49.1 | NS |
| Albumin (g/L) | 4.0 ± 0.5 | 4.18 ± 0.428 | 4.25 ± 0.52 | 0.040 | 4.2 ± 0.5 | 4.2 ± 0.4 | NS | 4.2 ± 0.4 | 4.4 ± 0.4 | 0.030 | 4.1 ± 0.4 | 4.3 ± 0.5 | 0.020 | 4.2 ± 0.4 | 4.0 ± 0.6 | NS |
| Creatinine (mg/dL) | 0.8 ± 0.2 | 0.79 ± 0.16 | 0.81 ± 0.2 | NS | 0.8 ± 0.2 | 0.8 ± 0.1 | NS | 0.8 ± 0.2 | 0.8 ± 0.2 | NS | 0.8 ± 0.2 | 0.8 ± 0.2 | NS | 0.7 ± 0.2 | 0.8 ± 0.22 | NS |
| HCV viral load (IU/mL) | 3.3 × 106± 6 × 106 | 3.5 × 106± 5.9 × 106 | 3.2 × 106± 5.9 × 106 | NS | 3.5 × 106± 6.2 × 106 | 3.8 × 106± 8.9 × 106 | NS | 2.8 × 106± 3.8 × 106 | 3 × 106± 4.1 × 106 | NS | 4.1 × 106± 9.5 × 106 | 2.3 × 106± 2.6 × 106 | NS | 3.3 × 106± 4.7 × 106 | 3 × 106± 4 × 106 | NS |
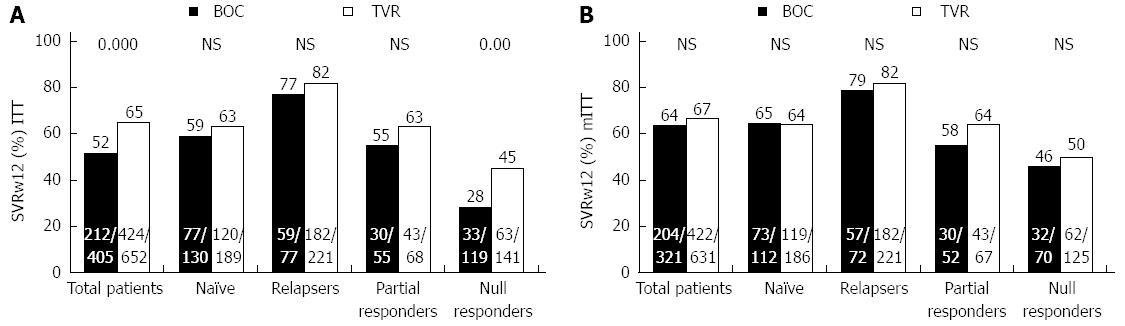
The SVR results (Figure 2) were obtained by grouping the patients by ITT (n = 1057) (Figure 2A) and by mITT (n = 952) (Figure 2B). In the group treated with BOC (n = 405), 99% (n = 401) were given the lead-in treatment, and of these, 20% (81/401) did not respond well; whereas in the patients treated with TVR (n = 652), only 14% (n = 90) were given the lead-in treatment, and of these, 23% (n = 21) responded poorly. By ITT, among the all patients group those treated with TVR achieved a higher SVR rate than those given BOC (65% vs 52%, P < 0.0001) as did the NR group (45% vs 28%, P = 0.005). By contrast, there were no significant differences in the TN, R or PR groups (P > 0.05). As expected, the analysis by mITT revealed no significant differences in any of the study groups (Figure 2B).
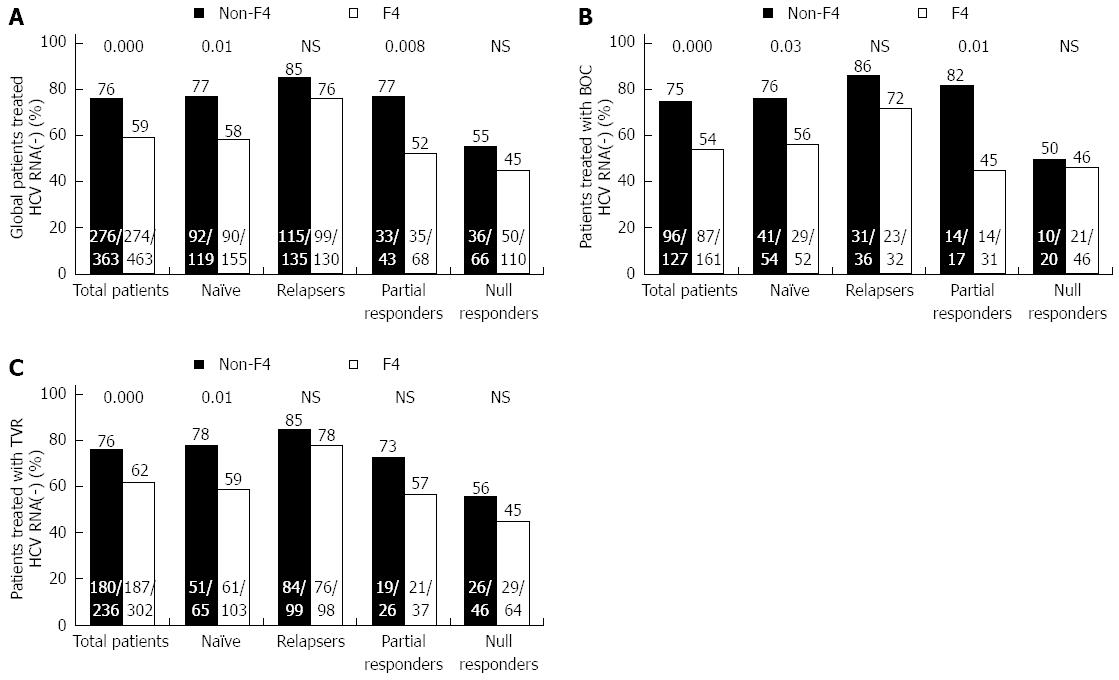
In response to treatment by type of fibrosis (non-F4 vs F4), the non-F4 patients achieved a higher SVR rate in the TN and PR patients (P = 0.01 and P = 0.008, respectively) (Figure 3A). The same pattern was observed in the patients given BOC (P = 0.03 and P = 0.01) (Figure 3B). However, among the patients given TVR, there were only differences among the TN patients (P = 0.01) (Figure 3C).
In the univariate and multivariate analysis (Table 3), taking into account the baseline characteristics of patients by ITT and by response to antiviral therapy, we found that the type of prior response, IL28B, liver fibrosis, GGT and treatment with PIs were all significantly associated with SVR rates. When the analysis was performed using the propensity score, treatment with PIs lost statistical significance (OR = 1.3, 95%CI: 0.8-2.05, P = 0.89). This outcome was confirmed in the group of patients by mITT, for whom treatment with PIs was not associated with SVR rates (OR = 1.1, 95%CI: 0.7-1.7, P = 0.77) (Table 3).
| ITT | mITT | |||||||||||
| Univariate | Multivariate | Univariate | Multivariate | |||||||||
| OR | 95%CI | P value | OR | 95%CI | P value | OR | 95%CI | P value | OR | 95%CI | P value | |
| Previous response1 | ||||||||||||
| R | 2.6 | 1.8-3.7 | 0.000 | 1.8 | 1.09-3.1 | 0.022 | 2.4 | 1.6-3.5 | 0.000 | 1.9 | 1.1-3.3 | 0.019 |
| PR | 0.9 | 0.6-1.3 | NS | 0.9 | 0.5-1.7 | NS | 0.9 | 0.6-1.3 | NS | 0.8 | 0.4-1.6 | NS |
| NR | 0.4 | 0.2-0.5 | 0.000 | 0.6 | 0.37-0.9 | 0.330 | 0.5 | 0.3-0.7 | 0.000 | 0.7 | 0.39-1.1 | NS |
| IL28B2 | ||||||||||||
| CC | 3.6 | 2.2-5.9 | 0.000 | 3.07 | 1.5-5.9 | 0.001 | 3.2 | 1.9-5.4 | 0.000 | 3.0 | 1.4-6.0 | 0.002 |
| CT | 1.8 | 1.2-2.6 | 0.003 | 1.6 | 1.001-2.6 | 0.049 | 1.8 | 1.2-2.8 | 0.004 | 1.6 | 0.9-2.7 | NS |
| Fibrosis3 | ||||||||||||
| Non-F4 | 2.1 | 1.6-2.8 | 0.000 | 2.0 | 1.33-3.0 | 0.001 | 2.26 | 1.7-3.04 | 0.000 | 2.1 | 1.4-3.3 | 0.000 |
| Treatment4 | ||||||||||||
| TVR | 1.7 | 1.3-2.1 | 0.000 | 1.5 | 1.02-2.2 | 0.038 | 1.1 | 0.9-1.5 | NS | 1.1 | 0.7-1.7 | NS |
| LogGGT | 0.19 | 0.12-0.3 | 0.000 | 0.3 | 0.18-0.6 | 0 | 0.2 | 0.15-0.23 | 0.000 | 0.4 | 0.2-0.7 | 0.004 |
| LogViral load | 0.8 | 0.6-1.003 | NS | 0.7 | 0.5-0.99 | 0.048 | 0.8 | 0.6-1.003 | NS | 0.7 | 0.4-1.1 | NS |
Twenty eight percent of the all patients group (263/952 patients, mITT) had to discontinue treatment, for various reasons, the most common of which was non-viral response (51%), followed by serious adverse effects (31%) (Table 4). By type of treatment (BOC vs TVR), there were no statistically significant differences. Non-viral response was greater in the TE than in the TN patients (57% vs 40%, P = 0.01), while there were more deaths in the TN group (8% vs 2%, P = 0.02). The following causes of death were recorded: two patients, cardiovascular problems; one patient, lung neoplasm unrelated to treatment; two patients, severe infection (pneumonia and salmonellosis respectively); one patient with non-F4 stage, hepatic decompensation; four patients with very advanced liver cirrhosis, multi-organ failure caused by severe anaemia, hepatic decompensation, hepatic encephalopathy, infection and digestive haemorrhage; one patient, unknown cause unrelated to treatment.
| All patients (n = 952) | TN (n = 298) | TE (n = 654) | |||||||||||
| All patients (n = 952) | TN (n = 298) | TE (n = 654) | P value | BOC (n = 321) | TVR (n = 631) | P value | BOC (n = 112) | TVR (n = 186) | P value | BOC (n = 209) | TVR (n = 445) | P value | |
| Stop treatment | 263/952 (28) | 97/298 (33) | 166/654 (25) | 0.022 | 88/321 (27) | 175/631 (28) | NS | 33/112 (30) | 64/186 (34) | NS | 55/209 (26) | 111/445 (25) | NS |
| Non-VR | 133/263 (51) | 39/97 (40) | 94/166 (57) | 0.010 | 48/88 (54) | 85/175 (49) | NS | 11/33 (33) | 28/64 (44) | NS | 37/55 (67) | 54/111 (51) | NS |
| Severe AE | 83/263 (31) | 31/97 (32) | 52/166 (31) | NS | 22/88 (25) | 61/175 (35) | NS | 10/33 (30) | 21/64 (33) | NS | 12/55 (22) | 40/111 (36) | NS |
| Abandonment | 6/263 (2) | 2/97 (2) | 4/166 (2) | NS | 4/88 (4) | 2/175 (1) | NS | 2/33 (6) | 0/64 (0) | NS | 2/55 (4) | 2/111 (2) | NS |
| Infection | 6/263 (2) | 2/97 (2) | 4/166 (2) | NS | 4/88 (4) | 2/175 (1) | NS | 2/33 (6) | 0/64 (0) | NS | 2/55 (4) | 2/111 (2) | NS |
| Exitus | 11/263 (4) | 8/97 (8) | 3/166 (2) | 0.020 | 3/88 (3) | 8/175 (5) | NS | 2/33 (6) | 6/64 (9) | NS | 1/55 (2) | 2/111 (2) | NS |
| Other | 14/263 (5) | 5/97 (5) | 9/166 (5) | NS | 3/88 (3) | 11/175 (6) | NS | 2/33 (6) | 3/64 (5) | NS | 1/55 (2) | 8/111 (7) | NS |
The adverse haematologic effects observed are shown in Table 5. With respect to neutrophils (n = 943), 25% of the patients had severe neutropaenia (grade 3-4), this being more common in the patients given BOC (33% vs 20%, P≤ 0.0001). Thrombocytopaenia occurred in the same proportion in patients given BOC and in those given TVR, although it was higher in the F4 than in the non-F4 patients (26% vs 8%, P≤ 0.0001). No significant differences were observed in the degree of anaemia among the patients treated with BOC or TVR; however, the fact that the proportion of patients with severe anaemia (grade 3-4) was greater in the TVR patients (10% vs 8%, P > 0.05), led to more of these patients receiving transfusions (P = 0.001) and EPO (P≤ 0.0001). Severe anaemia was also more frequent in the F4 patients than in the non-F4 patients (11% vs 7%, P = 0.025).
| All patients | Boceprevir | Telaprevir | P value | Non-F4 | F4 | P value | |
| Neutropaenia | n = 943 | n = 360 | n = 583 | n = 369 | n = 505 | ||
| Grade 0-1-2: | 707 (75) | 241 (67) | 466 (80) | 0 | 289 (78) | 370 (73) | NS |
| > 750 μL | |||||||
| Grade 3-4: | 236 (25) | 119 (33) | 117 (20) | 80 (22) | 135 (27) | ||
| ≤ 750 μL | |||||||
| Thrombocytopaenia | n = 935 | n = 359 | n = 576 | n = 365 | n = 501 | ||
| Grade 0-1-2: | 0.000 | ||||||
| > 50.000 μL | 753 (81) | 293 (82) | 460 (80) | NS | 337 (92) | 371 (74) | |
| Grade 3-4: | |||||||
| ≤ 50.000 μL | 182 (20) | 66 (18) | 116 (20) | 28 (8) | 130 (26) | ||
| Anaemia | n = 1036 | n = 397 | n = 639 | n = 411 | n = 539 | ||
| Grade 0-1-2: | 943 (91) | 367 (92) | 576 (90) | NS | 384 (93) | 481 (89) | 0.025 |
| > 8 g/dL | |||||||
| Grade 3-4: | 93 (9) | 30 (8) | 63 (10) | 27 (7) | 58 (11) | ||
| < 8 g/dL | |||||||
| Transfusion | n = 1036 | n = 397 | n = 639 | 0.001 | n = 411 | n = 539 | NS |
| 150 (15) | 39 (10) | 111 (17) | 49 (12) | 85 (16) | |||
| EPO | n = 1033 | n = 396 | n = 637 | 0 | n = 410 | n = 539 | NS |
| 218 (21) | 60 (15) | 158 (25) | 75 (18) | 117 (22) | |||
| Onset of anaemia ( ≤ 9.5 g/dL) | 12 ± 8 | 15 ± 11 | 9.5 ± 6.1 | 0 | 11.3 ± 7.2 | 11.5 ± 9 | NS |
The data presented in this study reflect the experience of standard clinical practice, in a cohort of 1057 patients with CHC genotype 1, treated with BOC or TVR in triple therapy. We analyse the effectiveness and safety profiles of triple therapy with first-generation PIs in combination with PEG-IFN/RBV, in both TN and TE patients, most of whom presented an advanced grade of fibrosis. The inclusion of patients in one or other of the treatment arms was decided by the physician, and for this reason more R patients were given TVR than BOC, while more NR patients were given BOC than TVR. The baseline characteristics were very similar among the two groups of patients, although the presence of liver fibrosis may have led to different SVR rates in the TN and PR groups, to different levels of albumin in the R and PR groups, and to different levels of GGT in the NR patients. The remaining variables did not present significant differences.
The SVR rates obtained were similar to those reported in previous clinical trials[19,20]. Thus, the R patients presented the highest SVR rates and the NR patients, the lowest[21]. The SVR rates were similar in all the subgroups of patients, for BOC and TVR by ITT and mITT, with the sole exception of the NR group by ITT. In a prospective analysis of 511 patients with liver cirrhosis, treated previously (the CUPIC study)[12], the SVR rates were lower than those obtained in our cohort, probably because these patients had a more advanced stage of liver disease. However, in the CUPIC study the proportion of patients with no response to previous treatment was 8% vs 25% in our study. Regarding the TN patients treated with BOC or TVR, our observed rates of SVR were also lower than those observed in another prospective study of 621 TN patients, in which 266 patients were treated with BOC or TVR, obtaining SVR rates of 76.6% and 71.1% respectively[22]. In our study, the SVR rates in the TN patients were closer to those achieved in an observational study of a cohort of 835 American Veterans patients[23]. The high proportion of F4 patients (56%) in our study is comparable to the observations obtained from a study of over 1500 TN and TE patients with advanced liver fibrosis and treated with TVR[14]. Analysis of the rate of SVR in response to treatment, according to the degree of fibrosis, showed that non-F4 patients responded better than F4 patients, thus confirming the negative relationship between response to treatment with PIs and the increasing severity of liver fibrosis[7,12,20]. In our cohort, the SVR of the non-F4 patients in the TN and PR groups was significantly related to the degree of hepatic fibrosis. In the R and NR patients, although the rate of SVR was lower in the F4 patients, the differences were not significant. The R patients presented the highest rate of SVR, both in the BOC group and in the TVR group, especially when these patients were non-F4 (85%). On the other hand, the NR patients were the poorest responders to treatment, although the overall rate of SVR was 55% in the non-F4 and 45% in the F4 patients. In general, the non-F4 patients achieved a high rate of SVR; however, this was not the case among the F4 patients, which suggests that triple therapy with BOC or TVR is less effective in these patients, especially if they are NR. The data concerning patients with advanced fibrosis are important, because very few F3 and F4 patients were included in the clinical trials register.
With regard to effectiveness, bivariate and multivariate analysis identified the following factors as predictors of SVR, both by ITT and by mITT: relapsers[21], the IL28B CC genotype[24,25], the non-F4 stage and logGGT. The type of treatment with PIs was statistically significant in favour of TVR in the bivariate and multivariate analysis by ITT but not in that by mITT. To examine on an equal basis the characteristics of patients treated with two different PIs, propensity score statistical analysis was applied to the results obtained[17,18]. Following this process, the type of treatment, BOC or TVR, lost statistical significance as a predictor of SVR. Therefore, the effectiveness obtained is similar with both of these first-generation PIs.
One of the major drawbacks in using first-generation PIs in combination with PEG-IFN/RBV is the increased presence of adverse effects[7,20,26]. More than a quarter of the patients in this study stopped treatment, this proportion being higher in the TN than in the TE patients. The most important reason for interrupting the treatment was the absence of virologic response in the TE patients. In the case of the TN patients, treatment interruption was due to the death of the patient in a significant number of cases, but this was a consequence of several patients with well advanced hepatic cirrhosis having been included by one of the participating hospitals. The other reasons, such as severe adverse effects, abandonment or other infection, were similar in both TN and TE patients and there were no differences between BOC and TVR.
Our analysis of haematologic alterations revealed that treatment with TVR led to higher levels of neutropaenia than that with BOC. Furthermore, the TVR patients required a larger number of blood transfusions and greater use of erythropoietin, even though levels of anaemia were similar in the two groups of patients. The sharper fall in haemoglobin in the patients treated with TVR, taking into account that the onset of anaemia was weaker in this group, may have led to the increased need for transfusions and for erythropoietin. Regarding thrombocytopaenia, there were no differences between BOC and TVR in clinical practice despite the longer duration of treatment with BOC with respect to TVR. The analysis of adverse effects according to the stage of fibrosis showed that the F4 patients had a higher degree of thrombocytopaenia and severe anaemia than the non-F4 patients.
These results reflect the experience of actual clinical practice in a population of patients with advanced fibrosis, and highlight the importance of such studies in providing more realistic data about the treatment of HCV, on a broad scale. The effectiveness of first-generation PIs is generally high, except for NR patients. Future treatments, especially those based on interferon-free regimens, will radically change the use of these drugs, which currently have a very significant rate of adverse effects.
The participating hospitals and the Principal Investigators, from the Alhambra Spanish Study Group, thank all the following for their contributions: Politécnico La Fe University Hospital, Valencia (Berenguer M, Prieto M); Virgen del Rocío University Hospital, Sevilla (Sousa JM, Cuaresma M, Ferrer MT); San Cecilio University Hospital, Granada (Escolano E); Nuestra Señora de Valme University Hospital, Sevilla (Millán R); Costa del Sol Healthcare Agency, Marbella, Málaga (Aguilar VM); Fundación Alcorcón University Hospital, Alcorcón, Madrid (Alonso S, Gutiérrez ML); Virgen de las Nieves University Hospital, Granada (López MA, Nogueras F); Dr. Negrín University Hospital, Gran Canaria (Martín JM); Zaragoza Clinical Hospital (Montero J, Simón MA); University Hospital Río Hortega, Valladolid (Sánchez G); University Hospital Miguel Servet, Zaragoza (Fuentes J); University Hospital Nuestra Señora de la Candelaria, Tenerife (González A, Amoros A, Pérez F), Hospital Torrecárdenas, Almería (Casado M, Peláez G, González M); Virgen de la Victoria University Hospital, Málaga (Ortega A, García M, Andrade R); University Hospital Salamanca, Salamanca (Martín MI); Complejo Hospitalario Jaén, Jaén (Baeyens E); Arnau Villanova Hospital, Valencia (González O); Virgen de la Concha Hospital, Zamora (Rodríguez S, Martín E, Conde P); University General Hospital Morales Meseguer, Murcia (Hallal H); Hospital Poniente, Almería (Estévez M, Jordan T); Clinic Xerencia Hospital, Santiago de Compostela (Molina E); University Hospital Rafael Méndez de Lorca, Murcia (Jurado A, Llamoza C); Hospital San Jorge de Huesca (Bernal V, Cortés L); Hospital Juan Ramón Jiménez, Huelva (Jiménez F, Vázquez JM); University Hospital Donosti, San Sebastián (Almanzor E, Arenas J); Santa Ana Hospital, Motril (Sousa FL); Puerta del Mar Hospital, Cádiz (Macías MA).
Triple therapy with first generation NS3/4A protease inhibitors (PIs), including boceprevir (BOC) or telaprevir (TVR) accounted for the standard of care for patients with chronic hepatitis C (CHC) genotype 1 infection. Few clinical trials have been conducted in patients with advanced fibrosis. Moreover, the analyses of clinical records provide useful, necessary information to objectively evaluate the effectiveness and safety of new treatments in routine clinical practice against hepatitis C virus (HCV) infection.
The present results show, the experience of actual clinical practice in a population of 1057 Spanish patients from 38 hospitals with advanced fibrosis, and the highlight the importance of such studies in providing more realistic data about the treatment of HCV, on a broad scale. The effectiveness of first-generation PIs was generally high, except for null responders (NR) patients. Future treatments, especially those based on interferon (IFN)-free regimens, will radically change the use of these drugs, which currently have a very significant rate of adverse effects.
This study represents a significant contribution to knowledge of the effectiveness and safety of the first generation NS3/4A PIs in patients with advanced fibrosis in routine clinical practice and contributes to the new treatment with inhibitors of second generation NS3/4A and IFN-free treatment.
This report is the largest real-practice analysis in Spain; it represents a nationwide registry with 1057 patients treated with HCV antiviral therapy with triple therapy containing 1st generation PIs BOC and TVR. These results could be useful to the medical community, especially in those countries were access to new direct agents antiviral may be restricted or non-accessible.
This manuscript by Salmerón et al described the effectiveness and safety of PI-based triple therapy in real-world cohorts including large numbers of patients with advanced liver disease (56% of patients with cirrhosis). The effectiveness of first-generation PIs was generally high, except for NR patients, and according to the degree of fibrosis, the non-F4 patients responded better than F4 patients.
P- Reviewer: Jeffrey GP, Mihm U, Silva G S- Editor: Yu J L- Editor: A E- Editor: Ma S
| 1. | Mohd Hanafiah K, Groeger J, Flaxman AD, Wiersma ST. Global epidemiology of hepatitis C virus infection: new estimates of age-specific antibody to HCV seroprevalence. Hepatology. 2013;57:1333-1342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1770] [Cited by in RCA: 1846] [Article Influence: 153.8] [Reference Citation Analysis (3)] |
| 2. | Dienstag JL, Ghany MG, Morgan TR, Di Bisceglie AM, Bonkovsky HL, Kim HY, Seeff LB, Szabo G, Wright EC, Sterling RK. A prospective study of the rate of progression in compensated, histologically advanced chronic hepatitis C. Hepatology. 2011;54:396-405. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 131] [Cited by in RCA: 132] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 3. | Perz JF, Armstrong GL, Farrington LA, Hutin YJ, Bell BP. The contributions of hepatitis B virus and hepatitis C virus infections to cirrhosis and primary liver cancer worldwide. J Hepatol. 2006;45:529-538. [PubMed] |
| 4. | Fried MW, Shiffman ML, Reddy KR, Smith C, Marinos G, Gonçales FL, Häussinger D, Diago M, Carosi G, Dhumeaux D. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med. 2002;347:975-982. [PubMed] |
| 5. | Manns MP, McHutchison JG, Gordon SC, Rustgi VK, Shiffman M, Reindollar R, Goodman ZD, Koury K, Ling M, Albrecht JK. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet. 2001;358:958-965. [PubMed] |
| 6. | Hézode C, Forestier N, Dusheiko G, Ferenci P, Pol S, Goeser T, Bronowicki JP, Bourlière M, Gharakhanian S, Bengtsson L. Telaprevir and peginterferon with or without ribavirin for chronic HCV infection. N Engl J Med. 2009;360:1839-1850. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 849] [Cited by in RCA: 794] [Article Influence: 49.6] [Reference Citation Analysis (0)] |
| 7. | Jacobson IM, McHutchison JG, Dusheiko G, Di Bisceglie AM, Reddy KR, Bzowej NH, Marcellin P, Muir AJ, Ferenci P, Flisiak R. Telaprevir for previously untreated chronic hepatitis C virus infection. N Engl J Med. 2011;364:2405-2416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1905] [Cited by in RCA: 1861] [Article Influence: 132.9] [Reference Citation Analysis (0)] |
| 8. | Poordad F, McCone J, Bacon BR, Bruno S, Manns MP, Sulkowski MS, Jacobson IM, Reddy KR, Goodman ZD, Boparai N. Boceprevir for untreated chronic HCV genotype 1 infection. N Engl J Med. 2011;364:1195-1206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1996] [Cited by in RCA: 1980] [Article Influence: 141.4] [Reference Citation Analysis (0)] |
| 9. | Chopra A, Klein PL, Drinnan T, Lee SS. How to optimize HCV therapy in genotype 1 patients: management of side-effects. Liver Int. 2013;33 Suppl 1:30-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 32] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 10. | Hézode C, Fontaine H, Dorival C, Larrey D, Zoulim F, Canva V, de Ledinghen V, Poynard T, Samuel D, Bourlière M. Triple therapy in treatment-experienced patients with HCV-cirrhosis in a multicentre cohort of the French Early Access Programme (ANRS CO20-CUPIC) - NCT01514890. J Hepatol. 2013;59:434-441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 359] [Cited by in RCA: 362] [Article Influence: 30.2] [Reference Citation Analysis (0)] |
| 11. | Belperio PS, Hwang EW, Thomas IC, Mole LA, Cheung RC, Backus LI. Early virologic responses and hematologic safety of direct-acting antiviral therapies in veterans with chronic hepatitis C. Clin Gastroenterol Hepatol. 2013;11:1021-1027. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 12. | Hézode C, Fontaine H, Dorival C, Zoulim F, Larrey D, Canva V, De Ledinghen V, Poynard T, Samuel D, Bourliere M. Effectiveness of telaprevir or boceprevir in treatment-experienced patients with HCV genotype 1 infection and cirrhosis. Gastroenterology. 2014;147:132-142.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 201] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 13. | Available from: https: //www.msssi.gob.es/profesionales/farmacia/pdf/TRATAMIENTO_HEPATITIS_CRONICA_C.pdf 2012. |
| 14. | Colombo M, Fernández I, Abdurakhmanov D, Ferreira PA, Strasser SI, Urbanek P, Moreno C, Streinu-Cercel A, Verheyen A, Iraqi W. Safety and on-treatment efficacy of telaprevir: the early access programme for patients with advanced hepatitis C. Gut. 2014;63:1150-1158. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 38] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 15. | Calleja JL, Pascasio JM, Ruiz-Antorán B, Gea F, Bárcena R, Larrubia JR, Pérez-Álvarez R, Sousa JM, Romero-Gómez M, Solá R. Safety and efficacy of triple therapy with peginterferon, ribavirin and boceprevir within an early access programme in Spanish patients with hepatitis C genotype 1 with severe fibrosis: SVRw12 analysis. Liver Int. 2015;35:90-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 16. | Castéra L, Vergniol J, Foucher J, Le Bail B, Chanteloup E, Haaser M, Darriet M, Couzigou P, De Lédinghen V. Prospective comparison of transient elastography, Fibrotest, APRI, and liver biopsy for the assessment of fibrosis in chronic hepatitis C. Gastroenterology. 2005;128:343-350. [PubMed] |
| 17. | D’Agostino RB. Propensity scores in cardiovascular research. Circulation. 2007;115:2340-2343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 363] [Cited by in RCA: 377] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 18. | Austin PC. An Introduction to Propensity Score Methods for Reducing the Effects of Confounding in Observational Studies. Multivariate Behav Res. 2011;46:399-424. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6382] [Cited by in RCA: 7487] [Article Influence: 534.8] [Reference Citation Analysis (0)] |
| 19. | Bacon BR, Gordon SC, Lawitz E, Marcellin P, Vierling JM, Zeuzem S, Poordad F, Goodman ZD, Sings HL, Boparai N. Boceprevir for previously treated chronic HCV genotype 1 infection. N Engl J Med. 2011;364:1207-1217. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1306] [Cited by in RCA: 1308] [Article Influence: 93.4] [Reference Citation Analysis (0)] |
| 20. | Zeuzem S, Andreone P, Pol S, Lawitz E, Diago M, Roberts S, Focaccia R, Younossi Z, Foster GR, Horban A. Telaprevir for retreatment of HCV infection. N Engl J Med. 2011;364:2417-2428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1230] [Cited by in RCA: 1214] [Article Influence: 86.7] [Reference Citation Analysis (0)] |
| 21. | Bonnet D, Guivarch M, Bérard E, Combis JM, Remy AJ, Glibert A, Payen JL, Metivier S, Barange K, Desmorat H. Telaprevir- and boceprevir-based tritherapies in real practice for F3-F4 pretreated hepatitis C virus patients. World J Hepatol. 2014;6:660-669. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 22. | Mangia A, Cenderello G, Orlandini A, Piazzolla V, Picciotto A, Zuin M, Ciancio A, Brancaccio G, Forte P, Carretta V. Individualized treatment of genotype 1 naïve patients: an Italian multicenter field practice experience. PLoS One. 2014;9:e110284. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 23. | Backus LI, Belperio PS, Shahoumian TA, Cheung R, Mole LA. Comparative effectiveness of the hepatitis C virus protease inhibitors boceprevir and telaprevir in a large U.S. cohort. Aliment Pharmacol Ther. 2014;39:93-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 45] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 24. | Bruno S, Vierling JM, Esteban R, Nyberg LM, Tanno H, Goodman Z, Poordad F, Bacon B, Gottesdiener K, Pedicone LD. Efficacy and safety of boceprevir plus peginterferon-ribavirin in patients with HCV G1 infection and advanced fibrosis/cirrhosis. J Hepatol. 2013;58:479-487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 47] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 25. | Pol S, Aerssens J, Zeuzem S, Andreone P, Lawitz EJ, Roberts S, Younossi Z, Foster GR, Focaccia R, Horban A. Limited impact of IL28B genotype on response rates in telaprevir-treated patients with prior treatment failure. J Hepatol. 2013;58:883-889. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 48] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 26. | Wehmeyer MH, Eißing F, Jordan S, Röder C, Hennigs A, Degen O, Hüfner A, Hertling S, Schmiedel S, Sterneck M. Safety and efficacy of protease inhibitor based combination therapy in a single-center “real-life” cohort of 110 patients with chronic hepatitis C genotype 1 infection. BMC Gastroenterol. 2014;14:87. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |