Published online Aug 14, 2015. doi: 10.3748/wjg.v21.i30.9126
Peer-review started: January 16, 2015
First decision: March 10, 2015
Revised: April 6, 2015
Accepted: May 27, 2015
Article in press: May 27, 2015
Published online: August 14, 2015
Processing time: 213 Days and 18 Hours
AIM: To investigate the nature and origin of cardiac mucosa (CM).
METHODS: Biopsy samples from sixty-one individuals were included in this study. The specimens were taken “at”, “just below”, or “just above” the gastroesophageal junction, including the histologic squamocolumnar junction. Clinical data were obtained by reviewing electronic medical records for each patient. Patients with a history of stomach adenoma or carcinoma and esophageal carcinoma were excluded, and cases that were endoscopically suspicious of Barrett’s esophagus or a polyp were also ruled out. Histologic and endoscopic reviews were performed blinded to the patient’s clinical data. Histologic evaluation was conducted by two pathologists, and endoscopic review was performed by a endoscopist with wide experience in the field. Histologically, the columnar epithelium of squamocolumnar junction, presence and severity of acute and chronic inflammation, atrophy, intestinal metaplasia, and presence of carditis were evaluated. Endoscopically, reflux esophagitis was evaluated by Los Angeles (LA) classification, hiatal hernias were classified by Hill grade, and gastroesophageal flap valves were assessed.
RESULTS: Fifty-nine of the 61 (96.7%) patients were Korean; 65.6% (40/61) of the patients underwent endoscopy according to the schedule of the National Health Insurance Program as a screening inspection. Of these, only 20.0% (8/40) of cases had reflux symptoms. CM was present in 41/61 (67.2%) individuals, and its presence was associated with older age compared to oxyntocardiac mucosa/oxyntic mucosa (60.59 ± 2.02 years vs 51.55 ± 3.35 years; P = 0.018). The presence of CM was associated with endoscopic diagnosis of esophagitis according to the LA classification (P = 0.022). CM was associated with mononuclear cell infiltration and neutrophilic infiltration, which were statistically significant (P = 0.001, and P = 0.004, respectively). The inflammation of CM, “carditis”, showed a statistically significant association with endoscopic diagnosis of reflux esophagitis according to the LA classification (P = 0.008).
CONCLUSION: CM at the gastroesophageal junction is a common histologic finding in biopsy specimens, though not always present, and associated with gastroesophageal reflux disease and carditis severity.
Core tip: Incidence of gastroesophageal reflux disease and gastroesophageal junction (GEJ) adenocarcinomas is increasing in Asia, though with a lower prevalence than Western countries. The existence and origin of cardiac mucosa (CM) at the GEJ is debated, but most data were from a Western population. This study shows that CM at the GEJ is a common histologic finding in a chiefly Korean population, and 4.4% showed direct continuity of oxyntic mucosa and squamous epithelium even in single biopsy specimens. CM was associated with gastroesophageal reflux disease and carditis severity, suggesting CM may be an acquired structure and is associated with reflux stimuli, similar to results from Western populations.
- Citation: Kim A, Park WY, Shin N, Lee HJ, Kim YK, Lee SJ, Hwang CS, Park DY, Kim GH, Lee BE, Jo HJ. Cardiac mucosa at the gastroesophageal junction: An Eastern perspective. World J Gastroenterol 2015; 21(30): 9126-9133
- URL: https://www.wjgnet.com/1007-9327/full/v21/i30/9126.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i30.9126
The incidence of gastroesophageal reflux disease (GERD) and gastroesophageal junction (GEJ) adenocarcinomas is increasing worldwide, especially in Asia[1,2], though the prevalence remains lower than that in the United States and Europe. The existence and origin of cardiac mucosa (CM) at the GEJ is one of the most hotly debated topics among gastroenterologists and pathologists. There are two main opinions about the nature and the origin of the CM at the GEJ: one is that cardia and CM are a normal finding[3-5], and the other is that CM is not a normal mucosa but rather a metaplastic response to reflux stimuli[6-10]. In our previous study, we suggested that CM originated from the distal esophagus and the presence of CM is a marker of GERD in esophagectomy specimens in the Korean population[11]. In general, distal esophageal squamous epithelium, which cannot tolerate the acidic proteolytic environment, is consistently exposed to reflux damage during a patient’s lifetime[12]. The esophagus adapts to the reflux stimuli by metaplasia, converting into columnar epithelium. Furthermore, there are reports suggesting there is an association between CM and GERD[6,10,13], and still others showing different results associating carditis (inflammation of CM) and GERD[14-16]. Recently, the prospective histoGERD trial[17], which analyzed biopsy specimens, revealed that the presence of CM is associated with symptoms and histologic changes of GERD and endoscopic diagnosis of esophagitis. However, most of these data were from Western countries where there is a high prevalence of GERD. With this background, we investigated the histopathologic nature of GEJ and CM in a single institution in South Korea. Our data suggest that CM is an indicator of GERD.
The tissue samples of GEJ were obtained by endoscopic biopsy carried out at the Pusan National University Hospital, South Korea, from 2011 to June of 2014. To select the specimens from GEJs, a statistics program in an electronic medical record system of Pusan National University Hospital was used. First, using the search phrase “gastroesophageal junction”, we identified 345 applicable cases. Sixteen cases were excluded from the analysis because they had a history of esophageal cancer, stomach cancer, or stomach adenoma. The endoscopic findings were reviewed to precisely select cases in which samples were obtained only “at”, “just below”, or “just above” the GEJ, and to exclude cases that were clinically polyp lesions. This resulted in an additional 35 cases being excluded. Thirteen cases endoscopically suspicious of Barrett’s esophagus were also excluded. Finally, the histologic features were reviewed by two pathologists. Only samples containing histologic squamocolumnar junctions (SCJs) were included; thus, an additional 174 cases were excluded. Cases with only surface epithelium without a glandular portion were excluded, and samples with marked acute inflammation or ulcer were also excluded. Thus, the final study cohort consisted of 61 individuals (see Figure 1) with biopsy samples obtained from the GEJ with histologic SCJ, and proper amounts of glandular components.
All endoscopists had wide experience in the field. The esophagus, GEJ, and stomach of all patients were examined. For endoscopy, a single channel endoscope (GIF-H260 or GIF-Q260; Olympus Co. Ltd., Tokyo, Japan) was used. A highly experienced endoscopist, performing over 500 endoscopies per year for over a decade, reviewed all photographs captured during the endoscopy. In addition, the endoscopist was blind to the clinical and histologic data. The presence or absence of reflux esophagitis and hiatal hernia was evaluated, and the gastroesophageal flap valve (GEFV), which reflects reflux, was graded retrospectively according to the criteria indicated below.
The GEJ was defined as the oral side end of the fold, which is present continuously from the gastric lumen[18], as well as the end of the anal side of the fine longitudinal vessel, because the veins in the lower part of the esophagus were distributed uniformly, running parallel and longitudinally in the lamina propria[19,20].
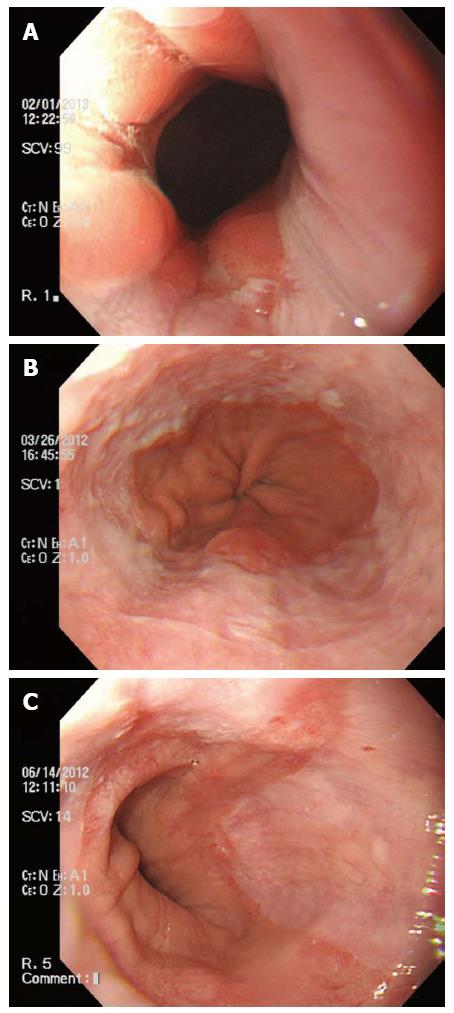
If esophagitis was present, it was graded according to the Los Angeles (LA) classification[21,22], which focuses not only on the extent of mucosal breaks but also on minimal changes (see Figure 2). All categories but 0, by the LA classification, were considered as reflux esophagitis.
Hiatal hernia was defined as a circular extension of the gastric mucosa above the diaphragmatic hiatus > 2 cm in the axial length[23].
The GEJ was viewed using a retroflexed endoscope during gastric inflation and GEFV was graded by the recently described grading system by Hill et al[24]. The GEFV is largely classified into two groups: the normal appearance (grades I and II) group and the reflux appearance (grades III and IV) group.
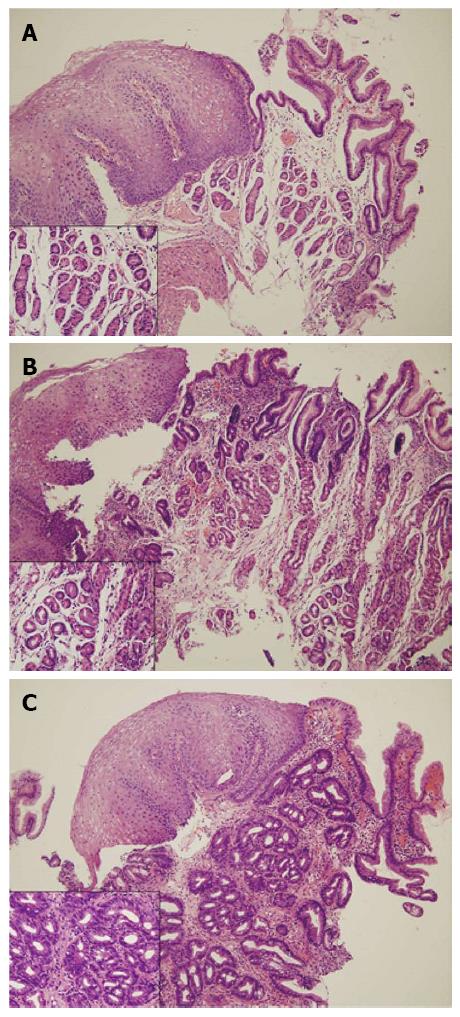
All samples were fixed in 10% buffered formalin, embedded in paraffin, cut at a minimum of four levels, and stained with hematoxylin and eosin. All biopsy samples were assessed by two pathologists who were blinded to clinical data and endoscopic findings. The columnar epithelium of the SCJ was classified according to the type of glandular component present (see Figure 3) as follows: (1) oxyntic mucosa (OM); (2) oxyntocardiac mucosa (OCM); or (3) CM. The presence and severity of inflammation, atrophy, and intestinal metaplasia were evaluated based on the updated Sydney system for evaluation of gastritis[25]. Cardiac mucosal inflammation (carditis) was defined by the presence of neutrophils in the lamina propria or glands (mild or more than mild infiltration of neutrophils by Sydney classification), or the presence of plasma cells, lymphocytes, and eosinophils in the lamina propria (moderate or more than moderate infiltration of mononuclear cells by the Sydney classification). The presence of pancreatic acinar cells, defined as small clusters or lobules of epithelial cells similar to pancreatic acinar cells in CM[26], was also evaluated.
Statistical comparisons were made between: (1) patients with CM at GEJ and patients with OM or OCM at GEJ; and (2) patients with carditis and patients without carditis. The data were analyzed for differences between groups by Student’s t or χ2 tests. Logistic regression was used for multivariate analysis. P < 0.05 was considered statistically significant. Statistical calculations were performed using SPSS version 10.0 for Windows (SPSS Inc., Chicago, IL, United States).
The biomedical statistical review of this study was performed by Jinmi Kim, PhD, a research professor in the Department of Biostatistics at the Clinical Trial Center, Pusan National University Hospital.
The patients ranged in age from 29 years to 80 years (mean age 57.62 years, median age 59.00 years). Fifty-nine of the 61 (96.7%) patients were Korean, and the other two patients were Russian. The presence of CM was significantly associated with older age compared with OM/OCM (60.59 ± 2.017 years vs 51.55 ± 3.353 years; P = 0.018).
CM was found in 41/61 cases (67.2%), and OCM and OM were found in 17/61 (27.9%) cases and 3/61 (4.9%) cases, respectively. These data indicate that in at least 32.8% of the cases, there was no circumferential presence of CM. Pancreatic acinar cells were found in 6/61 (9.8%) cases, and the esophageal gland duct was present in 1/61 (1.6%) cases.
Regarding the clinicopathologic significance of CM in terms of GERD, Table 1 describes the relationship between the type of mucosa and endoscopic findings. The presence of CM was significantly associated with endoscopic diagnosis of reflux esophagitis according to the modified LA classification (P = 0.022). Furthermore, Table 2 shows that the presence of CM was significantly associated with chronic inflammation (moderate or marked infiltration of mononuclear cells in lamina propria) and activity of inflammation (neutrophilic infiltration in lamina propria and/or glands) (P = 0.001 and P = 0.004, respectively). Table 3 shows the relationship between the presence of carditis and the endoscopic findings. The presence of carditis was associated with endoscopic diagnosis of reflux esophagitis according to the modified LA classification (P = 0.008). On multivariate analysis, mononuclear cell infiltration reflecting chronic inflammation was significantly associated with CM (P = 0.049), and neutrophilic infiltration, which indicates an acute inflammatory condition, was associated with presence of CM, though it was not statistically significant (P = 0.057) (Table 4).
| Assessment | Mucosa type | ||
| OM/OCM (n = 20) | CM (n = 41) | P value | |
| Los Angeles classification | |||
| N | 15 (75.0) | 18 (43.9) | 0.022 |
| M, A, B, C, D | 5 (25.0) | 23 (56.1) | |
| Hill grade | |||
| Normal (1, 2) | 16 (80.0) | 27 (65.9) | 0.255 |
| Reflux (3, 4) | 4 (20.0) | 14 (34.1) | |
| Hiatal hernia | |||
| Absent | 15 (75.0) | 22 (53.7) | 0.109 |
| Present | 5 (25.0) | 19 (46.3) | |
| Assessment | Mucosa type | ||
| OM/OCM (n = 20) | CM (n = 41) | P value | |
| Mononuclear cell infiltration | |||
| Mild | 11 (55.0) | 6 (14.6) | 0.001 |
| Moderate, marked | 9 (45.0) | 35 (85.4) | |
| Neutrophil infiltration | |||
| Absent | 17 (85.0) | 19 (46.3) | 0.004 |
| Mild, moderate, marked | 3 (15.0) | 22 (53.7) | |
| Intestinal metaplasia | |||
| Absent | 17 (85.0) | 28 (68.3) | 0.164 |
| Present | 3 (15.0) | 13 (31.7) | |
| Helicobacter pylori infection | |||
| Absent | 16 (80.0) | 33 (80.5) | 0.964 |
| Present | 4 (20.0) | 8 (19.5) | |
| Assessment | Carditis | ||
| Absent (n = 24) | Present (n = 37) | P value | |
| Los Angeles classification | |||
| N | 18 (75.0) | 15 (40.5) | 0.008 |
| M, A, B, C, D | 6 (25.0) | 22 (59.5) | |
| Hill grade | |||
| Normal (1, 2) | 20 (83.3) | 23 (62.2) | 0.077 |
| Reflux (3, 4) | 4 (16.7) | 14 (37.8) | |
| Hiatal hernia | |||
| Absent | 17 (70.8) | 20 (54.1) | 0.190 |
| Present | 7 (29.2) | 17 (45.9) | |
| Helicobacter pylori infection | |||
| Absent | 20 (83.3) | 29 (78.4) | 0.634 |
| Present | 4 (16.7) | 8 (21.6) | |
| Assessment | Adjusted odds ratio | 95%CI | P value |
| Mononuclear cell infiltration | 4.230 | 1.003-17.833 | 0.049 |
| Neutrophil infiltration | 4.296 | 0.958-19.267 | 0.057 |
| Los Angeles classification | 2.969 | 0.771-11.430 | 0.114 |
| Age | 1.038 | 0.989-1.089 | 0.130 |
Interestingly, the presence of pancreatic acinar cells was significantly associated with hiatal hernia (P = 0.039), but there was no association with the presence of pancreatic acinar cells and endoscopic reflux esophagitis, intestinal metaplasia, or Helicobacter pylori (H. pylori) infection (data not shown, P = 0.130, P = 0.163, P = 0.202, respectively). The esophageal gland duct was present in one case, and the duct was present among the glandular component.
The results of this study demonstrate that the presence of CM at the GEJ is significantly associated with the presence of GERD and severity of carditis. Multivariate analysis revealed that a chronic inflammatory condition is associated with presence of CM, suggesting that the presence of CM reflects the reflux damage and can thus be considered as an indicator of GERD. The data might indirectly support evidence of a rising incidence of GERD or adenocarcinomas of the GEJ and proximal stomach in Asia, though these results are based on a small retrospective dataset from a single institution.
We previously analyzed 30 esophagogastrectomy specimens[11] and found that CM was present circumferentially in 66.7% of cases. So, 33.3% of the cases had direct continuity of OM and squamous epithelium. In the present study, even with biopsy samples, three cases (4.9%) showed direct continuity of OM and squamous epithelium, even though the samples were not obtained systematically around the GEJ. A significant association between CM and older age was also observed.
The Korean Ministry of National Health and Welfare provide nationwide health medical examinations with national health insurance. This includes biennial endoscopic examinations targeting adults aged more than 40 years. In the current study, 65.6% of patients underwent endoscopy according to the schedule of the National Health Insurance Program as a screening inspection. Of these, only 20.0% (8/40) of cases had any reflux symptoms.
The cause of carditis is a controversial topic. Currently, it is thought to result from gastroesophageal reflux or a proximal extension of H. pylori infection from the remnant of the stomach. Der et al[14] reported that acute and chronic inflammation of the CM had different etiologic factors, being distal gastritis and H. pylori infection, and acid reflux, respectively. In addition, there are reports elucidating the etiology of carditis by status of H. pylori infection[15] and presence or absence of chronic gastritis of the remainder stomach[27]. The present study was performed retrospectively, and samples from the remainder stomach were not available for evaluation, so we could not evaluate the possibility of gastritis involving GEJ and the status of H. pylori infection of the remainder stomach. Although we could not determine the cause of carditis, all of these findings indirectly suggest that CM itself reflects the inflammatory condition associated with reflux stimuli. Regarding the significance of CM in GEJ, Chandrasoma et al[13] reported that the squamo-oxyntic gap (OCM ± CM ± intestinal epithelium between squamous epithelium and the OM) is equivalent to the columnar-lined esophagus, and its presence is an indicator of reflux, defining the presence of intestinal metaplasia within the squamo-oxyntic gap as Barrett’s esophagus. According to their criteria, 26.2% (16/61) of the cases in the present study can be classified as Barrett’s esophagus.
The origin and the significance of pancreatic acinar cells are variably reported in the literature as being a congenital structure[28] or metaplastic elements[29] related to GERD. In this study, the presence of pancreatic acinar cells was associated with hiatal hernia, but there was no association with endoscopic diagnosis of reflux esophagitis, reflux appearance of GEFV, carditis, or H. pylori infection (data not shown). The nature and significance of the pancreatic acinar cells at the GEJ should be further defined.
The results of this study provide data supporting the notion that the CM may be an acquired structure and is associated with reflux stimuli, similar to results derived from Western populations. This study has many limitations, mainly because we were unable to evaluate the histologic findings in the esophagus and remainder stomach because the study was performed retrospectively. Prospective, systematic, and multicenter studies are required to confirm the data presented herein.
In Asia, the prevalence of gastroesophageal reflux disease and gastroesophageal junction (GEJ) adenocarcinoma is lower than Western population, but the incidence is increasing. The existence and origin of cardiac mucosa (CM) at the GEJ is a hotly debated topic, and there are two main opinions about the nature and the origin of CM at the GEJ: that it is a normal congenital structure or a metaplastic response to reflux stimuli. However, most of the data are from Western countries.
Histologically, CM was a common finding but 3/61 (4.9%) cases had oxyntic mucosa (OM) even in a single biopsy sample, which means they had direct continuity of squamous epithelium and OM. The association between an inflammatory condition and presence of CM at the GEJ suggests that CM may be an acquired structure associated with reflux stimuli, which is a similar result to Western datasets.
The present study could not evaluate the possibility of extension of distal gastritis or impact of Helicobacter pylori infection, as the authors performed a retrospective study and the biopsy procedure was not performed systematically. However, the absence of CM (meaning oxyntocardiac mucosa or OM at the GEJ) even in single biopsy samples reinforces that CM is not located circumferentially, which indirectly suggests that CM may not be a normal congenital structure. Furthermore, the association between severity of inflammation and presence of CM supports the idea that the presence of CM at the GEJ can be an indicator of reflux stimuli. Also, according to the criteria used by Chandrasoma et al, 26.2% (16/61) of cases in the present study belong to Barrett’s esophagus, which may explain the rising incidence of gastroesophageal reflux disease and GEJ adenocarcinoma in Asia.
The presence of CM at the GEJ can be a histologic indicator of reflux stimuli. The results of this study indicate the need to perform a multicenter and prospective study to better elucidate the nature and origin of CM at GEJ.
The columnar epithelium of squamocolumnar junction was classified according to the type of glandular component: (1) OM composed entirely of parietal and chief cells without any mucous cells below the foveolar region; (2) oxyntocardiac mucosa, which contains a mixture of mucous cells and parietal cells; and (3) CM composed entirely of mucous cells without any parietal cells.
Well-written, it’s better to add demographic data of the patients and multivariate analysis.
P- Reviewer: Lamarca A S- Editor: Yu J L- Editor: AmEditor E- Editor: Zhang DN
| 1. | Kusano C, Gotoda T, Khor CJ, Katai H, Kato H, Taniguchi H, Shimoda T. Changing trends in the proportion of adenocarcinoma of the esophagogastric junction in a large tertiary referral center in Japan. J Gastroenterol Hepatol. 2008;23:1662-1665. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 193] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 2. | Park JJ, Kim JW, Kim HJ, Chung MG, Park SM, Baik GH, Nah BK, Nam SY, Seo KS, Ko BS. The prevalence of and risk factors for Barrett’s esophagus in a Korean population: A nationwide multicenter prospective study. J Clin Gastroenterol. 2009;43:907-914. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 53] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 3. | Kilgore SP, Ormsby AH, Gramlich TL, Rice TW, Richter JE, Falk GW, Goldblum JR. The gastric cardia: fact or fiction? Am J Gastroenterol. 2000;95:921-924. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 4. | Glickman JN, Fox V, Antonioli DA, Wang HH, Odze RD. Morphology of the cardia and significance of carditis in pediatric patients. Am J Surg Pathol. 2002;26:1032-1039. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 58] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 5. | De Hertogh G, Van Eyken P, Ectors N, Tack J, Geboes K. On the existence and location of cardiac mucosa: an autopsy study in embryos, fetuses, and infants. Gut. 2003;52:791-796. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 36] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 6. | Chandrasoma PT, Lokuhetty DM, Demeester TR, Bremmer CG, Peters JH, Oberg S, Groshen S. Definition of histopathologic changes in gastroesophageal reflux disease. Am J Surg Pathol. 2000;24:344-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 107] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 7. | Park YS, Park HJ, Kang GH, Kim CJ, Chi JG. Histology of gastroesophageal junction in fetal and pediatric autopsy. Arch Pathol Lab Med. 2003;127:451-455. [PubMed] |
| 8. | Chandrasoma PT, Der R, Ma Y, Dalton P, Taira M. Histology of the gastroesophageal junction: an autopsy study. Am J Surg Pathol. 2000;24:402-409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 141] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 9. | Oberg S, Peters JH, DeMeester TR, Chandrasoma P, Hagen JA, Ireland AP, Ritter MP, Mason RJ, Crookes P, Bremner CG. Inflammation and specialized intestinal metaplasia of cardiac mucosa is a manifestation of gastroesophageal reflux disease. Ann Surg. 1997;226:522-30; discussion 530-2. [PubMed] |
| 10. | Lenglinger J, Ringhofer C, Eisler M, Sedivy R, Wrba F, Zacherl J, Cosentini EP, Prager G, Haefner M, Riegler M. Histopathology of columnar-lined esophagus in patients with gastroesophageal reflux disease. Wien Klin Wochenschr. 2007;119:405-411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 11. | Kim A, Shin N, Lee HJ, Jo HJ, Kim JY, Kim YK, Park do Y, Park WY, I H, Kim GH. Histopathological features of the gastroesophageal junction: an Eastern view. Histol Histopathol. 2015;30:689-695. [PubMed] |
| 12. | Johnson LF, Demeester TR. Twenty-four-hour pH monitoring of the distal esophagus. A quantitative measure of gastroesophageal reflux. Am J Gastroenterol. 1974;62:325-332. [PubMed] |
| 13. | Chandrasoma P, Wijetunge S, Demeester SR, Hagen J, Demeester TR. The histologic squamo-oxyntic gap: an accurate and reproducible diagnostic marker of gastroesophageal reflux disease. Am J Surg Pathol. 2010;34:1574-1581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 35] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 14. | Der R, Tsao-Wei DD, Demeester T, Peters J, Groshen S, Lord RV, Chandrasoma P. Carditis: a manifestation of gastroesophageal reflux disease. Am J Surg Pathol. 2001;25:245-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 82] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 15. | Goldstein NS, Karim R. Gastric cardia inflammation and intestinal metaplasia: associations with reflux esophagitis and Helicobacter pylori. Mod Pathol. 1999;12:1017-1024. [PubMed] |
| 16. | Goldblum JR, Vicari JJ, Falk GW, Rice TW, Peek RM, Easley K, Richter JE. Inflammation and intestinal metaplasia of the gastric cardia: the role of gastroesophageal reflux and H. pylori infection. Gastroenterology. 1998;114:633-639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 176] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 17. | Langner C, Schneider NI, Plieschnegger W, Schmack B, Bordel H, Höfler B, Eherer AJ, Wolf EM, Rehak P, Vieth M. Cardiac mucosa at the gastro-oesophageal junction: indicator of gastro-oesophageal reflux disease? Data from a prospective central European multicentre study on histological and endoscopic diagnosis of oesophagitis (histoGERD trial). Histopathology. 2014;65:81-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 18. | Nandurkar S, Talley NJ. Barrett’s esophagus: the long and the short of it. Am J Gastroenterol. 1999;94:30-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 19. | Vianna A, Hayes PC, Moscoso G, Driver M, Portmann B, Westaby D, Williams R. Normal venous circulation of the gastroesophageal junction. A route to understanding varices. Gastroenterology. 1987;93:876-889. [PubMed] |
| 20. | Noda T. Angioarchitectural study of esophageal varices. With special reference to variceal rupture. Virchows Arch A Pathol Anat Histopathol. 1984;404:381-392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 46] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 21. | Lundell LR, Dent J, Bennett JR, Blum AL, Armstrong D, Galmiche JP, Johnson F, Hongo M, Richter JE, Spechler SJ. Endoscopic assessment of oesophagitis: clinical and functional correlates and further validation of the Los Angeles classification. Gut. 1999;45:172-180. [PubMed] |
| 22. | Hongo M. Minimal changes in reflux esophagitis: red ones and white ones. J Gastroenterol. 2006;41:95-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 92] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 23. | Kahrilas PJ, Kim HC, Pandolfino JE. Approaches to the diagnosis and grading of hiatal hernia. Best Pract Res Clin Gastroenterol. 2008;22:601-616. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 330] [Cited by in RCA: 254] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 24. | Hill LD, Kozarek RA, Kraemer SJ, Aye RW, Mercer CD, Low DE, Pope CE. The gastroesophageal flap valve: in vitro and in vivo observations. Gastrointest Endosc. 1996;44:541-547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 347] [Cited by in RCA: 304] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 25. | Dixon MF, Genta RM, Yardley JH, Correa P. Classification and grading of gastritis. The updated Sydney System. International Workshop on the Histopathology of Gastritis, Houston 1994. Am J Surg Pathol. 1996;20:1161-1181. [PubMed] |
| 26. | Schneider NI, Plieschnegger W, Geppert M, Wigginghaus B, Höss GM, Eherer A, Wolf EM, Rehak P, Vieth M, Langner C. Pancreatic acinar cells--a normal finding at the gastroesophageal junction? Data from a prospective Central European multicenter study. Virchows Arch. 2013;463:643-650. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 27. | Voutilainen M, Färkkilä M, Mecklin JP, Juhola M, Sipponen P. Chronic inflammation at the gastroesophageal junction (carditis) appears to be a specific finding related to Helicobacter pylori infection and gastroesophageal reflux disease. The Central Finland Endoscopy Study Group. Am J Gastroenterol. 1999;94:3175-3180. [PubMed] |
| 28. | Wang HH, Zeroogian JM, Spechler SJ, Goyal RK, Antonioli DA. Prevalence and significance of pancreatic acinar metaplasia at the gastroesophageal junction. Am J Surg Pathol. 1996;20:1507-1510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 43] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 29. | Doglioni C, Laurino L, Dei Tos AP, De Boni M, Franzin G, Braidotti P, Viale G. Pancreatic (acinar) metaplasia of the gastric mucosa. Histology, ultrastructure, immunocytochemistry, and clinicopathologic correlations of 101 cases. Am J Surg Pathol. 1993;17:1134-1143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 45] [Article Influence: 1.4] [Reference Citation Analysis (0)] |