Published online Aug 14, 2015. doi: 10.3748/wjg.v21.i30.9079
Peer-review started: March 11, 2015
First decision: April 24, 2015
Revised: May 9, 2015
Accepted: June 9, 2015
Article in press: June 10, 2015
Published online: August 14, 2015
Processing time: 161 Days and 21.4 Hours
AIM: To investigate the hepatoprotective effects and mechanisms of an extract of Salvia miltiorrhiza and Carthamus tinctorius in vivo.
METHODS: C57BL/6J mice were randomly assigned to five groups and intraperitoneally administered 0.9% saline, Salvia miltiorrhiza and Carthamus tinctorius extract [Danhong injection (DHI), 0.75 and 3 g/kg mixed extract] or reduced glutathione for injection (RGI, 300 mg/kg) for 30 min before exposure to lipopolysaccharide (LPS, 16 mg/kg). After intraperitoneal LPS stimulation for 90 min or 6 h, the mice were sacrificed by ether anaesthesia, and serum and liver samples were collected. Histological analysis (H&E) and terminal deoxynucleotidyl transferase-mediated dUTP nick end-labelling (TUNEL) staining were performed. Alanine transferase (ALT), aspartate transaminase (AST), total bilirubin (TBil), glutathione-S-transferase (GST), malondialdehyde (MDA), tumour necrosis factor (TNF)-α, interleukin (IL)-6, and caspase-3 levels were measured. Bax, Bcl-2, P-IκBα, IκBα, P-NF-κB p65, and NF-κB p65 protein levels were determined by Western blot. TNF-α, IL-6, caspase-3, Bax and Bcl-2 mRNA expression was measured by real-time reverse transcription-polymerase chain reaction (RT-PCR).
RESULTS: Hematoxylin-eosin staining and TUNEL results suggested that DHI (3 g/kg) treatment alleviated inflammatory and apoptotic (P < 0.01) injury in the liver of mice. DHI treatment dose-dependently blunted the abnormal changes in biochemical parameters such as ALT (72.53 ± 2.83 for 3 g/kg, P < 0.01), AST (76.97 ± 5.00 for 3 g/kg, P < 0.01), TBil (1.17 ± 0.10 for 3 g/kg, P < 0.01), MDA (0.81 ± 0.36 for 3 g/kg, P < 0.01), and GST (358.86 ± 12.09 for 3 g/kg, P < 0.01). Moreover, DHI (3 g/kg) remarkably decreased LPS-induced protein expression of TNF-α (340.55 ± 10.18 for 3 g/kg, P < 0.01), IL-6 (261.34 ± 10.18 for 3 g/kg, P < 0.01), and enzyme activity of caspase-3 (0.93 ± 0.029 for 3 g/kg, P < 0.01). The LPS-induced mRNA expression of TNF-α, IL-6 and caspase-3 was also decreased by DHI. Western blot analysis revealed that DHI antagonised LPS-stimulated decrease of Bcl-2 and increase of Bax protein expression. Furthermore, DHI inhibited LPS-induced IκBα and NF-κB p65 phosphorylation.
CONCLUSION: DHI may be a multi-function protectant against acute hepatic injury in mice through its anti-inflammatory, anti-oxidative and anti-apoptotic activities.
Core tip:Salvia miltiorrhiza and Carthamus tinctorius extract [Danhong injection (DHI)] effectively protected against hepatic injury. DHI intervention significantly reduced alanine transferase, aspartate transaminase, total bilirubin, malondialdehyde, glutathione-S-transferase, tumour necrosis factor-α, interleukin-6, and caspase-3 levels in an lipopolysaccharide (LPS)-induced mouse model of acute liver injury. Moreover, DHI antagonised LPS-induced Bcl-2 and Bax expression and inhibited IκBα and nuclear factor-κB p65 phosphorylation. These findings suggest that Salvia miltiorrhiza and Carthamus tinctorius extract (such as DHI) acts as a multi-function protectant against acute hepatic injury in mice through its anti-inflammatory, anti-oxidative and anti-apoptotic activities.
-
Citation: Gao LN, Yan K, Cui YL, Fan GW, Wang YF. Protective effect of
Salvia miltiorrhiza andCarthamus tinctorius extract against lipopolysaccharide-induced liver injury. World J Gastroenterol 2015; 21(30): 9079-9092 - URL: https://www.wjgnet.com/1007-9327/full/v21/i30/9079.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i30.9079
Substantial evidence suggests that Salvia miltiorrhiza Bge. (Lamiaceae) is hepatoprotective against hepatic toxicity and ischemia/reperfusion injury via anti-oxidative effects and improved microcirculation[1]. Similarly, Carthamus Red from Carthamus tinctorius L. (Composite) has hepatoprotective effects against CCl4-induced liver injury[2]. The combination of Salvia miltiorrhiza and Carthamus tinctorius extracts has been traditionally used for synergistic therapeutic effects on activating blood circulation and resolving stasis and to treat ischemic encephalopathy and coronary heart disease in the clinic[3-5]. As a classic prescription of the Salvia miltiorrhiza and Carthamus tinctorius herbal pair, Danhong injection (DHI) is composed of a 30% ethanol extract of the Salvia miltiorrhiza root and rhizome and an aqueous extract of the Carthamus tinctorius flower according to standard extraction processes. High-performance liquid chromatography (HPLC) fingerprinting of DHI has been widely performed. The primary active components of DHI include tanshinone IIA, danshensu, protocatechuic aldehyde, rosmarinic acid, hydroxysafflor yellow A, and salvianolic acid B[6-9]. Based on the literature, we proposed that the Salvia miltiorrhiza and Carthamus tinctorius extract (namely DHI in this study) may be beneficial for hepatic protection.
Hepatic diseases are a global human health problem, with high morbidity and mortality[10]. There is no effective treatment for fatal liver disease, such as hepatic failure. Although hepatic transplantation is associated with a high survival rate (50%-70%)[11], insufficient organ donation and high expenses limit its application. Therefore, the hepatoprotective effects of various drugs have been evaluated in animal models.
Lipopolysaccharide (LPS) is the major component of the outer membrane of Gram-negative bacteria, and the core-lipid A region is the toxic moiety of LPS. LPS impairs the liver by acting as a hepatotoxin[12,13]. The liver provides the first line of defence against bacteria and their products[14]. In animal models, various reagents, such as carbon tetrachloride[15], D-galactosamine/LPS[16] and concanavalin A[17], have been used to induce acute liver injury. In this study, we induced acute liver injury in mice by LPS alone; a 16 mg/kg dose of LPS is sufficient to cause lung, liver and kidney injury[18]. Simultaneously, we employed reduced glutathione for injection (RGI) as a positive control drug against LPS-induced acute liver injury. We investigated the hepatoprotective effects of DHI and explored the underlying mechanisms.
Male C57BL/6J mice (18-20 g) were purchased from Beijing HuaFuKang Bio-technology Co., Ltd and housed under standard laboratory conditions with controlled light (on at 7:00 am and off at 7:00 pm), comfortable temperature (24 °C ± 1 °C) and standard humidity (55% ± 5%). All mice in this study were used in accordance with the NIH Guide for the Care and Use of Laboratory Animals, and the protocol was approved by the Ethics Committee of Tianjin University of Traditional Chinese Medicine (TCM-2009-037-E10).
DHI was purchased from Shandong Buchang Pharmaceutical Co., Ltd (Jinan, China) (Drug approval number: Z20026866). LPS from Escherichia coli 0111:B4, anti-mouse IgG peroxidase conjugate and β-actin monoclonal antibody were obtained from Sigma-Aldrich Co. (St. Louis, United States). Interleukin (IL)-6 and tumour necrosis factor (TNF)-α mouse ELISA kits and anti-mouse Bax and Bcl-2 monoclonal antibodies were obtained from eBioscience (San Diego, United States). Mammalian Cell Lysis Kits, Bradford Protein Assay Kits and UNIQ-10 column Trizol total RNA extraction kits were obtained from Sangon Biological Engineering Technology & Services Co., Ltd (Shanghai, China). FastStart Universal SYBR Green Master (ROX) kits were purchased from Roche (Mannheim, Germany). Detection kits for alanine transferase (ALT), aspartate transaminase (AST), total bilirubin (TBil) and glutathione-S-transferase (GST) were purchased from Nanjing Jiancheng Bioengineering Institute (Nanjing, China). The assay kits for malondialdehyde (MDA), terminal deoxynucleotidyl transferase-mediated dUTP nick end-labelling (TUNEL), cell apoptosis detection, and Nuclear and Cytoplasmic Protein Extraction were purchased from Beyotime Institute of Biotechnology (Haimen, China). RGI was obtained from LvYe Pharmaceutical Co., Ltd (Yantai, China). The BCA Protein Assay Kit was purchased from Thermo Pierce (Rockford, United States). The caspase-3 colorimetric assay kit was obtained from Enzo Life Science (Farmingdale, United States). Antibodies against phospho-IκB-α, IκBα, phospho-NF-κB p65, NF-κB p65 and the peroxidase-conjugated secondary antibody were purchased from Cell Signaling Technology (Beverly, United States). Goat anti-rabbit IgG peroxidase conjugate was purchased from Calbiochem (Darmstadt, Germany).
According to the standard extraction process drafted by Shandong BuChang Pharmaceutical Co. Ltd and approved by the State Food and Drug Administration (SFDA) of China, Salvia miltiorrhiza and Carthamus tinctorius extracts were prepared as DHI. First, Salvia miltiorrhiza (750 g) was immersed in 7.5 L of 30% ethanol, extracted twice for 1 h at 50 °C, and filtered. Carthamus tinctorius (250 g) was mixed with the material remainder by filtration of the Salvia miltiorrhiza exact and immersed twice in 2.5 L water for 1 h at 35 °C. Second, the aqueous solution was mixed with the alcoholic extract and vacuum evaporated to a relative density of 1.0-1.2 (65 °C). Third, isotonic sodium chloride injection was added to adjust the pH level to 6-7. DHI was then prepared after filtration, potting and sterilisation.
The DHI quality control standard stipulated by SFDA of China is that the total amount of Danshensu (molecular formula: C9H10O5) and protocatechuic aldehyde (molecular formula: C7H6O3) should not be less than 0.5 mg in a 1-mL injection analysed by HPLC. In addition, the total flavonoids determined by visible spectrophotometry should not be less than 5.0 mg/mL as determined using rutin as a reference (molecular formula: C27H30O16)[3,19].
In the present study, we performed two parallel animal experiments. In each experiment, mice were randomly assigned to 5 groups (8 animals each): blank control; LPS (dissolved in sterile pyrogen-free saline solution, 16 mg/kg body weight); LPS + DHI (0.75 and 3 g/kg, relative to human clinical dosage); positive control, LPS + RGI (300 mg/kg). The DHI dosage representation was in g/kg (crude drugs weight/body weight). The quantities of the crude drugs were calculated such that the extract contained 750 g of Salvia miltiorrhiza and 250 g of Carthamus tinctorius per litre. Therefore, the g/kg dosage representation is equivalent to the mL/kg[3] found in many other references. Mice were intraperitoneally injected with 0.9% saline (for the control group), DHI and RGI 30 min prior to a single LPS injection (0.9% saline for the blank control group). Different volumes of DHI and RGI were dissolved in 0.9% saline to the same total volume of the maximum administration dosage. After LPS exposure for 90 min or 6 h, the mice were anaesthetised with ether, and blood was collected. Subsequent cardiac perfusion was performed prior to liver tissue collection to avoid influencing the liver tissue results. Serum was separated by centrifugation at 3000 rpm for 15 min at 4 °C. Both serum and liver tissues were frozen at -80 °C until use.
For histological analysis, liver specimens obtained after 90-min LPS exposure were fixed in 10% neutral-buffered formalin, routinely processed, and sectioned (5 μm thick). After haematoxylin and eosin (H&E) staining, live specimens were photographed under a light microscope (Olympus, Tokyo, Japan) at magnification × 200. For hepatocyte apoptosis detection, liver tissues were collected after 6-h LPS stimulation. The TUNEL assay was performed to label the 3’-end of fragmented DNA in liver sections according to the manufacturer’s instructions (Beyotime, China). Finally, slides were examined by fluorescence microscopy (Nikon, Japan), and quantitative statistical analysis was performed using NIH Image J software.
For ALT, AST and TBil detection, serum samples were collected after 6-h LPS stimulation. For IL-6 and TNF-α analysis, liver tissues were harvested from mice stimulated with LPS for 90 min. In addition, liver tissues collected for measuring MDA production and GST activity were stimulated with LPS for 6 h. Liver tissues were homogenised in phosphate-buffered saline (PBS) and centrifuged at 8000 rpm for 15 min at 4 °C. Supernatant aliquots were stored at -80 °C until use. All ALT, AST, TBil, IL-6, TNF-α, MDA, GST and caspase-3 measurements were performed using commercially available kits. Caspase-3 activity was determined using a modified protocol in which a standard curve was prepared with pNA diluted in buffer (assay buffer and lysis buffer were mixed at a ratio of 9:1). One unit was the amount of enzyme that cleaved 10-9 M Ac-DEVD-pNA colorimetric substrate per hour at 37 °C under saturated substrate concentrations.
Liver tissues were stimulated with LPS for 90 min for TNF-α and IL-6 expression analysis and for 6 h for the detection of caspase-3, Bcl-2 and Bax. Real-time RT-PCR was performed as described previously[12], and oligonucleotide primers used for mouse TNF-α, IL-6, caspase-3, Bcl-2, Bax and β-actin (an internal control) are listed in Table 1.
| Gene | Primer | Sequence (5’-3’) | PCR product (bp) |
| β-actin | Forward | TGTTACCAACTGGGACGACA | 165 |
| (NM_007393.3) | Reverse | GGGGTGTTGAAGGTCTCAAA | |
| TNF-α | Forward | TAGCCAGGAGGGAGAACAGA | 127 |
| (NM_013693.2) | Reverse | TTTTCTGGAGGGAGATGTGG | |
| IL-6 | Forward | TCCAGTTGCCTTCTTGGGAC | 140 |
| (NM_031168.1) | Reverse | GTGTAATTAAGCCTCCGACTTG | |
| Bax | Forward | CTGCAGAGGATGATTGCTGA | 174 |
| (NM_007527.3) | Reverse | GATCAGCTCGGGCACTTTAG | |
| Bcl-2 | Forward | GGACTTGAAGTGCCATTGGT | 127 |
| (NM_177410.2) | Reverse | AGCCCCTCTGTGACAGCTTA | |
| Caspase-3 | Forward | ATGGGAGCAAGTCAGTGGAC | 137 |
| (NM_009810.2) | Reverse | CGTACCAGAGCGAGATGACA |
For Bcl-2 and Bax analysis, livers were obtained after LPS treatment for 6 h. For NF-κB family detection, livers were collected after LPS treatment for 90 min. Livers were lysed using a Nuclear and Cytoplasmic Protein Extraction Kit (Beyotime Institute of Biotechnology, China) according to the manufacturer’s protocol. The protein concentration in the supernatant was determined using the BCA method. For Western blot analysis, equal quantities (20 μg) of protein were boiled for 5 min and subjected to SDS-PAGE, followed by electrophoretic transfer to polyvinylidene difluoride (PVDF) membranes. The membranes were blocked with TTBS (0.5% Tween 20, 10 mmol/L Tris-HCl, pH 7.5, 150 mmol/L NaCl) containing 5% non-fat milk for 1 h at room temperature, followed by incubation with antibodies against phospho-IκB-α, IκBα, phospho-NF-κB p65, NF-κB p65 (1:1000, Cell Signaling Technology, United States), Bax, Bcl-2 (1:1000, eBioscience, United States) or β-actin (1:5000, Sigma-Aldrich Co., United States) overnight at 4 °C. The membranes were washed and incubated with HRP-conjugated secondary antibodies against mouse (1:1000 for IκBα, Bax and Bcl-2, and 1:10000 for β-actin, Sigma-Aldrich Co., United States) or rabbit (1:1000 for phospho-IκB-α, phospho-NF-κB p65 and NF-κB p65, Calbiochem, Germany) for 1 h at room temperature. After washing, protein bands were detected using an ECL detection kit (Millipore, United States) and exposed to Kodak BioMax Light films. Films were quantified using NIH Image J software.
Statistical analyses were performed with Origin 7.5 software (MicroCal, United States). Data are expressed as the mean ± SE. Differences between the values of various experimental groups were assessed by one-way analysis of variance (ANOVA), and P-values less than 0.05 were considered statistically significant.
The statistical methods of this study were reviewed by Jing-Bo Zhai from Tianjin University of Traditional Chinese Medicine.
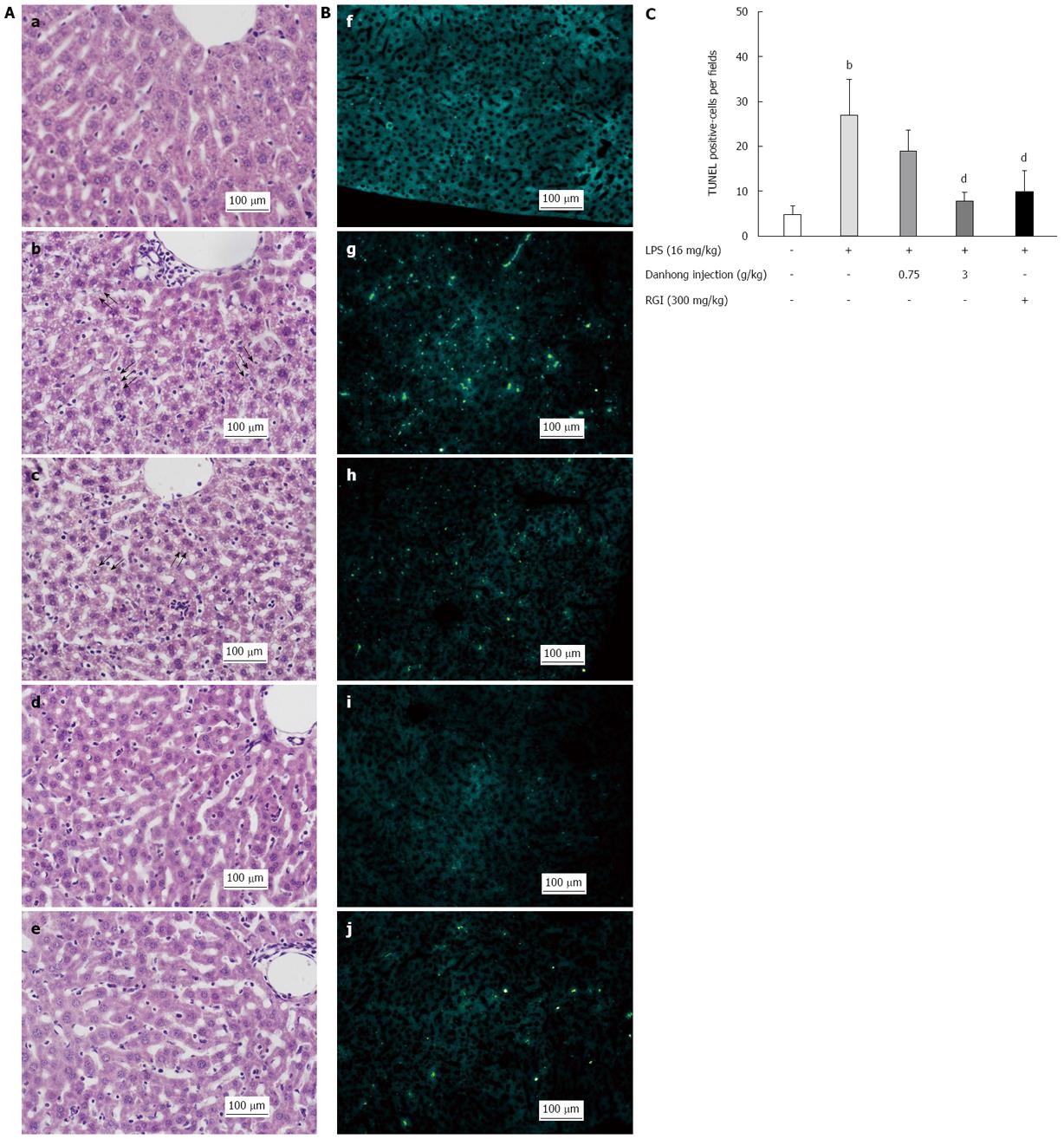
H&E staining analysis of liver sections (Figure 1A) revealed intact lobular architecture and normal hepatocyte structure in mice that did not receive LPS (a). However, upon LPS exposure (b), histological changes primarily characterised by fat degeneration occurred, which are indicated by arrows in Figure 1A. Moreover, hepatic cells became loosely and irregularly arranged. By contrast, DHI administration prior to LPS exposure (c, d) ameliorated these changes, as only slight fat degeneration or few inflammatory granulocyte and monocyte infiltrates were visible. RGI (e) also alleviated inflammatory injury, although not to the same extent as 3 g/kg DHI (d).
To confirm that DHI prevented hepatocyte apoptosis, we performed TUNEL assays on liver sections (Figure 1B). Following LPS treatment, mouse livers (g) displayed increased hepatocyte damage and abundant TUNEL-positive hepatocytes, whereas we did not observe any TUNEL-positive cells in the blank control group (f). When we administered DHI or RGI (h-j) prior to LPS, DHI (3 g/kg) and RGI both significantly alleviated LPS-induced apoptotic injury (P < 0.01, Figure 1C). Taken together, these results indicate that DHI attenuated massive LPS-induced hepatocyte apoptosis in a dose-dependent manner.
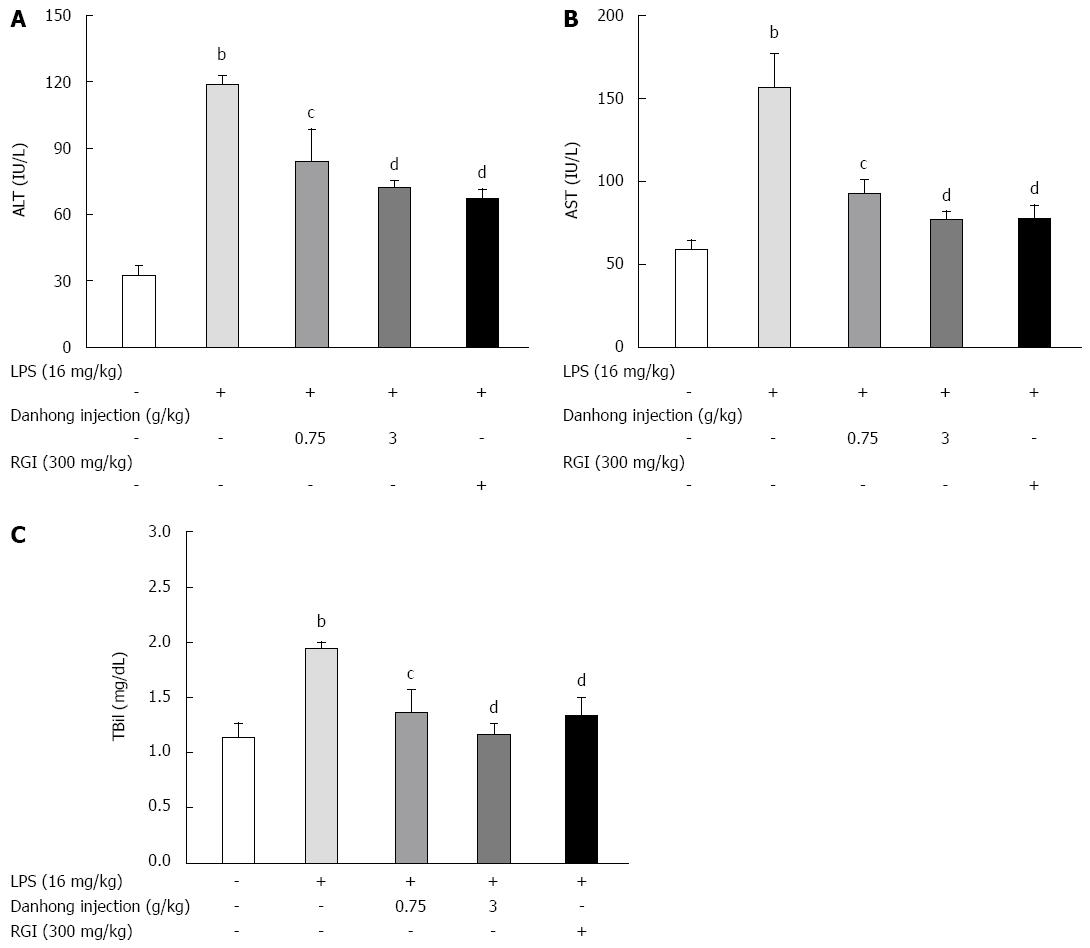
To examine hepatic function, we examined ALT and AST activities and TBil production. LPS alone increased ALT (A) and AST activity (B) and TBil production (C) by 363.3%, 265.7% and 170.1%, respectively, in mouse serum (Figure 2). However, DHI or RGI pre-treatment significantly and dose-dependently decreased these activities (P < 0.05 or P < 0.01).
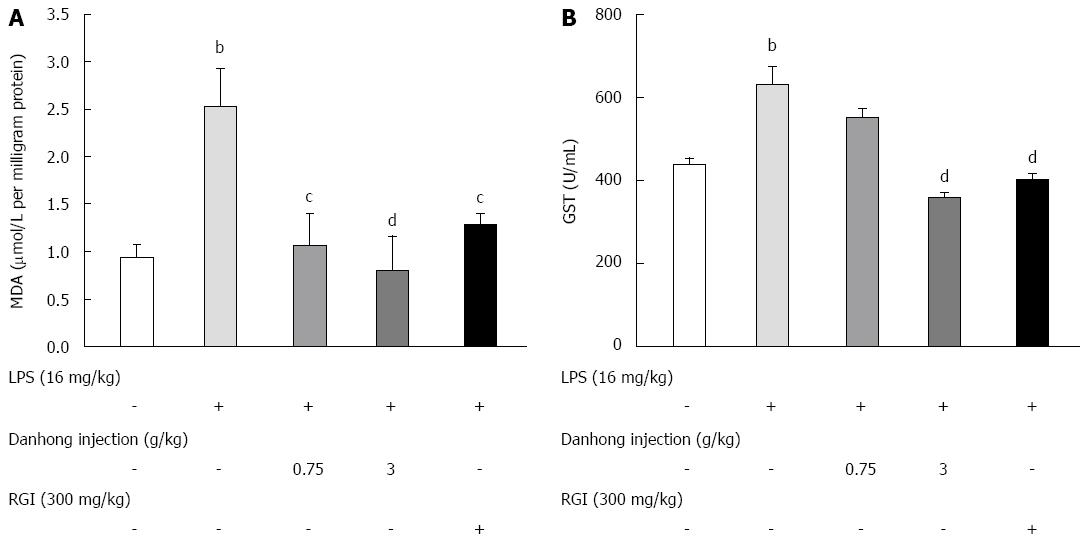
To assess the degree of oxidative stress, we measured MDA and GST levels. As shown in Figure 3, liver levels of MDA (A) and GST (B) increased by 2.68-fold and 1.44-fold, respectively, in mice stimulated with LPS or saline as a control. However, DHI or RGI administration prior to LPS markedly ameliorated oxidative injury, as evidenced by reductions in MDA (0.81 ± 0.36 for 3 g/kg, P < 0.01) and GST (358.86 ± 12.09 for 3 g/kg, P < 0.01) levels.
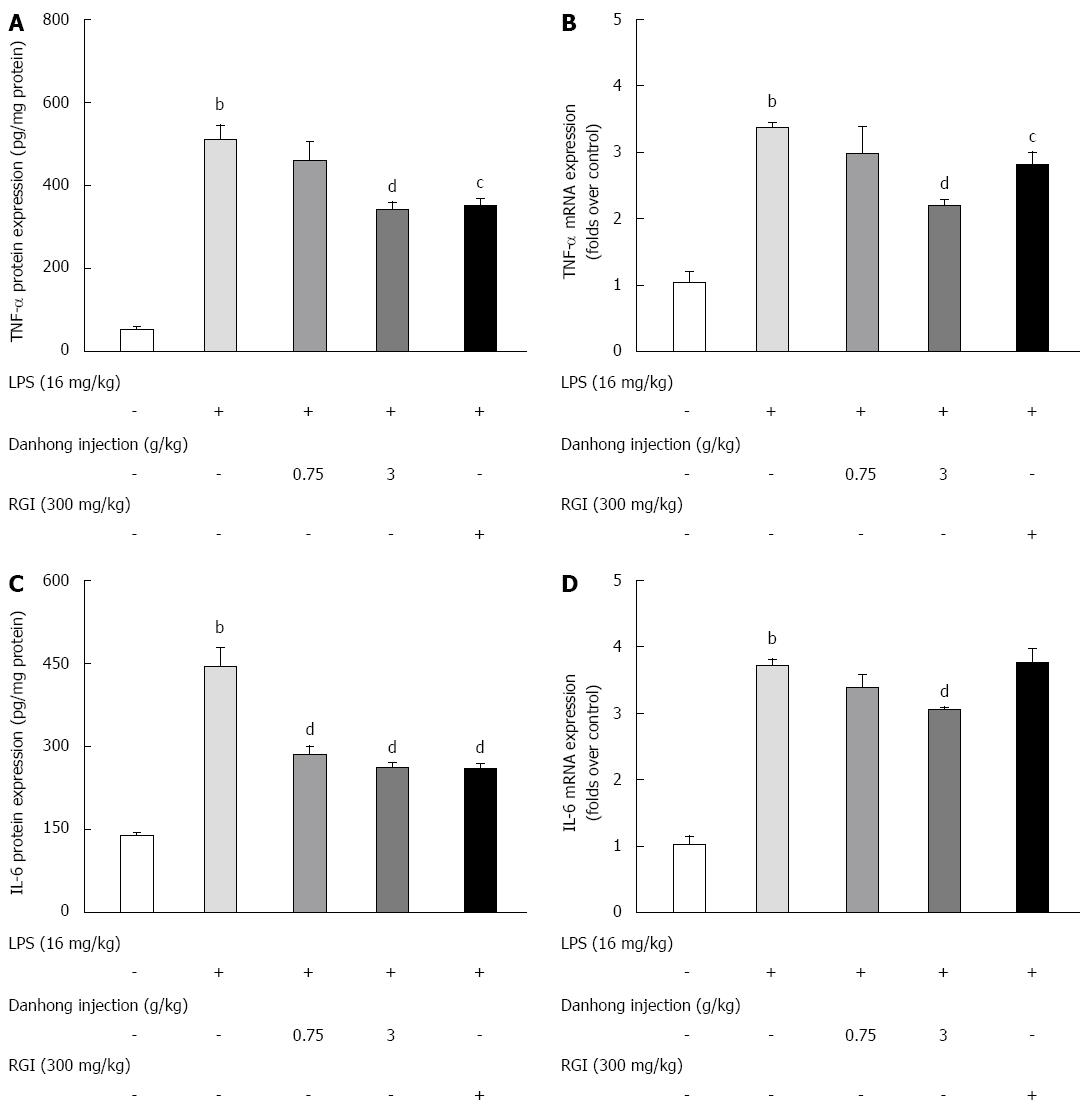
Compared to the LPS-alone group, DHI (3 g/kg) significantly reduced the LPS-induced increase in TNF-α protein (Figure 4A, P < 0.01) and mRNA expression (Figure 4B, P < 0.01). Similarly, LPS alone increased IL-6 protein and mRNA expression by 317.0% and 363.1%, respectively, compared to saline treatment. After DHI administration, IL-6 protein levels significantly decreased in a dose-dependent manner (Figure 4C, P < 0.01). However, only the high dose of DHI (3 g/kg) exhibited a marked inhibitory effect (P < 0.01) on IL-6 mRNA expression (Figure 4D, 3.06 ± 0.043, P < 0.01) and caspase-3 activity (0.93 ± 0.029, P < 0.01).
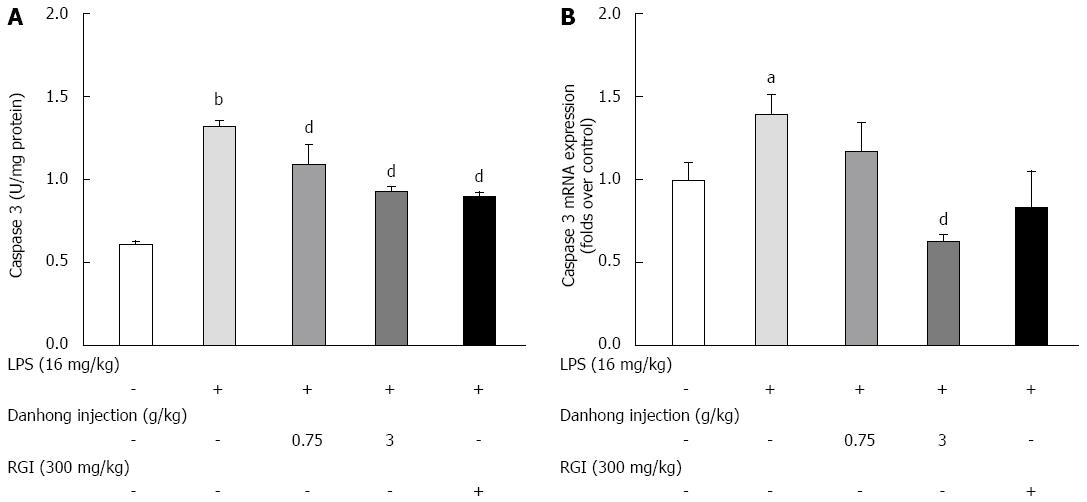
To further investigate the anti-apoptotic and hepatoprotective effects of DHI, we measured caspase-3 activity (Figure 5) by examining enzyme activity and mRNA expression. Compared to the blank control group, LPS significantly increased caspase-3 activity by 217.6%, whereas DHI markedly blunted caspase-3 activity (0.93 ± 0.029 for 3 g/kg, P < 0.01). DHI (3 g/kg) also significantly decreased caspase-3 mRNA expression compared to LPS treatment alone (0.63 ± 0.044, P < 0.01), but RGI treatment had no effect on caspase-3 mRNA expression compared to LPS treatment alone.
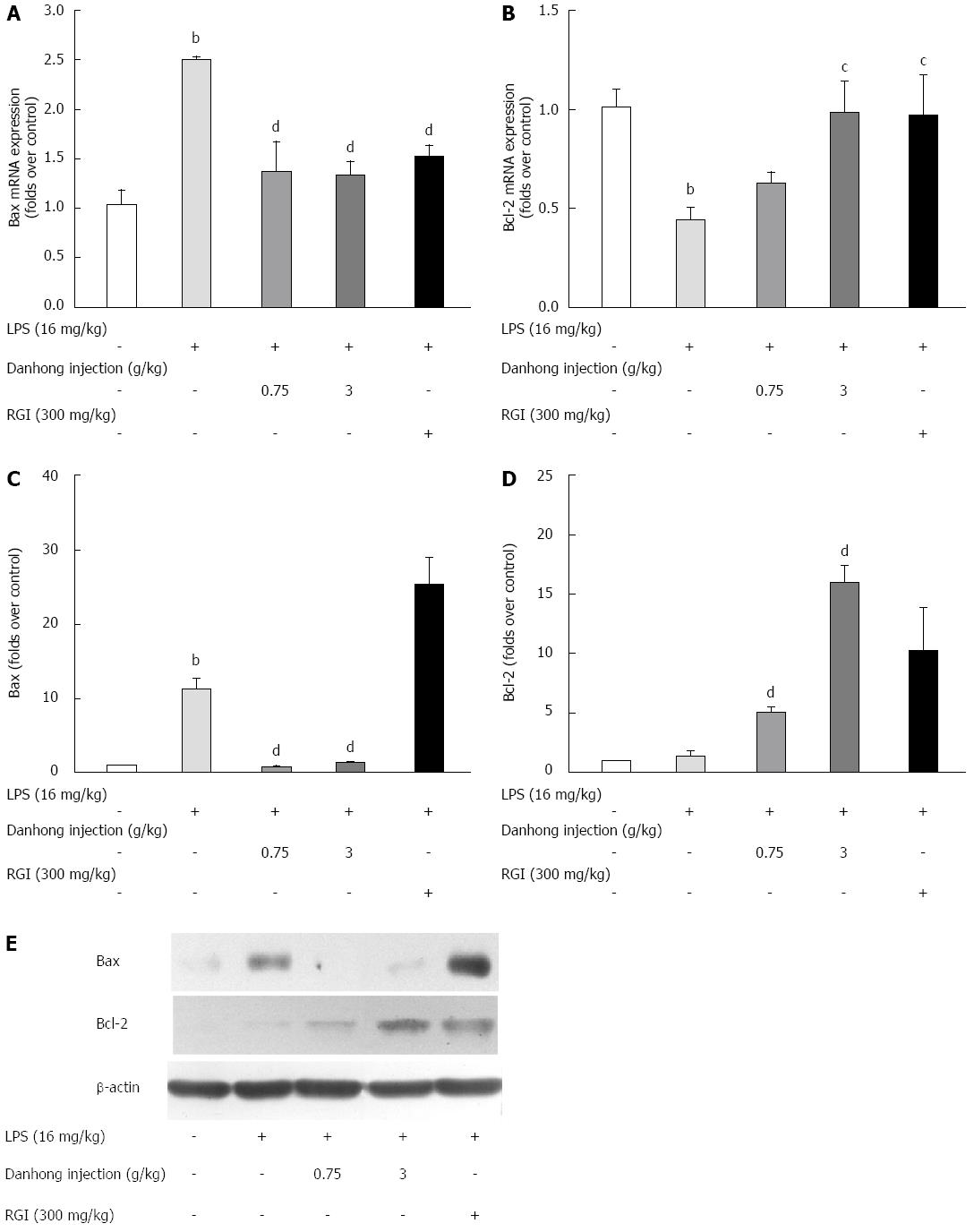
Real-time RT-PCR (Figure 6A and B) revealed that LPS treatment led to an increase in Bax mRNA expression (242.2%, P < 0.01) and a decrease in the mRNA expression of Bcl-2 (44.2%, P < 0.01), an integral membrane anti-apoptotic protein. However, the aberrant changes in Bax and Bcl-2 expression were alleviated by DHI administration (P < 0.05 or P < 0.01). Moreover, we analysed Bcl-2 and Bax protein expression by Western blot (Figure 6E). The relative optical density (Figure 6C and D) showed that LPS up-regulated Bax protein expression (P < 0.01). However, DHI antagonised this LPS-induced protein expression (P < 0.01). Moreover, DHI significantly increased Bcl-2 protein expression compared to LPS treatment alone (P < 0.01). In summary, DHI protected hepatocytes from apoptotic injury by balancing Bax and Bcl-2 expression.
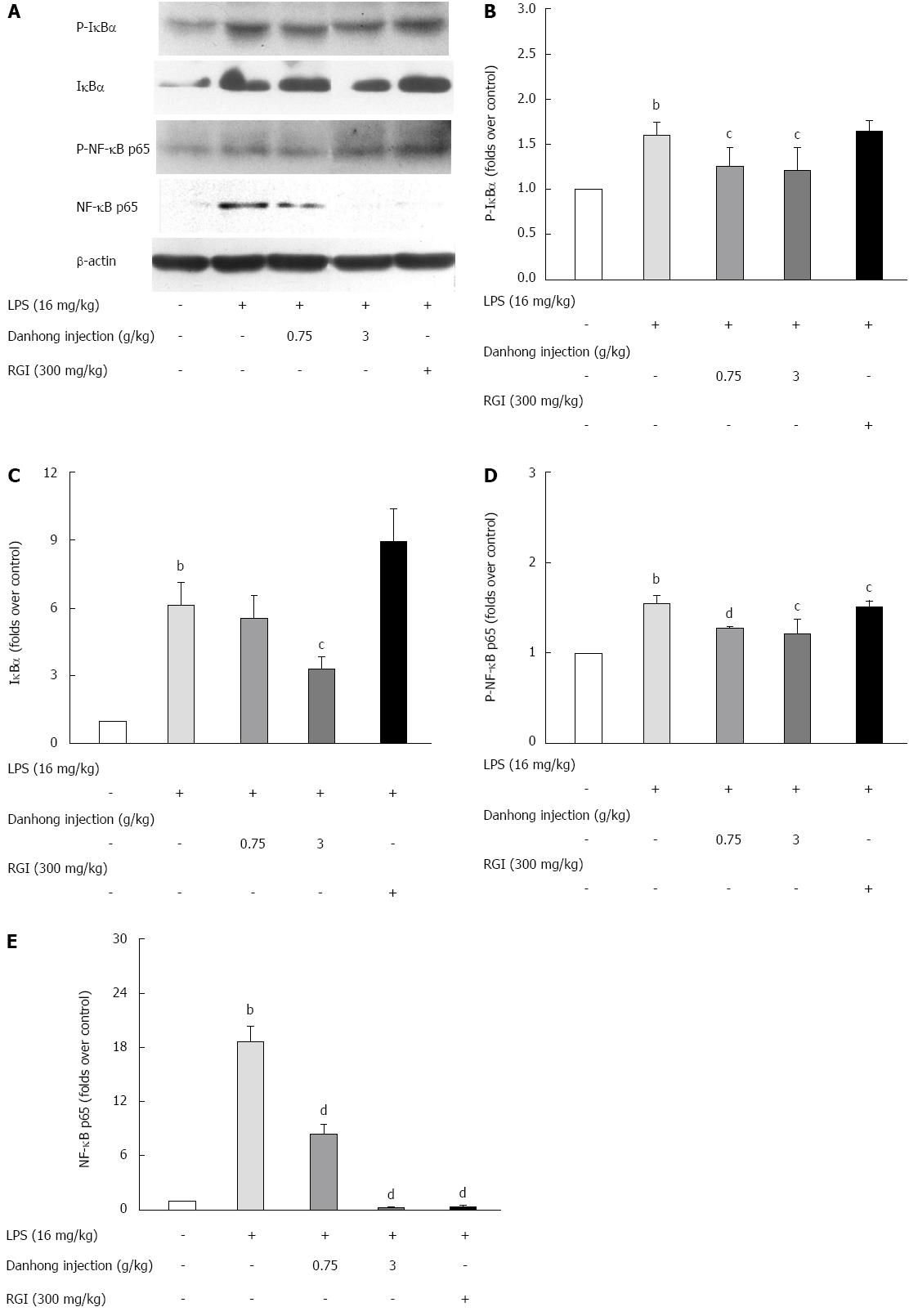
The NF-κB family plays a key role in pro-inflammatory and anti-apoptosis processes. Generally, NF-κB/Rel proteins enter the cytoplasm as homodimers or heterodimers in an inactive state in complexes with inhibitory IκB proteins. Upon LPS treatment (Figure 7), we observed IκBα degradation and phosphorylation, which revealed that DHI alleviated the LPS-induced phospho-IκBα up-regulation (P < 0.05). DHI (3 g/kg) significantly antagonised the LPS-induced IκBα increase (P < 0.05). To determine if NF-κB p65 translocation and phosphorylation occurred during acute liver injury, we examined the effects of DHI treatment on LPS-induced NF-κB p65 and phospho-NF-κB p65 by Western blot, which revealed that DHI significantly reduced the up-regulation of NF-κB p65 (P < 0.01) and phospho-NF-κB p65 (P < 0.05 or P < 0.01).
Studies have established that Salvia miltiorrhiza plays a vital role in vasorelaxation[20], scavenging oxygen free radicals, preventing lipid peroxidation, and combatting inflammation[21]. The primary pharmacological action of Carthamus tinctorius is to improve microcirculation, antagonise coagulation and thrombosis, and inhibit the inflammatory reaction[22-25]. Modern pharmacological studies have determined that Salvia miltiorrhiza and Carthamus tinctorius extracts in the form of a compound preparation (such as DHI, a classic preparation) are a potential anti-oxidative[26,27] agent and an anti-inflammatory drug[11]. The anti-inflammatory activity of DHI is primarily due to hydroxysafflor yellow A, and its anti-oxidative capacity relies on salvianolic acid B. Danshensu has the strongest anti-apoptotic effect[6]. To evaluate the safety of DHI, Wang et al[28] investigated its acute and chronic toxicity by intravenous injection, determining an LD50 of 39.5 mL/kg, more than 10-fold higher than the high dosage used here.
We performed novel pharmacological explorations of the effects of DHI on liver injury. H&E staining indicated that DHI largely ameliorated fat degeneration, indicating its hepatic protective capability. Second, DHI regulated ALT, AST, TBil and MDA levels and GST enzyme activities, suggesting that DHI repairs hepatic function and alleviates oxidative stress.
The regulation of liver function is dependent on communication between cells (hepatocytes, endothelial cells, Kupffer cells and others) and cytokines. TNF-α is required for the proliferation of normal hepatocytes and exerts an anti-apoptotic effect by inducing NF-κB[26,29]. However, excessive TNF-α may mediate hepatotoxicity by inducing apoptosis[30,31]. Furthermore, TNF-induced hepatocyte injury provides a vital signal for polymorphonuclear (PMN) transmigration from sinusoids into the parenchymal tissues, which leads to cellular necrosis[32]. IL-6 is secreted from activated macrophages and is a potent inducer of the hepatic acute phase response[33]. For mice with LPS-induced acute liver injury, DHI notably inhibited the increase in IL-6 and TNF-α protein and mRNA expression. These results confirm that DHI can improve hepatic function and protect the liver from oxidative stress and inflammatory damage.
The transcription factor NF-κB is involved in the regulation of many important cellular and physiological processes, such as growth, apoptosis, and the immune and inflammatory responses[33-35]. Further exploring the mechanisms of DHI in acute mouse liver injury, we observed that LPS induced the NF-κB signalling pathway and TNF-α secretion via IκBα phosphorylation triggered NF-κB p65 translocation to the nucleus stimulated the inflammatory cascade reaction. The released inflammatory mediators, such as TNF-α and IL-6, could then multiply the toxic effects of LPS on multiple organs and lead to multiple organ dysfunctions. However, DHI treatment inactivated the NF-κB pathway and protected against hepatic injury-induced deterioration.
Acute inflammation can also induce apoptotic injury[36]. Excess TNF-α is an important inducer of endotoxemia-induced liver injury and accelerates hepatocyte apoptosis[37]. To explore whether apoptosis is involved in LPS-induced liver injury, we performed TUNEL assays. Our results revealed that LPS administration led to striking hepatocyte apoptosis with focal concentration. However, DHI blunted the apoptotic response. Caspase-3 is indispensable for some typical characteristics of apoptosis, such as chromatin condensation and DNA fragmentation[38]. The detection of caspase-3 revealed that DHI treatment alleviated LPS-induced caspase-3 activity at both the enzymatic activity and mRNA expression level. We also propose that the reduced caspase-3 activity was due to a decrease in mRNA expression or the dual role of mRNA expression and enzyme activity. We did not observe a significant change in caspase-8 activity. The key anti-apoptotic protein Bcl-2 can inactivate caspases[35,39]. Further investigating the possible anti-apoptotic mechanism of DHI, we observed that DHI reduced Bax expression and increased Bcl-2 expression. As mentioned above, we propose that DHI inhibits hepatic apoptosis by balancing Bcl-2 and Bax levels.
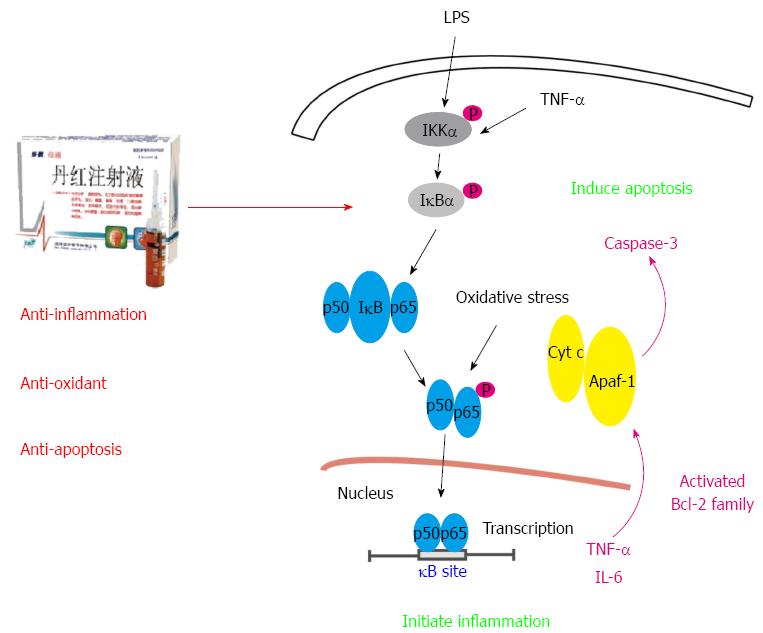
In conclusion, this study focused on the effect of Salvia miltiorrhiza and Carthamus tinctorius extract on hepatic protection. DHI is a popular Chinese medicine consisting of Salvia miltiorrhiza and Carthamus tinctorius. LPS injection activated the NF-κB signalling pathway, resulting in the subsequent release of cytokines, including TNF-α and IL-6. TNF-α secretion induces oxidative stress injury, which deteriorates the NF-κB-related signalling cascade. In addition, excessive TNF-α production stimulates Bcl-2 family activation to release cytochrome C, leading to caspase-3 activation and hepatocyte damage. DHI administration prior to LPS alleviated acute liver injury through its anti-oxidative capacity and anti-inflammatory effects by inhibiting NF-κB pathway activation and through its anti-apoptotic effect by balancing Bcl-2 and Bax levels (Figure 8).
Substantial evidence suggests that Salvia miltiorrhiza Bge. (Lamiaceae) is hepatoprotective against hepatic toxicity and ischemia/reperfusion injury via anti-oxidative effects and improved microcirculation. Similarly, falvonoids from Carthamus tinctorius L. (Composite) have hepatoprotective effects. The combination of Salvia miltiorrhiza and Carthamus tinctorius extracts (Danhong injection, DHI) has been traditionally used for synergistic therapeutic effects on activating blood circulation and resolving stasis and to treat ischemic encephalopathy and coronary heart disease in the clinic. The present study aimed to investigate the hepatoprotective effects of DHI and explore the underlying mechanisms.
Hepatic injury always companies with inflammatory infiltration, oxidative stress and apoptotic injury. Lipopolysaccharide (LPS) injection activates the nuclear factor (NF)-κB signalling pathway, resulting in the subsequent release of cytokines, including tumor necrosis factor (TNF)-α and interleukin (IL)-6. TNF-α secretion induces oxidative stress injury, which deteriorates the NF-κB-related signalling cascade. In addition, excessive TNF-α production stimulates Bcl-2 family activation to release cytochrome C, leading to caspase-3 activation and hepatocyte damage.
The present study first reported the protective effect of DHI on LPS-induced liver injury in mice. DHI may be a multi-function protectant against acute hepatic injury in mice through its anti-inflammatory, anti-oxidative and anti-apoptotic activities.
The results of the present study highlighted the protective effect of DHI on LPS-induced liver injury. It may be a potent drug for developing new therapeutic treatments for liver diseases.
LPS is the major component of the outer membrane of Gram-negative bacteria, and the core-lipid A region is the toxic moiety of LPS. LPS impairs the liver by acting as a hepatotoxin. The liver provides the first line of defence against bacteria and their products.
This is an interesting study in which authors indicated that Salvia mitiorrhiza and Carthanmus tinctorius extract (Danhong injection, DHI) protected from LPS-induced hepatic injury depending upon anti-inflammation, anti-oxidation and anti-apoptosis in mice. It may be valuable for new therapeutic treatments for liver diseases.
Animal care and use statement: The animal protocol was designed to minimize pain or discomfort to the animals. The animals were acclimatized to laboratory conditions (24 °C ± 1 °C, 12 h/12 h light/dark, 55% ± 5% humidity, ad libitum water) for two weeks prior to experimentation. Intraperitoneal injection administration was carried out with conscious animals, using syringes of 1 mL. All animals were given ether anesthesia for tissue and blood collection.
P- Reviewer: Kim Y S- Editor: Yu J L- Editor: Wang TQ E- Editor: Ma S
| 1. | You JS, Pan TL, Lee YS. Protective effects of Danshen (Salvia miltiorrhiza) on adriamycin-induced cardiac and hepatic toxicity in rats. Phytother Res. 2007;21:1146-1152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 2. | Wu S, Yue Y, Tian H, Li Z, Li X, He W, Ding H. Carthamus red from Carthamus tinctorius L. exerts antioxidant and hepatoprotective effect against CCl(4)-induced liver damage in rats via the Nrf2 pathway. J Ethnopharmacol. 2013;148:570-578. [PubMed] [DOI] [Full Text] |
| 3. | He Y, Wan H, Du Y, Bie X, Zhao T, Fu W, Xing P. Protective effect of Danhong injection on cerebral ischemia-reperfusion injury in rats. J Ethnopharmacol. 2012;144:387-394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 91] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 4. | Sun M, Zhang JJ, Shan JZ, Zhang H, Jin CY, Xu S, Wang YL. Clinical observation of Danhong Injection (herbal TCM product from Radix Salviae miltiorrhizae and Flos Carthami tinctorii) in the treatment of traumatic intracranial hematoma. Phytomedicine. 2009;16:683-689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 58] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 5. | Sun K, Fu C, Nie S, You Y. The index and improvement effect of using Danhong injection to patients with atherosclerosis symptoms of coronary heart disease (CHD). Pak J Pharm Sci. 2014;27:1699-1704. [PubMed] |
| 6. | Liu SY, Li WL, Qu HB, Zhao BC, Zhao T. [On-line monitoring of extraction process of danhong injection based on near-infrared spectroscopy]. Zhongguo Zhong Yao Zazhi. 2013;38:1657-1662. [PubMed] |
| 7. | Huang HX, Li WL, Qu HB, Zhao BC, Zhao T. [Implementation of extraction process trajectory for in-line quality control of danhong injection]. Zhongguo Zhong Yao Zazhi. 2013;38:1663-1666. [PubMed] |
| 8. | Guan Y, Yin Y, Zhu YR, Guo C, Wei G, Duan JL, Wang YH, Zhou D, Quan W, Weng Y. Dissection of mechanisms of a chinese medicinal formula: danhong injection therapy for myocardial ischemia/reperfusion injury in vivo and in vitro. Evid Based Complement Alternat Med. 2013;2013:972370. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 48] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 9. | Xie YY, Xiao X, Luo JM, Fu C, Wang QW, Wang YM, Liang QL, Luo GA. Integrating qualitative and quantitative characterization of traditional Chinese medicine injection by high-performance liquid chromatography with diode array detection and tandem mass spectrometry. J Sep Sci. 2014;37:1438-1447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 10. | Mizuhara H, O’Neill E, Seki N, Ogawa T, Kusunoki C, Otsuka K, Satoh S, Niwa M, Senoh H, Fujiwara H. T cell activation-associated hepatic injury: mediation by tumor necrosis factors and protection by interleukin 6. J Exp Med. 1994;179:1529-1537. [PubMed] |
| 11. | Terblanche J, Hickman R. Animal models of fulminant hepatic failure. Dig Dis Sci. 1991;36:770-774. [PubMed] |
| 12. | Nolan JP. The role of intestinal endotoxin in liver injury: a long and evolving history. Hepatology. 2010;52:1829-1835. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 197] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 13. | Gao LN, Cui YL, Wang QS, Wang SX. Amelioration of Danhong injection on the lipopolysaccharide-stimulated systemic acute inflammatory reaction via multi-target strategy. J Ethnopharmacol. 2013;149:772-782. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 38] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 14. | Kuhla A, Eipel C, Abshagen K, Siebert N, Menger MD, Vollmar B. Role of the perforin/granzyme cell death pathway in D-Gal/LPS-induced inflammatory liver injury. Am J Physiol Gastrointest Liver Physiol. 2009;296:G1069-G1076. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 40] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 15. | Son G, Iimuro Y, Seki E, Hirano T, Kaneda Y, Fujimoto J. Selective inactivation of NF-kappaB in the liver using NF-kappaB decoy suppresses CCl4-induced liver injury and fibrosis. Am J Physiol Gastrointest Liver Physiol. 2007;293:G631-G639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 104] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 16. | Masaki T, Chiba S, Tatsukawa H, Yasuda T, Noguchi H, Seike M, Yoshimatsu H. Adiponectin protects LPS-induced liver injury through modulation of TNF-alpha in KK-Ay obese mice. Hepatology. 2004;40:177-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 307] [Cited by in RCA: 310] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 17. | Knolle PA, Gerken G, Loser E, Dienes HP, Gantner F, Tiegs G, Meyer zum Buschenfelde KH, Lohse AW. Role of sinusoidal endothelial cells of the liver in concanavalin A-induced hepatic injury in mice. Hepatology. 1996;24:824-829. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 84] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 18. | Tuñón MJ, Alvarez M, Culebras JM, González-Gallego J. An overview of animal models for investigating the pathogenesis and therapeutic strategies in acute hepatic failure. World J Gastroenterol. 2009;15:3086-3098. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 152] [Cited by in RCA: 161] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 19. | SFDA. National Drugs surveillance administratative bureau standard WS-22110. (ZD-1220)-2002. 2002;. |
| 20. | Wang D, Fan G, Wang Y, Liu H, Wang B, Dong J, Zhang P, Zhang B, Karas RH, Gao X. Vascular reactivity screen of Chinese medicine danhong injection identifies Danshensu as a NO-independent but PGI2-mediated relaxation factor. J Cardiovasc Pharmacol. 2013;62:457-465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 35] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 21. | Zhang XP, Jiang J, Yu YP, Cheng QH, Chen B. Effect of Danshen on apoptosis and NF-κB protein expression of the intestinal mucosa of rats with severe acute pancreatitis or obstructive jaundice. Hepatobiliary Pancreat Dis Int. 2010;9:537-546. [PubMed] |
| 22. | Ling L, Lijia L, Tingzhu L. Effects of serum oxidation low density lipoprotein and c-reactive protein on coronary heart disease. Guizhou Yixue Za Zhi. 2004;28:588-589. |
| 23. | Li L. Effects of Danhong Injection on Vascular Endothelial Function and Inflammatory Factors in Patients with Unstable Angina Pectoris. Caoyao Yixue. 2011;30:319-321. |
| 24. | Guan GF, Huan XP, Wang LL, Du LL, Kong XH. Effects of Danhong injection on lipid metabolism and vascular endothelial function in rabbit model with experimental atherosclerosis. J ClinCardiol. 2007;23:304-306. |
| 25. | Zhao PX, Jiang S. [Effect of danhong injection on ET-1, sP-sel, and hs-CRP in patients with acute coronary syndrome undergoing percutaneous coronary intervention]. Zhongguo Zhong Xi Yi Jie He Zazhi. 2011;31:11-14. [PubMed] |
| 26. | Zhang X, Chen L, Zhang J, Tian H, Zhang X, Zhou Y, Wang Z, Wang K. Effect of salvia miltiorrhizae on apoptosis and NF-kappaB p65 expression in the liver of rats with severe acute pancreatitis or obstructive jaundice. J Gastroenterol Hepatol. 2009;24:841-852. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 27. | Guo H, Li MJ, Liu QQ, Guo LL, Ma MM, Wang SX, Yu B, Hu LM. Danhong injection attenuates ischemia/reperfusion-induced brain damage which is associating with Nrf2 levels in vivo and in vitro. Neurochem Res. 2014;39:1817-1824. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 41] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 28. | Wang BZ, Cao MR, Zhou WH. The toxicological study of Danhong. Zhongguo Shiyong Yiyao. 2008;3:27-29. |
| 29. | Strieter RM, Kunkel SL, Bone RC. Role of tumor necrosis factor-alpha in disease states and inflammation. Crit Care Med. 1993;21:S447-S463. [PubMed] |
| 30. | Liedtke C, Bangen JM, Freimuth J, Beraza N, Lambertz D, Cubero FJ, Hatting M, Karlmark KR, Streetz KL, Krombach GA. Loss of caspase-8 protects mice against inflammation-related hepatocarcinogenesis but induces non-apoptotic liver injury. Gastroenterology. 2011;141:2176-2187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 96] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 31. | Chawla RK, Watson WH, Eastin CE, Lee EY, Schmidt J, McClain CJ. S-adenosylmethionine deficiency and TNF-alpha in lipopolysaccharide-induced hepatic injury. Am J Physiol. 1998;275:G125-G129. [PubMed] |
| 32. | Bradham CA, Plümpe J, Manns MP, Brenner DA, Trautwein C. Mechanisms of hepatic toxicity. I. TNF-induced liver injury. Am J Physiol. 1998;275:G387-G392. [PubMed] |
| 33. | Jones JJ, Fan J, Nathens AB, Kapus A, Shekhman M, Marshall JC, Parodo J, Rotstein OD. Redox manipulation using the thiol-oxidizing agent diethyl maleate prevents hepatocellular necrosis and apoptosis in a rodent endotoxemia model. Hepatology. 1999;30:714-724. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 34. | Yudkin JS, Kumari M, Humphries SE, Mohamed-Ali V. Inflammation, obesity, stress and coronary heart disease: is interleukin-6 the link? Atherosclerosis. 2000;148:209-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1283] [Cited by in RCA: 1250] [Article Influence: 50.0] [Reference Citation Analysis (0)] |
| 35. | Li Q, Verma IM. NF-kappaB regulation in the immune system. Nat Rev Immunol. 2002;2:725-734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2972] [Cited by in RCA: 3213] [Article Influence: 139.7] [Reference Citation Analysis (0)] |
| 36. | Tak PP, Firestein GS. NF-kappaB: a key role in inflammatory diseases. J Clin Invest. 2001;107:7-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3011] [Cited by in RCA: 3171] [Article Influence: 132.1] [Reference Citation Analysis (0)] |
| 37. | Adams JM, Cory S. The Bcl-2 protein family: arbiters of cell survival. Science. 1998;281:1322-1326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3914] [Cited by in RCA: 3936] [Article Influence: 145.8] [Reference Citation Analysis (0)] |
| 38. | Shimizu S, Yamada Y, Okuno M, Ohnishi H, Osawa Y, Seishima M, Moriwaki H. Liver injury induced by lipopolysaccharide is mediated by TNFR-1 but not by TNFR-2 or Fas in mice. Hepatol Res. 2005;31:136-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 39. | Wang Y, Gao LN, Cui YL, Jiang HL. Protective effect of danhong injection on acute hepatic failure induced by lipopolysaccharide and d-galactosamine in mice. Evid Based Complement Alternat Med. 2014;2014:153902. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |