Published online Aug 14, 2015. doi: 10.3748/wjg.v21.i30.9055
Peer-review started: March 14, 2015
First decision: April 23, 2015
Revised: May 13, 2015
Accepted: June 26, 2015
Article in press: June 26, 2015
Published online: August 14, 2015
Processing time: 157 Days and 8.4 Hours
AIM: To assess the direct effects of ischemia on intestinal epithelial integrity. Furthermore, clinical efforts at mitigating the effect of hypoperfusion on gut permeability have focused on restoring gut vascular function.
METHODS: We report that, in the Caco-2 cell model of transepithelial transport, calcium glycerophosphate (CGP), an inhibitor of intestinal alkaline phosphatase F3, has a significant effect to preserve transepithelial electrical resistance (TEER) and to attenuate increases in mannitol flux rates during hypoxia or cytokine stimulation.
RESULTS: The effect was observable even at concentrations as low as 1 μmol/L. As celiac disease is also marked by a loss of gut epithelial integrity, the effect of CGP to attenuate the effect of the α-gliadin peptide 31-55 was also examined. In this instance, CGP exerted little effect of preservation of TEER, but significantly attenuated peptide induced increase in mannitol flux.
CONCLUSION: It appears that CGP treatment might synergize with other therapies to preserve gut epithelial integrity.
Core tip: This article presents a novel role for calcium glycerophosphate in preserving the gut epithelial integrity during hypoxia and in the presence of cytokines. Calcium glycerophosphate showed a significant time and concentration dependent effect to attenuate increased gut permeability caused by hypoxia, cytokine stimulation and α-gliadin peptide 31-55. The effect was observable even at concentrations as low as 1 μmol/L. A better understanding of this phenomenon would help in devising new preventive and therapeutic regimens.
- Citation: Datta P, Weis MT. Calcium glycerophosphate preserves transepithelial integrity in the Caco-2 model of intestinal transport. World J Gastroenterol 2015; 21(30): 9055-9066
- URL: https://www.wjgnet.com/1007-9327/full/v21/i30/9055.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i30.9055
Ischemia/reperfusion (I/R) injury is a broad area of medical significance. Stroke and coronary artery disease (CAD) are the most obvious examples of I/R injury. However, the fundamental problem is much broader, and includes gut ischemia consequent to splanchnic hypoperfusion. Chronic gut ischemia is not so dramatic as stroke or CAD, but may be more insidious. Splanchnic hypoperfusion is a consequence of endurance sports activity[1,2], and a significant element in the pathophysiology of congestive heart failure[3,4], shock[5], cardiac surgery[6] and hemodialysis[7]. Although the ultimate causes of hypoperfusion are different in each of these clinical situations, they share similar clinical consequences.
During simulated gut ischemia, there is extensive neutrophil recruitment to the post-capillary venule[8], a process that is dependent, at least in part, on long-chain fatty acyl CoA synthetase[9], likely acting by inhibiting cell adhesion protein palmitoylation[10,11]. The subsequent immune system activation increases synthesis of an array of cytokines, including tumor necrosis factor (TNF)α, interleukin (IL)β and interferon (IFN)γ. While these have been studied extensively in various pathologies involving splanchnic hypoperfusion[12,13], few studies have focused on assessment of the direct effects of either hypoxia or cytokine liberation on intestinal epithelial integrity[14,15]. Furthermore, clinical efforts at mitigating the effect of hypoperfusion on gut permeability have focused on restoring gut vascular function.
Gut epithelial integrity is dependent, at least in part, on sphingosine-1-phosphate (S1P) generation[16]. Earlier studies revealed that calcium glycerophosphate (CGP) is an inhibitor of intestinal F3 alkaline phosphatase[17]. As alkaline phosphatase catalyzes the conversion of S1P to sphingosine and inorganic phosphate[18], here we test the hypothesis that by inhibiting intestinal alkaline phosphatase, CGP might raise the S1P concentrations, thus helping to preserve intestinal integrity during ischemic insult.
Loss of intestinal epithelial integrity is caused by gluten peptides in genetically susceptible patients (celiac disease). Pancreatic proteases cleave α-gliadin to peptides that may be the direct cause of enteropathy. Previous studies have shown that the P31-55 cleavage product of α-gliadin increased Caco2 transepithelial permeability, although the cells remained intact and further hydrolytic processing of the peptide was observed[19]. Therefore the effect of CGP was investigated in the presence of these peptides, in anticipation that CGP would return the permeability toward normal.
All experiments were conducted on Caco-2 cells, a line of cells derived from a colorectal carcinoma, and obtained from American Type Culture Collection (Rockville MD). Upon prolonged culture, these cells express the characteristics of small intestinal enterocytes[20]. They are widely used as a model of transepithelial transport in the small intestine, and are considered a model for celiac disease[19,21,22]. Cells were grown on a transwell inserts (6-well, 12-well or 24-well, 0.4 micron pore size, PET Polyester, Corning) a permeable barrier system, in minimal essential media (MEM) supplemented with 20% fetal calf serum. The media volumes in the apical and basolateral chambers were 1.5 mL and 1 mL (6-well plates), 0.5 mL and 1 mL (12-well plates), and 0.1 mL and 0.5 mL (24-well plates), respectively.
CGP was purchased from Astha Laboratories Pvt Ltd, India. It was dissolved in 0.1% citric acid solution, filtered, and diluted in media to the desired concentrations. No change in pH was observed on addition of citric acid.
The tightness of the tight junctions in a transporting epithelium was evaluated by measuring the transepithelial electrical resistance (TEER). TEER measures the movement of ions across the cell (transcellular) and between cells (paracellular). In a very tight monolayer, transport is nearly all transcellular, hence the electrical resistance is high; the electrical resistance across the membrane was measured using the EVOM2 epithelial voltohmmeter (World Precision Instruments, Sarasota FL, United States). At 3 to 4 wk in culture, TEER was in the range of 600-700 Ωcm2. The resistance was measured by placing the electrodes directly in the transwell inserts with media present. Time 0 h indicates that TEER was measured immediately after the addition of various CGP concentrations. Subsequent TEER measurements were taken at various times, up to 24 h. Rates were calculated by taking the slope of the linear portion of the TEER or mannitol flux vs time curve (0 to 4 h). The time 0 TEER was measured for each well (baseline). Subsequent TEER measurements for that well were expressed as a percentage of the baseline measurement.
Measuring mannitol flux complemented the TEER results. Cells were seeded into 6 well plates, and [14C] labeled mannitol (10 μmol/L) was added to the apical media; 10 μL samples were removed from the basolateral media at intervals throughout the experiment. The rate of mannitol flux was determined by calculating the slope of dpm in the basolateral chamber as a function of time.
E-cadherin is the principle protein of tight junctions. The levels of E-cadherin were measured in cells by western blot analysis (primary antibody from BD biosciences). The cells were collected from the transwell inserts after the treatments and were lysed in the cell lysis buffer. The proteins were then separated according to the molecular weight through gel electrophoresis. Proteins were transferred to a membrane and incubated with E-Cad antibody and developed later with secondary antibody. The expression was compared to the control that had no treatment and no significant change in the expression was observed.
Calcium glycerophosphate has activity as a phosphatase inhibitor, inviting our hypothesis that CGP elevates S1P concentrations by inhibiting dephosphorylation. S1P concentrations were measured in Caco-2 cells using a commercially available ELISA kit (Echelon Catalog # K-1900).
Baseline values of mannitol flux and TEER were obtained in unstimulated cells, in the absence and presence of increasing concentrations of CGP.
The effect of hypoxia was measured for TEER and mannitol flux, in the presence and absence of CGP (taken as control in the experiment). Cells were grown to confluence on transwells, 0.4 micron pore size. Experiments in hypoxic conditions were conducted in a hypoxia chamber (Coy Laboratory Products, Grass Lake MI, United States), as detailed elsewhere[23]. Briefly, confluent cultures were placed in the chamber under a 95% N2/5% CO2 atmosphere and fed with serum and glucose free MEM, and bubbled with 95% N2/5% CO2. Under these conditions, the pO2 in the media is below 25 mmHg (6%). Normoxic conditions were the standard CO2 incubator and media containing glucose.
The effect of exogenous cytokines was measured for each parameter, in the presence and absence of CGP (taken as control in the experiment). Both hypoxia/ischemia and celiac disease are characterized by cytokine release, which increase monolayer permeability[5]. Cells were challenged with TNFα, ILβ and IFNγ (final concentrations: 10 ng/mL; 1 ng/mL; 100 ng/mL, respectively), then TEER, mannitol flux, E-Cadherin expression, and concentrations of S1P were measured.
The effect of α-gliadin 31-55 peptide was measured for each parameter. α-Gliadin is the offending protein initiating the symptoms of celiac disease in susceptible patients. Gastric and pancreatic proteases cleave α-gliadin to peptides, which are likely the direct cause of the enteropathy. The literature[19] suggests that the peptide comprised of α-gliadin amino acids 31-55 is a likely candidate. The peptide (Thermo Scientific, Custom synthesis) was added to the media at 100 μg/mL.
Data were analyzed by two-way analysis of variance followed by Dunnett’s or Tukey’s multiple comparison tests, as appropriate. This analysis was done using the GraphPad Prism 6 software.
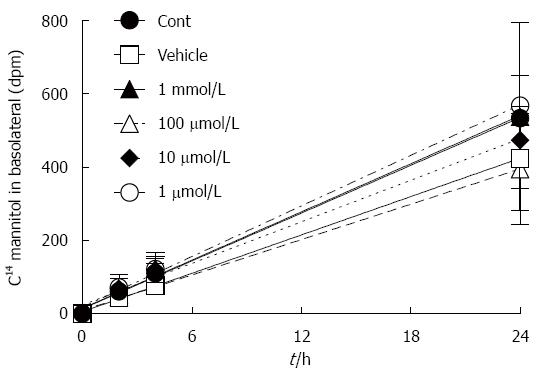
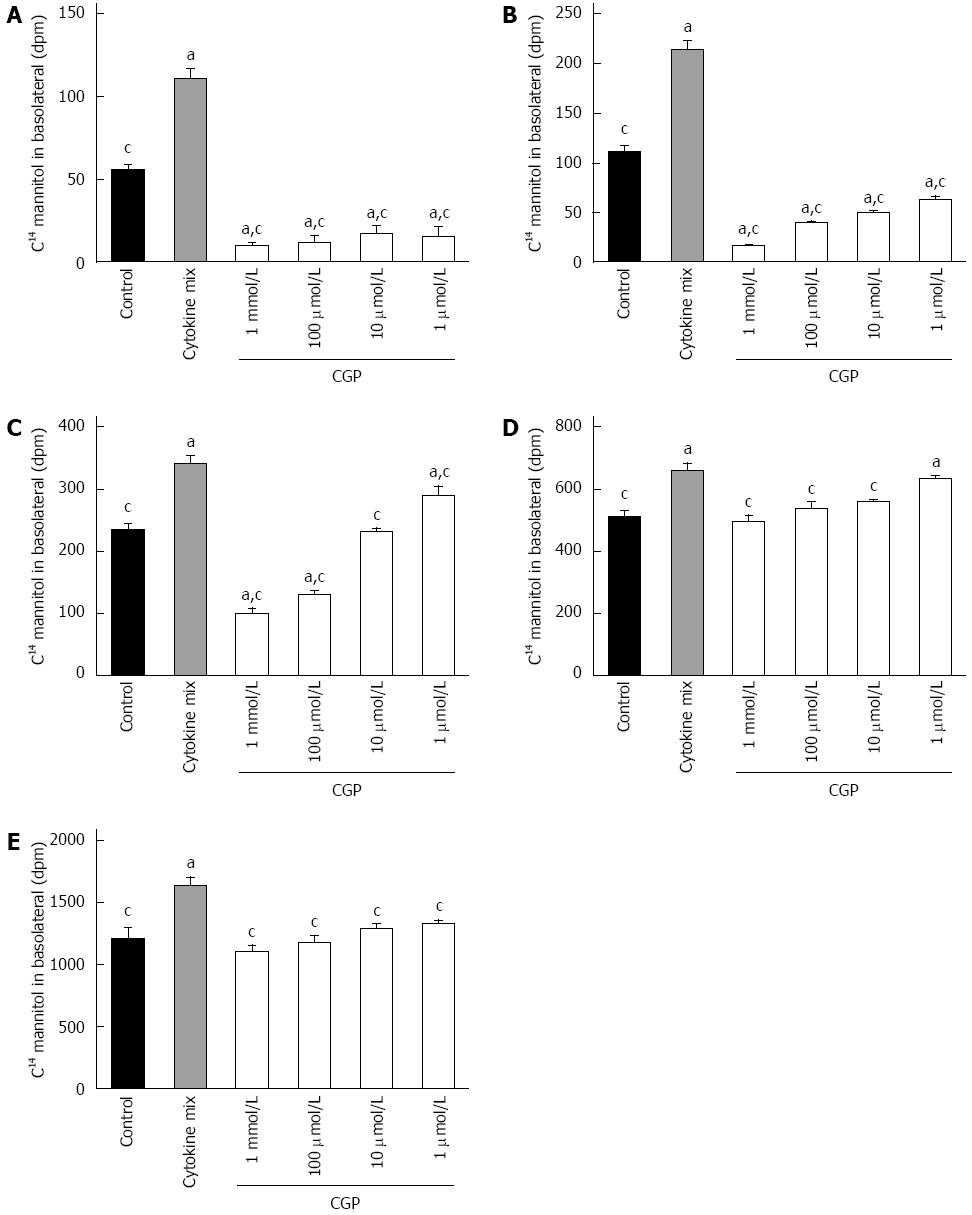
Dpm mannitol movement from the apical to the basolateral fluid under baseline conditions was graphed as a function of time (Figure 1). The slopes of these lines (mannitol flux) are presented in Table 1. CGP had no effect on baseline apical to basolateral mannitol flux. Since there was no significant difference among the treatment groups, a pooled rate of baseline mannitol flux was calculated for the first 4 h, and was 20 ± 1 dpm/h.
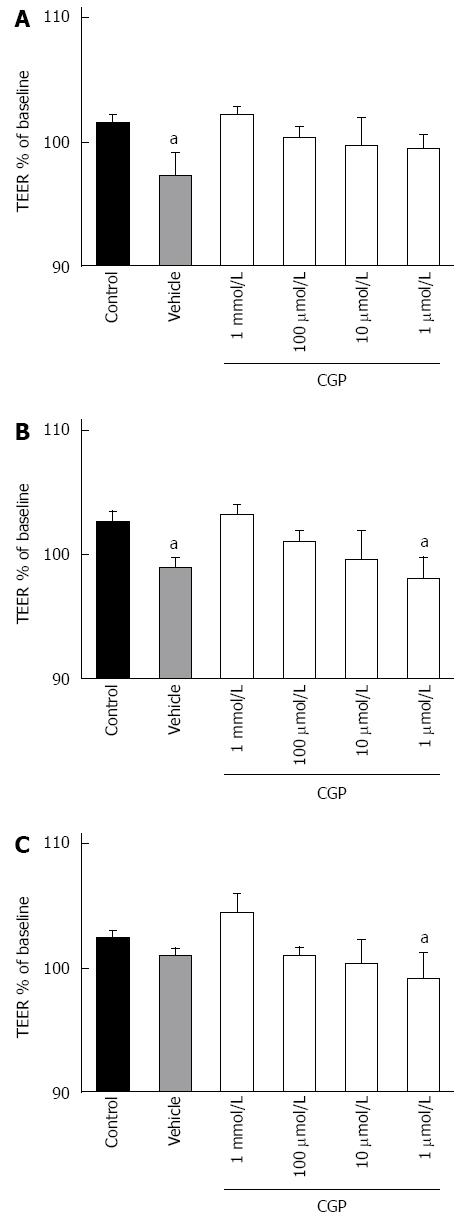
The effect of CGP on baseline TEER is shown in Figure 2. The slope of TEER as a function of time was calculated, and is presented in Table 1. Vehicle used here is citric acid solution (as CGP was dissolved in it) caused a small but statistically significant loss of TEER over time, an effect that was reversed by addition of CGP, in a concentration dependent fashion.
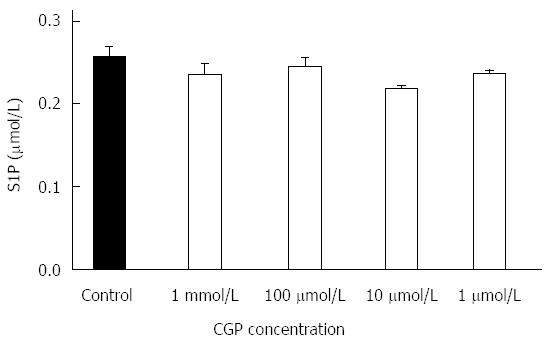
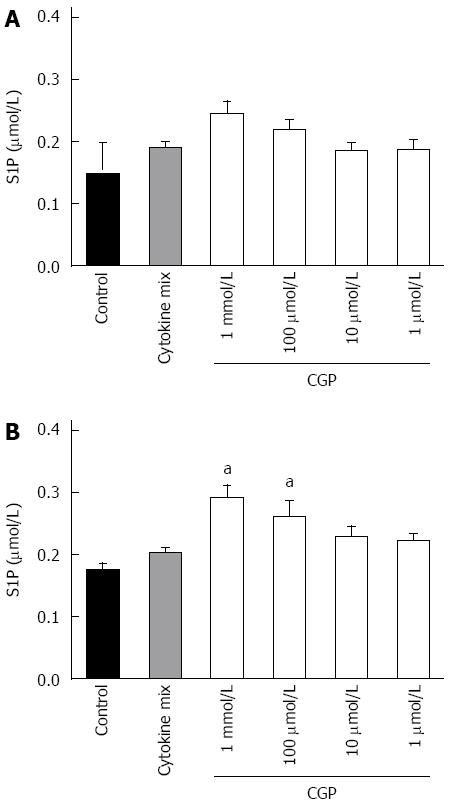
CGP had no effect on 24 h sphingosine 1-phosphate protein concentrations, as shown in Figure 3.
Hypoxia/ischemia is a state of diminished oxygen and nutrient supply such that cellular demands are not met. During hypoxia, intestinal epithelial permeability is increased[14]. We hypothesized that the permeability might be alleviated by CGP treatment. The hypothesis was tested by placing Caco-2 cells in a hypoxia chamber, as described in METHODS, and measuring both apical to basolateral mannitol flux and TEER.
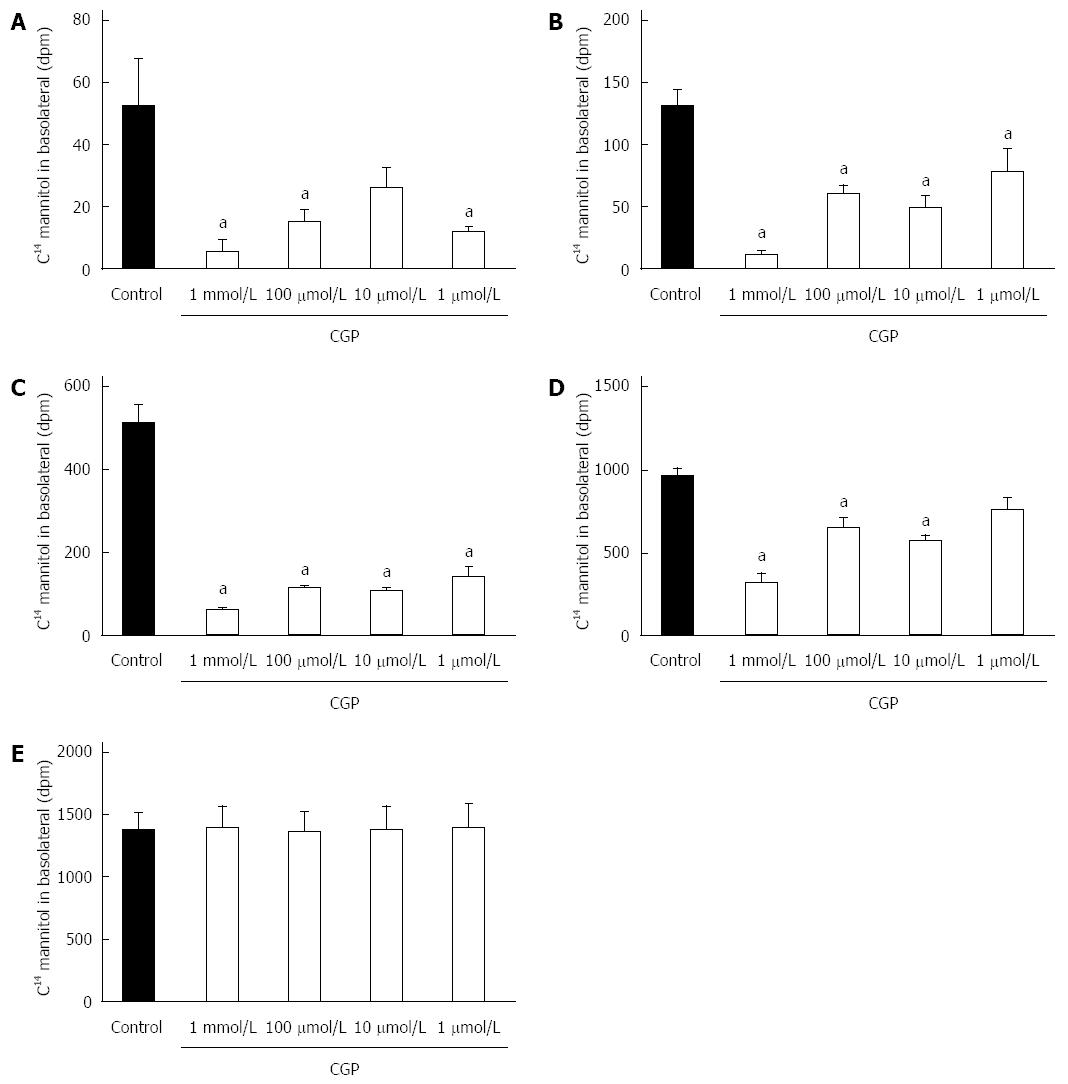
CGP significantly reduced the effect of hypoxia on apical to basolateral mannitol flux. The effect was both concentration and time dependent, and had disappeared by 5 h in all treatment groups (Figure 4). At 5 h, the mean apical to basolateral mannitol flux for all groups was 1380 ± 6 dpm (range 1358 to 1395 dpm). The rate of mannitol flux (dpm/h) was calculated for the first three hours of hypoxia, and results are shown in Table 2. CGP treatment reduced the rate of mannitol flux at all concentrations tested.
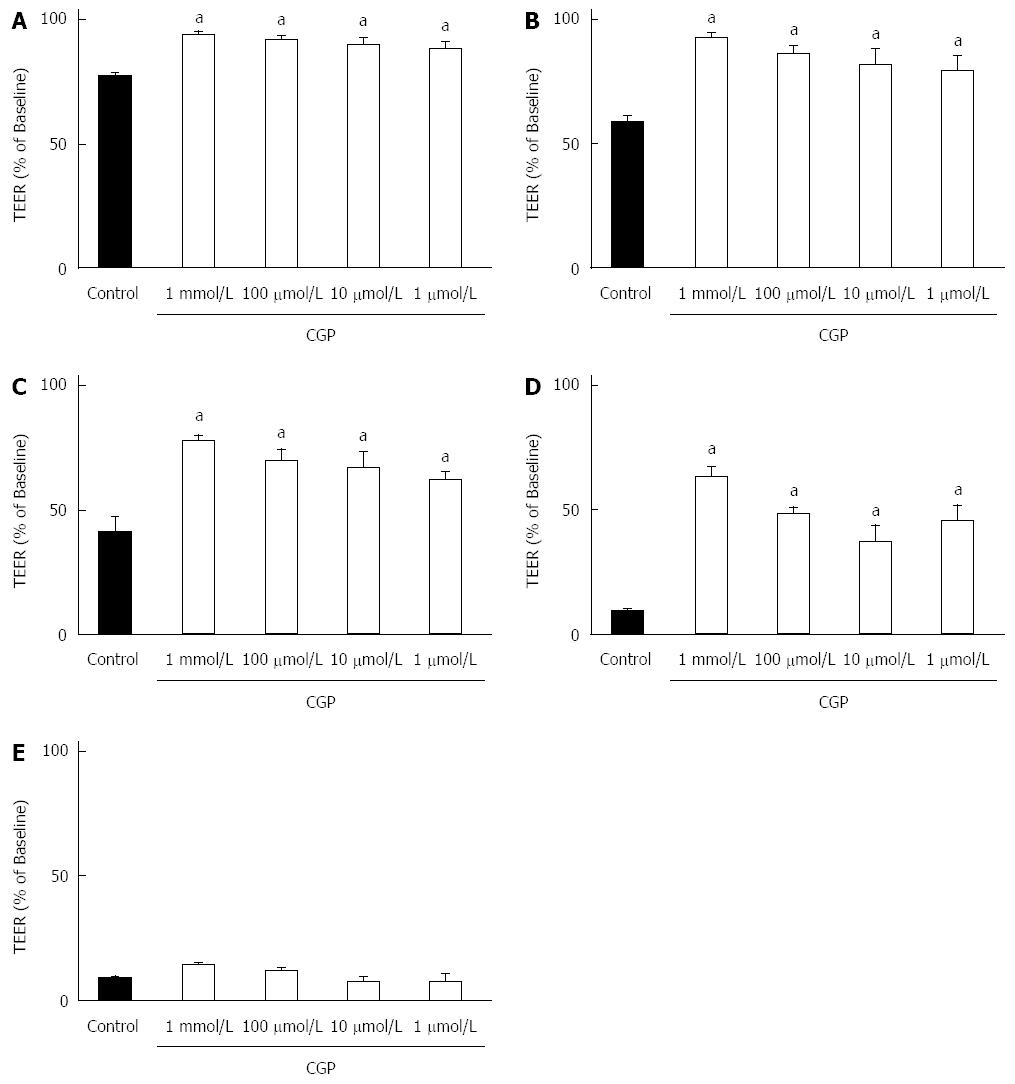
Figure 5 shows the effect of CGP on TEER during hypoxia. Baseline TEER values were > 600 Ωcm2 for all groups. As there is some variability in baseline TEER, all data have been expressed as a % of baseline TEER. After 1-h hypoxia, TEER in control cells was significantly decreased, and continued to drop for the duration of the experiment. CGP preserved TEER in a concentration and time dependent manner such that by 5 h there was no longer a discernable effect. The rate of decrease in TEER during the first three hours of hypoxia is shown in Table 2. The CGP treatment had a significant effect in reducing the rate of TEER loss at all concentrations tested.
In vivo, I/R injury is mediated, at least in part, by neutrophil recruitment[12], with subsequent release of pro-inflammatory cytokines[24] such as TNFα, ILβ and IFNγ. This inflammatory response was mimicked by adding a mixture of these cytokines to the culture media as described in Methods. As shown in Figure 6, cytokine alone increased mannitol flux. CGP decreased cytokine stimulated mannitol flux in a time and concentration dependent manner. The rate of mannitol flux (dpm/h) was calculated for the first 4 h of cytokine exposure, and is presented in Table 3.
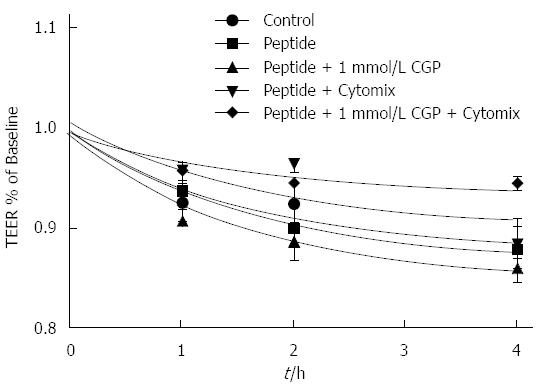
The onset of the effect of cytomix on TEER is slower than for mannitol flux. For the sake of clarity, Figure 7 shows only the 8 and 10 h results. Because there is considerable variability in the absolute TEER at baseline (0 h), values are expressed as a % of baseline. Cytomix does not begin to reduce TEER until 8 h exposure (upper panel). At 10 h, TEER in all CGP treated groups except 1 μmol/L was significantly greater than cytomix alone and not different from control (lower panel). By 24 h, all CGP effect is lost (data not shown). The rates of TEER decline are presented in Table 3.
The effect of CGP on S1P concentrations in the presence of cytomix is shown in Figure 8. Contrary to expectations, cytomix alone failed to decrease S1P. However, in the presence of cytomix, CGP significantly increased S1P concentrations, an effect that was particularly noted at 4 h. These data suggest that perhaps the effect of CGP on cytomix induced mannitol permeability is mediated by S1P. However, the data are insufficient to make any definitive conclusions regarding this mechanism.
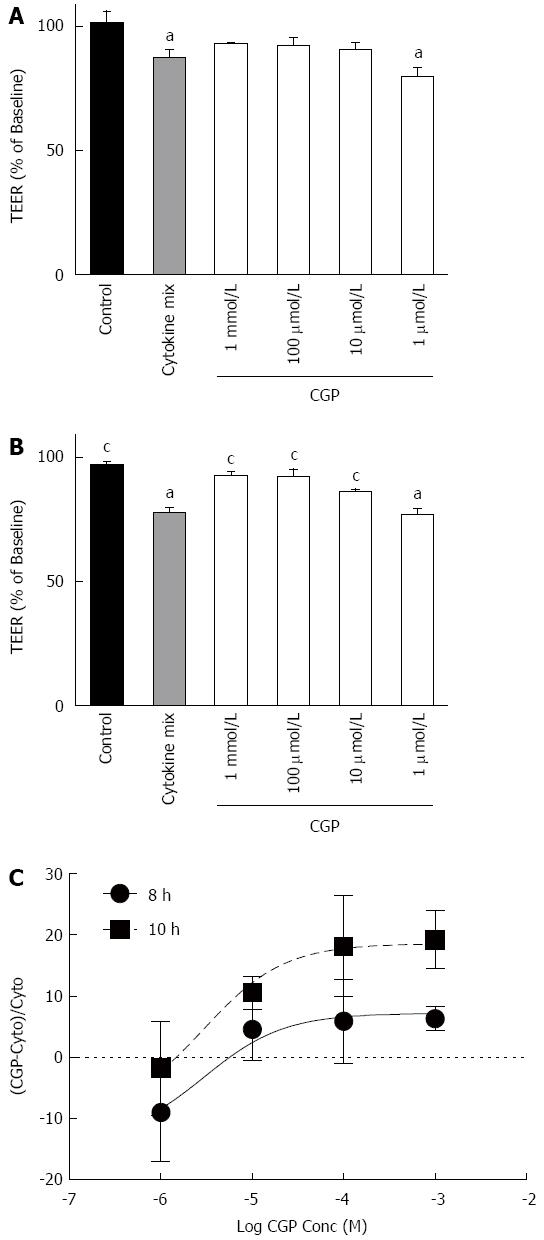
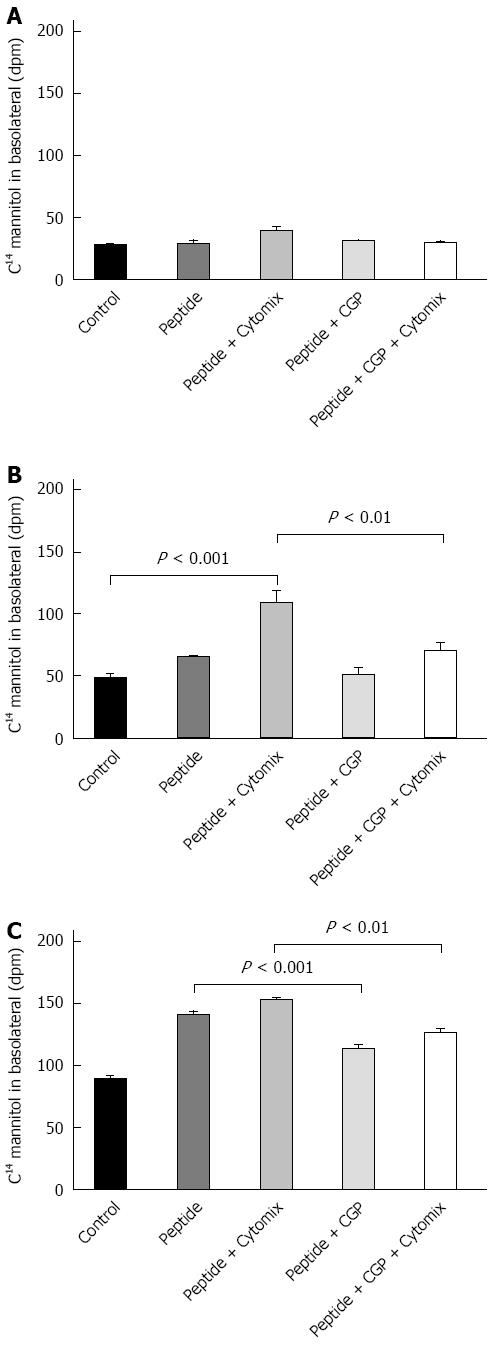
Although celiac disease is not caused by gut ischemia, α-gliadin stimulates cytokine release[25]. The α-gliadin peptide fragment 31-55 is the offending peptide initiating the inflammatory symptoms of celiac disease in susceptible patients[19]. The effect of the peptide fragment on mannitol flux and TEER were measured in the presence and absence of cytokine mix so as to mimic that inflammation. The results of these experiments are shown in Figure 9. At 1 h, there were no differences among the experimental groups. At 2 h, mannitol flux was significantly elevated in the peptide-cytomix treated cells as compared to control. When 1 mmol/L CGP was included with the peptide-cytomix, the mannitol flux at 2 h was not significantly different from control. At 4 h, mannitol flux in both the peptide and peptide + cytomix groups was significantly greater than control. CGP (1 mmol/L) significantly reduced both the peptide-induced and peptide + cytomix induced increases in mannitol flux. The rate of mannitol flux for each experimental group is shown in Table 4.
The effect of CGP on α-gliadin peptide induced reduction in TEER is shown in Figure 10. In contrast to the mannitol experiments, these data indicate that there is only a very small effect of CGP on TEER. Notably, though, the rate of TEER loss in the peptide+cytomix group was 0.0272% ± 0.00497% per hour. In the presence of 1 mmol/L CGP, this rate was significantly less, 0.0120% ± 0.00327% per hour (P = 0.019). At the end of the TEER experiments, the protein was assayed for E-cadherin, a major tight junction protein. Contrary to expectations, E-cadherin was 75.7% ± 8.6% of control (P = 0.0479) in the peptide+cytokine treated cells (data not shown). However, in the peptide+cytokine+CGP treated cells, E-cadherin levels were 101% ± 2.9% of control (P = 0.048 vs peptide+cytokine alone).
To our knowledge, prior studies of gut ischemia/reperfusion injury have focused on whole-animal or intact tissue studies. The present study demonstrates the direct effect of hypoxia/ischemia and cytokine stimulation on a model of intestinal epithelial transport, in the absence of the underlying muscularis and without the complicating factor of blood flow. Furthermore, the study shows that a pharmacologic agent, calcium glycerophosphate, applied directly to Caco-2 cells, attenuates the I/R induced increase in transepithelial permeability.
Under baseline conditions, CGP has, at most, only a small effect on transepithelial permeability. Furthermore, under baseline conditions, CGP does not appear to have any significant effect on S1P concentrations. In contrast, CGP has a significant time and concentration dependent effect to attenuate increased gut permeability caused by hypoxia, cytokine stimulation, and α-gliadin peptide 31-55.
It was somewhat surprising that the cytokine effect on mannitol flux was observed at an early time point, suggesting that the cytokine effect on mannitol flux may not require new protein synthesis. Rather, the time course over which CGP increases S1P in cytokine-stimulated cells suggests that the effect of CGP on mannitol flux may be linked to the increased S1P levels. However, the present data are insufficient to permit any firm conclusions.
Gut hypoperfusion is a ubiquitous complication of CHF[3,6], and contributes to poor nutritional status and CHF-induced cachexia[26]. In 2010, there were more than 1000000 hospital discharges for CHF, up from about 400000 in 1980, and the number continues to rise. The incidence of CHF is about 1% of the population over 65, with a 5 year survival rate of about 50%. The most recent estimate of CHF prevalence is more than 5.1 million persons, a number that is expected to rise to more than 8 million by 2030. In 2010, heart failure was the direct or contributing cause of about 280000 deaths in the United States[27]. To date, treatment of CHF associated gut ischemia has focused on improving the gut perfusion rate, itself dependent entirely on improved overall cardiac performance[28]. We have been unable to find evidence of therapies aimed directly at improving gut epithelial integrity. One could reasonably anticipate that modest improvements in cardiac performance (about all that can currently be achieved) would synergize with improved gut integrity, leading to an overall improvement in the status of the CHF patient.
Aside from CHF, gut ischemia has been identified as a complication of endurance exercise. In the United States, nearly 10 million persons run or jog 110 or more days per year and an additional 3.3 million ride a bicycle 110 or more days per year. A study of long-distance runners and cyclists found that the prevalence of exercise related lower GI symptoms was 71% for runners and 64% for cyclists[1]. The same study revealed that 5% of runners and 6% of cyclists experienced symptoms severe enough to require medication. These symptoms have been linked to splanchnic hypoperfusion[2] and are exacerbated by concomitant administration of non-steroidal anti-inflammatory drugs such as ibuprofen[29]. More recently, it has been suggested that ingestion of L-citrulline might be useful in mitigating the symptoms of exercise induced gut ischemia, most likely by increasing the availability of arginine to vascular endothelial nitric oxide synthase[30]. Like CHF, the proposed treatment rests on improving gut blood flow rather than by directly improving gut epithelial integrity.
Celiac disease is an autoimmune disorder of small intestine characterized by adaptive and innate immune response to gluten present in wheat and barley in genetically susceptible individuals. The enzyme transglutaminase 2 (TG2) modifies the gliadin protein, which results in an inflammatory response. TG2 inhibitors have therefore been suggested as identified drugs for this disease. However it is a debilitating condition, with limited treatment options. These include corticosteroids and monoclonal antibodies aimed at specific mediators of inflammation as well as severely restricted diets. The corticosteroids have significant and potentially damaging side effects, as do the monoclonal antibodies. The latter have the further disadvantage of requiring parenteral administration. Dietary therapies have limited efficacy, where the ubiquitous presence of hidden gluten makes its effective elimination nearly impossible. Our study revealed that use of CGP in the in-vitro cells lowered the mannitol flux increased by the presence of cytomix and peptides. These factors may make it worthwhile to pursue CGP as an adjunct to celiac dietary therapy, as CGP might limit the negative impact of hidden dietary gluten.
Calcium glycerophosphate has been used as a dietary supplement to neutralize excess gastric acid and as a palliative in interstitial cystitis, prostatitis and overactive bladder[31], and is sold under the brand name Prelief by AK Pharma Company. Each tablet contains 345 mg calcium glycerophosphate (65 mg of elemental calcium). Two tablets are recommended per day. The exact mechanism of action is unclear, but Duboqa and Di Trolio have reported that CGP (Prelief) significantly reduced urinary NMP-22, a marker associated with urothelial cell death, interstitial cystitis and bladder cancer (Multinational Interstitial Cystitis Association, Proceeding 2003).
This study adds value to its benefits, as it is found to attenuate the effect of hypoxia and cytokine induced increase in epithelial permeability. Our results suggest that it may act by increasing the concentration of S1P, although alternative mechanisms are possible. Additional research on its mechanism of action is required to further strengthen our hypothesis. So far there have not been any data reflecting this aspect of this action of calcium glycerophosphate.
Ischemia/reperfusion injury is a broad area of medical significance and only few studies have focused on assessment of the direct effects of ischemia on intestinal epithelial integrity. Calcium glycerophosphate (CGP) shows promising results in maintaining the gut epithelial integrity during hypoxia and in the presence of cytokines.
In the Caco-2 cell model of transepithelial transport, CGP, an inhibitor of intestinal alkaline phosphatase F3, has shown to have a significant effect to preserve transepithelial electrical resistance (TEER) and to attenuate increases in mannitol flux rates during hypoxia or cytokine stimulation.
In this article a novel role of CGP has been described in preserving the gut epithelial integrity during hypoxia and in the presence of cytokines. CGP showed a significant time and concentration dependent effect to attenuate increased gut permeability caused by hypoxia, cytokine stimulation and α-gliadin peptide 31-55. The effect was observable even at concentrations as low as 1 μmol/L.
The study results suggest that CGP treatment has the potential to be used with other therapies to preserve gut epithelial integrity.
Epithelial junctions are the critical component of the intrinsic barrier that is disrupted in various medical conditions such as ischemia/reperfusion injury, celiac disease and during inflammation.
The authors examined the effects of Calcium glycerolphosphate on intestinal epithelial barrier under different experimental conditions. They used Caco-2 cell model of transpithelial transport and measured TEER and mannitol flux. This is an interesting article as it describes another aspect of CGP mechanism of action in preserving the transepithelial integrity.
P- Reviewer: Crenn PP, De Silva AP, Zhang L S- Editor: Ma YJ L- Editor: A E- Editor: Ma S
| 1. | Peters HP, Bos M, Seebregts L, Akkermans LM, van Berge Henegouwen GP, Bol E, Mosterd WL, de Vries WR. Gastrointestinal symptoms in long-distance runners, cyclists, and triathletes: prevalence, medication, and etiology. Am J Gastroenterol. 1999;94:1570-1581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 106] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 2. | van Wijck K, Lenaerts K, Grootjans J, Wijnands KA, Poeze M, van Loon LJ, Dejong CH, Buurman WA. Physiology and pathophysiology of splanchnic hypoperfusion and intestinal injury during exercise: strategies for evaluation and prevention. Am J Physiol Gastrointest Liver Physiol. 2012;303:G155-G168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 182] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 3. | Sandek A, Bauditz J, Swidsinski A, Buhner S, Weber-Eibel J, von Haehling S, Schroedl W, Karhausen T, Doehner W, Rauchhaus M. Altered intestinal function in patients with chronic heart failure. J Am Coll Cardiol. 2007;50:1561-1569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 363] [Cited by in RCA: 477] [Article Influence: 26.5] [Reference Citation Analysis (0)] |
| 4. | Krüger S, Kunz D, Graf J, Stickel T, Merx MW, Koch KC, Janssens U, Hanrath P. Endotoxin hypersensitivity in chronic heart failure. Int J Cardiol. 2007;115:159-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 5. | Arvidsson D, Rasmussen I, Almqvist P, Niklasson F, Haglund U. Splanchnic oxygen consumption in septic and hemorrhagic shock. Surgery. 1991;109:190-197. [PubMed] |
| 7. | Jakob SM, Ruokonen E, Vuolteenaho O, Lampainen E, Takala J. Splanchnic perfusion during hemodialysis: evidence for marginal tissue perfusion. Crit Care Med. 2001;29:1393-1398. [PubMed] |
| 8. | Prior AM, Zhang M, Blakeman N, Datta P, Pham H, Chen Q, Young LH, Weis MT, Hua DH. Inhibition of long chain fatty acyl-CoA synthetase (ACSL) and ischemia reperfusion injury. Bioorg Med Chem Lett. 2014;24:1057-1061. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 9. | Blakeman N, Chen Q, Tolson J, Rueter B, Diaz B, Casey B, Young LH, Weis MT. Triacsin C, a Fatty Acyl CoA Synthetase Inhibitor, Improves Cardiac Performance Following Global Ischemia. Am J Biomed Sci. 2012;4:249-261. |
| 10. | Sardjono CT, Harbour SN, Yip JC, Paddock C, Tridandapani S, Newman PJ, Jackson DE. Palmitoylation at Cys595 is essential for PECAM-1 localisation into membrane microdomains and for efficient PECAM-1-mediated cytoprotection. Thromb Haemost. 2006;96:756-766. [PubMed] |
| 11. | Sim DS, Dilks JR, Flaumenhaft R. Platelets possess and require an active protein palmitoylation pathway for agonist-mediated activation and in vivo thrombus formation. Arterioscler Thromb Vasc Biol. 2007;27:1478-1485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 12. | Esposito E, Mazzon E, Muià C, Meli R, Sessa E, Cuzzocrea S. Splanchnic ischemia and reperfusion injury is reduced by genetic or pharmacological inhibition of TNF-alpha. J Leukoc Biol. 2007;81:1032-1043. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 22] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 13. | Cavriani G, Domingos HV, Oliveira-Filho RM, Sudo-Hayashi LS, Vargaftig BB, de Lima WT. Lymphatic thoracic duct ligation modulates the serum levels of IL-1beta and IL-10 after intestinal ischemia/reperfusion in rats with the involvement of tumor necrosis factor alpha and nitric oxide. Shock. 2007;27:209-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 37] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 14. | Xu DZ, Lu Q, Kubicka R, Deitch EA. The effect of hypoxia/reoxygenation on the cellular function of intestinal epithelial cells. J Trauma. 1999;46:280-285. [PubMed] |
| 15. | Qiu Y, Yu M, Yang Y, Sheng H, Wang W, Sun L, Chen G, Liu Y, Xiao W, Yang H. Disturbance of intraepithelial lymphocytes in a murine model of acute intestinal ischemia/reperfusion. J Mol Histol. 2014;45:217-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 16. | Greenspon J, Li R, Xiao L, Rao JN, Sun R, Strauch ED, Shea-Donohue T, Wang JY, Turner DJ. Sphingosine-1-phosphate regulates the expression of adherens junction protein E-cadherin and enhances intestinal epithelial cell barrier function. Dig Dis Sci. 2011;56:1342-1353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 44] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 17. | Tardivel S, Razanamaniraka L, Porembska Z, Crouzoulon G, Fournier P, Dupuis Y. Homodimer and heterodimer forms of adult rat intestinal alkaline phosphatase. Life Sci. 1988;43:2059-2065. [PubMed] |
| 18. | Edsall LC, Spiegel S. Enzymatic measurement of sphingosine 1-phosphate. Anal Biochem. 1999;272:80-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 152] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 19. | Iacomino G, Fierro O, D’Auria S, Picariello G, Ferranti P, Liguori C, Addeo F, Mamone G. Structural analysis and Caco-2 cell permeability of the celiac-toxic A-gliadin peptide 31-55. J Agric Food Chem. 2013;61:1088-1096. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 20. | Jumarie C, Malo C. Caco-2 cells cultured in serum-free medium as a model for the study of enterocytic differentiation in vitro. J Cell Physiol. 1991;149:24-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 163] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 21. | Caputo I, Secondo A, Lepretti M, Paolella G, Auricchio S, Barone MV, Esposito C. Gliadin peptides induce tissue transglutaminase activation and ER-stress through Ca2+ mobilization in Caco-2 cells. PLoS One. 2012;7:e45209. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 40] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 22. | Rauhavirta T, Oittinen M, Kivistö R, Männistö PT, Garcia-Horsman JA, Wang Z, Griffin M, Mäki M, Kaukinen K, Lindfors K. Are transglutaminase 2 inhibitors able to reduce gliadin-induced toxicity related to celiac disease? A proof-of-concept study. J Clin Immunol. 2013;33:134-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 40] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 23. | Yang T, Roder KE, Bhat GJ, Thekkumkara TJ, Abbruscato TJ. Protein kinase C family members as a target for regulation of blood-brain barrier Na,K,2Cl-cotransporter during in vitro stroke conditions and nicotine exposure. Pharm Res. 2006;23:291-302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 24. | Anker SD, Volterrani M, Egerer KR, Felton CV, Kox WJ, Poole-Wilson PA, Coats AJ. Tumour necrosis factor alpha as a predictor of impaired peak leg blood flow in patients with chronic heart failure. QJM. 1998;91:199-203. [PubMed] |
| 25. | Larsen J, Dall M, Antvorskov JC, Weile C, Engkilde K, Josefsen K, Buschard K. Dietary gluten increases natural killer cell cytotoxicity and cytokine secretion. Eur J Immunol. 2014;44:3056-3067. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 26] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 26. | Sandek A, Valentova M, von Haehling S, Doehner W, Anker SD. The small intestine: a critical linkage in pathophysiology of cardiac cachexia. Int J Cardiol. 2011;146:277-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 27. | Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Blaha MJ, Dai S, Ford ES, Fox CS, Franco S. Executive summary: heart disease and stroke statistics--2014 update: a report from the American Heart Association. Circulation. 2014;129:399-410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1070] [Cited by in RCA: 1119] [Article Influence: 101.7] [Reference Citation Analysis (0)] |
| 28. | Anker SD, Negassa A, Coats AJ, Afzal R, Poole-Wilson PA, Cohn JN, Yusuf S. Prognostic importance of weight loss in chronic heart failure and the effect of treatment with angiotensin-converting-enzyme inhibitors: an observational study. Lancet. 2003;361:1077-1083. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 510] [Cited by in RCA: 516] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 29. | Van Wijck K, Lenaerts K, Van Bijnen AA, Boonen B, Van Loon LJ, Dejong CH, Buurman WA. Aggravation of exercise-induced intestinal injury by Ibuprofen in athletes. Med Sci Sports Exerc. 2012;44:2257-2262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 73] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 30. | van Wijck K, Wijnands KA, Meesters DM, Boonen B, van Loon LJ, Buurman WA, Dejong CH, Lenaerts K, Poeze M. L-citrulline improves splanchnic perfusion and reduces gut injury during exercise. Med Sci Sports Exerc. 2014;46:2039-2046. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 62] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 31. | Bassaly R, Downes K, Hart S. Dietary consumption triggers in interstitial cystitis/bladder pain syndrome patients. Female Pelvic Med Reconstr Surg. 2011;17:36-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 43] [Article Influence: 3.6] [Reference Citation Analysis (0)] |