Published online Jan 21, 2015. doi: 10.3748/wjg.v21.i3.988
Peer-review started: July 20, 2014
First decision: August 27, 2014
Revised: September 11, 2014
Accepted: October 20, 2014
Article in press: October 21, 2014
Published online: January 21, 2015
Processing time: 186 Days and 2.4 Hours
AIM: To investigate whether liver lobe volume and albumin (ALB) could predict the presence and severity of liver cirrhosis, and esophageal varices.
METHODS: Seventy-one cirrhotic patients with hepatitis B and 21 healthy individuals were enrolled in this study. All the participants underwent abdominal enhanced magnetic resonance imaging to measure each liver lobe volume, and biochemical workup for testing ALB and Child-Pugh class. All cirrhotic patients underwent upper gastrointestinal endoscopy to show the presence of cirrhotic esophageal varices. Right liver lobe volume (RV), left medial liver lobe volume (LMV), left lateral liver lobe volume (LLV), and caudate lobe volume (CV) were measured using enhanced magnetic resonance imaging. The ratios of RV to ALB (RV/ALB), LMV to ALB (LMV/ALB), LLV to ALB (LLV/ALB) and CV to ALB (CV/ALB) were calculated. Statistical analyses were performed to determine whether and how the combination of liver lobe volume measured using magnetic resonance imaging and albumin could predict the presence and severity of liver cirrhosis, and the presence of esophageal varices.
RESULTS: RV, LMV, LLV and CV decreased (r = -0.51-0.373; all P < 0.05), while RV/ALB increased (r = 0.424; P < 0.05), with the progress of Child-Pugh class of liver cirrhosis. RV, LMV, CV, LLV/ALB and CV/ALB could identify presence of liver cirrhosis; LLV and LMV could distinguish Child-Pugh class A from B; RV, LMV, LLV, CV, RV/ALB and LLV/ALB could distinguish class A from C; RV and LLV/ALB could differentiate B from C; and RV, RV/ALB and CV/ALB could identify presence of esophageal varices (all P < 0.05). Among these parameters, CV/ALB could best identify the presence of liver cirrhosis, with an area under receiver operating characteristic curve (AUC) of 0.860, a sensitivity of 82.0% and a specificity of 83.0%. LLV could best distinguish class A from B, with an AUC of 0.761, a sensitivity of 74.4% and a specificity of 73.1%. RV could best distinguish class A from C, with an AUC of 0.900, a sensitivity of 90.3% and a specificity of 84.5%. LLV/ALB could best distinguish class B from C, with an AUC of 0.900, a sensitivity of 93.8% and a specificity of 81.5%. RV/ALB could best identify esophageal varices, with an AUC of 0.890, a sensitivity of 80.0% and a specificity of 83.5%.
CONCLUSION: The combination of liver lobe volume and ALB has potential to identify presence and severity of cirrhosis, and presence of esophageal varices.
Core tip: We determined whether and how the combination of albumin and liver lobe volume (measured using magnetic resonance imaging) could predict the presence and severity of liver cirrhosis, and the presence of esophageal varices. The ratio of caudate lobe volume to albumin could identify the occurrence of cirrhosis, and that of left lateral liver lobe volume, right liver lobe volume, and the ratio of left lateral liver lobe volume to albumin could differentiate Child-Pugh class A from B, A from C, and B from C, respectively. The right liver lobe volume to albumin ratio could identify the presence of esophageal varices.
- Citation: Li H, Chen TW, Li ZL, Zhang XM, Li CJ, Chen XL, Chen GW, Hu JN, Ye YQ. Albumin and magnetic resonance imaging-liver volume to identify hepatitis B-related cirrhosis and esophageal varices. World J Gastroenterol 2015; 21(3): 988-996
- URL: https://www.wjgnet.com/1007-9327/full/v21/i3/988.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i3.988
Liver cirrhosis is a common condition that causes progressive liver dysfunction. In the early stages of cirrhosis, the liver is still compensating and application of adequate therapy can help prolong sufficient liver function. When the liver function decompensates, the patient is in end-stage liver disease and has a high risk of developing complications, such as gastrointestinal bleeding[1]. Therefore, it is important to follow up the progress of this disease and determine the stage of cirrhosis[2]. The modified Child-Pugh classification system has been confirmed as an independent prognostic factor for survival of cirrhotic patients, and can be utilized to adequately assess liver transplantation candidates[3,4].
The morphology of the liver changes with the progress of Child-Pugh classification. Previous studies reported that changes in liver lobe volume were positively correlated with prognosis and Child-Pugh classifications[4]. One interesting study focused on the correlation between the ratio of right liver lobe diameter to albumin and Child-Pugh classifications, and clarifying the significant correlation in classifying cirrhosis[5]. In addition, esophageal varices are one of the major complications of liver cirrhosis, with a risk of bleeding from varices of approximately 25%-35%[6]. Prophylactic endoscopic variceal ligation can decrease the incidence of first variceal bleeding and mortality in cirrhotic patients who have large varices[6]. Nevertheless, repeated endoscopic examinations are not accepted for patients and are expensive. As a safe, effective and repeatable noninvasive modality, magnetic resonance imaging (MRI) has increasingly been used to assess liver diseases[7,8]. Previous studies reported that liver volume indexes measured on MRI could be used as a method for grading the severity of cirrhosis[9,10]. To the best of our knowledge, there was no study focusing on the combination of liver lobe volume measured on MRI with albumin to assess the presence of cirrhosis and define its Child-Pugh classifications[11]. There were also no reports on the combination of liver lobe volume with albumin to determine the presence of esophageal varices in cirrhotic patients. Therefore, we aimed to determine how liver lobe volume and the ratio of the liver lobe volume to albumin could determine the presence and Child-Pugh class of liver cirrhosis, and the presence of esophageal varices.
The institutional human research review committee of our hospital approved this study. Written informed consent was obtained from each patient before the prospective study.
The study included 96 consecutive patients with confirmed liver cirrhosis between February 2012 and December 2013. The inclusion criteria were: (1) the diagnosis of cirrhosis in patients with hepatitis B was based on physical findings, laboratory investigations, image findings or histopathological findings, whenever available, according to the American Association for the Study of Liver Diseases practice guidelines on chronic hepatitis B (2007)[12]; (2) the patients underwent abdominal triple-phase enhanced MRI scans, biochemical workup and upper gastrointestinal endoscopy; and (3) image data showed patients without portal vein-emboli or hepatic carcinoma. This biochemical workup was used to achieve the Child-Pugh score calculation using five parameters including, albumin (ALB), ascites, bilirubin, prothrombin activity and encephalopathy[3]. The endoscopy was to demonstrate the presence of esophageal varices. The exclusion criteria were: (1) patients had a history of treatments for portal hypertension (n = 10); (2) patients had primary hematological disorders, such as lymphoma and leukemia (n = 2); and (3) patients had active alcohol abuse (less than six months of alcohol abstinence) (n = 13). Consequently, 71 patients (36 men and 35 women; age range, 31-76 years; median age, 59 years) were enrolled into this study. In this cohort, 33 patients (46.5%) had ascites, 15 patients (21.1%) had esophageal varices, 10 patients (14.1%) had both ascites and esophageal varices, and 13 patients (18.3%) had neither ascites nor esophageal varices. According to the Child-Pugh classification system, 27, 28 and 16 patients were categorized into Child-Pugh class A, B and C, respectively.
Additionally, 21 random consecutive healthy volunteers with no history of chronic liver disease (12 men and 9 women; median age 58 years; range: 38-70 years) who underwent upper abdominal triphasic enhancement MRI and biochemical workup at our institution served as the reference group.
Each participant underwent MRI scans supinely with a 3.0-T scanner (Signa Excite; GE Medical Systems, Milwaukee, WI, United States) in an 8-channel phased array body coil after the establishment of respiratory signals from the diaphragm to the inferior border of the spleen to cover the entire liver. The routine MRI sequences included spoiled gradient recalled T1- and fast recovery fast spin echo T2-weighted imaging. Subsequently, each patient received an injection of standard dose (0.2 mmol/kg of body weight) of gadodiamide (Magnevist; Bayer Healthcare, Germany) at a standard flow rate (3 mL/s) through a 21-gauge peripheral venous access followed by a 20-mL saline solution flush. After the previous injection, each participant underwent axial three-dimensional liver acquisition with volume acceleration (3D-LAVA), with a repetition time of 3.9 ms, an echo time of 1.8 ms, a field of view of 34 cm × 34 cm, a slice thickness of 5.0 mm, a slice gap of zero and a matrix of 256 mm × 224 mm.
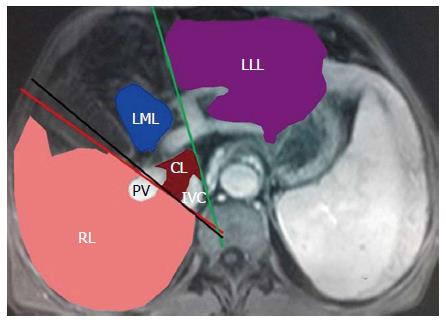
The analysis of the original MRI data was performed on a workstation (GE Advantage Workstation Version 4.4-09; Sun Microsystems, Palo Alto, CA, United States). The portal venous phase images were used for the above-mentioned analysis because the boundary of each liver lobe could be traced more clearly on the portal venous phase than on arterial or delayed phase[13]. As depicted in the Goldsmith and Woodburne system[14], the liver comprises four lobes including left lateral and medial lobes, right lobe and caudate lobe (Figure 1). Each liver lobe volume was measured retrospectively and independently by two experienced abdominal radiologists (Tian-wu Chen and Hang Li), without the knowledge of clinical data. On each axial 3D-LAVA image, liver lobe contour was manually drawn, excluding the inferior vena cava and gallbladder, and the cross-sectional area of each liver lobe was automatically calculated by the software[15]. This previous data analysis on each contiguous transverse level was repeated until the entire liver lobe was covered. Right liver lobe volume (RV), left medial liver lobe volume (LMV), left lateral liver lobe volume (LLV), and caudate lobe volume (CV) were acquired by the sum of the corresponding liver lobe areas × section thickness[15]. Based on each liver lobe volume and albumin, the ratios of RV to albumin (RV/ALB), of LMV to albumin (LMV/ALB), of LLV to albumin (LLV/ALB), and of CV to albumin (CV/ALB) were calculated.
The MRI data of the 71 cirrhotic patients were randomly chosen to test the interobserver variability of the measurements. In the 71 cirrhotic patients, the interobserver agreement in liver lobe volume measurements between the two independent observers was assessed using coefficient of variation coefficient of variation (mean ± SD, × 100)[16]. When coefficient of variation was less than 10%, interobserver variability was considered to be small, and the averaged value of the two observers’ measurements was regarded as the final liver lobe volume parameter[17]. If coefficient of variation exceeded 10%, the previous observers made two further measurements and an average of the four measurements was used as the final liver lobe volume parameter.
The relationship between each liver lobe volume parameter and Child-Pugh class was tested by Spearman’s rank correlation analyses. The Mann-Whitney U test was used to compare liver lobe volume parameters among Child-Pugh classifications, with Bonferroni correction for multigroup comparisons. The two independent samples test was performed to compare each liver lobe volume parameter between patients with and without esophageal varices. If there were significant positive findings in any liver lobe volume parameter classified by Child-Pugh classifications, receiver-operating characteristic (ROC) analysis was performed to determine if the cutoff values of liver lobe volume parameter could help identify the presence and the Child-Pugh class of cirrhosis. When statistically positive findings were found in the comparison of any liver lobe volume parameter between patients with and without esophageal varices, ROC analysis was performed to determine if the cutoff values of the liver lobe volume parameters could help predict the presence of esophageal varices. P values < 0.05 were accepted as significant.
The mean coefficient of variation in each liver lobe volume parameter measurement and the numbers of patients with coefficient of variation less than 10% and exceeding 10% are shown in Table 1. For two observers’ measurements of each liver lobe volume parameter in the 71 cirrhotic patients, the interobserver variability was low when the coefficient of variation was less than 10%, and the averaged value of each liver lobe volume parameter obtained by the two observers was used for subsequent analyses. For the two observers’ measurements of RV in 12 patients, LMV in 10 patients, LLV in eight patients, CV in two patients and RV/ALB in one patient, the coefficient of variation exceeded 10%; therefore, two additional measurements were obtained and an average of the four measurements was used as the final liver lobe volume parameter.
| Liver lobe volume parameters | Mean coefficient of variation (range) | ≤ 10% (n) | > 10% (n) |
| RV | 7.5% (2%-14%) | 59 | 12 |
| LMV | 8.6% (3%-15%) | 61 | 10 |
| LLV | 8.2% (3%-13%) | 63 | 8 |
| CV | 6.0% (2%-11%) | 69 | 2 |
| RV/ALB | 5.5% (1%-11%) | 70 | 1 |
| LMV/ALB | 6.4% (1%-10%) | 71 | 0 |
| LLV/ALB | 6.2% (2%-10%) | 71 | 0 |
| CV/ALB | 4.0% (2%-9%) | 71 | 0 |
The possible clinical data, including the gender, age, body weight, body mass index, and liver lobe volume parameters of all the participants are shown in Table 2. Cirrhotic patients were more likely to have lower RV (P < 0.001) and LMV (P = 0.001), and larger CV (P = 0.001), LLV/ALB (P < 0.001) and CV/ALB (P < 0.001) than the healthy volunteers. RV, LMV, CV, LLV/ALB and CV/ALB could identify the presence of liver cirrhosis. However, no significant differences were found in gender (P = 0.756), age (P = 0.135), body weight (P = 0.08), body mass index (P = 0.056), LLV (P = 0.06), RV/ALB (P = 0.631) and LMV/ALB (P = 0.564) between cirrhotic patients and healthy volunteers.
| No cirrhosis | Child-Pugh class of cirrhosis | |||
| (n = 21) | Class A (n = 27) | Class B (n = 28) | Class C (n = 16) | |
| Gender (M/F) | 12/9 | 12/15 | 13/15 | 11/5 |
| Age | 56.23 ± 13.02 | 59.43 ± 12.93 | 54.57 ± 12.59 | 53.56 ± 16.13 |
| Body weight (kg) | 65.42 ± 5.34 | 60.53 ± 3.20 | 57.61 ± 2.05 | 55.33 ± 1.53 |
| BMI (kg/m2) | 23.15 ± 0.54 | 22.42 ± 0.45 | 21.25 ± 0.31 | 19.41 ± 0.24 |
| RV (mm3) | 806.45 ± 198.891 | 649.60 ± 123.46 | 586.98 ± 137.283 | 470.58 ± 46.034 |
| LMV (mm3) | 234.29 ± 70.341 | 193.23 ± 47.052 | 161.27 ± 43.04 | 147.47 ± 83.754 |
| LLV (mm3) | 215.51 ± 133.63 | 279.60 ± 95.332 | 218.69 ± 35.47 | 208.49 ± 36.174 |
| CV (mm3) | 20.28 ± 9.351 | 34.36 ± 10.46 | 29.15 ± 12.23 | 22.41 ± 10.944 |
| ALB (g/L) | 45.27 ± 3.46 | 37.82 ± 4.07 | 33.24 ± 2.56 | 26.76 ± 3.23 |
| RV/ALB | 17.59 ± 4.31 | 16.98 ± 3.03 | 18.61 ± 4.12 | 20.45 ± 3.554 |
| LMV/ALB | 5.52 ± 1.73 | 5.16 ± 1.39 | 5.15 ± 1.45 | 6.43 ± 3.81 |
| LLV/ALB | 5.14 ± 3.411 | 7.29 ± 2.95 | 7.08 ± 1.263 | 9.19 ± 1.404 |
| CV/ALB | 0.48 ± 0.241 | 0.87 ± 0.28 | 0.95 ± 0.43 | 0.96 ± 0.45 |
RV (r = -0.519, P < 0.001), LMV (r = -0.415, P = 0.007), LLV (r = -0.437, P = 0.002) and CV (r = -0.373, P = 0.01) decreased, while RV/ALB (r = 0.424; P = 0.005) increased, with progressive Child-Pugh class of cirrhosis. Spearman’s rank correlation analyses could not be performed to assess the correlations of LMV/ALB, LLV/ALB or CV/ALB with Child-Pugh class of cirrhosis because no upward or downward trend was found in these parameters, as shown in Table 2. LLV (P = 0.002) and LMV (P = 0.004) could distinguish class A from B; RV (P <0.001), LMV (P = 0.019), LLV (P = 0.001), CV (P = 0.001), RV/ALB (P = 0.001) and LLV/ALB (P = 0.015) could distinguish class A from C; and RV (P = 0.001) and LLV/ALB (P < 0.001) could differentiate class B from C.
We only predicted the esophageal varices rather than gastric varices because esophageal varices are one of the major complications of liver cirrhosis, with the risk of bleeding from varices of approximately 25%-35%[5]. Comparison of each liver lobe volume parameter between cirrhotic patients with and without esophageal varices is illustrated in Table 3. As shown by the two independent samples test, RV in patients without esophageal varices was larger than in those with esophageal varices (P < 0.001). RV/ALB (P < 0.001); CV/ALB (P = 0.04) in patients with esophageal varices were larger than in those without esophageal varices.
| Parameters | Esophageal varices | |
| No (n = 46) | Yes (n = 25) | |
| RV (mm3) | 687.85 ± 175.731 | 534.87 ± 85.86 |
| LMV (mm3) | 190.01 ± 63.70 | 167.18 ± 66.70 |
| LLV (mm3) | 544.26 ± 98.74 | 216.05 ± 39.04 |
| CV (mm3) | 27.52 ± 12.83 | 27.61 ± 8.54 |
| RV/ALB | 16.98 ± 3.361 | 21.26 ± 3.01 |
| LMV/ALB | 5.26 ± 1.68 | 5.96 ± 2.96 |
| LLV/ALB | 6.91 ± 2.77 | 7.78 ± 1.92 |
| CV/ALB | 0.78 ± 0.411 | 0.97 ± 0.31 |
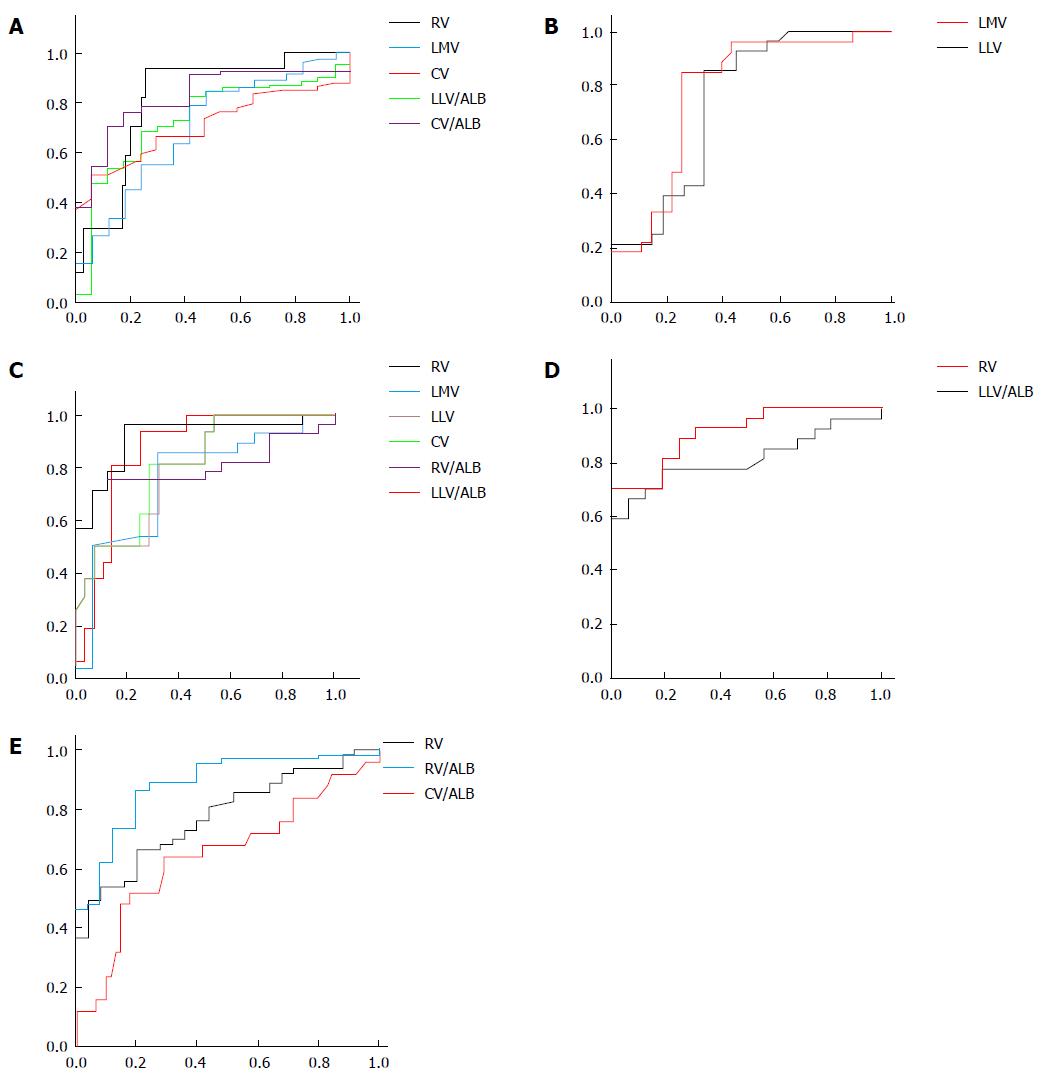
In this study, ROC analyses of liver lobe volume parameters were performed to discriminate between patients with and without liver cirrhosis, and distinguish Child-Pugh class A from B, A from C, and B from C. The ROC analyses were also carried out to differentiate between patients with and without esophageal varices. The AUC, cutoff values, satisfactory sensitivity and specificity for the previous differentiations are shown in Table 4. Among these parameters, CV/ALB (AUC = 0.86), LLV (AUC = 0.761), RV (AUC = 0.9) and LLV/ALB (AUC = 0.9) were the best noninvasive factors to distinguish cirrhotic patients from healthy participants (Figure 2A), Child-Pugh class A from B (Figure 2B), A from C (Figure 2C), and B from C (Figure 2D), respectively. RV/ALB (AUC = 0.890) was the best predictor for identifying the presence of esophageal varices in cirrhotic patients (Figure 2E). The best liver lobe volume parameters for differentiating the presence of cirrhosis and Child-Pugh classification, and predicting the presence of esophageal varices, are shown in Table 5.
| Parameters | Cut-off | Differentiations | AUC | Sensitivity | Specificity |
| RV (mm3) | 692.3 | No cirrhosis vs cirrhosis | 0.816 | 70.6% | 75% |
| 508.9 | Class A vs C | 0.900 | 90.3% | 84.5% | |
| 522.2 | Class B vs C | 0.803 | 70.0% | 88% | |
| 579.45 | No varices vs varices | 0.780 | 71.4% | 70.0% | |
| LMV (mm3) | 201.3 | No cirrhosis vs cirrhosis | 0.754 | 70.6% | 77.0% |
| 181.1 | Class A vs B | 0.728 | 68.0% | 71.0% | |
| 155.4 | Class A vs C | 0.751 | 82.1% | 75.0% | |
| LLV (mm3) | 233.2 | Class A vs B | 0.761 | 74.4% | 73.1% |
| 224.9 | Class A vs C | 0.792 | 82.1% | 75.0% | |
| CV (mm3) | 23.8 | No cirrhosis vs cirrhosis | 0.756 | 69.0% | 65.0% |
| 25.1 | Class A vs C | 0.806 | 85.7% | 69.0% | |
| RV/ALB | 19.9 | Class A vs C | 0.801 | 68.8% | 79.6% |
| 20.46 | No varices vs varices | 0.890 | 80.0% | 83.5% | |
| LLV/ALB | 0.9 | No cirrhosis vs cirrhosis | 0.763 | 70.6% | 71.0% |
| 8.3 | Class A vs C | 0.752 | 68.8% | 65.5% | |
| 7.5 | Class B vs C | 0.900 | 93.8% | 81.5% | |
| CV/ALB | 0.6 | No cirrhosis vs cirrhosis | 0.860 | 82.0% | 83.0% |
| 0.825 | No varices vs varices | 0.673 | 64.0% | 67.0% |
| Parameter | Cut-off | Differentiations | Sensitivity | Specificity |
| CV/ALB | 0.6 | No cirrhosis vs cirrhosis | 82.0% | 83.00% |
| RV (mm3) | 508.9 | Class A vs C | 90.3% | 84.5% |
| LLV/ALB | 7.5 | Class B vs C | 93.8% | 81.5% |
| LLV (mm3) | 233.2 | Class A vs B | 74.4% | 73.1% |
| RV/ALB (mm3) | 20.46 | No varices vs varices | 80.0% | 83.5% |
Most of published studies have investigated the value of various imaging methods for diagnosing liver cirrhosis and evaluating related complications[3,10,18]. MRI could provide satisfactory quality of three-dimensional reconstruction images and clear anatomy of each liver lobe[19]. The most significant intra- and extra-hepatic changes related to this condition are atrophy of the right liver lobe, hypertrophy of the caudate lobe and the lateral segment of the left lobe, the presence of ascites, decreased albumin, and varicose veins[10,20]; therefore, we investigated the utility of the liver lobe volume obtained on MRI and the ratio of the liver lobe volume to albumin to determine the presence and Child-Pugh class of liver cirrhosis, and to identify the presence of esophageal varices.
As shown in this study, RV, LMV, LLV, and CV decreased, while RV/ALB increased, with progressive Child-Pugh class of cirrhosis. Regarding the variation of liver lobe volume, patients with compensated cirrhosis typically exhibit hypertrophy of the caudate lobe and the lateral segment of the left lobe, and atrophy of the right lobe and medial segment of the left lobe when the healthy liver progresses to the stage of compensated cirrhosis[10,21]. The pathological mechanism may be that the right portal vein branch enters directly into the parenchyma of the right liver lobe[22]. In cases of cirrhosis, hepatic fibrosis and cirrhosis nodules cause compression and irregular stenoses of the intrahepatic branches of this portal vein, and reduce flow through the right portal branch, resulting in the obvious atrophy of right liver lobe. Conversely, the portal branch runs through the falciform ligament, which is still outside the liver parenchyma, before entering the left liver lobe, resulting in a relatively greater blood supply to the lateral segment. Hypertrophy of the caudate lobe can be explained similarly in that most portal branches (78%) distributed in the caudate lobe arise from the bifurcation of the portal vein and have a shorter intrahepatic course than the vessels in the right lobe[2]. The cause of atrophy of the medial liver lobe may be that the left portal branch inflow to this lobe decreases, as does the right portal branch flow because of the diminishing compensatory hepatic function of the lateral liver lobe and the caudate lobe.
As cirrhosis progresses to decompensated cirrhosis, hypertrophy of the lateral liver lobe and the caudate lobe reaches a maximum, and then the two hypertrophied liver lobes begin to atrophy, with further progressive Child-Pugh class. As regards RV/ALB, our results agreed with those of Alempijevic, who found that the ratio of right liver lobe diameter on ultrasonography to serum albumin was significantly correlated with Child-Pugh class[6]. Based on the more and more obvious decrease of the albumin with the progressive Child-Pugh class of cirrhosis, we presumed that albumin reduced more obviously than RV, leading to the increase of RV/ALB.
As shown by the Mann-Whitney tests, RV, LMV, CV, LLV/ALB and CV/ALB could identify the presence of liver cirrhosis. Clinically, the Child-Pugh Class A patients usually show a good median survival term without orthotopic liver transplantation. The Child-Pugh Class C patients are considered conventional candidates for the procedure. Child-Pugh Class B patients can be considered a heterogeneous group, as their clinical condition may remain stable for more than a year or may rapidly deteriorate[23]. Therefore, it was important to differentiate the Child-Pugh classification. Our study indicated that LLV and LMV could distinguish class A from B; RV, LMV, LLV, CV, RV/ALB and LLV/ALB could distinguish class A from C; and RV and LLV/ALB could differentiate class B from C. Additionally, we also performed ROC analysis to determine how to use RV, LMV, LLV, CV, RV/ALB, LLV/ALB and CV/ALB to identify the occurrence and Child-Pugh class of liver cirrhosis for the first time. Among these parameters, CV/ALB could be the best factor to identify the presence of liver cirrhosis, with an AUC of more than 0.8. LLV, RV and LLV/ALB could best distinguish class A from B, with an AUC of more than 0.75; class A from C, with an AUC of 0.9; and class B from C with an AUC of 0.9, respectively. A previous study reported that liver volume could reflect the liver functional reserve, similar to the Child-Pugh class[24,25]. Our findings further indicate that LLV, RV and LLV/ALB could best reflect the decrease of liver functional reserve from class A from B, A from C, and class B from C.
In addition, cirrhosis can result in esophageal varices, which may induce fulminant massive hemorrhage of the upper gastrointestinal tract. Two recent studies reported that the ratio of right liver lobe diameter on ultrasonography to albumin could be a noninvasive parameter providing accurate information pertinent to determination of presence of esophageal varices[6,26]. As demonstrated in this study, RV, LMV and LLV were larger, and CV, RV/ALB, LMV/ALB, LLV/ALB and CV/ALB were lower in cirrhotic patients with esophageal varices than without esophageal varices. For the first time, we performed the ROC analysis of the previous parameters to predict the presence of esophageal varices, and found that RV/ALB could be the best parameter to predict the presence of esophageal varices, with an AUC of 0.89.
There is a limitation to our study. The sample size was relatively small. In particularly, the healthy control group was small while the cirrhotic group included both compensated and decompensated patients. Moreover, a large number of patients presented with clinical decompensation (ascites) but had no sign of esophageal varices on upper endoscopy, and the possible reason to explain this limitation may be attribute to the small sample size of the compensated patients. Despite this limitation, our study indicated that liver lobe volume parameters could help differentiate the presence of cirrhosis and its Child-Pugh class, and could identify the presence of esophageal varices. We will perform further studies with larger samples to confirm the results.
In conclusion, we confirmed that RV, LMV, LLV and CV decreased, while RV/ALB increased with Child-Pugh class of cirrhosis. CV/ALB could be used to identify the occurrence of cirrhosis, and LLV, RV and LLV/ALB could be recommended for differentiating Child-Pugh class A from B, A from C, and B from C, respectively. RV/ALB could help identify the presence of esophageal varices in cirrhotic patients. The findings could be helpful for the selection of appropriate liver lobe volume parameters to identify the presence and Child-Pugh class of cirrhosis, and the presence of esophageal varices for choosing appropriate treatment.
It is important to follow up the progress of liver cirrhosis and determine the stage of this disease. The modified Child-Pugh classification system has been confirmed as an independent prognostic factor for survival of cirrhotic patients. Previous studies reported that the changes in liver lobe volume were positively correlated with prognosis of Child-Pugh classes. There was an interesting study focusing on the correlation of the ratio of right liver lobe diameter to albumin with Child-Pugh class. In addition, esophageal varices are one of the major complications of liver cirrhosis, with the risk of bleeding from varices. However, how liver lobe volume and the ratio of liver lobe volume to albumin could determine the Child-Pugh class of liver cirrhosis and the presence of esophageal varices remained unclear.
Liver lobe volume measured on magnetic resonance imaging (MRI) or the ratio of right liver lobe diameter to albumin correlates with the Child-Pugh class of liver cirrhosis. However, whether liver lobe volume and the ratio of each liver lobe volume to albumin could predict the Child-Pugh class of liver cirrhosis and the presence of esophageal varices has not been determined.
The authors investigated the association of liver lobe volume measured on magnetic resonance imaging and the ratio of each liver lobe volume to albumin with Child-Pugh class of liver cirrhosis and with the presence of esophageal varices. They utilized receiver-operating characteristic curve analysis to identify the Child-Pugh class of cirrhosis and the presence of esophageal varices.
The authors found that liver lobe volume measured on magnetic resonance imaging and the ratio of each liver lobe volume to albumin could predict the presence, and Child-Pugh class, of liver cirrhosis, and the presence of esophageal varices. The ratio of caudate lobe volume to albumin could be used to identify the occurrence of cirrhosis. The left lateral liver lobe volume, right liver lobe volume, and the ratio of left lateral liver lobe volume to albumin could be recommended for differentiating Child-Pugh class A from B, A from C, and B from C, respectively. The ratio of right liver lobe volume to albumin could be recommended as an indicator for identifying the presence of esophageal varices in cirrhotic patients.
The modified Child-Pugh classification system of liver cirrhosis is considered the cornerstone in prognostic evaluation of cirrhotic patients, and contains five variables, including serum levels of bilirubin and albumin, prothrombin time, ascites, and encephalopathy, and allows categorization of patients into Child-Pugh Class A, B and C.
The authors study the potential of combination of liver lobe volumes (measured by MRI) and albumin levels in the identification of liver cirrhosis severity and esophageal varices in patients affected by hepatitis B virus. In addition, the authors observed an interesting correlation between radiological and biochemical parameters for the prediction of “presence of cirrhosis”, “Child-Pugh stage of the disease” and “presence of esophageal varices”.
P- Reviewer: Higuera-de la Tijera MF, Kovacs SJ, Lisotti A, Maroni L, Raggi CMF S- Editor: Qi Y L- Editor: Stewart G E- Editor: Liu XM
| 1. | Tsochatzis EA, Bosch J, Burroughs AK. Liver cirrhosis. Lancet. 2014;383:1749-1761. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1139] [Cited by in RCA: 1315] [Article Influence: 119.5] [Reference Citation Analysis (0)] |
| 2. | Ito K, Mitchell DG, Hann HW, Outwater EK, Kim Y, Fujita T, Okazaki H, Honjo K, Matsunaga N. Progressive viral-induced cirrhosis: serial MR imaging findings and clinical correlation. Radiology. 1998;207:729-735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 37] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 3. | Durand F, Valla D. Assessment of the prognosis of cirrhosis: Child-Pugh versus MELD. J Hepatol. 2005;42 Suppl:S100-S107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 376] [Cited by in RCA: 426] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 4. | Zhou XP, Lu T, Wei YG, Chen XZ. Liver volume variation in patients with virus-induced cirrhosis: findings on MDCT. AJR Am J Roentgenol. 2007;189:W153-W159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 72] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 5. | Alempijevic T, Bulat V, Djuranovic S, Kovacevic N, Jesic R, Tomic D, Krstic S, Krstic M. Right liver lobe/albumin ratio: contribution to non-invasive assessment of portal hypertension. World J Gastroenterol. 2007;13:5331-5335. [PubMed] |
| 6. | Psilopoulos D, Galanis P, Goulas S, Papanikolaou IS, Elefsiniotis I, Liatsos C, Sparos L, Mavrogiannis C. Endoscopic variceal ligation vs. propranolol for prevention of first variceal bleeding: a randomized controlled trial. Eur J Gastroenterol Hepatol. 2005;17:1111-1117. [PubMed] |
| 7. | Talwalkar JA, Yin M, Fidler JL, Sanderson SO, Kamath PS, Ehman RL. Magnetic resonance imaging of hepatic fibrosis: emerging clinical applications. Hepatology. 2008;47:332-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 244] [Cited by in RCA: 227] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 8. | Taouli B, Ehman RL, Reeder SB. Advanced MRI methods for assessment of chronic liver disease. AJR Am J Roentgenol. 2009;193:14-27. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 150] [Cited by in RCA: 133] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 9. | Ito K, Mitchell DG, Hann HW, Kim Y, Fujita T, Okazaki H, Honjo K, Matsunaga N. Viral-induced cirrhosis: grading of severity using MR imaging. AJR Am J Roentgenol. 1999;173:591-596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 43] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 10. | Ito K, Mitchell DG, Hann HW, Outwater EK, Kim Y. Compensated cirrhosis due to viral hepatitis: using MR imaging to predict clinical progression. AJR Am J Roentgenol. 1997;169:801-805. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 24] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 11. | North Italian Endoscopic Club for the Study and Treatment of Esophageal Varices. Prediction of the first variceal hemorrhage in patients with cirrhosis of the liver and esophageal varices. A prospective multicenter study. N Engl J Med. 1988;319:983-989. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 996] [Cited by in RCA: 841] [Article Influence: 22.7] [Reference Citation Analysis (1)] |
| 12. | Lok AS, McMahon BJ. Chronic hepatitis B. Hepatology. 2007;45:507-539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1794] [Cited by in RCA: 1778] [Article Influence: 98.8] [Reference Citation Analysis (0)] |
| 13. | Li H, Chen TW, Zhang XM, Li ZL, Zhang JL, Wang D, Li T, Wu JL, Guo X, Chen XL. Liver lobe volumes and the ratios of liver lobe volumes to spleen volume on magnetic resonance imaging for staging liver fibrosis in a minipig model. PLoS One. 2013;8:e79681. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 14. | Goldsmith NA, Woodburne RT. The surgical anatomy pertaining to liver resection. Surg Gynecol Obstet. 1957;105:310-318. [PubMed] |
| 15. | Mazonakis M, Damilakis J, Maris T, Prassopoulos P, Gourtsoyiannis N. Comparison of two volumetric techniques for estimating liver volume using magnetic resonance imaging. J Magn Reson Imaging. 2002;15:557-563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 52] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 16. | Hara AK, Burkart DJ, Johnson CD, Felmlee JP, Ehman RL, Ilstrup DM, Harmsen WS. Variability of consecutive in vivo MR flow measurements in the main portal vein. AJR Am J Roentgenol. 1996;166:1311-1315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 17. | Johnson CR, Khandelwal SR, Schmidt-Ullrich RK, Ravalese J, Wazer DE. The influence of quantitative tumor volume measurements on local control in advanced head and neck cancer using concomitant boost accelerated superfractionated irradiation. Int J Radiat Oncol Biol Phys. 1995;32:635-641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 90] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 18. | Alempijevic T, Kovacevic N. Right liver lobe diameter: albumin ratio: a new non-invasive parameter for prediction of oesophageal varices in patients with liver cirrhosis (preliminary report). Gut. 2007;56:1166-1167; authro reply 1167. [PubMed] |
| 19. | Chen XL, Chen TW, Li ZL, Zhang XM, Chen N, Zeng NL, Li H, Tang HJ, Pu Y, Li CP. Spleen size measured on enhanced MRI for quantitatively staging liver fibrosis in minipigs. J Magn Reson Imaging. 2013;38:540-547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 20. | Cho KC, Patel YD, Wachsberg RH, Seeff J. Varices in portal hypertension: evaluation with CT. Radiographics. 1995;15:609-622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 88] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 21. | Saygili OB, Tarhan NC, Yildirim T, Serin E, Ozer B, Agildere AM. Value of computed tomography and magnetic resonance imaging for assessing severity of liver cirrhosis secondary to viral hepatitis. Eur J Radiol. 2005;54:400-407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 34] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 22. | Zhou L, Chen TW, Zhang XM, Yang Z, Tang HJ, Deng D, Zeng NL, Wang LY, Chen XL, Li H. Liver dynamic contrast-enhanced MRI for staging liver fibrosis in a piglet model. J Magn Reson Imaging. 2014;39:872-878. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 23. | Abdalla EK, Denys A, Chevalier P, Nemr RA, Vauthey JN. Total and segmental liver volume variations: implications for liver surgery. Surgery. 2004;135:404-410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 186] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 24. | Keeffe EB. Summary of guidelines on organ allocation and patient listing for liver transplantation. Liver Transpl Surg. 1998;4:S108-S114. [PubMed] |
| 25. | Schiano TD, Bodian C, Schwartz ME, Glajchen N, Min AD. Accuracy and significance of computed tomographic scan assessment of hepatic volume in patients undergoing liver transplantation. Transplantation. 2000;69:545-550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 97] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 26. | Esmat S, Omarn D, Rashid L. Can we consider the right hepatic lobe size/albumin ratio a noninvasive predictor of oesophageal varices in hepatitis C virus-related liver cirrhotic Egyptian patients? Eur J Intern Med. 2012;23:267-272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 13] [Article Influence: 1.0] [Reference Citation Analysis (0)] |