Published online Jan 21, 2015. doi: 10.3748/wjg.v21.i3.1014
Peer-review started: June 4, 2014
First decision: July 9, 2014
Revised: July 29, 2014
Accepted: September 18, 2014
Article in press: September 19, 2014
Published online: January 21, 2015
Processing time: 231 Days and 3.9 Hours
Classic polyarteritis nodosa (PAN) that targets medium-sized muscular arteries and microscopic polyangiitis (MPA), characterized by inflammation of small-caliber vessels and the presence of circulating myeloperoxidase anti-neutrophil cytoplasmic antibodies (MPO-ANCA), are distinct clinicopathological entities of systemic vasculitis. A 66-year-old woman presented with fever, cholestasis and positive MPO-ANCA. Radiological examination showed a pancreatic mass compressing the bile duct. Therefore, we performed pancreatoduodenectomy. Histopathological examination revealed that necrotizing vasculitis predominantly affecting the medium-sized vessels, spared arterioles or capillaries in the pancreas, a finding consistent with PAN. Unexpectedly, renal biopsy revealed small-caliber vasculitis and glomerulonephritis, supporting MPA. The initial manifestation of a pancreatic mass associated with vasculitis has only been reported in 7 articles. Its diagnosis is challenging because no reliable clinico-radiological findings have been observed. Clinicians should be aware of such cases and early diagnosis followed by immunosuppression is mandatory. Our findings may reflect a polyangiitis overlap syndrome coexisting between pancreatic PAN and renal MPA.
Core tip: A 66-year-old woman presented with a pancreatic mass accompanied by fever, cholestasis and positive myeloperoxidase anti-neutrophil cytoplasmic antibodies. The resected pancreas showed extensive fibrosis associated with necrotizing vasculitis, targeting medium-sized vessels but sparing small-caliber vessels, a finding compatible with polyarteritis nodosa. Unexpectedly, renal biopsy revealed small-caliber vasculitis and glomerulonephritis, supporting microscopic polyangiitis. The initial manifestation of a pancreatic mass associated with vasculitis has only been reported in 7 articles. Although rare, vasculitis should be included in a differential diagnosis for pancreatic masses. Additionally, our findings may reflect a polyangiitis overlap syndrome coexisting between pancreatic polyarteritis nodosa and renal microscopic polyangiitis.
- Citation: Yokoi Y, Nakamura I, Kaneko T, Sawayanagi T, Watahiki Y, Kuroda M. Pancreatic mass as an initial manifestation of polyarteritis nodosa: A case report and review of the literature. World J Gastroenterol 2015; 21(3): 1014-1019
- URL: https://www.wjgnet.com/1007-9327/full/v21/i3/1014.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i3.1014
Systemic vasculitis is characterized by a variety of clinical manifestations and courses, depending upon the organ involved. Among the classifications for systemic vasculitis, the Chapel Hill consensus conference (CHCC) nomenclature is widely accepted[1,2]. Vasculitis affecting small-caliber blood vessels (arterioles, venules or capillaries) often accompanies anti-neutrophil cytoplasmic antibodies which are postulated to play a major pathological role in developing necrotizing vasculitis[3]. Such ANCA-associated vasculitis includes the following 3 clinicopathological variants: microscopic polyangiitis (MPA), granulomatosis with polyangiitis (GPA) and eosinophilic granulomatosis with polyangiitis[2]. Among them, MPA is characterized by non-granulomatous inflammation, few or no immune deposits (pauci-immune), glomerulonephritis and the presence of myeloperoxidase (MPO)-ANCA[2].
Another category of vasculitis, classic polyarteritis nodosa (PAN), targets small and medium-sized muscular arteries, spares small-caliber vessels and causes diffuse vascular inflammation, ischemia or rupture of affected organs[4]. Although PAN frequently complicates the skin, joints, kidneys and gastrointestinal system, initial and symptomatic involvement of the pancreatobiliary system has only been reported in rare cases[5-8].
Herein, we report a patient presenting with fever, cholestasis and a pancreatic mass compressing the bile duct as a clinical feature of PAN.
A 66-year-old woman presented with a 2 wk history of intermittent high-grade fever (approximately 39 °C). She did not report arthralgia, myalgia or abdominal symptoms. Approximately 1 mo before admission, she underwent tympanotomy for left otitis media. Her medical history was noncontributory. She denied alcohol and drug use. Laboratory examination showed elevated biliary enzyme levels, including an alkaline phosphatase level of 717 U/L (115-359 U/L), gamma glutamyl transpeptidase levels of 238 U/L (10-47 U/L) and C-reactive protein (CRP) levels of 8.30 mg/dL (< 0.30 mg/dL). Serum levels of amylase, aspartate aminotransferase, alanine aminotransferase, blood urea nitrogen, creatinine, carcinoembryonic antigen, carbohydrate antigen 19-9 and procalcitonin were normal. The levels of glycated hemoglobin were slightly elevated. Leukocytosis and eosinophilia were not present. Immunological data showed slight elevations of IgG [1902 mg/dL (820-1740 mg/dL)] and IgA [628 mg/dL (90-400 mg/dL)], but IgM and IgG4 levels were normal. Autoimmune investigations showed elevated MPO-ANCA levels [473 IU/mL (< 3.5 IU/mL)] in addition to a slight elevation of anti-nuclear antibodies (1:64) and rheumatoid factor. Proteinase 3-ANCA, serum hepatitis B surface antigen and hepatitis C virus antibodies were not detected. No bacteria grew on blood culture. Urinalysis revealed proteinuria (2+) and hematuria (2+) with hyaline casts.
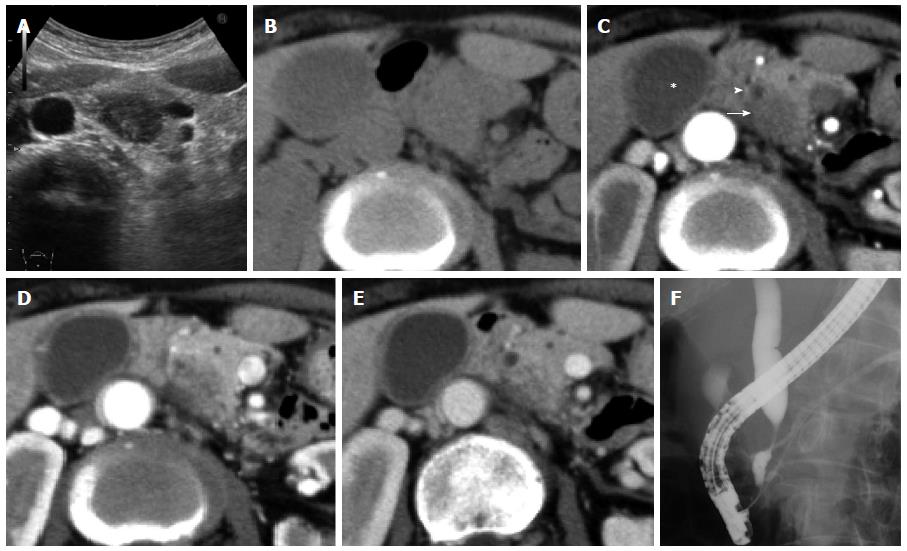
A hypoechoic 2.0 cm mass was observed in the pancreatic head on an abdominal ultrasonogram (Figure 1A). The corresponding lesion was an ill-defined hypodense mass with poor enhancement, observed by using a CT scan, and it compressed the distal common bile duct (CBD) and pancreatic duct (PD) (Figure 1B-E). The walls of the gallbladder and bile duct were thickened (Figure 1D, E). A chest CT scan showed slight changes, including bronchial dilation and peripheral inflammation with a centrilobular distribution. Angiographic reconstruction using a CT scan showed normal visceral arteries of the superior mesenteric artery (SMA) and celiac systems. Vascular stenosis or aneurysms were not detectable. Endoscopic retrograde cholangiopancreatography (ERCP) demonstrated a double duct sign with compression of the distal CBD and tortuous dilation of the PD (Figure 1F). Bile cytology and culture were negative according to the results obtained after using the sample via naso-biliary drainage.
We could not exclude the possibility of pancreatic cancer as a cause of the patient’s fever and therefore we performed a pylorus-preserving pancreatoduodenectomy. The pancreatic mass was soft on palpation and did not invade the adjacent tissues. Intraoperative ultrasonography revealed an ill-defined pancreatic mass with low echogenicity. The postoperative course was uneventful and the patient’s fever completely resolved with a reduction of CRP levels.
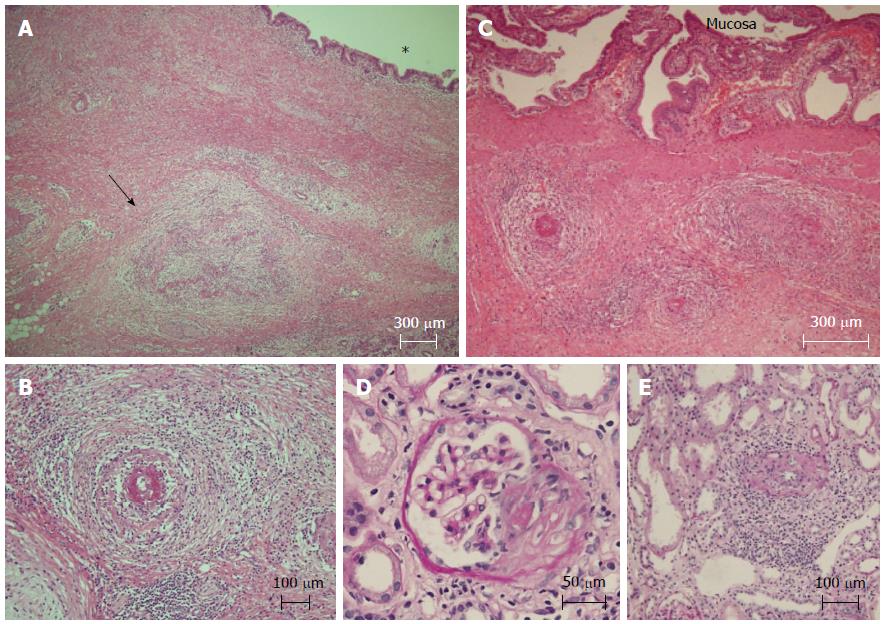
In the resected pancreas, the focal stenosis in the CBD was approximately 2 cm distal to the ampulla of Vater. There was marked fibrosis adjacent to the intrapancreatic CBD and PD (Figure 2A). The affected small to medium-sized arteries in the fibrosis were characterized by necrotizing arteritis with subintimal fibrinoid necrosis, disruption of the elastic laminae, perivascular fibrosis and inflammatory cell infiltration (Figure 2A, B). Vessel occlusion or thrombus was also observed (Figure 2A). Small-caliber vessels such as the arterioles, capillaries or venules were spared. Granulomatous inflammation and significant eosinophil infiltration were not found. The fibrotic lesion extended longitudinally towards the hepatic hilus along the bile duct. Necrotizing vasculitis was also observed in the walls of the proximal bile duct and gallbladder but their mucosal layers were well preserved (Figure 2C). The duodenum also showed arterial changes. These vascular changes were compatible with classical PAN.
To confirm systemic vasculitis, a renal needle biopsy was performed. Global sclerosis affected 20% of the glomeruli, whereas a cellular crescent was observed in 10% (Figure 2D). Interstitial fibrosis was observed in the tubulointerstitial area. Both active and healed stages of vasculitis were observed in the small arteries and capillaries (Figure 2E). Immune complexes were not detectable. These pathological findings were compatible with the renal changes of MPA according to the CHCC nomenclature[1,2].
Therapy with prednisone and cyclophosphamide was undertaken to induce remission of the systemic vasculitis. The patient has remained asymptomatic 6 mo after the operation.
In the present patient, a pancreatic mass accompanied by fever and cholestasis was observed; surgical removal successfully improved the patient’s clinical symptoms and data. Pathological study demonstrated extensive vascular injury in the pancreas, bile duct, gallbladder and duodenum. The affected vessels were small and medium-sized arteries and arterioles and capillaries were spared, a finding consistent with classic PAN[1,2,4]. Initial clinical manifestation of vasculitis in the pancreatobiliary system is uncommon, with only a few reports documenting pancreatitis or cholecystitis[7]. Other forms of pancreatic vasculitis, including mass formation, are extremely rare. The articles reporting a pancreatic mass associated with vasculitis were collected through a literature search with the words “vasculitis”, “pancreas”, “tumor” or “mass” in their title. Among them, 7 articles providing radiological and histopathological descriptions were reviewed (Table 1). Including our case, there were 3 PAN[5,6], 3 GPA[8-10] and 2 localized PAN[11,12]. The former 2 were major vasculitis presenting with a tumor-like lesion in the urogenital system and breast or kidney, respectively[6]. The median age was 62 years (range: 44-66 years), with a male predominance (5:3 ratio). Three patients were Japanese, 2 were white and 1 was Jewish. The symptoms were varied and nonspecific, including abdominal pain (5 patients), fever (3 patients), otitis media (2 patients) and jaundice (1 patient). All lesions were 2-3 cm in diameter and were localized in the head (6 patients), neck (1 patient) and both body and tail of the pancreas (1 patient). The gallbladder was also affected in 2 PAN patients. Among 4 cases analyzed, ANCA was positive in 3 (GPA, 2; PAN, 1). Use of glucocorticoids and a cytotoxic agent was effective in all cases if treated, otherwise rapid deterioration of necrotizing vasculitis was fatal, as shown in case 5. These findings indicate that early introduction of immunosuppressive treatment based on accurate diagnosis is crucial for a better outcome.
| Ref. | Age/sex/race etc. | Finaldiagnosis | Symptoms | Sitesinvolved | Prior diagnosis | Tumor sizeimaging findings | Diagnostic criteria | Outcome | |
| Pancreas | Patient | ||||||||
| Ito et al[11] | 44/M/ Japanese | Localized PAN | Epigastralgia | Head | No | ND ERCP: CBD stenosis | ND | Underwent PD | Discharged |
| O'Neil et al[8] | 62/M/ White | GPA | Jaundice Otitis media Nasal ulceration | Head Gallbladder | No | 3 cm CT: mass US: hypoechoic ERCP: CBD stenosis | ANCA (+) Needle biopsy: non diagnostic Renal biopsy: confirmed | Improved on CYC + CS | Improved on CYC + CS |
| Damani et al[5] | 46/F/ ND | PAN | Right upper abdominal pain | Neck | No | 2 cm US: hypoechoic CT: low attenuation, nonenhancing mass | Needle biopsy: non diagnostic Postoperative histopathology | Cholecystectomy Distal Px | Died (20 d) Various complication |
| Kariv et al[6] | 65/ M/ Jewish | PAN | Epigastralgia Weight loss Low grade fever | Head | No | 3 cm CT: mass | Needle biopsy: chronic pancreatitis | Underwent PD | Remission on CS |
| Matsubayashi et al[9] | 65/M/ Japanese | GPA | Left abdominal pain Constipation Low grade fever Tympanitis | Body and Tail | S/O GPA | ND CT: Enlargement of pancreas with sporadic low density lesions | 12PR3-ANCA (+) Autopsy | No | Died Hemorrhagic pneumonia Diffuse necrotizing pancreatitis |
| Tinazzi et al[10] | 48/F/ ND | GPA | Mid-epigastric pain | Head | No | 2 cm: US: Hypoechoic MRCP: Obstruction of pancreatic duct | Postoperative histopathology | Underwent PD | Improved on CYC + CS |
| Gonzalez-Gay et al[12] | 75/M/ White | Localized PAN | Epigastralgia | Head | No | ND | Postoperative histopathology | Underwent PD | Discharged |
| Our case | 66 /F/ Japanese | PAN Renal MPA | Otitis media Fever | Head Gallbladder Bile duct Duodenum | No | 2 cm US: Hypoechoic CT: Hypodense Non-enhancing | MPO-ANCA(+) Postoperative histopathology | Underwent PD | Improved on CYC + CS Discharged |
One of the obstacles in treatment strategy for a vasculitis-induced pancreatic mass is the difficulty in diagnosing it. Our review showed that 7 of 8 patients were diagnosed only after surgery or autopsy (Table 1). Besides neoplasm, the pancreatic mass can encompass a variety of diseases, such as an inflammatory pseudotumor (IPT). IPT includes autoimmune pancreatitis, groove pancreatitis and lipomatosis[13]. As shown in Table 1, regardless of different types of vasculitis, vasculitis-associated masses were hypoechoic and hypodense with poor encasement on a CT scan, making it difficult for differentiation from pancreatic cancer or IPT. For a focal pancreatic lesion, fine-needle biopsy is widely used with abdominal or endoscopic ultrasonography and it is useful in autoimmune pancreatitis[14]. However, fine-needle biopsy has potential sampling error problems; indeed, ultrasound or CT-guided needle biopsy failed to be diagnostic for pancreatic GPA (case 2) and PAN (cases 3 and 4). Negative findings do not exclude the possibility of malignancy and there is a risk of needle tract seeding or dissemination of tumor cells[15]. Thus, the diagnostic procedure is challenging. Some clinicians do away with the preoperative evaluation in patients with operable focal lesions of a clinically and radiologically suspicious malignancy. The common use of ANCA tests in the future would enhance preoperative diagnosis and avoid unnecessary radical operations.
Another interesting finding in this case was the coexistence of different entities of vasculitis, such as PAN in the pancreatobiliary system and MPA in the kidneys. The renal histopathological findings of small-caliber vessel (arteries and capillaries) vasculitis and positive MPO-ANCA supported the MPA diagnosis[2,3]. PAN and MPA had often been diagnosed together until the proposal of the CHCC nomenclature and distinguishing between these 2 entities is not clinically always straightforward[16]. Our case may represent the so-called polyangiitis overlap syndrome which is characterized by systemic vasculitis with features that overlap more than 1 type of vasculitis[17]. Alternatively, it is possibly a coincidence or part of the MAP or PAN spectrum. Renal MAP has been reported to complicate vasculitic disorders that can be attributed to PAN, such as a rupture of branch of the celiac[18] or SMA system[19] and coronary angiitis[20].
In conclusion, we encountered a patient with a pancreatic mass associated with PAN. A literature review revealed that pancreatic masses have been reported in 7 patients with primary vasculitis. Because of its rarity and lack of reliable discrimination from pancreatic cancer, clinicians should be aware of such cases and that early diagnosis followed by immunosuppressive treatment is mandatory.
A 66-year-old woman presented with a pancreatic mass accompanied by fever.
An inflammatory pseudotumor and pancreatic neoplasms, including cancer.
Laboratory examination showed elevated levels of biliary enzymes (alkaline phosphatase and gamma glutamyl transpeptidase), C-reactive protein and myeloperoxidase-anti nuclear cytoplasmic antibodies.
An abdominal computed tomography revealed an ill-defined 2.0 cm pancreatic mass with poor enhancement compressing the distal common bile duct (CBD) and pancreatic duct, as well as the thickened walls of the CBD and gallbladder.
The resected pancreas revealed extensive fibrosis associated with necrotizing vasculitis targeting medium-sized vessels and sparing small-caliber vessels.
The patient underwent surgical resection followed by immunosuppression after pathological diagnosis of polyarteritis nodosa.
A pancreatic mass as an initial manifestation of vasculitis is extremely rare, with only 7 cases reported in the literature.
The case emphasizes that vasculitis should be included in the differential diagnosis of a pancreatic mass accompanied by fever.
Although immunosuppression is the optimal treatment for a vasculitis-associated pancreatic tumor, the diagnosis is challenging because of its rarity and lack of discrimination from pancreatic cancer.
P- Reviewer: Ranieri G S- Editor: Qi Y L- Editor: Roemmele A E- Editor: Wang CH
| 1. | Jennette JC, Falk RJ, Andrassy K, Bacon PA, Churg J, Gross WL, Hagen EC, Hoffman GS, Hunder GG, Kallenberg CG. Nomenclature of systemic vasculitides. Proposal of an international consensus conference. Arthritis Rheum. 1994;37:187-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2763] [Cited by in RCA: 2408] [Article Influence: 77.7] [Reference Citation Analysis (0)] |
| 2. | Jennette JC, Falk RJ, Bacon PA, Basu N, Cid MC, Ferrario F, Flores-Suarez LF, Gross WL, Guillevin L, Hagen EC. 2012 revised International Chapel Hill Consensus Conference Nomenclature of Vasculitides. Arthritis Rheum. 2013;65:1-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4416] [Cited by in RCA: 4256] [Article Influence: 354.7] [Reference Citation Analysis (0)] |
| 3. | Kallenberg CG. Pathogenesis of ANCA-associated vasculitides. Ann Rheum Dis. 2011;70 Suppl 1:i59-i63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 98] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 4. | Fauci AS, Haynes B, Katz P. The spectrum of vasculitis: clinical, pathologic, immunologic and therapeutic considerations. Ann Intern Med. 1978;89:660-676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 908] [Cited by in RCA: 760] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 5. | Damani NN, Asch MR, Redston M. The diagnostic challenge of vasculitis in a patient presenting with acute cholecystitis and a focal pancreatic mass: case report. Can Assoc Radiol J. 1997;48:179-182. [PubMed] |
| 6. | Kariv R, Sidi Y, Gur H. Systemic vasculitis presenting as a tumorlike lesion. Four case reports and an analysis of 79 reported cases. Medicine (Baltimore). 2000;79:349-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 51] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 7. | Pagnoux C, Mahr A, Cohen P, Guillevin L. Presentation and outcome of gastrointestinal involvement in systemic necrotizing vasculitides: analysis of 62 patients with polyarteritis nodosa, microscopic polyangiitis, Wegener granulomatosis, Churg-Strauss syndrome, or rheumatoid arthritis-associated vasculitis. Medicine (Baltimore). 2005;84:115-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 280] [Cited by in RCA: 256] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 8. | O’Neil KM, Jones DM, Lawson JM. Wegener’s granulomatosis masquerading as pancreatic carcinoma. Dig Dis Sci. 1992;37:702-704. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 9. | Matsubayashi H, Seki T, Niki S, Mizumura Y, Taguchi Y, Moriyasu F, Go K. Wegener’s granulomatosis with onset of acute pancreatitis and rapid progress. A case report. Pancreatology. 2001;1:263-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 10. | Tinazzi I, Caramaschi P, Parisi A, Faccioli N, Capelli P, Biasi D. Pancreatic granulomatous necrotizing vasculitis: a case report and review of the literature. Rheumatol Int. 2007;27:989-991. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 11. | Ito M, Sano K, Inaba H, Hotchi M. Localized necrotizing arteritis. A report of two cases involving the gallbladder and pancreas. Arch Pathol Lab Med. 1991;115:780-783. [PubMed] |
| 12. | Gonzalez-Gay MA, Vazquez-Rodriguez TR, Miranda-Filloy JA, Pazos-Ferro A, Garcia-Rodeja E. Localized vasculitis of the gastrointestinal tract: a case report and literature review. Clin Exp Rheumatol. 2008;26:S101-S104. [PubMed] |
| 13. | Adsay NV, Basturk O, Klimstra DS, Klöppel G. Pancreatic pseudotumors: non-neoplastic solid lesions of the pancreas that clinically mimic pancreas cancer. Semin Diagn Pathol. 2004;21:260-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 53] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 14. | Kanno A, Ishida K, Hamada S, Fujishima F, Unno J, Kume K, Kikuta K, Hirota M, Masamune A, Satoh K. Diagnosis of autoimmune pancreatitis by EUS-FNA by using a 22-gauge needle based on the International Consensus Diagnostic Criteria. Gastrointest Endosc. 2012;76:594-602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 99] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 15. | Hirooka Y, Goto H, Itoh A, Hashimoto S, Niwa K, Ishikawa H, Okada N, Itoh T, Kawashima H. Case of intraductal papillary mucinous tumor in which endosonography-guided fine-needle aspiration biopsy caused dissemination. J Gastroenterol Hepatol. 2003;18:1323-1324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 148] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 16. | Basu N, Watts R, Bajema I, Baslund B, Bley T, Boers M, Brogan P, Calabrese L, Cid MC, Cohen-Tervaert JW. EULAR points to consider in the development of classification and diagnostic criteria in systemic vasculitis. Ann Rheum Dis. 2010;69:1744-1750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 110] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 17. | Leavitt RY, Fauci AS. Polyangiitis overlap syndrome. Classification and prospective clinical experience. Am J Med. 1986;81:79-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 107] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 18. | Ito Y, Tanaka A, Sugiura Y, Sezaki R. An autopsy case of intraabdominal hemorrhage in microscopic polyangiitis. Intern Med. 2011;50:1501-1502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 19. | Ueda S, Matsumoto M, Ahn T, Adachi S, Oku K, Takagi M, Fukui H, Yoshikawa M. Microscopic polyangiitis complicated with massive intestinal bleeding. J Gastroenterol. 2001;36:264-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 13] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 20. | Shah AS, Din JN, Payne JR, Dhaun N, Denvir MA, Mills NL. Coronary angiitis and cardiac arrest in antineutrophil cytoplasmic-antibody associated systemic vasculitis. Circulation. 2011;123:e230-e231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |