Published online Jan 21, 2015. doi: 10.3748/wjg.v21.i3.1009
Peer-review started: May 10, 2014
First decision: June 10, 2014
Revised: June 23, 2014
Accepted: July 22, 2014
Article in press: July 22, 2014
Published online: January 21, 2015
Processing time: 256 Days and 0.6 Hours
The effectiveness of hepatitis C treatment has improved with the development of interferon (IFN), and it has drastically improved with the development of peg-interferon-α (PEG-IFN) in combination with ribavirin (RBV) and, more recently, with the addition of a protease inhibitor. Simeprevir, which is a second-generation protease inhibitor, has shown clinically favorable safety and tolerability profiles. Simeprevir received its first global approval in Japan in September 2013 for the treatment of genotype 1 chronic hepatitis C in combination with PEG-IFN and RBV. One serious adverse event associated with IFN therapy is interstitial pneumonitis, which can be fatal. We experienced a patient with interstitial pneumonitis that was induced by simeprevir with PEG-IFN and RBV therapy for chronic hepatitis C in the early stages of therapy (8 wk after initiating therapy). This is the first case report of interstitial pneumonitis with simeprevir with PEG-IFN and RBV in the world. In addition, it is very interesting that the onset of interstitial pneumonitis was earlier than that in conventional PEG-IFN and RBV therapy. This finding suggests that simeprevir augments the adverse event. We present this case report in light of relevant literature on interstitial pneumonitis with conventional PEG-IFN and RBV therapy.
Core tip: Simeprevir is recently being used as a protease inhibitor for hepatitis C. Several reports have indicated that simeprevir has clinically favorable safety and tolerability profiles. However, this is the first report of interstitial pneumonitis that was induced by simeprevir with peg-interferon and ribavirin therapy for chronic hepatitis C. Therefore, it is necessary to carefully observe the presence of respiratory symptoms in patients receiving this treatment.
- Citation: Tamaki K, Okubo A. Simeprevir with peginterferon and ribavirin induced interstitial pneumonitis: First case report. World J Gastroenterol 2015; 21(3): 1009-1013
- URL: https://www.wjgnet.com/1007-9327/full/v21/i3/1009.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i3.1009
Approximately 150 million people worldwide are infected with the hepatitis C virus (HCV). HCV can lead to chronic hepatitis, liver cirrhosis, and hepatocellular carcinoma; the treatment of patients with HCV typically includes interferon (IFN) therapy.
The treatment of HCV has evolved over the past 20 years. Before 2011, the standard treatment was a combination of IFN alpha-polyethylene glycol [peg-IFN-α (PEG-IFN)], given as a weekly injection, and oral ribavirin (RBV). In patients infected with HCV genotype 1, the most common genotype worldwide, the standard treatment is a combination of PEG-IFN and RBV for 48 wk. This treatment results in only 40%-50% sustained virological response (SVR)[1-3].
The effectiveness of treatment has improved with the development of IFN, and it has drastically improved with the development of PEG-IFN in combination with RBV and, more recently, with the addition of a protease inhibitor.
The first direct-acting antivirals (DAAs), the NS3-4A serine protease inhibitors boceprevir and telaprevir, improved the rate of SVR; however, their toxicity in combination with PEG-IFN and RBV limited their overall efficacy[4]. In Japan, telaprevir or simeprevir is recently being used as a protease inhibitor for hepatitis C. Compared with PEG-IFN and RBV therapy, telaprevir-based triple therapy (with PEG-IFN and RBV) has a high frequency and severe dermatological and hematological adverse events (anemia)[5,6].
Several reports have indicated that simeprevir has clinically favorable safety and tolerability profiles. The report from the OPERA-1 study described adverse events in treatment-naïve patients graded as 1 or 2 in severity. The most common adverse events reported for recipients of simeprevir were fatigue, nausea, asthenia, diarrhea, bone pain, and dry skin[7]. One serious adverse event associated with IFN therapy includes interstitial pneumonitis, which can be fatal. Therefore, it is necessary to carefully observe the presence of respiratory symptoms (i.e., cough, dyspnea) in patients receiving this treatment.
Here, we present a case of interstitial pneumonitis that was induced by simeprevir with PEG-IFN and RBV therapy for chronic hepatitis C and began in the early stages of therapy (8 wk after the initiation of therapy). This is first case report of interstitial pneumonitis with simeprevir with PEG-IFN and RBV in the world.
In addition, the onset of interstitial pneumonitis was earlier than that of interstitial pneumonitis in conventional PEG-IFN and RBV therapy. This suggests simeprevir augments this adverse event. We report our case with relevant literature on interstitial pneumonitis with conventional PEG-IFN and RBV therapy.
A 70-year-old female was diagnosed with chronic hepatitis C in 1994, and she underwent treatment at the outpatient clinic of our hospital (Table 1). She had no history of autoimmune or pulmonary disease. In 2011, she was treatment naïve when she began a 72-wk PEG-IFNα-2A + RBV therapy. HCV RNA became undetectable at week 16, and it remained undetectable throughout the remaining treatment. However, a relapse occurred 2 mo later during a follow-up examination.
| Parameters | Value |
| WBC | 5600/μL |
| RBC | 481 × 104/μL |
| Hb | 14.1 g/dL |
| Plt | 15.2 × 104/μL |
| PT-INR | 1.17 |
| AST | 63 IU/L |
| ALT | 48 IU/L |
| TP | 7.6 g/dL |
| Alb | 3.7 g/dL |
| TBIL | 1.3 mg/dL |
| ALP | 554 IU/L |
| γ-GTP | 27 IU/L |
| CRP | < 0.10 mg/dL |
| LDH | 285 U/L |
| UA | 4.6 mg/dL |
| BUN | 5.8 mg/dL |
| Cr | 0.7 mg/dL |
| Na | 141 mEq/L |
| K | 4.2 mEq/L |
| HBsAg | (-) |
| HCVAb | (+) |
| HCV-RNA | 5.6 LogIU/mL |
| Genotype | 1B |
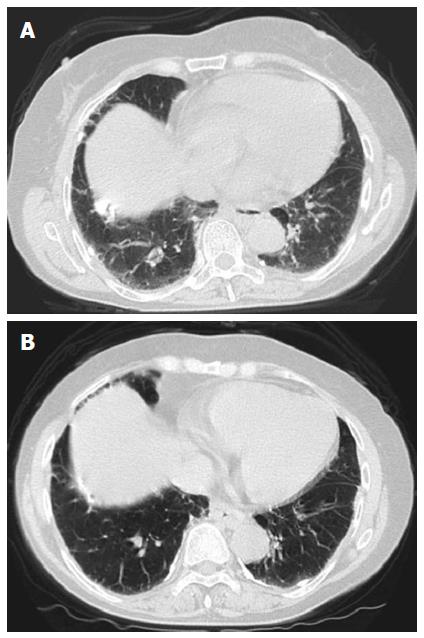
From January 2014, she was treated with triple therapy that included simeprevir, PEG-IFNα-2A, and RBV. The HCV RNA level immediately decreased at week 1 to 2.2 log10 IU/mL, and HCV RNA was undetectable at week 4. There were no adverse events or abnormalities other than mild anemia and neutropenia identified with hemogram and biochemistry at week 7. However, she visited our hospital for dyspnea on effort and a mild dry cough that appeared at approximately week 8. Her basal oxygen saturation was 98% (room air); however, her physical examination revealed a bibasailar mild, fine crackle, and high-resolution computed tomography (CT) revealed bilateral ground-glass opacities (Figure 1A). In addition, her KL-6 was elevated to 3021 U/mL.
Based on these findings, we diagnosed her with interstitial pneumonitis caused by simeprevir with PEG-IFNα-2A and RBV therapy. Interstitial pneumonitis was grade 2 on the World Health Organization grading scale (Table 2). Following her diagnosis, we immediately discontinued the treatment and administered 20 mg of oral prednisolone daily.
| Positive | Negative | |
| Incidence | 11 | 113 |
| Incidence rate | 0.026% | 0.27% |
| Dead | 1 | 12 |
| Dead/incidence | 9.1% | 10.6% |
After beginning prednisolone therapy, her respiratory symptoms gradually improved, and the ground-glass opacities improved on chest CT (Figure 1B). Prednisolone was then gradually tapered down by 5 mg every 3 d, and her respiratory symptoms disappeared. She had maintained persistent undetectable HCV RNA and achieved normal ALT levels at a follow-up visit 2 mo after the end of the treatment.
Simeprevir received its first global approval in Japan in September 2013 for the treatment of genotype 1 chronic hepatitis C in combination with PEG-IFN and RBV. Simeprevir is administered as a pill once daily. It is a small-molecule macrocyclic drug that targets and selectively inhibits HCV NS3/4A serine protease, thereby blocking the enzyme that enables HCV to replicate in host cells[8].
Compared with a placebo, treatment with simeprevir, PEG-IFN, and RBV resulted in significantly higher SVR rates in the overall patient population[9]. In a Japanese study, SVR at 12 wk after the end of treatment was achieved in 88.6% of simeprevir-treated patients and 61.7% of placebo-treated patien[10]. In Phase I and phase II studies conducted in Japan, the rate of adverse events was reported to be 97.7% (426/436). The most common adverse events and their incidence were as follows: rash (46.6%), pruritus (24.1%), hyperbilirubinemia (22.2%), constipation (6.7%), and photosensitivity reaction (1.8%). However, no interstitial pneumonitis was reported in these studies. Nearly all patients receiving simeprevir with PEG-IFN and RBV experience adverse events that can be serious. Fatigue and flu-like symptoms are common, and psychiatric symptoms, weight loss, seizures, peripheral neuropathy, and bone marrow suppression can also occur. RBV causes hemolysis and skin complications and is teratogenic[11]. The adverse events associated with PEG-IFNα-2A that were described previously in a Japanese Welfare Ministry report include an interstitial pneumonia incidence rate of 0.3%. The shortest onset time was 11 wk, and the longest reported onset time was 38 wk (mean 16 wk). This is the first case of interstitial pneumonia with triple therapy (simeprevir with PEG-IFNα-2A and RBV). This case was observed in week 8; this was earlier than the cases of interstitial pneumonitis with PEG-IFNα-2A observed previously. Moreover, the immunological and pharmacokinetic properties of PEG-IFNα-2a, with the longest half-life, may act to trigger the pathophysiologic mechanisms of interstitial pneumonitis. The mechanism of this adverse event remains unclear; however, it is considered idiosyncratic and is probably related to the IFN immunomodulatory activity that includes the induction of enzymes, suppression of cell proliferation, enhancement of macrophage phagocytic activity, inhibition of suppressor T cells, and liberation of proinflammatory cytokines[12-14]. Although co-administration of RBV does not affect the pharmacokinetics of PEG-IFN, these drugs altogether have enhanced toxicity. In addition, clinically relevant drug-drug interactions between PEG-IFN alfa-2a (40 KD) and agents metabolized via the hepatic P450 system are unlikely to occur[15].
Simeprevir inhibits OATP1B1/3 and P-glycoprotein transporters. Co-administration of simeprevir with drugs that are substrates of OATP1B1/3 and P-glycoprotein transport may result in increased plasma concentrations of these drugs[16]. Therefore, it is possible that a combination of drugs will result in greater pulmonary toxicity. However, further studies are required to confirm this possibility. The fact that reports of pneumonitis associated with RBV monotherapy and simeprevir monotherapy have not been published till date enhances the probability of interstitial pneumonitis induced by PEG-IFN in the present case.
In spite of the additional efficacy and safety of PEG-IFN, which is largely used in combination with simeprevir to treat chronic hepatitis C, we report a case of interstitial pneumonitis related to PEG-IFNα-2a, notifying physicians about this pulmonary nonspecific adverse event with potential severity, given the insufficiency of publications regarding this risk during the treatment of chronic hepatitis C.
In most cases, symptoms of pneumonitis are reversible after cessation of treatment with IFN and RBV. There is no consensus with regard to the treatment of interstitial pneumonitis induced with IFN and RBV. Upon review of the literature, three options are possible. The first option is to stop the combination treatment of HCV and wait until the disease resolves, which was done in a limited number of cases. The second option is to administer steroids, although the dosage and route of administration regimes vary widely. The third option, i.e., adding azathioprine to steroids in therapy-resistant relapsing cases, may be beneficial for resolving interstitial pneumonitis[17]. A shorter overall treatment duration is acceptable in patients with chronic HCV infection because it reduces the exposure to PEG-IFN and RBV, thereby resulting in a reduced incidence of adverse events[18-20].
In conclusion, interstitial pneumonitis with triple therapy, including simeprevir, PEG-IFNα-2A, and RBV, is rare but can be fatal. Larger and longer studies are required to assess the efficacy and safety of simeprevir for HCV infection.
A 70-year-old female with interstitial pneumonitis that was induced by simeprevir with peg-interferon-α (PEG-IFN) and ribavirin (RBV) therapy for chronic hepatitis C.
The patient had dyspnea on effort and a mild dry cough.
Anemia is associated with the use of RBV, respiratory infections, bactrerial pneumonia.
Laboratory tests showed elevated KL-6 (3021 U/mL) suggesting interstitial pneumonitis.
The patient’s chest computed tomography revealed bilateral ground-glass opacities.
The authors immediately discontinued triple therapy (simeprevir with PEG-IFN and RBV) and administered 20 mg of oral prednisolone daily.
Drug-induced lung injury may involve the airways, lung parenchyma, mediastinum, pleura, pulmonary vasculature, and the most common form of drug-induced lung toxicity is drug-induced interstitial pneumonitis.
This is the first report of interstitial pneumonitis that was induced by simeprevir with PEG-IFN and RBV therapy for chronic hepatitis C.
The case is well documented showing enough data to sustain the diagnosis of interstitial pneumonitis developed by the patient after 8 wk of treatment.
P- Reviewer: Hegade VS, Larrubia JR S- Editor: Ma YJ L- Editor: A E- Editor: Zhang DN
| 1. | Fried MW, Shiffman ML, Reddy KR, Smith C, Marinos G, Gonçales FL, Häussinger D, Diago M, Carosi G, Dhumeaux D. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med. 2002;347:975-982. [PubMed] |
| 2. | Hadziyannis SJ, Sette H, Morgan TR, Balan V, Diago M, Marcellin P, Ramadori G, Bodenheimer H, Bernstein D, Rizzetto M. Peginterferon-alpha2a and ribavirin combination therapy in chronic hepatitis C: a randomized study of treatment duration and ribavirin dose. Ann Intern Med. 2004;140:346-355. [PubMed] |
| 3. | Manns MP, McHutchison JG, Gordon SC, Rustgi VK, Shiffman M, Reindollar R, Goodman ZD, Koury K, Ling M, Albrecht JK. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet. 2001;358:958-965. [PubMed] |
| 4. | deLemos AS, Chung RT. Hepatitis C treatment: an incipient therapeutic revolution. Trends Mol Med. 2014;20:315-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 38] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 5. | Torii H, Sueki H, Kumada H, Sakurai Y, Aoki K, Yamada I, Ohtsuki M. Dermatological side-effects of telaprevir-based triple therapy for chronic hepatitis C in phase III trials in Japan. J Dermatol. 2013;40:587-595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 6. | Matthews SJ, Lancaster JW. Telaprevir: a hepatitis C NS3/4A protease inhibitor. Clin Ther. 2012;34:1857-1882. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 29] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 7. | Lenz O, de Bruijne J, Vijgen L, Verbinnen T, Weegink C, Van Marck H, Vandenbroucke I, Peeters M, Simmen K, Fanning G. Efficacy of re-treatment with TMC435 as combination therapy in hepatitis C virus-infected patients following TMC435 monotherapy. Gastroenterology. 2012;143:1176-1178.e1-e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 34] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 8. | Vaidya A, Perry CM. Simeprevir: first global approval. Drugs. 2013;73:2093-2106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 34] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 9. | Zeuzem S, Berg T, Gane E, Ferenci P, Foster GR, Fried MW, Hezode C, Hirschfield GM, Jacobson I, Nikitin I. Simeprevir increases rate of sustained virologic response among treatment-experienced patients with HCV genotype-1 infection: a phase IIb trial. Gastroenterology. 2014;146:430-441.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 174] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 10. | Hayashi N, Izumi N, Kumada H, Okanoue T, Tsubouchi H, Yatsuhashi H, Kato M, Ki R, Komada Y, Seto C. Simeprevir with peginterferon/ribavirin for treatment-naïve hepatitis C genotype 1 patients in Japan: CONCERTO-1, a phase III trial. J Hepatol. 2014;61:219-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 127] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 11. | Izumi N, Hayashi N, Kumada H, Okanoue T, Tsubouchi H, Yatsuhashi H, Kato M, Ki R, Komada Y, Seto C. Once-daily simeprevir with peginterferon and ribavirin for treatment-experienced HCV genotype 1-infected patients in Japan: the CONCERTO-2 and CONCERTO-3 studies. J Gastroenterol. 2014;49:941-953. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 74] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 12. | Tilg H. New insights into the mechanisms of interferon alfa: an immunoregulatory and anti-inflammatory cytokine. Gastroenterology. 1997;112:1017-1021. [PubMed] |
| 13. | Borden EC, Parkinson D. A perspective on the clinical effectiveness and tolerance of interferon-alpha. Semin Oncol. 1998;25:3-8. [PubMed] |
| 14. | Dalgard O, Bjøro K, Hellum K, Myrvang B, Bjøro T, Haug E, Bell H. Thyroid dysfunction during treatment of chronic hepatitis C with interferon alpha: no association with either interferon dosage or efficacy of therapy. J Intern Med. 2002;251:400-406. [PubMed] |
| 15. | Brennan BJ, Xu ZX, Grippo JF. Effect of peginterferon alfa-2a (40KD) on cytochrome P450 isoenzyme activity. Br J Clin Pharmacol. 2013;75:497-506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 17. | Slavenburg S, Heijdra YF, Drenth JP. Pneumonitis as a consequence of (peg)interferon-ribavirin combination therapy for hepatitis C: a review of the literature. Dig Dis Sci. 2010;55:579-585. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 38] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 18. | Kwo PY. Response-guided therapy for HCV. Gastroenterol Hepatol (N Y). 2011;7:43-45. [PubMed] |
| 19. | McEwan P, Yuan Y, Litauen G, Kim R. Cost benefit analysis of response guided therapy: dynamic disease Markov modeling for patients with chronic hepatitis (HCV) by fibrosis stages. J Hepatol. 2011;54 (S461); Abs1167. |
| 20. | Reddy KR, Lin F, Zoulim F. Response-guided and -unguided treatment of chronic hepatitis C. Liver Int. 2012;32 Suppl 1:64-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |