Published online Aug 7, 2015. doi: 10.3748/wjg.v21.i29.8888
Peer-review started: January 16, 2015
First decision: March 10, 2015
Revised: March 26, 2015
Accepted: May 4, 2015
Article in press: May 4, 2015
Published online: August 7, 2015
Processing time: 205 Days and 1.7 Hours
AIM: To elucidate anticancer effects of transcatheter arterial infusion chemotherapy (TAI) in patients with hepatocellular carcinoma (HCC).
METHODS: Data from a total of 95 patients with HCC who received TAI were analyzed retrospectively. The efficacy of TAI was evaluated according to the Response Evaluation Criteria in Cancer of the Liver. Overall survival was calculated from the date of initial treatment to the date of death or last follow-up. Survival curves were calculated by the Kaplan-Meier method, and differences in survival were evaluated by the log rank test. Clinical variables that were identified as statistically different by a univariate analysis were included into the Cox proportional hazard regression model for multivariate analysis. A prognostic index based on the regression coefficients derived from variables identified by the multivariate analysis was constructed. Stratification of the patients was conducted using this prognostic index.
RESULTS: The patient group was comprised of 76 men and 19 women with an average age of 68 years (range: 37-82 years). Six patients (6.3%) showed complete response and 18 patients (18.9%) showed partial response, for an overall response rate of 25.2%. The median overall survival was 27.6 mo, and the proportions of survivors at 1, 2, and 5 years were 67.4%, 54.0%, and 17.4%, respectively. Multivariate analysis demonstrated that no prior transcatheter arterial chemoembolization, lactate dehydrogenase < 230 IU/L, and performance status of 0 were the independent favorable prognostic factors. The development of a 0-3-point prognostic score index was based on the sum of these three prognostic factors. Subsequently, the patients were categorized into three groups: those with a good (prognostic index = 0-1; n = 54), intermediate (prognostic index = 2; n = 26), or poor (prognostic index = 3; n = 15) prognosis. The median survival times in these three groups were 41.0, 21.2, and 6.8 mo, respectively (P < 0.01).
CONCLUSION: Our simple prognostic index may be helpful for management of patients in determining treatment strategies for advanced HCC in the era of molecularly targeted therapy.
Core tip: Transcatheter arterial infusion chemotherapy is one of the therapeutic approaches for hepatocellular carcinoma. In this study, multivariate Cox regression analyses demonstrated that no prior transcatheter arterial chemoembolization, lactate dehydrogenase < 230 IU/L, and performance status of 0 were the independent favorable prognostic factors. The prognostic index based on a combination of these three prognostic factors successfully categorized the patients into three groups with good, intermediate, or poor prognoses. This index may assist in the prediction of response to transcatheter arterial infusion chemotherapy in patients with hepatocellular carcinoma.
- Citation: Suzuki E, Chiba T, Ooka Y, Ogasawara S, Tawada A, Motoyama T, Kanogawa N, Saito T, Yoshikawa M, Yokosuka O. Transcatheter arterial infusion for advanced hepatocellular carcinoma: Who are candidates? World J Gastroenterol 2015; 21(29): 8888-8893
- URL: https://www.wjgnet.com/1007-9327/full/v21/i29/8888.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i29.8888
Hepatocellular carcinoma (HCC) is the sixth most common malignancy in the world[1,2]. Treatment options, such as resection, liver transplantation, and local ablative treatments, offer a chance of cure and improved life expectancy[3-7]. Transcatheter arterial chemoembolization (TACE) exhibits a marked antitumor effect in HCC, and meta-analyses have demonstrated that TACE improves the survival of patients with unresectable HCC and preserved hepatic function[8,9]. Recently, the multikinase inhibitor, sorafenib, has been also established as standard treatment for advanced HCC[10,11], but the survival rate remains unsatisfactory[12].
Transcatheter arterial infusion (TAI) is often used for the treatment of intermediate and advanced HCC, especially in Japan, because high concentration of the anticancer drugs can be delivered to tumors with less toxicity[13,14]. However, there is no evidence within phase III trials that show a survival benefit. In a randomized controlled trial of TACE and TAI with zinostatin stimalamer and lipiodol, TAI yielded results comparable to those of TACE with respect to survival[15]. In addition, sorafenib has been used in TACE-refractory cases, but the survival advantage of this agent is modest. If TAI can be used as an alternative treatment option, the addition of embolization may not be necessary for HCC patients, and TAI may result in longer survival when compared with sorafenib. Although the identification of prognostic factors might help with appropriate patient selection for TAI, only a few reports have investigated these factors[16,17].
The present study was conducted to investigate the antitumor efficacy of the treatment, as well as to identify the prognostic factors in patients with advanced HCC receiving TAI. In addition, a prognostic index is proposed to assist with determining treatment strategies in patients with HCC.
Between February 2000 and October 2010, 95 patients with advanced HCC were treated with TAI using various chemotherapy regimens at Chiba University Hospital, Japan. HCC was diagnosed on the basis of histologic examination or imaging studies such as distinctive findings on CT and/or angiography, with elevated serum levels of serum alpha-fetoprotein or des-γ-carboxy prothrombin. In principle, to assess the extent and size of the tumors before treatment, chest X-ray, ultrasonography, CT, and angiography of the abdomen were performed. Written informed consent was obtained from all the patients prior to the start of the treatment. This retrospective analysis was approved by the Ethics Committee of Chiba University.
TAI was performed by selectively introducing a catheter into the artery feeding the tumor and injecting anticancer drugs in a similar fashion to TACE[18,19]. Generally, the indication for TAI at our institution was as follows: (1) repeated TACE could not control the HCC progression; and (2) selective TACE could not be performed because of multilobar tumor expansion. Anticancer agents used for TAI were as follows: (1) epirubicin was selected as first-line treatment before cisplatin approval; (2) cisplatin-based regimens were used before miriplatin approval, but not in patients with chronic kidney disease; and (3) miriplatin was selected as first-line treatment after miriplatin approval. The dose of the drug and whether or not the port is implanted, were determined based on the tumor size and liver function. After the treatment, follow-up examinations, including CT, ultrasonography, tumor marker measurement, and serum biochemistry, were generally performed first at 1-3 mo after the treatment completion, and subsequently every 3-4 mo. The transcatheter arterial treatments were repeated when disease progression by imaging studies or clinical deterioration of the patient’s general condition occurred.
The antitumor effect was assessed by CT or magnetic resonance imaging at 3 mo after treatment according to the Response Evaluation Criteria in Cancer of the Liver[20]. Lipiodol accumulation in the tumor was regarded as representing necrotic tissue. Complete response was defined as 100% size reduction or 100% necrosis of all tumors, and partial response was defined as > 50% reduction and/or necrosis in the sum of all measurable tumors. Progressive disease was defined as more than 25% tumor growth in the sum of all lesions and/or the appearance of any new lesions. Stable disease was considered as any effect that did not qualify for classification as complete or partial response or progressive disease.
Variables were chosen based on previous investigations or our own clinical experience. Each of the variables was divided into two clinically meaningful subgroups. Overall survival was calculated from the date of initial treatment to the date of death or last follow-up. Survival curves were calculated by the Kaplan-Meier method. Univariate and multivariate analyses were calculated by Cox proportional hazards regression model. Only variables identified as statistically different by a univariate analysis were included in the multivariate analysis. A prognostic index based on the regression coefficients derived from all variables identified by the multivariate analysis was constructed. Stratification of the patients was based on this prognostic index. All P values shown in this report are of the two-tailed type. Differences at P < 0.05 were considered to be statistically significant.
The characteristics of all the 95 patients are shown in Table 1. The numbers of patients with Barcelona Clinic Liver Cancer stage A, B, C, and D were 6 (6.3%), 58 (61.1%), 27 (28.4%), and 4 (4.2%), respectively. Chemotherapy regimens of TAI are shown in Table 2. Thirty-five of 95 patients received hepatic arterial infusion chemotherapy using an implanted port-catheter system.
| Variables | Value |
| Sex, male/female | 76/19 |
| Age (yr): median (range) | 68 (37-82) |
| ECOG-PS: 0/1/2 | 62/28/5 |
| Etiology HBV/HCV/Others | 72/12/11 |
| Child-Pugh: A/B/C | 49/42/4 |
| Maximum tumor diameter (mm): median (range) | 30.0 (10.0-160.0) |
| Tumor distribution: unilateral/bilateral | 22/73 |
| Portal vein tumor thrombosis: yes/no | 19/76 |
| AFP (ng/mL), median (range) | 86.0 (1.9-5856.0) |
| DCP (mAU/mL), median (range) | 117 (0-204030) |
| Previous treatment | |
| Surgical resection | 4 |
| PEI | 25 |
| RFA | 8 |
| TACE | 34 |
| None | 24 |
| Regimen | No. of patients |
| Cisplatin | 35 |
| Carboplatin | 21 |
| Epirubicin | 13 |
| SMANCS | 12 |
| Miriplatin | 6 |
| 5-fluorouracil | 5 |
| Doxorubicin | 2 |
| Pirarubicin | 1 |
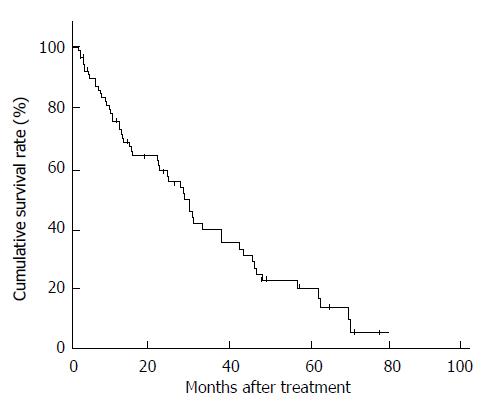
Six patients (6.3%) showed a complete response and 18 patients (18.9%) showed a partial response, for an overall response rate of 25.2%. The median survival time was 27.6 mo, and the proportions of survivors at 1, 2, and 5 years were 67.4%, 54.0%, and 17.4%, respectively (Figure 1).
The results of univariate analysis and multivariate analysis using the Cox proportional hazard model are shown in Table 3. Among the factors, platelet ≥ 10.0 × 104/mm3, total bilirubin < 1.0 mg/dL, lactate dehydrogenase (LDH) < 230 IU/L, performance status (PS) of 0, prothrombin time ≥ 70%, and no prior TACE, were significantly associated with longer survival time (all P < 0.05). In the multivariate analyses, only those variables identified as significant by the univariate analysis were examined. No prior TACE, serum LDH < 230 IU/L and PS of 0 were significantly associated with favorable survival.
| Variable | Univariate analysis | Multivariate analysis | ||
| HR (95%CI) | P value | HR (95%CI) | P value | |
| Sex (male) | 1.20 (0.60-2.37) | 0.62 | ||
| Age (≥ 70 yr) | 0.94 (0.59-1.70) | 0.81 | ||
| ECOG-PS (1 or 2) | 2.81 (1.58-5.02) | < 0.01 | 2.38 (1.29-4.39) | < 0.01 |
| Platelet count (≥ 10.0 × 104/mm3) | 2.07 (1.22-3.53) | < 0.01 | ||
| AST (≥ 70 IU/L) | 1.55 (0.92-2.61) | 0.10 | ||
| ALT (≥ 53 IU/L) | 1.23 (0.73-2.09) | 0.44 | ||
| LDH (≥ 230 IU/L) | 1.92 (1.14-3.22) | 0.01 | 2.03 (1.19-3.45) | < 0.01 |
| Total bilirubin (≥ 1.0 mg/dL) | 1.88 (1.11-3.18) | 0.02 | ||
| Albumin (≥ 3.0 g/dL) | 0.80 (0.43-1.48) | 0.47 | ||
| Prothrombin (≥ 70%) | 0.48 (0.26-0.88) | 0.02 | ||
| Maximum tumor diameter (≥ 30 mm) | 1.27 (0.74-2.16) | 0.39 | ||
| Tumor distribution (bilateral) | 1.12 (0.62-2.02) | 0.71 | ||
| Portal vein tumor thrombosis | 1.78 (0.45-2.19) | 0.14 | ||
| AFP (≥ 100 mg/ml) | 1.25 (0.74-2.11) | 0.40 | ||
| DCP (≥ 1000 mAU/mL) | 1.20 (0.67-1.45) | 0.33 | ||
| Prior TACE | 2.38 (1.34-4.24) | < 0.01 | 2.34 (1.28-4.28) | < 0.01 |
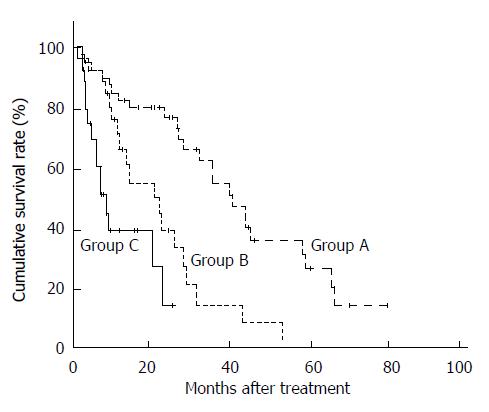
To apply these findings clinically, a prognostic index was evaluated according to the regression coefficients derived from the three significant variables identified by multivariate analysis: prognostic index = score for prior TACE (0 for no, 1 for yes) + score for LDH (0 for < 230 IU/L, 1 for ≥ 230 IU/L) + score for PS (0 for PS 0, 1 for PS 1 or 2). The index values ranged from 0 to 3. The patients were then stratified into three groups according to the prognostic index, as follows: good prognosis group (prognostic index = 0-1; n = 54) (equivalent to patients with none or one of the three prognostic factors); intermediate prognosis group (prognostic index = 2; n = 26) (equivalent to patients with two of the three prognostic factors); poor prognosis group (prognostic index = 3; n = 15) (equivalent to patients with all of the three prognostic factors). The survival curves of these groups are shown in Figure 2. The median survival times in the good, intermediate, and poor prognosis groups were 41.0, 21.2, and 6.8 mo, respectively, with significant differences among the three groups (P < 0.01).
The aim of this study was to investigate the anticancer activity of TAI and identify prognostic factors in patients with HCC receiving TAI. In this study, the median survival time and survival rates at two years in the current study were 27.6 mo and 54.0%, respectively. These results were comparable to those of TAI reported previously[16] and appeared to be favorable in comparison with those in conventional TACE performed approximately ten years ago[8,21]. However, recent studies[22-24] revealed that a new chemoembolization technique with drug eluting beads achieved longer survival than was observed in the present study.
Of importance, no prior TACE, LDH < 230 IU/L, and PS of 0 were found to be favorable prognostic factors by multivariate analysis. Many patients receiving TAI already have experienced TACE. One reason for unsatisfactory results is that HCC might acquire resistance to cytotoxic agents. LDH level correlates the tumor burden and the invasive potential of tumor. A high LDH level is associated with poor response to the therapy and recurrence of cancer in many malignancies[25,26]. PS is widely used as an assessment of the physical condition of the patient and is an important prognostic factor in patients with a variety of cancers[27,28]. For clinical application of these findings, we proposed a prognostic index based on the independent prognostic factors identified in this study. Patients are classified into three groups: those with good, intermediate, or poor prognoses. The median survival times in these three groups were 41.0, 21.2, and 6.8 mo, respectively. This index is easily calculated. Patients in the good prognosis group may obtain favorable response with TAI alone, with results comparable to those of TACE previously reported in Japan[29,30]. Even in TACE-refractory cases, TAI may be superior to sorafenib treatment if appropriate candidates are identified. In contrast, patients in the poor prognosis group are recommended for more aggressive treatments or best supportive care because of the extremely short median survival (6.8 mo).
The Barcelona Clinic Liver Cancer staging is widely used worldwide; in this staging system, TACE and sorafenib are the standard treatments for intermediate and advanced HCC, respectively[5]. Because of the lack of randomized controlled trials, TAI has not been recognized as an effective treatment. The combination of TAI with molecular targeted therapy may improve the survival for patients with advanced HCC.
This study has several limitations. First, the anticancer drugs used in TAI varied because no standard anticancer regimen has been established for TAI. Second, this study was retrospective and therefore has some inherent biases, such as selection criteria.
In conclusion, TAI exhibited antitumor effects and resulted in favorable survival in some patients with HCC. The prognostic factors identified and the proposed index based on these factors may be useful for predicting life expectancy, determining treatment strategies, and designing future clinical trials for advanced HCC in the era of molecularly targeted therapy.
Transcatheter arterial infusion chemotherapy (TAI) is one of the therapeutic approaches for hepatocellular carcinoma (HCC).
In this study, the efficacy of TAI is re-evaluated. Additionally, the prognostic index was developed to determine who are candidates for TAI.
The prognostic index was developed based on the results of multivariate analysis, as follows: prognostic index = score for prior transcatheter arterial chemoembolization (0 for no, 1 for yes) + score for lactate dehydrogenase (0 for < 230 IU/L, 1 for ≥ 230 IU/L) + score for performance status (0 for a status of 0, and 1 for a status of 1 or 2).
The prognostic index described in the current study may be helpful for management of patients via determining treatment strategies for advanced HCC.
TAI is an interventional radiologic treatment to deliver anticancer drugs to intrahepatic tumors.
The manuscript reported the efficacy of TAI on advanced HCC and investigated related prognostic factors by Cox regression analyses. The manuscript is well written and is significant to HCC management. As there is no other treatment control, such as TACE, related reports need to be cited and discussed in discussion section.
P- Reviewer: Hu B S- Editor: Yu J L- Editor: AmEditor E- Editor: Zhang DN
| 1. | Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69-90. [PubMed] |
| 2. | Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893-2917. [PubMed] |
| 3. | Bruix J, Sherman M. Management of hepatocellular carcinoma. Hepatology. 2005;42:1208-1236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4333] [Cited by in RCA: 4506] [Article Influence: 225.3] [Reference Citation Analysis (0)] |
| 4. | Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020-1022. [PubMed] |
| 5. | European Association for Study of Liver; European Organisation for Research and Treatment of Cancer. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. Eur J Cancer. 2012;48:599-641. [PubMed] |
| 6. | Kudo M, Izumi N, Kokudo N, Matsui O, Sakamoto M, Nakashima O, Kojiro M, Makuuchi M. Management of hepatocellular carcinoma in Japan: Consensus-Based Clinical Practice Guidelines proposed by the Japan Society of Hepatology (JSH) 2010 updated version. Dig Dis. 2011;29:339-364. [PubMed] |
| 7. | Omata M, Lesmana LA, Tateishi R, Chen PJ, Lin SM, Yoshida H, Kudo M, Lee JM, Choi BI, Poon RT. Asian Pacific Association for the Study of the Liver consensus recommendations on hepatocellular carcinoma. Hepatol Int. 2010;4:439-474. [PubMed] |
| 8. | Llovet JM, Bruix J. Systematic review of randomized trials for unresectable hepatocellular carcinoma: Chemoembolization improves survival. Hepatology. 2003;37:429-442. [PubMed] |
| 9. | Cammà C, Schepis F, Orlando A, Albanese M, Shahied L, Trevisani F, Andreone P, Craxì A, Cottone M. Transarterial chemoembolization for unresectable hepatocellular carcinoma: meta-analysis of randomized controlled trials. Radiology. 2002;224:47-54. [PubMed] |
| 10. | Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378-390. [PubMed] |
| 11. | Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S, Kim JS, Luo R, Feng J, Ye S, Yang TS. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10:25-34. [PubMed] |
| 12. | Ogasawara S, Chiba T, Ooka Y, Kanogawa N, Motoyama T, Suzuki E, Tawada A, Kanai F, Yoshikawa M, Yokosuka O. Efficacy of sorafenib in intermediate-stage hepatocellular carcinoma patients refractory to transarterial chemoembolization. Oncology. 2014;87:330-341. [PubMed] |
| 13. | Chang AE, Schneider PD, Sugarbaker PH, Simpson C, Culnane M, Steinberg SM. A prospective randomized trial of regional versus systemic continuous 5-fluorodeoxyuridine chemotherapy in the treatment of colorectal liver metastases. Ann Surg. 1987;206:685-693. [PubMed] |
| 14. | Reed ML, Vaitkevicius VK, Al-Sarraf M, Vaughn CB, Singhakowinta A, Sexon-Porte M, Izbicki R, Baker L, Straatsma GW. The practicality of chronic hepatic artery infusion therapy of primary and metastatic hepatic malignancies: ten-year results of 124 patients in a prospective protocol. Cancer. 1981;47:402-409. [PubMed] |
| 15. | Okusaka T, Kasugai H, Shioyama Y, Tanaka K, Kudo M, Saisho H, Osaki Y, Sata M, Fujiyama S, Kumada T. Transarterial chemotherapy alone versus transarterial chemoembolization for hepatocellular carcinoma: a randomized phase III trial. J Hepatol. 2009;51:1030-1036. [PubMed] |
| 16. | Ikeda M, Maeda S, Ashihara H, Nagahama H, Tanaka M, Sasaki Y. Transcatheter arterial infusion chemotherapy with cisplatin-lipiodol suspension in patients with hepatocellular carcinoma. J Gastroenterol. 2010;45:60-67. [PubMed] |
| 17. | Yamasaki T, Kimura T, Kurokawa F, Aoyama K, Ishikawa T, Tajima K, Yokoyama Y, Takami T, Omori K, Kawaguchi K. Prognostic factors in patients with advanced hepatocellular carcinoma receiving hepatic arterial infusion chemotherapy. J Gastroenterol. 2005;40:70-78. [PubMed] |
| 18. | Okimoto K, Ogasawara S, Chiba T, Ooka Y, Oobu M, Azemoto R, Kanogawa N, Motoyama T, Suzuki E, Tawada A. Efficacy of transcatheter arterial chemoembolization with miriplatin-lipiodol water-soluble contrast agent emulsion in patients with hepatocellular carcinoma. Anticancer Res. 2013;33:5603-5609. [PubMed] |
| 19. | Tawada A, Chiba T, Ooka Y, Kanogawa N, Saito T, Motoyama T, Ogasawara S, Suzuki E, Kanai F, Yoshikawa M. Transarterial chemoembolization with miriplatin plus epirubicin in patients with hepatocellular carcinoma. Anticancer Res. 2015;35:549-554. [PubMed] |
| 20. | Kudo M, Kubo S, Takayasu K, Sakamoto M, Tanaka M, Ikai I, Furuse J, Nakamura K, Makuuchi M. Response Evaluation Criteria in Cancer of the Liver (RECICL) proposed by the Liver Cancer Study Group of Japan (2009 Revised Version). Hepatol Res. 2010;40:686-692. [PubMed] |
| 21. | Lo CM, Ngan H, Tso WK, Liu CL, Lam CM, Poon RT, Fan ST, Wong J. Randomized controlled trial of transarterial lipiodol chemoembolization for unresectable hepatocellular carcinoma. Hepatology. 2002;35:1164-1171. [PubMed] |
| 22. | Raoul JL, Sangro B, Forner A, Mazzaferro V, Piscaglia F, Bolondi L, Lencioni R. Evolving strategies for the management of intermediate-stage hepatocellular carcinoma: available evidence and expert opinion on the use of transarterial chemoembolization. Cancer Treat Rev. 2011;37:212-220. [PubMed] |
| 23. | Burrel M, Reig M, Forner A, Barrufet M, de Lope CR, Tremosini S, Ayuso C, Llovet JM, Real MI, Bruix J. Survival of patients with hepatocellular carcinoma treated by transarterial chemoembolisation (TACE) using Drug Eluting Beads. Implications for clinical practice and trial design. J Hepatol. 2012;56:1330-1335. [PubMed] |
| 24. | Malagari K, Pomoni M, Moschouris H, Bouma E, Koskinas J, Stefaniotou A, Marinis A, Kelekis A, Alexopoulou E, Chatziioannou A. Chemoembolization with doxorubicin-eluting beads for unresectable hepatocellular carcinoma: five-year survival analysis. Cardiovasc Intervent Radiol. 2012;35:1119-1128. [PubMed] |
| 25. | Furuse J, Okusaka T, Ohkawa S, Nagase M, Funakoshi A, Boku N, Yamao K, Yamaguchi T, Sato T. A phase II study of uracil-tegafur plus doxorubicin and prognostic factors in patients with unresectable biliary tract cancer. Cancer Chemother Pharmacol. 2009;65:113-120. [PubMed] |
| 26. | Chen J, Sun MX, Hua YQ, Cai ZD. Prognostic significance of serum lactate dehydrogenase level in osteosarcoma: a meta-analysis. J Cancer Res Clin Oncol. 2014;140:1205-1210. [PubMed] |
| 27. | Suzuki E, Furuse J, Ikeda M, Okusaka T, Nakachi K, Mitsunaga S, Ueno H, Morizane C, Kondo S, Shimizu S. Treatment efficacy/safety and prognostic factors in patients with advanced biliary tract cancer receiving gemcitabine monotherapy: an analysis of 100 cases. Oncology. 2010;79:39-45. [PubMed] |
| 28. | Nakachi K, Furuse J, Ishii H, Suzuki E, Yoshino M. Prognostic factors in patients with gemcitabine-refractory pancreatic cancer. Jpn J Clin Oncol. 2007;37:114-120. [PubMed] |
| 29. | Takayasu K, Arii S, Ikai I, Omata M, Okita K, Ichida T, Matsuyama Y, Nakanuma Y, Kojiro M, Makuuchi M. Prospective cohort study of transarterial chemoembolization for unresectable hepatocellular carcinoma in 8510 patients. Gastroenterology. 2006;131:461-469. [PubMed] |
| 30. | Ikeda M, Arai Y, Park SJ, Takeuchi Y, Anai H, Kim JK, Inaba Y, Aramaki T, Kwon SH, Yamamoto S. Prospective study of transcatheter arterial chemoembolization for unresectable hepatocellular carcinoma: an Asian cooperative study between Japan and Korea. J Vasc Interv Radiol. 2013;24:490-500. [PubMed] |