Published online Aug 7, 2015. doi: 10.3748/wjg.v21.i29.8826
Peer-review started: January 1, 2015
First decision: March 10, 2015
Revised: April 10, 2015
Accepted: June 10, 2015
Article in press: June 10, 2015
Published online: August 7, 2015
Processing time: 221 Days and 0.5 Hours
AIM: To investigate the mechanistic action of brewers’ rice in regulating the Wnt/nuclear factor-kappa B (NF-κB)/Nrf2-signaling pathways during colon carcinogenesis in male Sprague-Dawley rats.
METHODS: Male Sprague-Dawley rats were randomly divided into the following five groups (six rats in each group): (G1) normal, (G2) azoxymethane (AOM) alone, (G3) AOM + 10% (weight (w)/weight (w)) brewers’ rice, (G4) AOM + 20% (w/w) brewers’ rice, and (G5) AOM + 40% (w/w) brewers’ rice. They were intraperitoneally administered 15 mg/kg body weight of AOM in saline once weekly over a two-week period and treated with an American Institute of Nutrition (AIN)-93G diet containing 10%, 20%, and 40% (w/w) brewers’ rice. The mRNA levels of glycogen synthase kinase 3β (GSK3β), β-catenin, key inflammation markers, nuclear factor E2-related factor 2 (Nrf2), and heme oxygenase-1 (HO-1)-dependent transcriptional activity were assessed by quantitative real-time polymerase chain reaction analyses. The colon superoxide dismutase, malondialdehyde, and nitric oxide levels were also analyzed to assess the antioxidant effect of these treatments. The results were analyzed using one-way analysis of variance (ANOVA), and a P value of < 0.05 was considered significant.
RESULTS: The overall analyses demonstrated that the dietary administration of brewers’ rice in AOM-induced rat colon carcinogenesis resulted in the transcriptional upregulation of GSK3β, inducible nitric oxide synthase (iNOS), Nrf2, and HO-1. We discovered that the dietary administration of brewers’ rice downregulated the β-catenin and NF-κB mRNA levels. A significant reduction in β-catenin expression was found in the groups administered with 20% (0.611 ± 0.034) and 40% (0.436 ± 0.045) (w/w) brewers’ rice compared with that of the group treated with AOM alone (1.000 ± 0.064) (P < 0.05). The NF-κB expression was significantly lower between the AOM-alone group (1.000 ± 0.048) and those groups fed with diets containing 10% (w/w) brewers’ rice (0.255 ± 0.022), 20% (w/w) brewers’ rice (0.450 ± 0.045), or 40% (w/w) brewers’ rice (0.541 ± 0.027) (P < 0.05). Brewers’ rice improved the antioxidant levels, indicating that brewers’ rice can enhance effective recovery from oxidative stress induced by AOM.
CONCLUSION: Our results provide evidence that brewers’ rice can suppress colon cancer via the regulation of Nrf2 expression and the inhibition of the Wnt/NF-κB signaling pathways.
Core tip: This study demonstrates that a treatment with 40% (w/w) brewers’ rice modulated the Wnt signaling pathway. Feeding 20% (w/w) brewers’ rice markedly improved the antioxidant level. These results strongly imply the potential use of brewers’ rice in future applications to combat oxidative stress and colon cancer.
- Citation: Tan BL, Norhaizan ME, Huynh K, Yeap SK, Hazilawati H, Roselina K. Brewers’ rice modulates oxidative stress in azoxymethane-mediated colon carcinogenesis in rats. World J Gastroenterol 2015; 21(29): 8826-8835
- URL: https://www.wjgnet.com/1007-9327/full/v21/i29/8826.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i29.8826
Colorectal cancer has become the third most prevalent cancer after lung and breast cancers and contributes to nearly 10% of the total cases of cancer and approximately 8% of total cancer deaths worldwide[1]. It represents the third and second most commonly diagnosed cancer in males and females, respectively, with more than 1.2 million new cancer cases and 608700 deaths in 2008 worldwide[2].
The deregulation of Wnt/β-catenin signaling has been demonstrated to be associated with cancer, particularly colorectal cancer[3]. Chronic infection and inflammation promote the expression of nuclear factor-kappa B (NF-κB)[4] and inflammatory-associated genes, such as inducible nitric oxide synthase (iNOS)[5]. The NF-κB pathway is associated with colorectal cancer, and the inhibition of NF-κB activation can reduce chemoresistance[6]. The nuclear factor E2-related factor 2 (Nrf2) transcription factor signaling pathway has become a target for chemoprevention. A previous study reported that Nrf2 regulates the expression of numerous detoxifying and antioxidant enzymes toward oxidative or electrophilic stress[7].
The health benefits of natural products have led to their recognition as sources of remedy[8]. Most studies have indicated that cancers may be prevented or delayed by treatment with natural dietary products or synthetic compounds[9]. Rice (Oryza sativa L.), an essential cereal crop grown in Asia, has become a major source of carbohydrates in the daily diet. Epidemiological studies have demonstrated that whole grain foods are recognized to be important for providing protection against cancer[10]. Brewers’ rice, known locally as temukut, consists of broken rice, rice bran, and rice germ, which is a waste product of the rice industry. The production of brewers’ rice during rice milling has been described in a previous report[11].
Our earlier study showed that the dietary administration of brewers’ rice can reduce the risk of azoxymethane (AOM)-induced colon carcinogenesis in rats through the downregulation of β-catenin and cyclooxygenase (COX-2)[12]. However, the molecular mechanism underlying these effects remains obscure. We hypothesized that brewers’ rice may provide chemopreventive or chemotherapeutic effects against colorectal cancer via regulation of multiple signaling pathways. The present study sets out to determine whether brewers’ rice confers suppressive effects on the gene expression of β-catenin and key inflammation markers, such as NF-κB and iNOS, which are particularly critical in the development of colon cancer. Glycogen synthase kinase 3β (GSK3β), a destruction complex that modulates the degradation of β-catenin, was also evaluated. Moreover, the potential roles of brewers’ rice in the regulation of Nrf2-dependent transcriptional activity were assessed during AOM-induced colon tumorigenesis in male Sprague-Dawley rats. Nrf2 and heme oxygenase-1 (HO-1) were evaluated to determine the effect of brewers’ rice in carcinogen metabolism against detoxification. The colon superoxide dismutase (SOD), malondialdehyde (MDA), and nitric oxide (NO) levels were also analyzed to assess the antioxidant effect of these treatments.
AOM and phosphate-buffered saline (PBS) were purchased from Sigma-Aldrich (St. Louis, MO, United States). RNA ShieldTM reagent was obtained from Zymo Research Corp. (Irvine, CA, United States). HiYieldTM Total Ribonucleic Acid (RNA) Mini Kit (Tissue) was purchased from Real Biotech Corporation (Banqiao City, Taipei County, Taiwan). High Capacity RNA-to-cDNA Kit and SYBR® Select Master Mix (CFX) were purchased from Applied Biosystems (Foster City, CA, United States). Specific primers were purchased from Sigma-Aldrich (St. Louis, MO, United States). Griess Reagent Kit was obtained from InvitrogenTM (Carlsbad, CA, United States). All other chemicals and reagents used were of analytical grade and bought from Sigma-Aldrich (St. Louis, MO, United States).
Freshly milled brewers’ rice samples from rice variety MR 219 were obtained from the BERNAS Milling Plant at Seri Tiram Jaya, Selangor, Malaysia. The stabilization of brewers’ rice was conducted as previously reported by Tan et al[13].
This study was conducted following the guidelines approved by the Institutional Animal Care and Use Committee (IACUC) of the Faculty of Medicine and Health Sciences, Universiti Putra Malaysia (UPM) Serdang, Selangor (IACUC protocol number: UPM/FPSK/PADS/BR-UUH/00461). A total of 30 four-week-old male Sprague-Dawley rats (Rattus norwegicus) were housed in a well-ventilated room at 25 to 27 °C with 50% ± 10% relative humidity and 12-h light/dark cycles. Hygienic conditions were maintained by weekly changes of woodchip beds. The animals were acclimatized for seven days and administered an American Institute of Nutrition (AIN)-93G diet and water ad libitum. The animals were randomly divided into the following five groups (six rats in each group): (G1) normal, (G2) AOM alone, (G3) AOM + 10% (weight (w)/weight (w)) brewers’ rice, (G4) AOM + 20% (w/w) brewers’ rice, and (G5) AOM + 40% (w/w) brewers’ rice. Beginning at six weeks of age, the rats were intraperitoneally given injections of AOM at a dose of 15 mg/kg body weight once weekly over a two-week period, whereas the rats in the normal group were given normal saline (vehicle control). The control groups (G1 and G2) were fed an AIN-93G diet, and the G3, G4, and G5 groups were given an AIN-93G diet containing 10%, 20%, and 40% (w/w) brewers’ rice, respectively. The experimental diets were prepared weekly and kept at 4 °C. The composition of the experimental diet (Table 1) was adjusted according to the nutrient content of brewers’ rice with respect to moisture (11.36% ± 0.12%), ash (1.56% ± 0.26%), protein (9.01% ± 0.27%), fat (1.95% ± 0.11%), total available carbohydrates (72.42% ± 1.25%), and total dietary fiber (5.32% ± 0.04%) contents[12]. After twenty weeks of treatment, the animals were sacrificed after anesthesia with diethyl ether, and the colon tissue was removed, rinsed with PBS, opened longitudinally, and fixed with RNA ShieldTM reagent or stored at -20 °C for further analyses.
| Ingredients (g/1000 g diet) | Group | ||||
| G1 | G2 | G3 | G4 | G5 | |
| Brewers’ rice | - | - | 100.0 | 200.0 | 400.0 |
| Corn starch | 397.5 | 397.5 | 315.3 | 233.2 | 68.9 |
| Casein | 200.0 | 200.0 | 191.0 | 182.0 | 164.0 |
| Maltodextrin | 132.0 | 132.0 | 132.0 | 132.0 | 132.0 |
| Sucrose | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 |
| Soybean oil | 70.0 | 70.0 | 68.1 | 66.1 | 62.2 |
| Powdered cellulose | 50.0 | 50.0 | 44.7 | 39.4 | 28.7 |
| AIN-93G mineral mix | 35.0 | 35.0 | 33.4 | 31.9 | 28.8 |
| AIN-93G vitamin mix | 10.0 | 10.0 | 10.0 | 10.0 | 10.0 |
| L-cystine | 3.0 | 3.0 | 3.0 | 3.0 | 3.0 |
| Choline bitartrate | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 |
| tert-butylhydroquinone | 0.014 | 0.014 | 0.014 | 0.014 | 0.014 |
The extraction of total RNA from colon tissue was performed using the HiYield Total RNA Mini Kit (Tissue). Initially, colon tissue disruption and homogenization were performed according to the manufacturer’s protocols. The colon tissue was homogenized in a mixture of 100 μL of lysis buffer, 400 μL of RB buffer, and 4 μL of β-mercaptoethanol. The sample was then incubated for 5 min at room temperature and centrifuged at 15680 ×g for 5 min. The supernatant was passed through the filter column and the collection tube. After centrifugation at 93 ×g for 1 min, 400 μL of 70% ethanol was added and passed through the RB column. After adding 400 μL of W1 Buffer and 600 μL of wash buffer, the RNA was eluted with 50 μL of RNase-free water and kept at -80 °C. Two microliters of nuclease-free water was added to the pedestal for a blank sample. After that, 1 μL of RNA sample was added. The RNA concentration was measured at 260 nm using a nanophotometer. Two micrograms of total RNA per 20 μL was reverse-transcribed using the High Capacity RNA-to-cDNA Kit, according to the manufacturer’s protocols. The reverse transcription reaction was performed using an Authorized Thermal Cycler. The reaction was performed at 37 °C for 60 min followed by 95 °C for 5 min to denature the enzyme and then maintained at 4 °C. The cDNA was then ready for use as a template for the amplification of real-time polymerase chain reaction (PCR).
The nucleotide primer sequences of rat origin were obtained from the National Center for Biotechnology Information Gene Bank (Table 2). The specific primers were validated for amplification specificity, amplification efficiency over a concentration range and consistency with the amplification efficiency of housekeeping genes. The mRNA levels of GSK3β, β-catenin, NF-κB, iNOS, Nrf2, and HO-1 were assayed using SYBR® Select Master Mix, CFX in a final volume of 20 μL, according to the manufacturer’s protocols. Initially, the cDNA template, primers, and kit contents (SYBR® Select Master Mix (CFX) and RNase-free water) were thawed on ice. Upon thawing, the reaction mix was prepared and thoroughly mixed. The qPCR reaction was then analyzed based on the following conditions: (1) uracil-DNA glycosylase (UDG) activation at 50 °C for 120 s (1 cycle); and (2) DNA polymerase activation at 95 °C for 120 s (1 cycle); denaturation at 95 °C for 2 s (40 cycles); and annealing/extension at 60 °C for 30 s (40 cycles). All samples and controls were determined in triplicate using an Eco™ Real-Time PCR system, and the v4.0.7.0 software (Illumina, Inc., San Diego, CA, United States) was used for data analysis. The fold inductions of the samples were compared with the control (AOM-alone group). Beta-actin (ACTB), β-2 microglobulin (B2M), and ribosomal protein, large, P1 (RPLP1) were used as housekeeping genes to normalize the expressions of the target genes.
| Primer name[Accession number] | Oligonucleotides (5’-3’)Sequence |
| GSK3β | F: GGGCACCAGAGCTGATCTTT |
| [NM_032080] | R: GCCGAAAGACCTTCGTCCA |
| Beta-catenin | F: CGTGGAAGCTGGTGGGATG |
| [AF121265] | R: TTCCTGCTTAGTCGCTGCAT |
| NF-κB | F: AGAGGATGTGGGGTTTCAGG |
| [NM_001276711.1] | R: GCTGAGCATGAAGGTGGATG |
| iNOS | F: GTACCCTCAGTTCTGTGCCT |
| [NM_012611.3] | R: TGTTGCGTTGGAAGTGTAGC |
| Nrf2 | F: TCTGACTCCGGCATTTCACT |
| [NM_031789.2] | R: CCCCAGAAGAATGTGTTGGC |
| HO-1 | F: CTAGAGCAGGACATGGCCTT |
| [NM_012580.2] | R: GCCTTCTGCGCAATCTTCTT |
| ACTB1 | F: CCACCCGCGAGTACAACC |
| [NM_031144.3] | R: TCAGGATGCCTCTCTTGCTC |
| B2M1 | F: CCCACCCTCATGGCTACTTC |
| [NM_012512.2] | R: GATGAAAACCGCACACAGGC |
| RPLP11 | F: CAAGGTGCTCGGTCCTTCC |
| [NM_001007604.2] | R: GAGCCTTTGCAAACAAGCCA |
The colon tissues of rats were homogenized in ice-cold PBS. Supernatants were collected by centrifugation at 370 ×g and 4 °C for 5 min and stored at -80 °C for the SOD[14], MDA[15], and NO[16] assays.
The SOD levels in the colon homogenates were analyzed following the inhibition of the reduction of nitroblue tetrazolium (NBT). Tissue supernatant was mixed with 0.1 mol/L of ethylenediaminetetraacetic acid (EDTA), 0.15 mg/mL of sodium cyanide, 1.5 mmol/L of NBT, 0.12 mmol/L of riboflavin, and 0.067 mol/L of phosphate buffer in a 300 μL volume. The sample absorbance was read at 560 nm, and the percentage of SOD inhibition was compared with that of the blank. The concentration of the sample was calculated using the amount of protein required to achieve 50% inhibition and expressed as U/mg of protein.
Lipid peroxidation was determined by measuring the thiobarbituric acid reactive substance (TBARS) levels. An aliquot of 100 μL of the supernatant was diluted with 400 μL of PBS and added with 12.5 μL of butylated hydroxytoluene (BHT, 8.8 mg/mL) and 250 μL of trichloroacetic acid (TCA, 30%). The mixture was vortexed, allowed to stand for 2 h at 4 °C and centrifuged at 2000 ×g for 15 min. The supernatant was boiled for 15 min with 37.5 μL of 0.1 mol/L EDTA and 125 μL of thiobarbituric acid (TBA, 1%). After cooling at room temperature, the absorbance of the pink-colored product was measured at 532 and 600 nm using an ELISA Reader (BioTek Instruments, Inc., Tigan Street, Winooski, United States). An aqueous solution of tetramethoxypropane was used as the standard. The MDA level was expressed as nmol MDA/g of protein and determined using a standard curve.
NO production in the colon was evaluated using a colorimetric Griess Reagent Kit, according to the manufacturer’s protocols. A 100-μL aliquot of the colon supernatant was loaded in the microtiter plate, and 20 μL of Griess reagent [0.1% of N-(1-naphthyl)ethylenediamine dihydrochloride and 1% of sulfanilic acid in 5% phosphoric acid] and 80 μL of deionized water were then added. The absorbance was measured at 540 nm using an ELISA Reader (BioTek Instruments, Inc., Tigan Street, Winooski, United States).
Data are expressed as mean ± SD, and statistical analyses were performed using one-way analysis of variance (ANOVA). Differences with P < 0.05 were considered significant. The statistical analyses were performed using the Statistical Package for Social Science (SPSS) version 19.0.
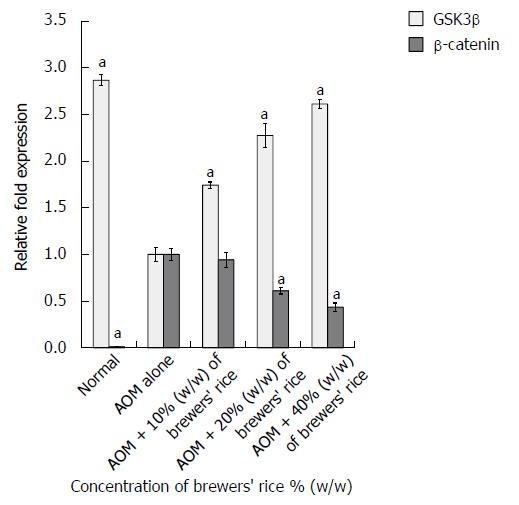
In the current study, we determined the GSK3β mRNA level of the control (normal and AOM alone) groups and the treatment groups through quantitative real-time PCR analyses. We observed that the normal group (2.866 ± 0.058) had the highest GSK3β mRNA level compared with the brewers’ rice-fed groups (Figure 1). The administration of brewers’ rice significantly increased the transcription of the GSK3β gene compared with AOM alone (P < 0.05). These findings clearly demonstrated that the dietary administration of brewers’ rice in AOM-induced rat colon carcinogenesis resulted in a dose-dependent increase in the GSK3β mRNA level.
As shown in Figure 1, our results showed that the colonic tumors in the groups treated with AOM alone had the highest β-catenin mRNA levels, whereas the administration of 20% (0.611 ± 0.034) and 40% (0.436 ± 0.045) (w/w) brewers’ rice markedly decreased the β-catenin mRNA levels. A significant reduction in β-catenin expression was found in the groups administered with 20% and 40% (w/w) brewers’ rice compared with the group treated with AOM alone (P < 0.05). In brewers’ rice-treated AOM-injected colon tumorigenesis rats, the phosphorylation and degradation of β-catenin increased in a dose-dependent manner. A very low β-catenin amount was observed in the normal group (0.011 ± 0.003) (Figure 1).
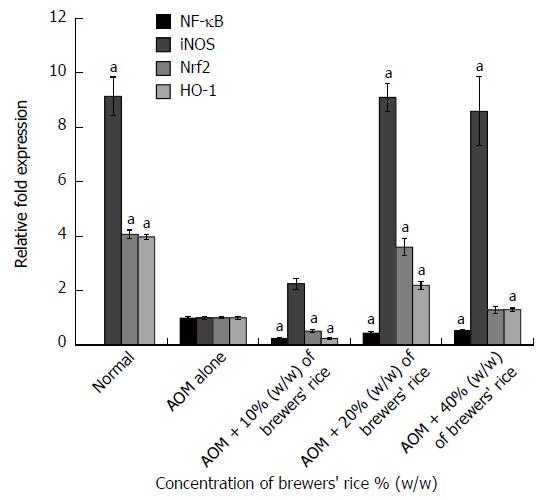
We hypothesized that brewers’ rice downregulates the expression of NF-κB. As expected, none of the rats exhibited NF-κB expression in the normal colon mucosa (Figure 2). The overall analysis indicated that the colon tissue in the group treated with AOM alone presented the highest NF-κB expression (1.000 ± 0.048) compared with the groups treated with brewers’ rice. A significant reduction in the gene expression of NF-κB was also observed in the rats of the groups treated with brewers’ rice compared with the group treated with AOM alone (P < 0.05). This finding revealed that the administration of brewers’ rice resulted in the inhibition of NF-κB expression, and the maximum effect was obtained with 10% (w/w) brewers’ rice (0.255 ± 0.022).
In the present study, we observed a high expression of iNOS mRNA in the normal colon mucosa (9.134 ± 0.708). The data presented in this study demonstrated that the groups administered with 20% (9.090 ± 0.519) and 40% (8.582 ± 1.261) (w/w) brewers’ rice exhibited significantly upregulated iNOS mRNA levels compared with the group treated with AOM alone (P < 0.05) (Figure 2).
As shown in Figure 2, in the normal group, which was administered saline but not treated with brewers’ rice, prominent Nrf2 gene expression was observed in the normal colon mucosa (4.068 ± 0.155). Our results showed that treatment with 20% (w/w) brewers’ rice (3.596 ± 0.308) effectively activated the gene expression of Nrf2 compared with AOM alone (Figure 2).
In the present study, we showed that the group treated with 20% (2.196 ± 0.150) and 40% (1.295 ± 0.063) (w/w) brewers’ rice exhibited upregulated HO-1 expression in colonic tumor tissue. Expectedly, we found that the normal group (3.967 ± 0.094) presented the highest expression of HO-1 (Figure 2).
The changes in the colon SOD, MDA, and NO activities after the dietary administration of brewers’ rice on AOM-induced colon carcinogenesis are summarized in Table 3. The SOD levels in the two treatment groups [20% (61.71 ± 2.36 U/mg of protein) and 40% (61.29 ± 4.32 U/mg of protein) (w/w) of brewers’ rice] were significantly elevated compared with that of the group treated with AOM alone (43.43 ± 2.96 U/mg of protein) (P < 0.05) (Table 3).
| Group | SOD (U/mg of protein) | MDA (nmol/g of protein) | NO (μmol/mg of protein) |
| Normal | 60.93 ± 5.23a | 5.36 ± 0.23a | 414.64 ± 11.59a |
| AOM alone | 43.43 ± 2.96 | 18.01 ± 1.43 | 798.46 ± 30.45 |
| AOM + 10% (w/w) of brewers’ rice | 43.85 ± 6.32 | 17.34 ± 3.16 | 622.70 ± 15.62a |
| AOM + 20% (w/w) of brewers’ rice | 61.71 ± 2.36a | 14.24 ± 0.58a | 533.40 ± 40.43a |
| AOM + 40% (w/w) of brewers’ rice | 61.29 ± 4.32a | 8.14 ± 1.42a | 619.35 ± 15.04a |
In addition to the effects on the SOD level, our findings showed that the highest MDA level was obtained in the group treated with AOM alone (18.01 ± 1.43 nmol/g of protein) compared with the groups treated with brewers’ rice. A significant reduction in the MDA level was found in the two treatment groups (20% (14.24 ± 0.58 nmol/g of protein) and 40% (8.14 ± 1.42 nmol/g of protein) (w/w) of brewers’ rice) compared with that of the group treated with AOM alone (18.01 ± 1.43 nmol/g of protein) (P < 0.05). These findings indicated that the dietary administration of brewers’ rice resulted in reductions in the MDA level in a dose-dependent manner, and the maximum effect was obtained with a concentration of 40% (w/w) brewers’ rice (8.14 ± 1.42 nmol/g of protein) (Table 3).
Consistent with the high levels of MDA observed in colon tumors, we also observed the highest NO level in the group treated with AOM alone (798.46 ± 30.45 μmol/mg of protein) compared with those of the other treatment groups (Table 3). After twenty weeks of treatment with brewers’ rice, the NO level was reduced. The suppressive effect of brewers’ rice on NO was notable in rats that received 20% (w/w) brewers’ rice (533.40 ± 40.43 μmol/mg of protein).
The current study is an extension of our earlier work, which determined that brewers’ rice was an effective dietary agent for the reduction of tumor incidence and multiplicity in rat colons induced with AOM[12]. We also determined that brewers’ rice markedly suppressed β-catenin expression in both the cytoplasm and the nucleus[12]. In the present study, male Sprague-Dawley rats were given different doses [10%, 20%, and 40% (w/w)] of brewers’ rice. A dosage of 10% (w/w) brewers’ rice was administered as suggested by a previous study performed by Boateng et al[17] on rice bran and rice germ. This dosage has been reported to reduce tumor formation. Moreover, higher concentrations [20% and 40% (w/w) brewers’ rice] were also used to determine the dose-dependent effect of brewers’ rice as a dietary agent in a rat colon cancer experimental model. Our earlier study reported that the highest dose [40% (w/w) brewers’ rice] was well-tolerated and did not suppress the growth of rats[12].
Targeting Wnt signaling upstream of T-cell factor (TCF)/β-catenin signaling is a critical therapeutic option. In the β-catenin destruction complex, GSK3β is one of the crucial components that modulates the degradation or accumulation of β-catenin in the nucleus. To ascertain whether brewers’ rice modulated GSK3βvia Wnt/β-catenin signaling, the GSK3β mRNA level was analyzed in the colon of rats induced with AOM. Overall, treatment with brewers’ rice resulted in an increase in the GSK3β mRNA level, and the maximum effect was obtained with 40% (w/w) brewers’ rice. To further verify whether the mechanisms of action of GSK3β observed in the colons of rats injected with AOM suppressed β-catenin expression, the mRNA level of β-catenin in response to brewers’ rice was further analyzed.
The Wnt/β-catenin pathway plays a vital role in tissue homeostasis and cancer susceptibility. The dysregulation of β-catenin and other Wnt molecules results in the nuclear localization of β-catenin, stimulation of Wnt target genes, and tumor formation[18]. Mutations in the β-catenin gene are usually found in AOM-induced colon tumorigenesis in rats and mice[19]. These findings, which are supported by the current data, further indicate that the activation of the β-catenin gene plays a vital role in the development of colon tumors in rats. The finding that the depletion of β-catenin suppresses tumor incidence and multiplicity in brewers’ rice-treated AOM-induced colon tumorigenesis suggests that brewers’ rice may become a potential strategy for the therapeutic control of Wnt/β-catenin signaling in colon cancer. In the present study, treatments with brewers’ rice resulted in increased GSK3β and decreased β-catenin, and the maximum effect was observed with 40% (w/w) brewers’ rice. The effects observed in the treatment with 40% (w/w) brewers’ rice could be explained by its higher concentrations of active compounds in brewers’ rice, which may confer better functional properties in the regulation of Wnt/β-catenin signaling pathway. A very low β-catenin mRNA level observed in the normal group was consistent with the findings reported by Barker et al[20], who found that Wnt/β-catenin signaling played an essential role in intestinal development, which is specific for the intestinal and mammary epithelia. A previous study also demonstrated that most of the β-catenin protein was present at very low amounts in the cytoplasm or nucleus of normal cells[21]. Cytoplasmic β-catenin was maintained at a low level for tissue homeostasis, particularly in strongly proliferative, self-renewing tissues, such as the skin and gut[22]. However, Wnt pathway mutations are not the only factors that promote the activation of β-catenin[23]. A study reported that NF-κB also plays a crucial role in colorectal and colitis-associated tumorigenesis[24]. Aberrant NF-κB stimulation has been identified in more than 50% of colorectal and colitis-associated tumors[25]. Thus, the expression levels of NF-κB in response to brewers’ rice were evaluated in the colons of rats induced with AOM.
The NF-κB family is a group of inducible transcription factors that are involved in immune and inflammatory responses and inhibit cell apoptosis. A previous study revealed that cancer cells with activated NF-κB are resistant against chemotherapeutics and ionizing radiation and that suppression of NF-κB activity markedly increases the sensitivity of cells to chemotherapeutic agents[26]. The inhibition of NF-κB transcriptional activity resulting from the administration of brewers’ rice was further supported by Biswas et al[27] and Xie et al[28], who found that phenolic compounds inhibited NF-κB in cell cultures and promoted anti-inflammatory and antioxidant responses. Although the maximum effect was observed in 10% (w/w) brewers’ rice, there was no significant difference between groups fed with 10% (w/w) brewers’ rice and groups fed with diets containing 20% (w/w) brewers’ rice or 40% (w/w) brewers’ rice (P > 0.05). The reason for the lack of any clear dose-dependence effects remains to be elucidated. One of the possible reasons may be due to the efficiency of brewers’ rice involved in the inhibition of NF-κB transcriptional activity reached with 10% (w/w) brewers’ rice. Collectively, the data presented in this study suggest that brewers’ rice may modulate colon tumor development through NF-κB signaling. In addition to the effects observed in Wnt and NF-κB signaling, the role of iNOS in the suppression of colon tumorigenesis elicited by brewers’ rice remains unknown. Therefore, we further determined the chemoprevention mechanism of iNOS on brewers’ rice in this model.
NO is produced during transcription and translation via iNOS, and once active, iNOS synthesizes high NO levels until substrate depletion[29]. However, our study shows contradictory results. It is possible that multiple cellular factors affect the sensitivity of NO, like specific NO metabolism pathways and interactions with other free radicals. The sensitivity of NO may also be associated with the expression of apoptosis-associated proteins, including Bcl-2, Bax, and Fas[30]. Excessive NO production can decrease the concentration of DNA repair enzymes and inhibit apoptosis through the nitrosylation of caspases[31]. The upregulation of iNOS mRNA levels in the current study was consistent with the results obtained by Radomski et al[32] and Dong et al[33], who reported that the expression of iNOS was inversely associated with metastatic activity in human colon cancer and murine melanoma (K-1735) cells. This finding was further supported by Shi et al[34], who demonstrated that iNOS overexpression not only attenuated the proliferation and metastasis of human renal cell carcinomas and murine fibrosarcoma but also induced apoptosis. However, the study conducted by Shi et al[34] contradicted the results reported by Sheng et al[35] and Di Popolo et al[36], who demonstrated that elevated iNOS mRNA and protein levels partially contributed to the inhibition of apoptosis in colon cancer cells. Therefore, the activation of iNOS at the mRNA level may play a critical role in growth inhibition and apoptosis in a human colorectal cancer (HT-29) cell line, as determined in our earlier studies[13,37]. A previous study showed that Nrf2 enhanced the basal expression of cytoprotective genes and suppressed cytokine-mediated inflammation[38]. Thus, the expression of Nrf2 in AOM-induced colon tissue was evaluated to determine whether brewers’ rice could modulate Nrf2 at the mRNA level.
Nrf2, which belongs to the Cap‘n’Collar family of basic region-leucine zipper transcription factors, was shown to be a key element in the antioxidant response element (ARE)-mediated transcriptional machinery[39]. Nrf2 plays a crucial role in the regulation of phase II detoxifying and antioxidant enzymes via AREs[40]. To determine whether brewers’ rice decreased colorectal cancer by modulating the antioxidant-mediated pathway, we examined the transcription of Nrf2. Treatment with 20% and 40% (w/w) brewers’ rice effectively activated the gene expression of Nrf2 and may be associated with the modulation of xenobiotic-metabolizing enzymes and responsible for the balance of carcinogen metabolism against detoxification[41].
Nrf2 is stimulated by an oxidative signal in the cytoplasm, which allows its translocation to the nucleus where it interacts with DNA ARE regions and promotes the expression of cytoprotective enzymes, such as glutathione S-transferase (GST), SOD, HO-1, and NADPH-quinone oxidase (NQO) (ARE-regulated genes)[42]. Our findings indicated that the manipulation of brewers’ rice in colonic tumor leads to changes in the gene expression of Nrf2-regulated HO-1, further suggesting that brewers’ rice is a positive regulator of Nrf2 signaling. The transcriptional downregulation of β-catenin and NF-κB in carcinogen-injected rats after treatment with a brewers’ rice diet was hypothesized because the carcinogen metabolism may have been shifted via Nrf2 and HO-1 in the colon. Our present study suggests that the possible chemopreventive mechanisms of brewers’ rice against colon carcinogenesis may be associated with both the phase I and II drug-metabolizing enzymes regulated by Nrf2, thus resulting in the detoxification of AOM and the rapid metabolism of AOM by P450. Collectively, this finding suggests that brewers’ rice may represent a promising natural dietary agent for the transcriptional downregulation of β-catenin and NF-κB and the upregulation of Nrf2 and HO-1 levels.
In addition to the effects observed in Nrf2 and HO-1 activation, the upregulation of Nrf2 and HO-1 activities in rats administered brewers’ rice indicated that brewers’ rice may be associated with an antioxidant enzyme. Therefore, the effect of treatments with brewers’ rice on the SOD, MDA, and NO activities in AOM-injected rats was examined. The decreased SOD levels in the group treated with AOM alone illustrated that the defense mechanism may have been overwhelmed to alleviate the amount of superoxide produced by the carcinogen. The observed effect may also be due to the impairment of antioxidant enzymes, which act as safeguards for cells during reactive oxygen species (ROS) detoxification[43]. This finding implies that the group treated with AOM alone, in which carcinogenesis was induced but no brewers’ rice treatment was administered, exhibited a reduction in SOD activity associated with a decreased antioxidative capacity. The group treated with AOM alone presented an increased MDA level and subsequently, increased lipid peroxidation, which was evident by the accumulation of β-catenin. Taken together, these findings suggest that the increased SOD and decreased MDA formation observed in the groups treated with brewers’ rice may be associated with a high total phenolic content and the bioactive compounds present in brewers’ rice, as reported by Tan et al[13]. Overall, the data obtained in this study suggest that brewers’ rice has the potential to increase SOD levels and reduce the activities of MDA and NO.
The transcriptional inhibition of β-catenin and NF-κB activities may lead to a suppression of colon cancer development, which implies that the observed effects can likely be attributed to the dietary compositions present in brewers’ rice. Most studies have demonstrated the additive and/or synergistic effects of some phytochemicals and nutrients[44-46]. Therefore, in the current study, rather than isolated compounds, brewers’ rice was administered to the rats. Results from our earlier study indicated that brewers’ rice consisted of a phenolic antioxidant, phytic acid, vitamin E, and γ-oryzanol[13]. The synergistic/additive activities of these components in brewers’ rice may contribute to a negative regulation of the Wnt and NF-κB signaling pathways to induce the phosphorylation and degradation of β-catenin and NF-κB expression, as observed in the present study. In addition to the effects observed in the Wnt and NF-κB signaling pathways, it is plausible that the bioactive constituents present in brewers’ rice facilitates the modulation of Nrf2 and Nrf2-regulated HO-1 expression, which subsequently enhances the antioxidant enzyme to mediate oxidative stress in the carcinogen-treated brewers’ rice-fed groups. In conclusion, this study provides clear evidence that brewers’ rice offers great potential against colorectal cancer via the regulation of Nrf2 expression and the inhibition of the Wnt and NF-κB signaling pathways. However, this study has been limited to the use of brewers’ rice in male Sprague-Dawley rats, and the duration of the treatment was only twenty weeks. Therefore, further studies are warranted in long-term animal studies or human clinical trials to confirm these findings. Uncontrolled signaling through the wingless/Wnt pathway and overexpression of NF-κB have been reported to play crucial roles in the development of colorectal cancer. Nrf2 is responsible in the regulation of phase II detoxification and antioxidant enzymes. Our findings showed that the dietary administration of 40% (w/w) brewers’ rice modulated the Wnt signaling pathway. Feeding 20% (w/w) brewers’ rice improved the antioxidant level, which indicated that brewers’ rice can effectively enhance recovery from oxidative stress induced by AOM. Taken together, these results strongly imply the potential use of brewers’ rice in future applications to combat oxidative stress and colon carcinogenesis.
We acknowledge BERNAS, Seri Tiram Jaya, Selangor, Malaysia for supplying the brewers’ rice sample and the laboratory staff of the Laboratory of Cancer Research (MAKNA) UPM for their technical assistance.
The deregulation of Wnt/β-catenin signaling and overexpression of nuclear factor-kappa B (NF-κB) has been associated with colorectal cancer. Studies have reported that natural products exert many beneficial health effects. Brewers’ rice, known locally as temukut, consists of broken rice, rice bran, and rice germ, which is a waste product produced in the rice industry. Although previous studies have demonstrated the anti-colon cancer activity of brewers’ rice, the molecular mechanisms underlying these effects have yet to be studied.
The authors aimed to investigate the mechanistic action of brewers’ rice in regulating the Wnt/NF-κB/Nrf2-signaling pathways and assess the antioxidant effect of these treatments during colon carcinogenesis in male Sprague-Dawley rats.
This is the first study demonstrating that brewers’ rice inhibited colon carcinogenesis via the modulation of multiple signaling pathways. The transcriptional inhibition of β-catenin and NF-κB activities and the activation of Nrf2 and HO-1 may be associated with the synergistic/additive effects of bioactive constituents present in brewers’ rice.
The authors hypothesize that brewers’ rice may provide chemopreventive or chemotherapeutic effects against colorectal cancer via the regulation of multiple signaling pathways. These findings suggest that brewers’ rice offers great potential against colorectal cancer via the regulation of Nrf2 expression and the inhibition of the Wnt/NF-κB signaling pathways.
Uncontrolled Wnt signaling pathway and overexpression of NF-κB have been reported to play a vital role in the development of colorectal cancer. Nrf2 is a key element in the ARE-mediated transcriptional machinery and plays a critical role in the regulation of phase II detoxification and antioxidant enzymes.
This is a very interesting study that attempts to elucidate the molecular mechanisms by which brewers′ rice could be a potential anticancer agent. This study is well-written, and its findings contribute to the understandings of the mechanisms through which rice may be beneficial in anticancer activity.
P- Reviewer: Agnihotri N, de Talamoni NGT, Koch TR, Paoluzi OA, Teramoto-Matsubara OT, Zhou T S- Editor: Ma YJ L- Editor: A E- Editor: Zhang DN
| 1. | American Cancer Society. Global Cancer Facts and Figures. 2nd ed. Atlanta: American Cancer Society 2011; 13-15. |
| 2. | Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23762] [Cited by in RCA: 25536] [Article Influence: 1824.0] [Reference Citation Analysis (7)] |
| 3. | Li VS, Ng SS, Boersema PJ, Low TY, Karthaus WR, Gerlach JP, Mohammed S, Heck AJ, Maurice MM, Mahmoudi T. Wnt signaling through inhibition of β-catenin degradation in an intact Axin1 complex. Cell. 2012;149:1245-1256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 627] [Cited by in RCA: 710] [Article Influence: 54.6] [Reference Citation Analysis (0)] |
| 4. | Pikarsky E, Porat RM, Stein I, Abramovitch R, Amit S, Kasem S, Gutkovich-Pyest E, Urieli-Shoval S, Galun E, Ben-Neriah Y. NF-kappaB functions as a tumour promoter in inflammation-associated cancer. Nature. 2004;431:461-466. [PubMed] |
| 5. | van der Woude CJ, Kleibeuker JH, Jansen PL, Moshage H. Chronic inflammation, apoptosis and (pre-)malignant lesions in the gastro-intestinal tract. Apoptosis. 2004;9:123-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 80] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 6. | Kunnumakkara AB, Diagaradjane P, Anand P, Harikumar KB, Deorukhkar A, Gelovani J, Guha S, Krishnan S, Aggarwal BB. Curcumin sensitizes human colorectal cancer to capecitabine by modulation of cyclin D1, COX-2, MMP-9, VEGF and CXCR4 expression in an orthotopic mouse model. Int J Cancer. 2009;125:2187-2197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 147] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 7. | Kensler TW, Wakabayashi N. Nrf2: friend or foe for chemoprevention? Carcinogenesis. 2010;31:90-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 378] [Cited by in RCA: 364] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 8. | Srujana TS, Babu KR, Rao BSS. Phytochemical investigation and biological activity of leaves extract of plant Boswellia Serrata. The Pharma Innovation. 2012;1:22-46. |
| 9. | Kong AN, Yu R, Hebbar V, Chen C, Owuor E, Hu R, Ee R, Mandlekar S. Signal transduction events elicited by cancer prevention compounds. Mutat Res. 2001;480-481:231-241. [PubMed] |
| 10. | Ward JL, Poutanen K, Gebruers K, Piironen V, Lampi AM, Nyström L, Andersson AA, Aman P, Boros D, Rakszegi M. The HEALTHGRAIN Cereal Diversity Screen: concept, results, and prospects. J Agric Food Chem. 2008;56:9699-9709. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 150] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 11. | Esa NM, Ling TB, Peng LS. By-products of rice processing: An overview of health benefits and applications. J Rice Res. 2013;1:107. [RCA] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 12. | Tan BL, Esa NM, Rahman HS, Hamzah H, Karim R. Brewers’ rice induces apoptosis in azoxymethane-induced colon carcinogenesis in rats via suppression of cell proliferation and the Wnt signaling pathway. BMC Complement Altern Med. 2014;14:304. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 13. | Tan BL, Norhaizan ME, Suhaniza HJ, Lai CC, Norazalina S, Roselina K. Antioxidant properties and antiproliferative effect of brewers’ rice extract (temukut) on selected cancer cell lines. Int Food Res J. 2013;20:2117-2124. |
| 14. | Ilouno LE, Shu EN, Igbokwe GE. An improved technique for the assay of red blood cell superoxide dismutase (SOD) activity. Clin Chim Acta. 1996;247:1-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 15. | Suhail M, Faizul-Suhail M. Oxidant-antioxidant status in pair-matched maternal and cord blood of normotensive and preeclamptic patients. Zhongguo Linchuang Yixue Yingxiang Zazhi. 2009;4:241-248. |
| 16. | He RR, Tsoi B, Lan F, Yao N, Yao XS, Kurihara H. Antioxidant properties of lutein contribute to the protection against lipopolysaccharide-induced uveitis in mice. Chin Med. 2011;6:38. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 39] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 17. | Boateng J, Verghese M, Panala V, Walker LT, Shackelford L. Protective effects of rice bran on chemically induced colon tumorigenesis may be due to synergistic/additive properties of bioactive components. Int J Cancer Res. 2009;5:153-166. [RCA] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 18. | Giles RH, van Es JH, Clevers H. Caught up in a Wnt storm: Wnt signaling in cancer. Biochim Biophys Acta. 2003;1653:1-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 247] [Cited by in RCA: 637] [Article Influence: 29.0] [Reference Citation Analysis (0)] |
| 19. | Takahashi M, Nakatsugi S, Sugimura T, Wakabayashi K. Frequent mutations of the beta-catenin gene in mouse colon tumors induced by azoxymethane. Carcinogenesis. 2000;21:1117-1120. [PubMed] |
| 20. | Barker N, Huls G, Korinek V, Clevers H. Restricted high level expression of Tcf-4 protein in intestinal and mammary gland epithelium. Am J Pathol. 1999;154:29-35. [PubMed] |
| 21. | Rubinfeld B, Albert I, Porfiri E, Fiol C, Munemitsu S, Polakis P. Binding of GSK3beta to the APC-beta-catenin complex and regulation of complex assembly. Science. 1996;272:1023-1026. [PubMed] |
| 22. | Schmalhofer O, Brabletz S, Brabletz T. E-cadherin, beta-catenin, and ZEB1 in malignant progression of cancer. Cancer Metastasis Rev. 2009;28:151-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 564] [Cited by in RCA: 623] [Article Influence: 38.9] [Reference Citation Analysis (0)] |
| 23. | Lu Z, Hunter T. Wnt-independent beta-catenin transactivation in tumor development. Cell Cycle. 2004;3:571-573. [PubMed] |
| 24. | Terzić J, Grivennikov S, Karin E, Karin M. Inflammation and colon cancer. Gastroenterology. 2010;138:2101-2114.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1333] [Cited by in RCA: 1507] [Article Influence: 100.5] [Reference Citation Analysis (0)] |
| 25. | Karin M, Greten FR. NF-kappaB: linking inflammation and immunity to cancer development and progression. Nat Rev Immunol. 2005;5:749-759. [PubMed] |
| 26. | Wang CY, Mayo MW, Baldwin AS. TNF- and cancer therapy-induced apoptosis: potentiation by inhibition of NF-kappaB. Science. 1996;274:784-787. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2069] [Cited by in RCA: 2067] [Article Influence: 71.3] [Reference Citation Analysis (0)] |
| 27. | Biswas SK, McClure D, Jimenez LA, Megson IL, Rahman I. Curcumin induces glutathione biosynthesis and inhibits NF-kappaB activation and interleukin-8 release in alveolar epithelial cells: mechanism of free radical scavenging activity. Antioxid Redox Signal. 2005;7:32-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 256] [Cited by in RCA: 247] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 28. | Xie C, Kang J, Ferguson ME, Nagarajan S, Badger TM, Wu X. Blueberries reduce pro-inflammatory cytokine TNF-α and IL-6 production in mouse macrophages by inhibiting NF-κB activation and the MAPK pathway. Mol Nutr Food Res. 2011;55:1587-1591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 87] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 29. | Hickey MJ, Granger DN, Kubes P. Inducible nitric oxide synthase (iNOS) and regulation of leucocyte/endothelial cell interactions: studies in iNOS-deficient mice. Acta Physiol Scand. 2001;173:119-126. [PubMed] |
| 30. | Xie K, Huang S. Contribution of nitric oxide-mediated apoptosis to cancer metastasis inefficiency. Free Radic Biol Med. 2003;34:969-986. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 78] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 31. | Rao CV. Nitric oxide signaling in colon cancer chemoprevention. Mutat Res. 2004;555:107-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 167] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 32. | Radomski MW, Jenkins DC, Holmes L, Moncada S. Human colorectal adenocarcinoma cells: differential nitric oxide synthesis determines their ability to aggregate platelets. Cancer Res. 1991;51:6073-6078. [PubMed] |
| 33. | Dong Z, Staroselsky AH, Qi X, Xie K, Fidler IJ. Inverse correlation between expression of inducible nitric oxide synthase activity and production of metastasis in K-1735 murine melanoma cells. Cancer Res. 1994;54:789-793. [PubMed] |
| 34. | Shi Q, Xiong Q, Wang B, Le X, Khan NA, Xie K. Influence of nitric oxide synthase II gene disruption on tumor growth and metastasis. Cancer Res. 2000;60:2579-2583. [PubMed] |
| 35. | Sheng H, Shao J, Morrow JD, Beauchamp RD, DuBois RN. Modulation of apoptosis and Bcl-2 expression by prostaglandin E2 in human colon cancer cells. Cancer Res. 1998;58:362-366. [PubMed] |
| 36. | Di Popolo A, Memoli A, Apicella A, Tuccillo C, di Palma A, Ricchi P, Acquaviva AM, Zarrilli R. IGF-II/IGF-I receptor pathway up-regulates COX-2 mRNA expression and PGE2 synthesis in Caco-2 human colon carcinoma cells. Oncogene. 2000;19:5517-5524. [PubMed] |
| 37. | Tan BL, Norhaizan ME, Yeap SK, Roselina K. Water extract of brewers’ rice induces antiproliferation of human colorectal cancer (HT-29) cell lines via the induction of apoptosis. Eur Rev Med Pharmacol Sci. 2015;19:1022-1029. [PubMed] |
| 38. | Hayes JD, McMahon M. NRF2 and KEAP1 mutations: permanent activation of an adaptive response in cancer. Trends Biochem Sci. 2009;34:176-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 647] [Cited by in RCA: 697] [Article Influence: 43.6] [Reference Citation Analysis (0)] |
| 39. | Chen C, Kong AN. Dietary chemopreventive compounds and ARE/EpRE signaling. Free Radic Biol Med. 2004;36:1505-1516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 184] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 40. | Shen G, Xu C, Hu R, Jain MR, Nair S, Lin W, Yang CS, Chan JY, Kong AN. Comparison of (-)-epigallocatechin-3-gallate elicited liver and small intestine gene expression profiles between C57BL/6J mice and C57BL/6J/Nrf2 (-/-) mice. Pharm Res. 2005;22:1805-1820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 113] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 41. | Perocco P, Bronzetti G, Canistro D, Valgimigli L, Sapone A, Affatato A, Pedulli GF, Pozzetti L, Broccoli M, Iori R. Glucoraphanin, the bioprecursor of the widely extolled chemopreventive agent sulforaphane found in broccoli, induces phase-I xenobiotic metabolizing enzymes and increases free radical generation in rat liver. Mutat Res. 2006;595:125-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 53] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 42. | Eggler AL, Gay KA, Mesecar AD. Molecular mechanisms of natural products in chemoprevention: induction of cytoprotective enzymes by Nrf2. Mol Nutr Food Res. 2008;52 Suppl 1:S84-S94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 87] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 43. | Du J, He D, Sun LN, Han T, Zhang H, Qin LP, Rahman K. Semen Hoveniae extract protects against acute alcohol-induced liver injury in mice. Pharm Biol. 2010;48:953-958. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 30] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 44. | Wedge DE, Meepagala KM, Magee JB, Smith SH, Huang G, Larcom LL. Anticarcinogenic activity of strawberry, blueberry, and raspberry extracts to breast and cervical cancer cells. J Med Food. 2001;4:49-51. [PubMed] |
| 45. | Liu RH. Health benefits of fruit and vegetables are from additive and synergistic combinations of phytochemicals. Am J Clin Nutr. 2003;78:517S-520S. [PubMed] |
| 46. | Tsuda H, Ohshima Y, Nomoto H, Fujita K, Matsuda E, Iigo M, Takasuka N, Moore MA. Cancer prevention by natural compounds. Drug Metab Pharmacokinet. 2004;19:245-263. [PubMed] |