Published online Aug 7, 2015. doi: 10.3748/wjg.v21.i29.8804
Peer-review started: April 16, 2015
First decision: May 18, 2015
Revised: June 9, 2015
Accepted: June 16, 2015
Article in press: June 16, 2015
Published online: August 7, 2015
Processing time: 116 Days and 0.7 Hours
Microscopic colitis is a common cause of chronic, nonbloody diarrhea. Microscopic colitis is more common in women than men and usually affects patients in their sixth and seventh decade. This article reviews the etiology and medical management of microscopic colitis. The etiology of microscopic colitis is unknown, but it is associated with autoimmune disorders, such as celiac disease, polyarthritis, and thyroid disorders. Smoking has been identified as a risk factor of microscopic colitis. Exposure to medications, such as non-steroidal anti-inflammatory drugs, proton pump inhibitors, and selective serotonin reuptake inhibitors, is suspected to play a role in microscopic colitis, although their direct causal relationship has not been proven. Multiple medications, including corticosteroids, anti-diarrheals, cholestyramine, bismuth, 5-aminosalicylates, and immunomodulators, have been used to treat microscopic colitis with variable response rates. Budesonide is effective in inducing and maintaining clinical remission but relapse rate is as high as 82% when budesonide is discontinued. There is limited data on management of steroid-dependent microscopic colitis or refractory microscopic colitis. Immunomodulators seem to have low response rate 0%-56% for patients with refractory microscopic colitis. Response rate 66%-100% was observed for use of anti-tumor necrosis factor (TNF) therapy for refractory microscopic colitis. Anti-TNF and diverting ileostomy may be an option in severe or refractory microscopic colitis.
Core tip: The etiology of microscopic colitis (MC) is unknown. There is a strong association with autoimmune disorders, smoking, and medications, such as non-steroidal anti-inflammatory drugs, proton pump inhibitors, and selective serotonin reuptake inhibitors. There are no societal guidelines on how to manage patients with MC. Data is strongest for the use of budesonide. Budesonide can rapidly induce clinical remission but relapse occurs frequently after discontinuation of budesonide. Anti-diarrheals may be used alone in mild MC or in conjunction with other therapies in moderate to severe MC. There is limited data on management of steroid-dependent or refractory MC but anti-TNF and diverting ileostomy may be options.
- Citation: Park T, Cave D, Marshall C. Microscopic colitis: A review of etiology, treatment and refractory disease. World J Gastroenterol 2015; 21(29): 8804-8810
- URL: https://www.wjgnet.com/1007-9327/full/v21/i29/8804.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i29.8804
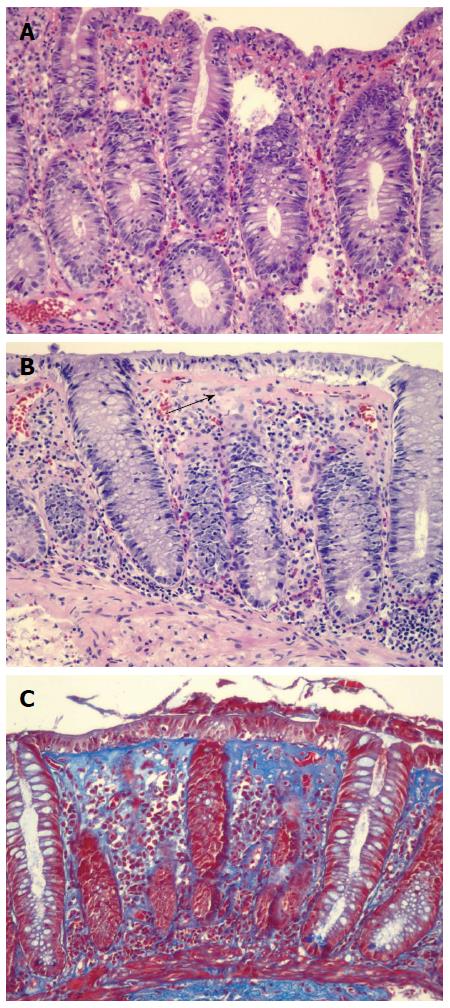
Microscopic colitis (MC) is a common cause of chronic, nonbloody diarrhea. Up to 10%-20% of chronic diarrhea is thought to be secondary to MC. The incidence of MC in Sweden, Spain, and Iceland from the 1990s ranged from 1.1 to 5.2 per 100000 person-years[1]. In a population based cohort study conducted from 1985 to 2001 in Olmsted County, Minnesota, the incidence of CC was 3.1 per 100000 and that of LC was 5.5 per 100000[1]. Poisson regression analysis showed that the incidence of MC increased over time, and the incidence of MC by the end of 2001 was 19.6 per 100000[1]. MC typically affects patients in their 50-60 s and occurs more frequently in women than men. The diagnosis is made by both clinical history and endoscopic biopsies. While chronic watery diarrhea is the most common symptom, some patients with MC may also experience abdominal pain, fecal incontinence, and/or weight loss. Colonoscopy generally reveals normal colonic mucosa but colonic biopsy shows classic histological features: > 20 intraepithelial lymphocytes per 100 epithelial cells in lymphocytic colitis (LC) and 10-20 μm of a thickened subepithelial collagen band in collagenous colitis (CC) (Figure 1). Inflammation in the lamina propria, with mainly mononuclear cells, may be seen in CC. Other etiologies, such as celiac disease, inflammatory bowel disease, and infectious colitis should be ruled out.
The etiology of MC is unknown. There is a strong association with autoimmune disorders, such as celiac disease, polyarthritis, and thyroid disorders (Table 1). Up to twenty to 60% of patients with LC and 17%-40% of patients with CC have autoimmune disease[2]. In fact, histological features of MC in the colon are present in 30% of patients with celiac disease. While no specific genetic mutations have been identified as direct cause of MC, some studies have found common genetic abnormalities. For instance, there is an increased prevalence of human leukocyte antigen (HLA) DR3 DQ2 allele in patients with MC, and metalloproteinase 9 gene variations have been associated with CC[3].
| Autoimmune disorder (type 1 diabetes, rheumatoid arthritis, celiac disease, thyroid disorders) |
| Nonsteroidal anti-inflammatory drugs |
| Proton pump inhibitors |
| Serotoin reuptake inhibitors |
| Statins |
| Beta-blockers |
| Smoking |
Smoking is a risk factor for MC. In a prospective, case-control study conducted from 2007 to 2010 in Spain involving 255 patients, smoking was significantly associated with LC and CC (OR = 3.8 in LC, OR = 2.4 in CC)[4]. Swedish study conducted by Vigren et al[5] also showed that smoking is associated with CC. Thirty-seven percent of patients with CC were smokers as compared to only 17% of patients in the control group (OR = 2.95). Subgroup analysis showed that the association of smoking with CC was most notable in the age group 16-44; 75% of patients in this age group were smokers as compared to 15% in the control group (odd ratio: 16.54). Smokers develop MC earlier than nonsmokers by a median of 14 years[6].
Medications are often implicated as a cause of MC. Non-steroidal anti-inflammatory drugs (OR = 4.6 in LC, OR = 3.8 in CC), proton pump inhibitors (OR = 2.7 in LC, OR = 6.4 in CC), selective serotonin reuptake inhibitors (OR = 17.5 in LC), and beta-blockers (OR = 3.6 in CC) are strongly associated with MC (Table 1)[4]. A population-based case-control study using a Dutch primary care database assessed the risk of MC from exposure to medications compared with community control as well as with colonoscopy control where subjects had negative colonoscopy and negative histology for MC[7]. This study showed that current use of proton pump inhibitor (OR = 10.6) and nonsteroidal anti-inflammatory drugs (OR = 5.6) significantly increased the risk of MC[7]. Diclofenac was the most commonly used non-steroidal anti-inflammatory drug, and omeprazole was the most commonly used proton pump inhibitor in this study. Selective serotonin reuptake inhibitors, beta-blockers, and ACE inhibitors increased the risk of MC when compared with community controls, but not when compared with colonoscopy controls[7].
A more recent theory centers around bacterial translocation in the gastrointestinal tract. Bacterial antigens or toxins are suspected to increase inflammatory mediators in the colonic mucosa, leading to increased mucosal permeability, increased cytokines, degradation of the collagen matrix, and dysregulation of intestinal subepithelial myofibroblasts. No specific organisms have been identified in causing or exacerbating MC. Analysis of colonic biopsies of patients with MC demonstrated an increased amount of interferon-gamma, tumor necrosis factor alpha, and interleukin-1β in patients with MC as compared to patients without MC who underwent routine screening/surveillance colonoscopy, suggesting role of Th1 immune response in MC[8]. There was a trend towards increased level of interleukin-13 in patients with MC but interleukin-13 levels were not significantly different between MC and non-MC patients[8]. There was no difference in interleukin-8 level between the two groups[8]. Mucosal mRNA levels of interferon-gamma and interleukin-15 were 100 times greater and tumor necrosis factor alpha was 60 times greater in patients with MC as compared to patients with irritable bowel syndrome with diarrhea predominance, also supporting the role of Th1 immune response, which may be triggered by an unknown luminal antigen and ultimately leads to MC in susceptible individuals[9].
There are no society guidelines on how to best manage patients with MC. While some provider will start with the most benign recommendations including lifestyle changes and work their way up to anti-diarrheals and steroids, others will go right to the most effective medications.
As an initial approach, a thorough history should be taken in order to corroborate a temporal relationship between starting a new medication and the onset of symptoms. If suspected, offending medications should be discontinued if possible. Smoking cessation should also be encouraged.
There are no randomized controlled trials comparing efficacy of antidiarrheals against placebo or other medical therapy for MC. A retrospective registry review of 163 patients with CC showed a 71% efficacy rate (49 out of 69 patients) of loperamide[10]. Consensus is that anti-diarrheals may be used alone in mild MC or in conjunction with other therapies in moderate to severe MC to reduce frequency of diarrhea.
In an animal study, bismuth subsalicylate and bismuth subcitrate enemas significantly reduced the macroscopic and microscopic appearance of the 2, 4, 6-trinitrobenzene sulfonic acid (TNBS)-induced colitis in rats compared to TNBS-induced rats treated with saline enemas[11]. TNBS-induced colitis is a model for chronic inflammation and ulceration seen in inflammatory bowel disease rather than MC; therefore, this animal study does not directly demonstrate benefit of bismuth in MC. However, its effect on reduction of microscopic injury scores can be used to infer its benefit in MC. A prospective study of 12 patients with MC treated with bismuth subsalicylate 262 mg/d for 8 wk showed a 92% rate of clinical remission and a 75% rate of histological resolution of MC[12]. Mean time to response was 2 wk. At 7-28 mo follow-up, 75% of patients remained in remission.
There are no randomized controlled trials demonstrating efficacy of cholestyramine for MC. In a small retrospective study involving 27 patients with CC, 12 out of 27 (44%) patients were found to have abnormal bile acid absorption. Eleven out of these 12 patients (92%) reported improvement in diarrhea within one week when treated with bile acid binding agents (cholestyramine 4 g 2-3 times per day, or colestipol 5 g 2-3 times per day if the patients could not tolerate the smell or taste of cholestyramine)[13]. Ten out of 15 (67%) patients with CC and normal bile acid absorption reported improvement in diarrhea when treated with bile acid binding agents[13]. Overall, 78% of patients with CC experienced improvement in diarrhea with bile acid binding agents. In a retrospective registry review of patients with CC, cholestyramine was effective in 26 out of 44 patients (59%)[10].
In a study of 64 patients with MC treated with mesalamine 2.4 g/d vs mesalamine 2.4 g/d plus cholestyramine 4 g/d for 6 mo, the rates of remission were similar between the two groups. Eighty four percent of patients experienced clinical remission within the first 2 wk of treatment[14]. Clinical remission occurred earlier in patients treated with mesalamine plus cholestyramine than those treated with mesalamine alone.
Other studies have demonstrated little benefit of aminosalicylates. In a double-blinded, randomized placebo-controlled trial of 92 patients comparing efficacy of budesonide, mesalamine, and placebo, interim analysis found mesalamine to be less effective than placebo in inducing clinical remission and the review board recommended closure of this study arm[15]. The rate of clinical remission was 44% in mesalamine group and 59.5% in placebo group at 8 wk[15].
A number of medications have been studied to induce clinical remission, and the evidence is strongest for the use of budesonide. A prospective, double-blinded, randomized controlled trial comparing induction of budesonide (9 mg/d for 4 wk, 6 mg/d for 2 wk, alternating doses of 6 and 3 mg/d for 2 wk) followed by maintenance dose of budesonide (alternating 6 and 3 mg/d) vs placebo for 1 year showed that maintenance of clinical remission rate was higher in budesonide group (61.4%) than in the placebo group (16.7%)[16]. Clinical remission rate after induction period was 84.5% and the median time to remission was 10.5 d[16].
Similar randomized controlled trials comparing induction of budesonide followed by maintenance with budesonide vs placebo have shown high clinical remission rate 77%-96% in budesonide group (Table 2). A Cochrane review by Chande et al[17] showed that the pooled odds ratio: for inducing clinical response with budesonide was 12.32 (95%CI: 5.53-27.46) and for maintaining clinical response was 8.82 (95%CI: 3.19-24.37)[17]. The number needed to treat was 2 for each outcome. Additionally, patients treated with budesonide had a higher rate of complete response than those treated with prednisone (82.5% vs 52.9%; OR = 4.18; 95%CI: 1.3-13.5) and were less likely to recur than those treated with prednisone (HR = 0.38; 95%CI: 0.18-0.85; P = 0.02)[18].
| Number of patients | Mean age (yr) | Treatment | Remission rate1 | Relapse rate2 | |
| Miehlke et al[31], 2008 | 48 | 57.5 | Budesonide 9 mg/d for 6 wk, followed by budesonide 6 mg/d vs placebo for 6 mo | Short-term 96% | 26% vs 65% placebo, P = 0.022 |
| Long-term 74% vs 35% placebo, P = 0.008 | |||||
| Bonderup et al[19], 2009 | 34 | 62.8 | Budesonide 9 mg/d for 6 wk, followed by budesonide 6 mg/d vs placebo for 24 wk | Long-term | 53% |
| 76.5% vs 12% placebo, P < 0.001 | |||||
| Miehlke et al[15], 2014 | 92 | 58.8 | Budesonide 9 mg/d vs placebo for 8 wk | At 8 wk, | 35% |
| 80% vs 59.5% placebo, P = 0.072 | |||||
| Münch et al[16], 2014 | 92 | 56.7 | Budesonide 9 mg/d for 8 wk, followed by budesonide 4.5 mg/d vs placebo for 6 mo | Short-term 84.5% | 82.1% vs 12.5% placebo |
| Long-term | |||||
| 61.4% vs 16.7% placebo, P < 0.001 |
A multicentered, randomized controlled trial of 92 patients comparing budesonide, mesalamine, and placebo for MC showed that budesonide was more effective than mesalamine (80% vs 44%, P = 0.0035) and placebo (80% vs 59.5%, P = 0.072) in inducing clinical remission at 8 wk[15]. Histological remission rate was the highest in patients treated with budesonide (87%) as compared to mesalamine (45%) and placebo (50%)[15]. The rates of adverse events were similar among budesonide, mesalamine, and placebo groups (47%, 68%, 54%)[15]. The most frequent adverse events were nasopharyngitis, headaches, and dyspepsia.
Although budesonide has been shown to rapidly induce clinical response, relapse occurs frequently after discontinuation of budesonide. Relapse rate is estimated to be as high as 26%-82% (Table 2). Median time to relapse after stopping active treatment was 39 d[19]. Patients with baseline diarrhea frequency > 5 per day (HR = 1.67), duration of diarrhea > 12 mo (HR = 1.82), and absence of budesonide maintenance therapy (HR = 2.73) were found to be at highest risk for relapse[20]. Other factors associated with relapse were advanced age (P = 0.047), a higher number of bowel movements per day at randomization after induction period (P = 0.009), and a higher number of bowel movements per day at baseline (P = 0.041)[16].
Immunomodulators, such as azathioprine, 6-mercaptopurine, and methotrexate, have been tried in patients with refractory MC or steroid dependent MC (Table 3). There are no randomized controlled trials studying efficacy of these medications for MC, and data on use of these medications for refractory MC is limited.
| Number of patients | Mean age (yr) | Treatment | Duration of therapy | Response rate | |
| Münch et al[22], 2013 | 46 | 59 | Azathioprine or 6-MP 2 mg/kg per day | Variable, 1-57 mo | 41% |
| Pardi et al[30], 2001 | 9 | 62 | Azathioprine or 6-MP 2 mg/kg per day | 26 mo | 56% |
| Münch et al[21], 2013 | 9 | 62 | Methotrexate 15-25 mg/wk | 12 wk | 0% |
| Esteve et al[25], 2011 | 4 | 59 | Infliximab 5 mg/kg at 0, 2, 6 wk then every 6-8 wk intravenously | Variable, 5-14 mo | 75% |
| Münch et al[26], 2012 | 3 | 55 | Adalimumab induction1 then 40 mg subcutaneously every 2 wk | 6 wk | 66% |
| Pola et al[24], 2013 | 1 | 58 | Infliximab 5 mg/kg at 0, 2, 6 wk then every 8 wk intravenously | 6 mo | 100% |
In a small study of 9 patients with CC who were intolerant or nonresponsive to budesonide, the patients were treated with methotrexate 15-25 mg/wk subcutaneously for 12 wk. Four of 9 patients discontinued methotrexate due to adverse effects (nausea, allergic reaction, worsening diarrhea), and none of the patients achieved clinical remission at week 12[21].
A multicentered European study showed 41% overall response rate to thiopurines (azathioprine or 6-mercaptopurine) in patients who were steroid dependent or failed other medical therapy, such as loperamide, cholestyramine, budesonide, mesalamine, and methotrexate[22]. Nine patients with steroid intolerance, refractoriness, or dependence were treated with azathioprine 2 mg/kg per day and were followed for 26 mo. Eight out of 9 patients (89%) had complete (56%) or partial (33%) response to azathioprine and were able to discontinue corticosteroids. One out of 9 patients (11%) had persistent severe diarrhea and required an ileostomy.
There are no randomized controlled trials studying efficacy of anti-tumor necrosis factor (TNF) for refractory MC, but there are multiple case reports of clinical response after induction therapy with either infliximab or adalimumab (Table 3). Aram et al[23] reported a patient with lymphocytic enterocolitis who failed corticosteroids, antibiotics, cholestyramine, azathioprine, and tincture of opium. This patient was treated with infliximab 5 mg/kg at 0, 2 and 6 wk with resolution of diarrhea. There was a report of a patient with steroid-dependent MC who failed to respond to diphenoxylate/atropine, loperamide, bismuth, mesalamine, cholestyramine, octreotide, alosetron, tincture of opium, and Boswellia serrata extract was treated with infliximab 5 mg/kg and methotrexate 12.5 mg per week. After 6 mo, the patient remained asymptomatic and free of corticosteroids[24].
Several small case series also demonstrated improvement. Long-term remission (more than 1 year) was achieved in 3 out of 4 patients with refractory MC who were treated with anti-TNF[25]. One patient developed Stevens Johnson syndrome after starting infliximab and relapsed when switched to adalimumab; therefore, anti-TNF was discontinued. The patient underwent colectomy. In another series, three patients with MC who failed loperamide, cholestyramine, budesonide, and methotrexate were treated with adalimumab. All 3 patients achieved clinical remission at week 6 but 1 patient stopped therapy due to adverse effects (vomiting, abdominal pain)[26].
There are case reports of patients successfully undergoing colectomy or diverting ileostomy for refractory and severe MC. The observational trend from these case reports is that both symptoms and histology improve after surgery but can recur when bowel continuation is restored.
In one case report, a 33 year-old patient with 5 years of chronic diarrhea from CC not responsive to Asacol and prednisone underwent total protocolectomy followed by ileal pouch anal anastomosis. At 2 year follow-up, she was having multiple bowel movements per day but diarrhea resolved and she was able to return to full-time job[27].
In another, a 59 year-old patient with CC who previously failed loperamide, prednisolone, budesonide, 5-aminosalicylic acid, cholestyramine, and norfloxacin underwent loop ileostomy[28]. Two to 4 mo after loop ileostomy, colonic biopsies showed resolution of subepithelial damage, but her clinical course was complicated by Clostridium difficile infection and problems with the stoma. One year later, the patient improved clinically and loop ileostomy was closed. The patient started budesonide 6mg daily after bowel restoration but relapsed with symptoms of CC.
In a series of patients, nine patients with MC refractory to medical therapy (sulfasalazine, mepacrine, corticosteroids, mesalamine, cholestyramine, loperamide, metronidazole) underwent ileostomy in between 1981-1992[29]. All patients experienced clinical and histological remission. The ileostomy was taken down in 5 patients after a diversion period of 4-15 mo. Diarrhea recurred in 4 out of 5 of these patients and 3 of them underwent additional surgery.
MC is characterized by chronic, watery diarrhea and subepithelial changes. Conservative therapy, such as discontinuation of offending mediations and trial of loperamide and/or bismuth, may be successful in treating non-severe MC. Budesonide can rapidly induce clinical remission and can maintain remission, but relapse occurs frequently after withdrawal of therapy. Methotrexate and mesalamine do not appear to be effective as monotherapy for MC. There is limited data to support the use of immunomodulators in refractory or steroid-dependent MC. Anti-TNF or ileostomy may be an option for severe, refractory MC; however, their efficacy has not been proven in randomized controlled trials yet, and their risk and benefits need to be discussed in detail with the patient prior to recommending these therapy.
P- Reviewer: Gunaltay S, Kawakami K S- Editor: Yu J L- Editor: A E- Editor: Ma S
| 1. | Pardi DS, Loftus EV, Smyrk TC, Kammer PP, Tremaine WJ, Schleck CD, Harmsen WS, Zinsmeister AR, Melton LJ, Sandborn WJ. The epidemiology of microscopic colitis: a population based study in Olmsted County, Minnesota. Gut. 2007;56:504-508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 181] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 2. | Langner C, Aust D, Ensari A, Villanacci V, Becheanu G, Miehlke S, Geboes K, Münch A. Histology of microscopic colitis-review with a practical approach for pathologists. Histopathology. 2015;66:613-626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 109] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 3. | Madisch A, Hellmig S, Schreiber S, Bethke B, Stolte M, Miehlke S. Allelic variation of the matrix metalloproteinase-9 gene is associated with collagenous colitis. Inflamm Bowel Dis. 2011;17:2295-2298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 4. | Fernández-Bañares F, de Sousa MR, Salas A, Beltrán B, Piqueras M, Iglesias E, Gisbert JP, Lobo B, Puig-Diví V, García-Planella E. Epidemiological risk factors in microscopic colitis: a prospective case-control study. Inflamm Bowel Dis. 2013;19:411-417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 25] [Reference Citation Analysis (0)] |
| 5. | Vigren L, Sjöberg K, Benoni C, Tysk C, Bohr J, Kilander A, Larsson L, Ström M, Hjortswang H. Is smoking a risk factor for collagenous colitis? Scand J Gastroenterol. 2011;46:1334-1339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 67] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 6. | Münch A, Langner C. Microscopic colitis: clinical and pathologic perspectives. Clin Gastroenterol Hepatol. 2015;13:228-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 54] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 7. | Masclee GM, Coloma PM, Kuipers EJ, Sturkenboom MC. Increased risk of microscopic colitis with use of proton pump inhibitors and non-steroidal anti-inflammatory drugs. Am J Gastroenterol. 2015;110:749-759. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 93] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 8. | Barmeyer C, Erko I, Fromm A, Bojarski C, Allers K, Moos V, Zeitz M, Fromm M, Schulzke JD. Ion transport and barrier function are disturbed in microscopic colitis. Ann N Y Acad Sci. 2012;1258:143-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 9. | Tagkalidis PP, Gibson PR, Bhathal PS. Microscopic colitis demonstrates a T helper cell type 1 mucosal cytokine profile. J Clin Pathol. 2007;60:382-387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 79] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 10. | Bohr J, Tysk C, Eriksson S, Abrahamsson H, Järnerot G. Collagenous colitis: a retrospective study of clinical presentation and treatment in 163 patients. Gut. 1996;39:846-851. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 256] [Cited by in RCA: 267] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 11. | Peterson TC, Cleary CE, Shaw AM, Malatjalian DA, Veldhuyzen van Zanten SJ. Therapeutic role for bismuth compounds in TNBS-induced colitis in the rat. Dig Dis Sci. 2000;45:466-473. [PubMed] |
| 12. | Fine KD, Lee EL. Efficacy of open-label bismuth subsalicylate for the treatment of microscopic colitis. Gastroenterology. 1998;114:29-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 113] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 13. | Ung KA, Gillberg R, Kilander A, Abrahamsson H. Role of bile acids and bile acid binding agents in patients with collagenous colitis. Gut. 2000;46:170-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 144] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 14. | Calabrese C, Fabbri A, Areni A, Zahlane D, Scialpi C, Di Febo G. Mesalazine with or without cholestyramine in the treatment of microscopic colitis: randomized controlled trial. J Gastroenterol Hepatol. 2007;22:809-814. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 68] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 15. | Miehlke S, Madisch A, Kupcinskas L, Petrauskas D, Böhm G, Marks HJ, Neumeyer M, Nathan T, Fernández-Bañares F, Greinwald R. Budesonide is more effective than mesalamine or placebo in short-term treatment of collagenous colitis. Gastroenterology. 2014;146:1222-1230.e1-2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 85] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 16. | Münch A, Bohr J, Miehlke S, Benoni C, Olesen M, Ost A, Strandberg L, Hellström PM, Hertervig E, Armerding P, Stehlik J, Lindberg G, Björk J, Lapidus A, Löfberg R, Bonderup O, Avnström S, Rössle M, Dilger K, Mueller R, Greinwald R, Tysk C, Ström M; on behalf of the BUC-63 investigators. Low-dose budesonide for maintenance of clinical remission in collagenous colitis: a randomised, placebo-controlled, 12-month trial. Gut. 2014;Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 61] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 17. | Chande N, MacDonald JK, McDonald JW. Interventions for treating microscopic colitis: a Cochrane Inflammatory Bowel Disease and Functional Bowel Disorders Review Group systematic review of randomized trials. Am J Gastroenterol. 2009;104:235-241; quiz 234, 242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 63] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 18. | Gentile NM, Abdalla AA, Khanna S, Smyrk TC, Tremaine WJ, Faubion WA, Kammer PP, Sandborn WJ, Loftus EV, Pardi DS. Outcomes of patients with microscopic colitis treated with corticosteroids: a population-based study. Am J Gastroenterol. 2013;108:256-259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 59] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 19. | Bonderup OK, Hansen JB, Teglbjaerg PS, Christensen LA, Fallingborg JF. Long-term budesonide treatment of collagenous colitis: a randomised, double-blind, placebo-controlled trial. Gut. 2009;58:68-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 90] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 20. | Miehlke S, Hansen JB, Madisch A, Schwarz F, Kuhlisch E, Morgner A, Teglbjaerg PS, Vieth M, Aust D, Bonderup OK. Risk factors for symptom relapse in collagenous colitis after withdrawal of short-term budesonide therapy. Inflamm Bowel Dis. 2013;19:2763-2767. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 21. | Münch A, Bohr J, Vigren L, Tysk C, Ström M. Lack of effect of methotrexate in budesonide-refractory collagenous colitis. Clin Exp Gastroenterol. 2013;6:149-152. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 33] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 22. | Münch A, Fernandez-Banares F, Munck LK. Azathioprine and mercaptopurine in the management of patients with chronic, active microscopic colitis. Aliment Pharmacol Ther. 2013;37:795-798. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 45] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 23. | Aram G, Bayless TM, Chen ZM, Montgomery EA, Donowitz M, Giardiello FM. Refractory lymphocytic enterocolitis and tumor necrosis factor antagonist therapy. Clin Gastroenterol Hepatol. 2010;8:391-394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 24. | Pola S, Fahmy M, Evans E, Tipps A, Sandborn WJ. Successful use of infliximab in the treatment of corticosteroid dependent collagenous colitis. Am J Gastroenterol. 2013;108:857-858. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 25. | Esteve M, Mahadevan U, Sainz E, Rodriguez E, Salas A, Fernández-Bañares F. Efficacy of anti-TNF therapies in refractory severe microscopic colitis. J Crohns Colitis. 2011;5:612-618. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 75] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 26. | Münch A, Ignatova S, Ström M. Adalimumab in budesonide and methotrexate refractory collagenous colitis. Scand J Gastroenterol. 2012;47:59-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 49] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 27. | Williams RA, Gelfand DV. Total proctocolectomy and ileal pouch anal anastomosis to successfully treat a patient with collagenous colitis. Am J Gastroenterol. 2000;95:2147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 28. | Münch A, Söderholm JD, Wallon C, Ost A, Olaison G, Ström M. Dynamics of mucosal permeability and inflammation in collagenous colitis before, during, and after loop ileostomy. Gut. 2005;54:1126-1128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 38] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 29. | Järnerot G, Tysk C, Bohr J, Eriksson S. Collagenous colitis and fecal stream diversion. Gastroenterology. 1995;109:449-455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 168] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 30. | Pardi DS, Loftus EV, Tremaine WJ, Sandborn WJ. Treatment of refractory microscopic colitis with azathioprine and 6-mercaptopurine. Gastroenterology. 2001;120:1483-1484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 75] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 31. | Miehlke S, Madisch A, Bethke B, Morgner A, Kuhlisch E, Henker C, Vogel G, Andersen M, Meier E, Baretton G. Oral budesonide for maintenance treatment of collagenous colitis: a randomized, double-blind, placebo-controlled trial. Gastroenterology. 2008;135:1510-1516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 98] [Article Influence: 5.8] [Reference Citation Analysis (0)] |