Published online Aug 7, 2015. doi: 10.3748/wjg.v21.i29.8769
Peer-review started: February 26, 2015
First decision: April 23, 2015
Revised: May 18, 2015
Accepted: June 26, 2015
Article in press: June 26, 2015
Published online: August 7, 2015
Processing time: 165 Days and 19.9 Hours
5-aminolevulinic acid (ALA) is a naturally occurring amino acid that is a protoporphyrin IX (PpIX) precursor and a next-generation photosensitive substance. After exogenous administration of ALA, PpIX specifically accumulates in cancer cells owing to the impaired metabolism of ALA to PpIX in mitochondria, which results in a red fluorescence following irradiation with blue light and the formation of singlet oxygen. Fluorescence navigation by photodynamic diagnosis (PDD) using ALA provides good visualization and detection of gastric cancer lesions and is a potentially valuable diagnostic tool for gastric cancer for evaluating both the surgical resection margins and extension of the lesion. Furthermore, PDD using ALA might be used to detect peritoneal metastases during preoperative staging laparoscopy, where it could provide useful information for the selection of a therapeutic approach. Another promising application for this modality is in the evaluation of lymph node metastases. Photodynamic therapy (PDT) using ALA to cause selective damage based on the accumulation of a photosensitizer in malignant tissue is expected to be a non-invasive endoscopic treatment for superficial early gastric cancer. ALA has the potential to be used not only as a diagnostic agent but also as a therapeutic drug, resulting in a new strategy for cancer diagnosis and therapy. Here, we review the current use of PDD and PDT in gastric cancer and evaluate its future potential beyond conventional modalities combined with a light energy upconverter, a light-emitting diode and near-infrared rays as light sources.
Core tip: 5-Aminolevulinic acid (ALA) is a naturally occurring amino acid that is a protoporphyrin IX precursor and a next-generation photosensitive substance. Fluorescence navigation by photodynamic diagnosis (PDD) using ALA is a potentially valuable diagnostic tool for gastric cancer for evaluating both the surgical resection margins and the extension of the lesion. Furthermore, PDD using ALA might be useful to detect peritoneal metastases during preoperative staging laparoscopy and evaluation of lymph node metastases. Photodynamic therapy using ALA to cause selective damage based on the accumulation of a photosensitizer in malignant tissue is expected to become a non-invasive endoscopic treatment for superficial early gastric cancer.
- Citation: Namikawa T, Yatabe T, Inoue K, Shuin T, Hanazaki K. Clinical applications of 5-aminolevulinic acid-mediated fluorescence for gastric cancer. World J Gastroenterol 2015; 21(29): 8769-8775
- URL: https://www.wjgnet.com/1007-9327/full/v21/i29/8769.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i29.8769
5-Aminolevulinic acid (ALA) is a naturally occurring amino acid derivative that acts as an endogenous substrate and precursor to protoporphyrin IX (PpIX). PpIX is a heme precursor in the biosynthetic pathway that emits a strong red fluorescence upon excitation with blue light[1,2]. Since ALA is analogous to amino acids, rapid absorption can be expected following ingestion, followed by being immediately metabolized to heme in normal cells. In various cancer cells, exogenous administration of excessive amounts of ALA increases the cellular level of PpIX, resulting in a higher accumulation of PpIX in cancer cells than in normal cells. However the mechanism of preferential accumulation of PpIX remains unclear[3-5].
Gastric cancer remains one of the leading causes of cancer-related deaths and is the third most common cancer worldwide[6]. Although surgery is the main treatment for operable gastric cancer, most patients who present with inoperable advanced or metastatic disease require palliative treatment, including chemotherapy or radiotherapy, in combination with novel molecular-targeted drugs that induce antibody-dependent cellular cytotoxicity[7-9]. Fortunately, early gastric cancer (EGC), in which the cancer cells are confined to the gastric mucosa or submucosa, regardless of lymph node metastasis, has an excellent outcome with surgical curative resection[10,11]. A less invasive therapy using endoscopy, such as endoscopic mucosal resection (EMR) or endoscopic submucosal dissection (ESD), is considered a favorable treatment for EGC without lymph node metastasis because it is able to resect a target lesion en bloc with preservation of the entire stomach[12,13].
Recent advances in limited treatments, including EMR, ESD and minimally invasive surgery, can improve the quality of life for patients with EGC[11,14]. However, sufficient resection margins are necessary to prevent the reappearance of EGC, as inadequate resections that do not maintain surgical margins free of cancer can lead to disease recurrence[10,11]. Recently, fluorescence imaging using photosensitive molecules such as ALA or indocyanine green (ICG) has been developed, and it is being applied as a navigating tool for various fields of surgery[15,16].
Exogenously administered ALA is incorporated by cells and is used to synthesize a naturally fluorescent substance, PpIX, which also exhibits photoactivity. When PpIX is excited by irradiation of a specific wavelength, mainly visible blue light of 375-475 nm, it emits red fluorescence, and this property can be harnessed to accurately identify cancer cells, which accumulate PpIX. This so-called photodynamic diagnosis (PDD) is a relatively new modality that is based on tumor-specific accumulation of 5-ALA-induced PpIX[3,15,16].
PDD imaging systems were recently improved to enable detection of malignant lesions in the brain, lung and esophagus based on systemic administration of the photosensitive substance Photofrin[15]. However, Photofrin has considerable adverse effects, such as strong phototoxic skin reactions and increases in serum aminotransaminase. Accordingly, ALA is clinically recognized as an effective and safe substrate for detecting various cancers owing to the low risk of side effects [3,15,17-19].
We have used an endoscopic PDD system (Karl Storz, Tuttlingen, Germany) comprised of a CCU Tricam SLII/3CCD CH Tricam-P PDD, D-Light C, and HOPKINSII Straight Forward Telescope 30° (Karl Storz)[5,15,16,20]. The D-Light C light source (300 W xenon arc lamp, Karl Storz) is equipped with a band-pass filter designed to transmit blue light (excitation wavelength, 375-445 nm), and the CCU Tricam SLII/3CCD CHTricam-P PDD video camera system is equipped with a long-pass filter designed to exclude blue light for fluorescence imaging (fluorescence emission wavelength, 600-740 nm). This PDD system has the advantage that it can switch instantly between the blue light mode for fluorescence imaging and the white light mode for conventional observation. In our studies, ALA is dissolved in 50 mL of a 5% glucose solution, and 1.0 g of this solution is given orally 3-4 h before the intraoperative PDD observation. Patients are shielded from direct sunlight for 24 h to avoid phototoxicity. In our experience, no special precautions have been necessary during ALA-PDD, such as liver support or light shielding, and no adverse events have thus far been encountered.
Several studies have used PDD using ALA (ALA-PDD) for the diagnosis and treatment of gastric cancer, including the application of this approach for staging laparoscopy[16,20-22]. Table 1 summarizes previous clinical reports of ALA-mediated fluorescence used for gastric cancer, including both PDD and photodynamic therapy (PDT)[16,20-25]. Among these studies, we recently examined the clinical usefulness of ALA-PDD during surgery for gastric cancer[16]. Our findings indicate that there is a difference in the ALA-PDD-positive rate between intestinal- and diffuse-type gastric cancers.
| Study | Year | Number of patients | Clinical application | Results |
| Mayinger et al[22] | 1999 | 4 | Feasibility study of PDD during endoscopy | All malignant lesions exhibited fluorescence during PDD |
| Kishi et al[20] | 2012 | 13 | Detection of peritoneal metastases | The tumor detection rate was higher in PDD than white light (72% vs 39%) |
| Murayama et al[21] | 2012 | 13 | Detection of peritoneal metastases | The accuracy of the fluorescence imaging was greater than that of white-light imaging |
| Koizumi et al[23] | 2013 | 14 | Detection of metastatic lymph nodes | The sensitivity, specificity, and accuracy of ALA-PDD were 70.8%, 96.7%, and 92.4%, respectively |
| Namikawa et al[16] | 2014 | 21 | Feasibility study of PDD during surgery | The sensitivity, specificity, and accuracy of ALA-PDD were 57.7%, 100%, and 66.7%, respectively |
| Nakamura et al[24] | 2014 | 5 | Evaluation of high-resolution magnifying videoendoscopy for PDD and PDT | PDD and PDT were successfully and safely performed, and CR was obtained in 71.4% of cases |
| Kishi et al[25] | 2014 | 52 | Detection of peritoneal metastases | ALA-PDD detected peritoneal metastases in 21% of the patients, while 46% of the patients had no evidence of dissemination on white-light examination |
Oligopeptide transporters (PEPT), such as peptide transporter 1 (PEPT1) and PEPT2, are involved in the cellular uptake of ALA[26-29]. ALA-mediated PpIX accumulation in tumors is associated with the expression of particular proteins, such as PEPT1, PEPT2, ferrochelatase, and ATP-binding cassette transporter G2 (ABCG2)[29-31]. Hagiya et al[29] reported that high expression of PEPT1 and low expression of ABCG2 correlated with ALA-induced accumulation of PpIX. Furthermore, Kobuchi et al[30] reported that ALA-induced PpIX production and cellular photosensitivity correlated negatively with the expression of the PpIX transporter ABCG2 but not with that of PEPT1, PEPT2 or ferrochelatase in a cancer cell line. These differences in the PpIX biosynthesis pathway might involve diagnostic findings of different histological types of gastric cancer[29,30,32].
Regarding the future direction of ALA-PDD for gastric cancer, it could be used as a tool for evaluating surgical resection margins and thereby assist with pathological diagnosis during surgery. Sometimes, a situation is encountered in which decisions must be made for proceeding during surgery for the treatment of gastric cancer, such as determining the extent of the cancer in cases with indistinct margins[33]. In these cases, ALA-PDD might provide useful information to judge the margins that would be sufficient for resection of the tumor.
Magnifying endoscopy is useful for determining the extent of intramucosal spread of differentiated EGC, and also provides more precise endoscopic diagnoses[34,35]. In addition, ALA-PDD might be an effective diagnostic procedure during endoscopic therapies such as EMR or ESD, in addition to early detection of gastric cancer. To detect any extension of the cancer in these limited surgeries[36], new endoscopic diagnostic procedures for EGC based on magnifying endoscopes and image-enhanced endoscopies, such as narrow-band imaging (NBI) and flexible spectral imaging color enhancement (FICE), have been developed[34,35,37]. In this regard, ALA-PDD could provide additional information to that obtained with these conventional endoscopic modes, and thus might prove valuable for evaluating the extent of the lesion.
Peritoneal metastasis from a primary gastric cancer is the most frequent type of distant metastasis and post-surgical recurrence in advanced gastric cancer with serosa-invading tumors, and is an incurable condition with poor prognosis[38,39]. Numerous attempts with new approaches have been made for gastric cancer staging, because accurate staging of gastric cancer, and particularly the diagnosis of peritoneal dissemination, is a prerequisite for determining the most appropriate therapy[7,8,25,39]. Although staging laparoscopy is used frequently in the management of patients with advanced gastric cancer to prevent unnecessary laparotomy[40,41], it has limitations in visualizing the dissemination of cancer nest[42-45].
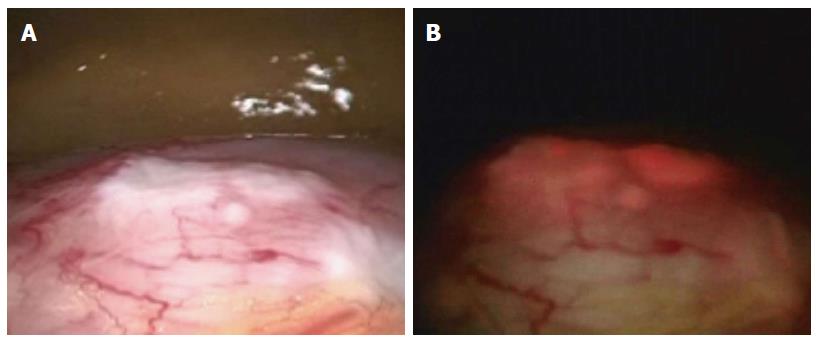
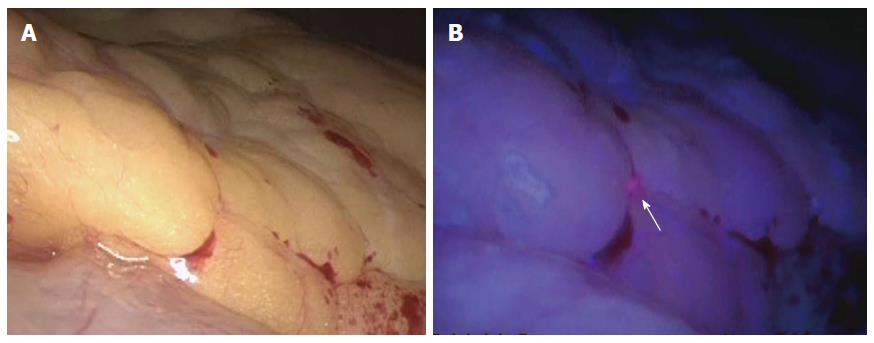
Staging laparoscopy with ALA-PDD is safe and improves the diagnostic accuracy for peritoneal metastases in patients with gastric cancer[20,21,25]. Kishi et al[20,25] examined the usefulness of ALA-PDD with staging laparoscopy in patients with serosa-invading advanced gastric cancer, comparing the detection sensitivity with that obtained using conventional white light. They demonstrated that the tumor detection rate of 72% using ALA-induced fluorescence was significantly higher than that achieved using white light (39%) in a mouse model of peritoneal metastases, which involved 8 mice with 729 peritoneal nodes. In addition, three metastatic lesions that were invisible under white light were detected under ALA-induced fluorescence in 13 patients undergoing staging laparoscopy. Furthermore, they correlated the ALA-PDD results with those from peritoneal fluid cytology and molecular diagnostic testing in 52 patients with advanced gastric cancer[26]. Twenty-four of the 52 patients (46%) had no macroscopic evidence of peritoneal metastases on white light examination; however, ALA-PDD detected dissemination in 5 of these 24 patients (21%).
Murayama et al[21] assessed the diagnostic capability of fluorescence laparoscopy in 13 patients with advanced gastric cancer using ALA for peritoneal dissemination, and for small superficial liver metastases that are difficult to identify by computed tomography scanning. Five of the 13 patients demonstrated peritoneal metastases, and one patient demonstrated superficial liver micrometastases by fluorescence laparoscopy using ALA.
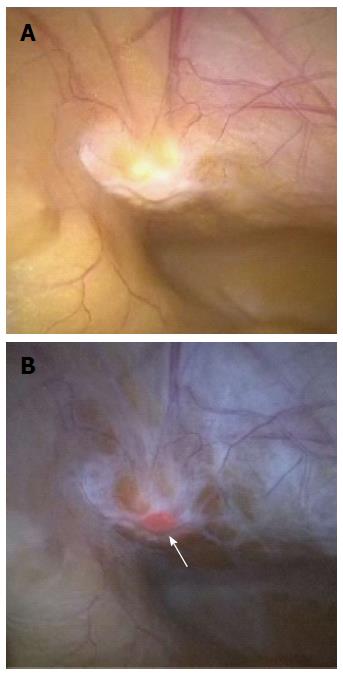
We have also carried out staging laparoscopy using ALA-PDD in patients with serosa-invading gastric cancer and confirmed good visualization of peritoneal metastases (Figures 1, 2, 3). Thus, ALA-PDD in combination with staging laparoscopy is a promising procedure for improving the reliability of detection of invisible metastases in advanced gastric cancer.
Lymph node metastases are one of the most important prognostic factors in gastric cancer, and precise diagnosis of these metastases is essential for selecting the most appropriate therapeutic strategy. Koizumi et al[23] examined the feasibility of using ALA-PDD to detect metastatic foci in 144 excised lymph nodes that were obtained from 14 gastric cancer patients. In total, 121 lymph nodes were diagnosed in agreement with the results of histopathological examination, and the overall diagnostic accuracy of gross fluorescence inspection without regard to fluorescence patterns was 84%. The authors concluded that ALA-PDD is a feasible and direct approach for detecting metastatic lymph nodes in gastric cancer patients using a simple apparatus. One difficulty with ALA-PDD in this application is that fluorescence imaging is only obtained from the outside of the lymph nodes, because human lymph nodes are surrounded by abundant connective tissues, causing a lower depth of penetration of blue light into the tissues. If deep observations were possible, diagnosis of lymph node metastasis would be possible in the full surgical field in situ, and thus it could be possible to select individual lymph nodes for extraction[21]. To resolve these issues, further investigations are needed.
ALA has been used successfully, not only to diagnose, but also to treat various tumors[44,45]. PDT is defined as the use of photodynamic agents that are biochemically activated by light, to cause tissue damage in the treatment of disease[46]. During PDT, emission of the excitation light, which falls within the absorption wavelengths of PpIX, results in the generation of reactive oxygen species (ROS) that induce apoptosis within the irradiated cells[47-49]. One advantage of ALA-PDT is that the lack of PpIX in normal cells potentially allows for a tumor-specific PDT with minimal adverse effects, and because tissue penetration by the excitation light is limited, the cytotoxic effects of PDT are also limited to the superficial tissue[50]. Therefore, ALA-PDT is expected to become an important non-invasive endoscopic treatment for superficial EGC[50,51].
Loh et al[46] demonstrated selective photosensitization of gastric mucosa with sparing of the other tissue layers of the stomach with ALA-PDT using a rat model. Although red light at a peak wavelength of approximately 635 nm is often used as the excitation light source in ALA-PDT[52,53], Hino et al[50] reported the efficacy of a light-emitting diode (LED) as an irradiation source for ALA-PDT in a mouse model of peritoneally disseminated gastric cancer. They demonstrated differences in anticancer effects, including ROS generation and cytotoxic effects, among three LED sources, which were violet at a peak wavelength of 410 nm, green at a peak wavelength of 525 nm, and red at a peak wavelength of 635 nm. The violet and green LEDs had the same anticancer effects, which were significantly greater than those of the red LED[50]. Thus, violet light has a greater cytotoxic effect, while red and infrared light penetrate deeper within biological tissues for in vivo clinical applications[51].
The near-infrared (NIR) window, composed of wavelengths between 700 and 1000 nm, provides the greatest depth of penetration in biological tissues, while blue or red lights yield low tissue penetrability and therefore are limited to surface cancer applications. To this end, Shimoyama et al[51] demonstrated a potentially novel PDT in a human gastric cancer cell line using NIR-irradiated lanthanide nanoparticles (LNP), which are excited by NIR-emitting visible light and administered using ALA. In addition to the tissue penetrability advantage, these early studies indicated possible additional advantages of LNP-sensitizer conjugates in reducing the background fluorescence, photobleaching, and photoblinking properties that are generally associated with such techniques[51,54].
ALA-PDD is a promising and safe diagnostic modality for determining tumor extent and for detecting metastatic lesions in gastric cancer. Furthermore, PDT has potential advantages in terms of minimizing the procedure invasiveness. Further investigations, including a prospective randomized controlled trial, are needed to verify the usefulness of the ALA-mediated fluorescence technology for gastric cancer.
P- Reviewer: Kim GH, Park WS, Peng SY, Shimada H, Verlato G S- Editor: Ma YJ L- Editor: A E- Editor: Ma S
| 1. | Kelty CJ, Brown NJ, Reed MW, Ackroyd R. The use of 5-aminolaevulinic acid as a photosensitiser in photodynamic therapy and photodiagnosis. Photochem Photobiol Sci. 2002;1:158-168. [PubMed] |
| 2. | Inoue K, Karashima T, Kamada M, Shuin T, Kurabayashi A, Furihata M, Fujita H, Utsumi K, Sasaki J. Regulation of 5-aminolevulinic acid-mediated protoporphyrin IX accumulation in human urothelial carcinomas. Pathobiology. 2009;76:303-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 49] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 3. | Loh CS, Vernon D, MacRobert AJ, Bedwell J, Bown SG, Brown SB. Endogenous porphyrin distribution induced by 5-aminolaevulinic acid in the tissue layers of the gastrointestinal tract. J Photochem Photobiol B. 1993;20:47-54. [PubMed] |
| 4. | Krieg RC, Messmann H, Rauch J, Seeger S, Knuechel R. Metabolic characterization of tumor cell-specific protoporphyrin IX accumulation after exposure to 5-aminolevulinic acid in human colonic cells. Photochem Photobiol. 2002;76:518-525. [PubMed] |
| 5. | Hinnen P, de Rooij FW, van Velthuysen ML, Edixhoven A, van Hillegersberg R, Tilanus HW, Wilson JH, Siersema PD. Biochemical basis of 5-aminolaevulinic acid-induced protoporphyrin IX accumulation: a study in patients with (pre)malignant lesions of the oesophagus. Br J Cancer. 1998;78:679-682. [PubMed] |
| 6. | Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9172] [Cited by in RCA: 9956] [Article Influence: 995.6] [Reference Citation Analysis (0)] |
| 7. | Koizumi W, Narahara H, Hara T, Takagane A, Akiya T, Takagi M, Miyashita K, Nishizaki T, Kobayashi O, Takiyama W. S-1 plus cisplatin versus S-1 alone for first-line treatment of advanced gastric cancer (SPIRITS trial): a phase III trial. Lancet Oncol. 2008;9:215-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1320] [Cited by in RCA: 1421] [Article Influence: 83.6] [Reference Citation Analysis (0)] |
| 8. | Boku N, Yamamoto S, Fukuda H, Shirao K, Doi T, Sawaki A, Koizumi W, Saito H, Yamaguchi K, Takiuchi H. Fluorouracil versus combination of irinotecan plus cisplatin versus S-1 in metastatic gastric cancer: a randomised phase 3 study. Lancet Oncol. 2009;10:1063-1069. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 449] [Cited by in RCA: 474] [Article Influence: 29.6] [Reference Citation Analysis (0)] |
| 9. | Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, Lordick F, Ohtsu A, Omuro Y, Satoh T. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376:687-697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5541] [Cited by in RCA: 5300] [Article Influence: 353.3] [Reference Citation Analysis (3)] |
| 10. | Sano T, Sasako M, Kinoshita T, Maruyama K. Recurrence of early gastric cancer. Follow-up of 1475 patients and review of the Japanese literature. Cancer. 1993;72:3174-3178. [PubMed] |
| 11. | Okabayashi T, Kobayashi M, Nishimori I, Sugimoto T, Namikawa T, Onishi S, Hanazaki K. Clinicopathological features and medical management of early gastric cancer. Am J Surg. 2008;195:229-232. [PubMed] |
| 12. | Ono H, Kondo H, Gotoda T, Shirao K, Yamaguchi H, Saito D, Hosokawa K, Shimoda T, Yoshida S. Endoscopic mucosal resection for treatment of early gastric cancer. Gut. 2001;48:225-229. [PubMed] |
| 13. | Gotoda T. Endoscopic resection of early gastric cancer. Gastric Cancer. 2007;10:1-11. [PubMed] |
| 14. | Namikawa T, Hiki N, Kinami S, Okabe H, Urushihara T, Kawahira H, Fukushima N, Kodera Y, Yumiba T, Oshio A. Factors that minimize postgastrectomy symptoms following pylorus-preserving gastrectomy: assessment using a newly developed scale (PGSAS-45). Gastric Cancer. 2015;18:397-406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 24] [Article Influence: 2.4] [Reference Citation Analysis (1)] |
| 15. | Inoue K, Fukuhara H, Shimamoto T, Kamada M, Iiyama T, Miyamura M, Kurabayashi A, Furihata M, Tanimura M, Watanabe H. Comparison between intravesical and oral administration of 5-aminolevulinic acid in the clinical benefit of photodynamic diagnosis for nonmuscle invasive bladder cancer. Cancer. 2012;118:1062-1074. [PubMed] |
| 16. | Namikawa T, Inoue K, Uemura S, Shiga M, Maeda H, Kitagawa H, Fukuhara H, Kobayashi M, Shuin T, Hanazaki K. Photodynamic diagnosis using 5-aminolevulinic acid during gastrectomy for gastric cancer. J Surg Oncol. 2014;109:213-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 17. | Utsuki S, Miyoshi N, Oka H, Miyajima Y, Shimizu S, Suzuki S, Fujii K. Fluorescence-guided resection of metastatic brain tumors using a 5-aminolevulinic acid-induced protoporphyrin IX: pathological study. Brain Tumor Pathol. 2007;24:53-55. [PubMed] |
| 18. | Fukuhara H, Inoue K, Satake H, Tamura K, Karashima T, Yamasaki I, Tatsuo I, Kurabayashi A, Furihata M, Shuin T. Photodynamic diagnosis of positive margin during radical prostatectomy: preliminary experience with 5-aminolevulinic acid. Int J Urol. 2011;18:585-591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 39] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 19. | Mayinger B, Neidhardt S, Reh H, Martus P, Hahn EG. Fluorescence induced with 5-aminolevulinic acid for the endoscopic detection and follow-up of esophageal lesions. Gastrointest Endosc. 2001;54:572-578. [PubMed] |
| 20. | Kishi K, Fujiwara Y, Yano M, Inoue M, Miyashiro I, Motoori M, Shingai T, Gotoh K, Takahashi H, Noura S. Staging laparoscopy using ALA-mediated photodynamic diagnosis improves the detection of peritoneal metastases in advanced gastric cancer. J Surg Oncol. 2012;106:294-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 49] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 21. | Murayama Y, Ichikawa D, Koizumi N, Komatsu S, Shiozaki A, Kuriu Y, Ikoma H, Kubota T, Nakanishi M, Harada Y. Staging fluorescence laparoscopy for gastric cancer by using 5-aminolevulinic acid. Anticancer Res. 2012;32:5421-5427. [PubMed] |
| 22. | Mayinger B, Reh H, Hochberger J, Hahn EG. Endoscopic photodynamic diagnosis: oral aminolevulinic acid is a marker of GI cancer and dysplastic lesions. Gastrointest Endosc. 1999;50:242-246. [PubMed] |
| 23. | Koizumi N, Harada Y, Murayama Y, Harada K, Beika M, Yamaoka Y, Dai P, Komatsu S, Kubota T, Ichikawa D. Detection of metastatic lymph nodes using 5-aminolevulinic acid in patients with gastric cancer. Ann Surg Oncol. 2013;20:3541-3548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 32] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 24. | Nakamura T, Oinuma T, Yamagishi H, Masuyama H, Terano A. Evaluation of a novel high-resolution magnifying videoendoscope that is capable of photodynamic diagnosis and therapy for gastric cancer. Photodiagnosis Photodyn Ther. 2015;12:115-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 25. | Kishi K, Fujiwara Y, Yano M, Motoori M, Sugimura K, Ohue M, Noura S, Marubashi S, Takahashi H, Sakon M. Diagnostic laparoscopy with 5-aminolevulinic-acid-mediated photodynamic diagnosis enhances the detection of peritoneal micrometastases in advanced gastric cancer. Oncology. 2014;87:257-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 27] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 26. | Rodriguez L, Batlle A, Di Venosa G, MacRobert AJ, Battah S, Daniel H, Casas A. Study of the mechanisms of uptake of 5-aminolevulinic acid derivatives by PEPT1 and PEPT2 transporters as a tool to improve photodynamic therapy of tumours. Int J Biochem Cell Biol. 2006;38:1530-1539. [PubMed] |
| 27. | Novotny A, Xiang J, Stummer W, Teuscher NS, Smith DE, Keep RF. Mechanisms of 5-aminolevulinic acid uptake at the choroid plexus. J Neurochem. 2000;75:321-328. [PubMed] |
| 28. | Döring F, Walter J, Will J, Föcking M, Boll M, Amasheh S, Clauss W, Daniel H. Delta-aminolevulinic acid transport by intestinal and renal peptide transporters and its physiological and clinical implications. J Clin Invest. 1998;101:2761-2767. [PubMed] |
| 29. | Hagiya Y, Endo Y, Yonemura Y, Takahashi K, Ishizuka M, Abe F, Tanaka T, Okura I, Nakajima M, Ishikawa T. Pivotal roles of peptide transporter PEPT1 and ATP-binding cassette (ABC) transporter ABCG2 in 5-aminolevulinic acid (ALA)-based photocytotoxicity of gastric cancer cells in vitro. Photodiagnosis Photodyn Ther. 2012;9:204-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 92] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 30. | Kobuchi H, Moriya K, Ogino T, Fujita H, Inoue K, Shuin T, Yasuda T, Utsumi K, Utsumi T. Mitochondrial localization of ABC transporter ABCG2 and its function in 5-aminolevulinic acid-mediated protoporphyrin IX accumulation. PLoS One. 2012;7:e50082. [PubMed] |
| 31. | Robey RW, Steadman K, Polgar O, Bates SE. ABCG2-mediated transport of photosensitizers: potential impact on photodynamic therapy. Cancer Biol Ther. 2005;4:187-194. [PubMed] |
| 32. | Inoue K, Fukuhara H, Kurabayashi A, Furihata M, Tsuda M, Nagakawa K, Fujita H, Utsumi K, Shuin T. Photodynamic therapy involves an antiangiogenic mechanism and is enhanced by ferrochelatase inhibitor in urothelial carcinoma. Cancer Sci. 2013;104:765-772. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 34] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 33. | Namikawa T, Kobayashi M, Kitagawa H, Okabayashi T, Dabanaka K, Okamoto K, Sugimoto T, Toi M, Hanazaki K. Early gastric cancer with widespread duodenal invasion within the mucosa. Dig Endosc. 2010;22:223-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 34. | Nakayoshi T, Tajiri H, Matsuda K, Kaise M, Ikegami M, Sasaki H. Magnifying endoscopy combined with narrow band imaging system for early gastric cancer: correlation of vascular pattern with histopathology (including video). Endoscopy. 2004;36:1080-1084. [PubMed] |
| 35. | Yao K, Oishi T, Matsui T, Yao T, Iwashita A. Novel magnified endoscopic findings of microvascular architecture in intramucosal gastric cancer. Gastrointest Endosc. 2002;56:279-284. [PubMed] |
| 36. | Abe N, Takeuchi H, Ooki A, Nagao G, Masaki T, Mori T, Sugiyama M. Recent developments in gastric endoscopic submucosal dissection: towards the era of endoscopic resection of layers deeper than the submucosa. Dig Endosc. 2013;25 Suppl 1:64-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 52] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 37. | Osawa H, Yoshizawa M, Yamamoto H, Kita H, Satoh K, Ohnishi H, Nakano H, Wada M, Arashiro M, Tsukui M. Optimal band imaging system can facilitate detection of changes in depressed-type early gastric cancer. Gastrointest Endosc. 2008;67:226-234. [PubMed] |
| 38. | Nashimoto A, Akazawa K, Isobe Y, Miyashiro I, Katai H, Kodera Y, Tsujitani S, Seto Y, Furukawa H, Oda I. Gastric cancer treated in 2002 in Japan: 2009 annual report of the JGCA nationwide registry. Gastric Cancer. 2013;16:1-27. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 312] [Cited by in RCA: 372] [Article Influence: 31.0] [Reference Citation Analysis (0)] |
| 39. | Yoon H, Lee DH. New approaches to gastric cancer staging: beyond endoscopic ultrasound, computed tomography and positron emission tomography. World J Gastroenterol. 2014;20:13783-13790. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 22] [Cited by in RCA: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 40. | Cardona K, Zhou Q, Gönen M, Shah MA, Strong VE, Brennan MF, Coit DG. Role of repeat staging laparoscopy in locoregionally advanced gastric or gastroesophageal cancer after neoadjuvant therapy. Ann Surg Oncol. 2013;20:548-554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 41. | Karanicolas PJ, Elkin EB, Jacks LM, Atoria CL, Strong VE, Brennan MF, Coit DG. Staging laparoscopy in the management of gastric cancer: a population-based analysis. J Am Coll Surg. 2011;213:644-651, 651.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 63] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 42. | Muntean V, Mihailov A, Iancu C, Toganel R, Fabian O, Domsa I, Muntean MV. Staging laparoscopy in gastric cancer. Accuracy and impact on therapy. J Gastrointestin Liver Dis. 2009;18:189-195. [PubMed] |
| 43. | Wong J, Coit D. Detection of gastric cancer peritoneal metastases by peritoneal lavage: Current limitations and future perspectives. Surgery. 2012;152:1-4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 44. | Saito M, Yano K, Kamigaki T, Goto S. A patient with scirrhous stomach cancer treated with combination of hyperthermotherapy and 5-aminolevulinic acid (ALA). Anticancer Res. 2013;33:2957-2963. [PubMed] |
| 45. | Ishizuka M, Abe F, Sano Y, Takahashi K, Inoue K, Nakajima M, Kohda T, Komatsu N, Ogura S, Tanaka T. Novel development of 5-aminolevurinic acid (ALA) in cancer diagnoses and therapy. Int Immunopharmacol. 2011;11:358-365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 187] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 46. | Loh CS, Bedwell J, MacRobert AJ, Krasner N, Phillips D, Bown SG. Photodynamic therapy of the normal rat stomach: a comparative study between di-sulphonated aluminium phthalocyanine and 5-aminolaevulinic acid. Br J Cancer. 1992;66:452-462. [PubMed] |
| 47. | Dolmans DE, Fukumura D, Jain RK. Photodynamic therapy for cancer. Nat Rev Cancer. 2003;3:380-387. [PubMed] |
| 48. | Brown SB, Brown EA, Walker I. The present and future role of photodynamic therapy in cancer treatment. Lancet Oncol. 2004;5:497-508. [PubMed] |
| 49. | Ito H, Tamura M, Matsui H, Majima HJ, Indo HP, Hyodo I. Reactive oxygen species involved cancer cellular specific 5-aminolevulinic acid uptake in gastric epithelial cells. J Clin Biochem Nutr. 2014;54:81-85. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 50. | Hino H, Murayama Y, Nakanishi M, Inoue K, Nakajima M, Otsuji E. 5-Aminolevulinic acid-mediated photodynamic therapy using light-emitting diodes of different wavelengths in a mouse model of peritoneally disseminated gastric cancer. J Surg Res. 2013;185:119-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 39] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 51. | Shimoyama A, Watase H, Liu Y, Ogura S, Hagiya Y, Takahashi K, Inoue K, Tanaka T, Murayama Y, Otsuji E. Access to a novel near-infrared photodynamic therapy through the combined use of 5-aminolevulinic acid and lanthanide nanoparticles. Photodiagnosis Photodyn Ther. 2013;10:607-614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 52. | Otake M, Nishiwaki M, Kobayashi Y, Baba S, Kohno E, Kawasaki T, Fujise Y, Nakamura H. Selective accumulation of ALA-induced PpIX and photodynamic effect in chemically induced hepatocellular carcinoma. Br J Cancer. 2003;89:730-736. [PubMed] |
| 53. | Chen X, Zhao P, Chen F, Li L, Luo R. Effect and mechanism of 5-aminolevulinic acid-mediated photodynamic therapy in esophageal cancer. Lasers Med Sci. 2011;26:69-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 54. | Mai HX, Zhang YW, Si R, Yan ZG, Sun LD, You LP, Yan CH. High-quality sodium rare-earth fluoride nanocrystals: controlled synthesis and optical properties. J Am Chem Soc. 2006;128:6426-6436. [PubMed] |