Published online Jul 14, 2015. doi: 10.3748/wjg.v21.i26.8215
Peer-review started: February 9, 2015
First decision: April 13, 2015
Revised: May 7, 2015
Accepted: May 27, 2015
Article in press: May 27, 2015
Published online: July 14, 2015
Processing time: 157 Days and 18.4 Hours
Solitary duodenal Peutz-Jeghers (PJ)-type hamartomatous polyps are rare and considered a different disease entity than classic PJ syndrome. We describe the case of an 89-year-old man admitted to our emergency department with symptoms of acute cholangitis, liver dysfunction, and slight jaundice. Magnetic resonance imaging showed multiple signal voids, reflecting choledocholithiasis, and an oval-shaped tumor in the common bile duct (CBD). Following endoscopic retrograde cholangiopancreatography, the patient was diagnosed with a lower CBD tumor 20 mm in diameter. Endoscopic sphincterotomy was performed for choledocholithotomy, resulting in the expulsion of a large tumor with a stalk connected to the papilla of Vater. The tumor was successfully excised en bloc by endoscopic snare papillectomy. Histopathologic examination showed that the tumor was a PJ-type hamartomatous polyp. No mucocutaneous pigmentation of the skin was evident and the patient’s family history was negative. Solitary duodenal PJ-type hamartomatous polyps are usually diagnosed incidentally during endoscopy for other indications because most of these tumors are asymptomatic or have nonspecific presentations. To our knowledge, this is the first reported solitary PJ-type polyp with intra-CBD growth treated by endoscopic snare papillectomy.
Core tip: Solitary duodenal Peutz-Jeghers (PJ)-type hamartomatous polyps are rare. This polyp is usually diagnosed incidentally during endoscopy because its presentation is nonspecific. Thus, it is interesting that acute cholangitis was shown as initial symptoms of PJ-type hamartomatous polyp due to intra-common bile duct growth. This is the first reported solitary duodenal PJ-type hamartomatous polyp treated by endoscopic snare papillectomy. PJ-type polyps should be distinguished as solitary or an incomplete type of PJ syndrome. These polyps should be treated by endoscopic or surgical resection due to their malignant potential.
- Citation: Suzuki K, Higuchi H, Shimizu S, Nakano M, Serizawa H, Morinaga S. Endoscopic snare papillectomy for a solitary Peutz-Jeghers-type polyp in the duodenum with ingrowth into the common bile duct: Case report. World J Gastroenterol 2015; 21(26): 8215-8220
- URL: https://www.wjgnet.com/1007-9327/full/v21/i26/8215.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i26.8215
Hamartomatous polyps are usually associated with Peutz-Jeghers syndrome (PJS)[1], which is characterized by gastrointestinal polyposis and mucocutaneous pigmentation. Solitary hamartomatous polyps have been considered a variant or a separate disease entity without the features of PJS[2]. Solitary duodenal hamartomas are even less common. These lesions are usually accompanied by nonspecific symptoms, with most diagnosed incidentally during endoscopy. We describe the successful use of endoscopic snare papillectomy to remove a solitary Peutz-Jeghers (PJ)-type polyp on the papilla of Vater. To our knowledge, this is the first patient to be described with a solitary PJ-type hamartomatous polyp showing ingrowth into the common bile duct (CBD).
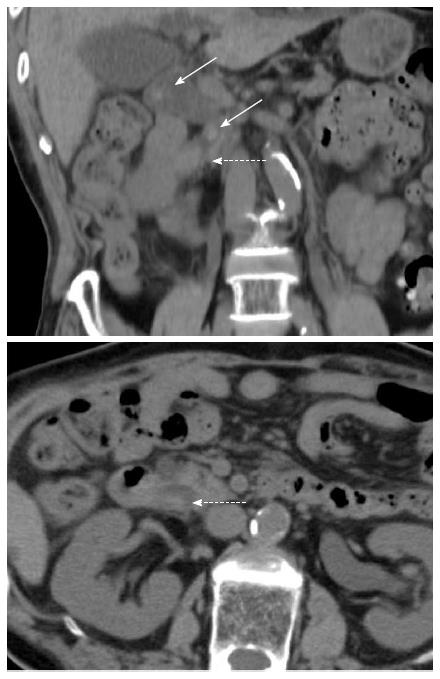
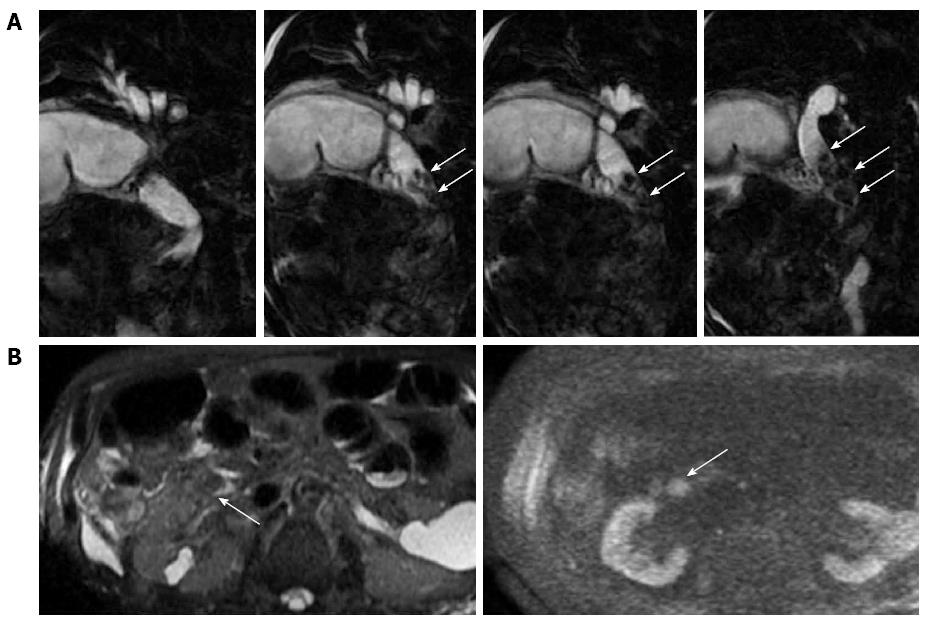
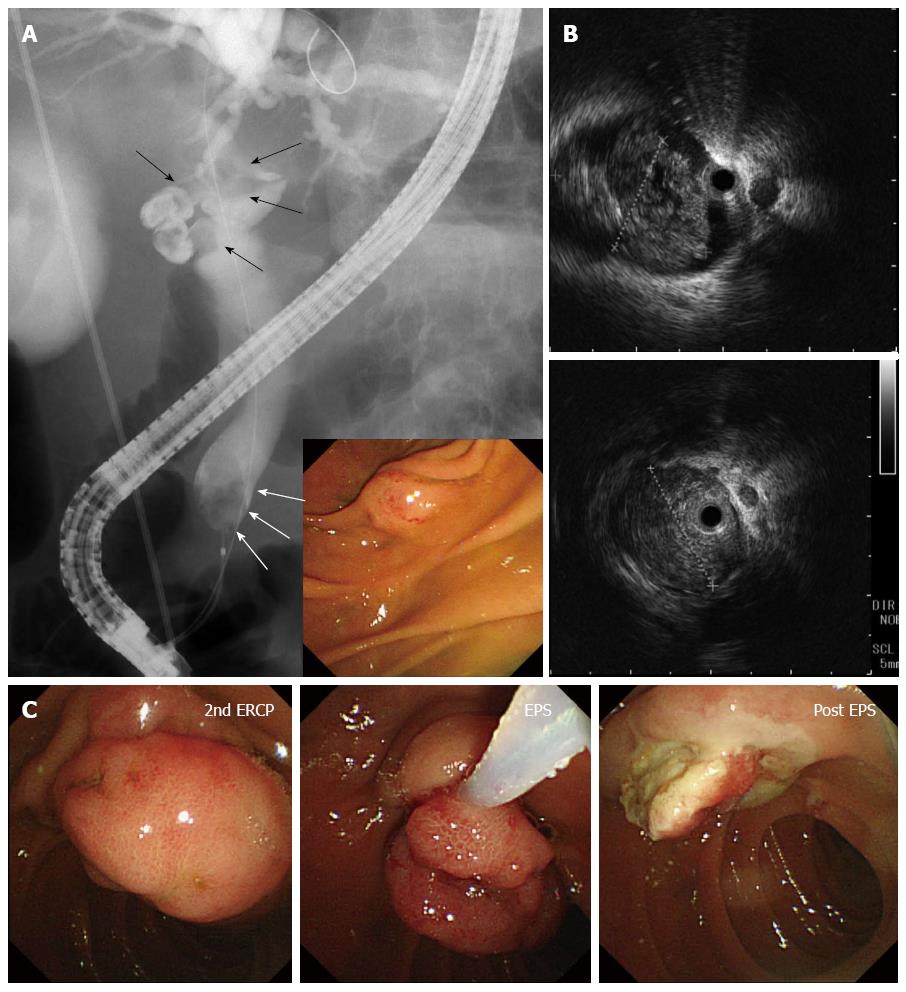
An 89-year-old man with a chief compliant of left brachium paresis was referred to our hospital and admitted to the emergency department. The patient had no significant medical history except for hypertension, atrial fibrillation, and chronic gastritis, and no significant family history. On admission, he showed evidence of an inflammatory reaction, with elevated WBC (10310/μL) and C-reactive protein (4.62 mg/dL). Liver dysfunction and slight jaundice were also observed, with elevated levels of aspartate transaminase (152 IU/L), alanine transaminase (68 IU/L), lactate dehydrogenase (551 IU/L), gamma-glutamyl transpeptidase (84 IU/L), and total bilirubin (2.32 mg/dL). Tumor markers, including carcinoembryonic antigen, cancer antigen 19-9 and alpha-fetoprotein, were within normal limits. Plain abdominal computed tomography (CT) demonstrated choledocholithiasis with multiple calcified stones in the dilated CBD and a tumor in the lower CBD (Figure 1). Magnetic resonance imaging with cholangiopancreatography showed multiple signal voids in the CBD reflecting tiny stones (Figure 2A). An oval-shaped tumor 20 mm in diameter in the lower CBD was detected by high-intensity signals on T2-weighted and diffusion-weighted imaging (Figure 2B). Following a diagnosis of jaundice due to choledocholithiasis and a CBD tumor, endoscopic retrograde cholangiopancreatography was performed. The lateral view showed a slight swelling of the papilla of Vater (Figure 3A), as well as multiple filling defects in the CBD. The largest defect, with a diameter of 4 cm, was located at the inferior extremity of the CBD without flexibility (Figure 3A). Intraductal ultrasound showed soft tissue echogenicity, suggesting that the defect was a neoplasm growing into the CBD (Figure 3B). Endoscopic sphincterotomy was performed and a plastic tube stent was inserted. A second endoscopic retrograde cholangiopancreatography, performed one week later, showed inversion and exclusion of the tumor (Figure 3C). The tumor, which had a stalk growing from the major duodenal papilla, was excised en bloc by endoscopic snare papillectomy (Figure 3C).
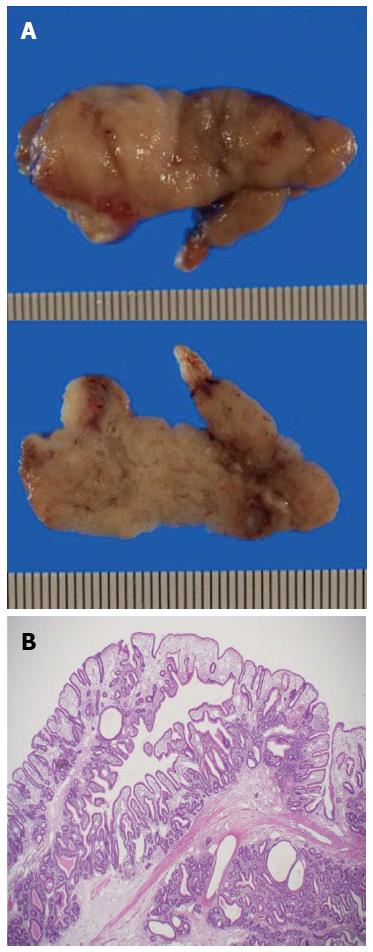
Histopathologic examination of the resected specimen showed a large polypoid neoplasm, 4.8 cm × 1.9 cm × 1.8 cm in size, with a lobular surface and epithelialized with intestinal type mucosa (Figure 4A). The polyp contained irregular hyperplastic crypts with focal cystic dilatation, especially in the lower layer, and branching bundles of smooth muscle extending from the muscularis mucosae. The epithelium was hyperplastic in nature but not dysplastic, resulting in a diagnosis of PJ-type hamartomatous polyp (Figure 4B).
The patient had no mucocutaneous pigmentation or family history of PJS. Upper gastrointestinal endoscopy and colonoscopy did not reveal any other PJ-type polyps. Thus, the final diagnosis was solitary PJ-type hamartomatous polyp of the major duodenal papilla.
PJS is a rare autosomal dominant disorder associated with mucocutaneous pigmentation (buccal mucosa, lips, fingers, and toes) and a family history of this condition[3]. Solitary PJ-type polyps, defined as hamartomatous polyps without any of the clinical symptoms of PJS, are characterized histologically by tree-like branching of smooth muscle fibers, with a core of smooth muscle, covered by mucosal tissue of near-normal appearance[4]. It is truly difficult to determine whether these solitary duodenal hamartomatous polyps are an incomplete type or initial clinical manifestation of PJS[2]. This entity may be differentiated from PJS based on information from previous studies, including: the lack of perioral or perianal mucocutaneous pigmentation and lack of family history; symptom onset usually occurring past the sixth and seventh decades of life; and lower risk of local cancer development than hamartomatous polyps in patients with PJS. The findings in our patient are consistent with these characteristics.
A search of case reports on the MEDLINE database up to 2014 using the terms “solitary”, “duodenum”, and “hamartomatous polyp”, but without PJS, identified 19 patients in 13 studies published in English with solitary-PJ type polyps in the duodenum, including the patient described here[2,5-15] (Table 1). Age at onset ranged from 23-89 years (mean ± SD: 60.3 ± 20.1 years). The most frequent polyp site was the second portion of the duodenum, followed by the duodenal bulb. Maximum polyp diameter ranged from 5-70 mm (mean ± SD: 26.4 ± 16.6 mm). The predominant presentation was asymptomatic, with other patients having nonspecific symptoms. Most patients underwent endoscopic resection, although some patients with large polyps (> 50 mm) required surgery. Of the 19 polyps, four showed malignant transformation.
| Case | Author | Year | Age | Sex | Location | Size (mm) | Symptoms | Treatment | Malignancy |
| 1 | Bott | 1986 | 23 | M | 4th | 50 × 40 | GI bleeding | Surgery | None |
| 2 | Naitoh | 1988 | 56 | F | 3rd | 30 × 15 | NA | Endoscopy | None |
| 3 | Tanaka | 1990 | 41 | M | 3rd | 25 × 18 | Asymptomatic | Endoscopy | None |
| 4 | - | - | 82 | F | 2nd | 25 × 20 | Asymptomatic | Endoscopy | None |
| 5 | Acea Nebril | 1993 | 63 | F | 1st | 50 × 35 | Anemia | Surgery | None |
| 6 | Ichiyoshi | 1996 | 84 | F | 2nd | 25 × 20 | GI bleeding | Endoscopy | Yes |
| 7 | Oncel | 2003 | 68 | F | 3rd | 25 | GI bleeding | Endoscopy | None |
| 8 | - | - | 53 | M | 2nd | 5 | Dyspepsia | Endoscopy | None |
| 9 | Kitaoka | 2004 | 22 | F | 1st | 30 | Asymptomatic | Endoscopy | None |
| 10 | Itaba | 2006 | 87 | F | 2nd | 18 | Abdominal pain | Endoscopy | None |
| 11 | 56 | M | 2nd | 12 | CEA elevation | Endoscopy | None | ||
| 12 | Suzuki | 2008 | 59 | F | 2nd | 15 × 15 | Epigastric discomfort | Endoscopy | None |
| 13 | - | - | 68 | F | 2nd | 10 × 8 | Epigastralgia | Endoscopy | Yes |
| 14 | - | - | 60 | F | 1st | 10 × 10 | Asymptomatic | Endoscopy | None |
| 15 | Jamaludin | 2009 | 46 | M | 1st | 70 × 40 | Dyspepsia | Surgery | Yes |
| 16 | Kantarciogle | 2009 | 28 | F | 2nd | 25 × 15 | Acute pancreatitis | Endoscopy | None |
| 17 | Sekino | 2011 | 84 | M | 1st | 14 | Asymptomatic | Endoscopy | Yes |
| 18 | - | - | 76 | M | 2nd | 15 | Asymptomatic | Endoscopy | None |
| 19 | Our case | 2014 | 89 | M | 2nd | 48 × 19 × 18 | Acute cholangitis | Endoscopy | None |
Duodenal solitary PJ-type hamartomatous polyp is usually diagnosed incidentally during gastroduodenal endoscopy. Its presentation is nonspecific and resembles common conditions, such as peptic ulcer disease[13]. Our patient was the first to report acute cholangitis and jaundice as the chief complaints. Moreover, this was the first solitary PJ-type hamartomatous polyp showing ingrowth into the CBD, although a solitary hamartomatous polyp in the CBD was reported in a patient without PJS after hepatoduodenostomy[16]. The mechanism of ingrowth into the CBD in our patient is undetermined. However, a polyp that initially developed on the papilla of Vater may have invaginated accidentally into the CBD while still tiny due to peristaltic motion. This polyp may then have grown in the CBD.
The characterization of PJ-type polyps as having a low risk of cancer is unclear. To our knowledge, 4 of 19 such lesions showed malignant transformation. The mean age of patient with malignancy was 70.5 years, higher than that of all 19 patients (60.3 years). In contrast, the maximum diameter may be nonindicative of malignancy, as patient 13 had a small malignant polyp only 10 mm in diameter. There is no definitive guideline for treatment of solitary duodenal PJ-type hamartomatous polyps. In general, as with other common gastroduodenal polyps, solitary PJ-type polyps with features of large size, sessile type, presence of induration, or with suspicion of submucosal invasion should be considered for surgical resection. As the differential diagnosis by morphologic features is difficult, endoscopic biopsies must be performed for the selection of an appropriate therapeutic strategy. When the biopsy is malignant, surgery should be performed according to the depth of tumor invasion. Endoscopic ultrasonography is useful. Meanwhile, comprehensive and cautious decision of surgical indication is required by consideration of patient characteristics, such as age, past history, and general condition.
In conclusion, associated signs and symptoms should be evaluated in patients with duodenal PJ-type hamartomatous polyps. In addition, patients should undergo upper intestinal endoscopy, colonoscopy, and whole-body screening. Solitary PJ-type hamartomatous polyps should be distinguished as solitary or as an incomplete type of PJS. These polyps should be treated by endoscopic or surgical resection.
An 89-year-old male with solitary duodenal Peutz-Jeghers (PJ)-type hamartomatous polyp, which had grown into the common bile duct (CBD).
Expulsion of a large tumor with a stalk connected to the papilla of Vater after endoscopic snare papillectomy.
Papillary-expanding-type cholangiocarcinoma; polypoid type carcinoma of the papilla of Vater.
WBC, 10310/μL; C-reactive protein, 4.62 mg/dL; aspartate transaminase, 152 IU/L; alanine transaminase, 68 IU/L; lactate dehydrogenase, 551 IU/L; gamma-glutamyl transpeptidase, 84 IU/L; total bilirubin, 2.32 mg/dL; inflammatory reaction with liver dysfunction and slight jaundice.
Computed tomography and magnetic resonance imaging revealed choledocholithiasis with multiple calcified stones in the dilated CBD and a tumor in the lower CBD.
Histopathologic examination showed a large polypoid neoplasm with a lobular surface and epithelialized with intestinal type mucosa containing irregular hyperplastic crypts with focal cystic dilatation, and branching bundles of smooth muscle extending from the muscularis mucosae.
The tumor with a stalk growing from the major duodenal papilla, which was inverted and excluded from the CBD, was excised en bloc by endoscopic snare papillectomy.
There are a few cases of solitary duodenal PJ-type hamartomatous polyps reported in the literature, however, the present case is the first to report showing ingrowth into the CBD with acute cholangitis and jaundice.
Endoscopic snare papillectomy is an endoscopic excision technique for a benign and/or low-grade malignant tumor of the papilla of the Vater, using a snare loop to enable en bloc resection.
This case report not only represents the diagnosis and therapeutic strategy for a solitary duodenal PJ-type hamartomatous polyp, but also introduces the usefulness of endoscopic snare papillectomy for a large pedunculated polyp adjacent to the papilla of Vater.
This article presents a rare case of solitary duodenal PJ-type hamartomatous polyp grown from the CBD, and also addresses a critical clinical question by integrating an extended review of literatures.
P- Reviewer: Armakolas A S- Editor: Yu J L- Editor: AmEditor E- Editor: Liu XM
| 1. | Kopacova M, Tacheci I, Rejchrt S, Bures J. Peutz-Jeghers syndrome: diagnostic and therapeutic approach. World J Gastroenterol. 2009;15:5397-5408. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 135] [Cited by in RCA: 127] [Article Influence: 7.9] [Reference Citation Analysis (1)] |
| 2. | Acea Nebril B, Taboada Filgueira L, Parajó Calvo A, Gayoso García R, Gómez Rodríguez D, Sánchez González F, Sogo Manzano C. Solitary hamartomatous duodenal polyp; a different entity: report of a case and review of the literature. Surg Today. 1993;23:1074-1077. [PubMed] |
| 3. | Jeghers H, Mckusick VA, Katz KH. Generalized intestinal polyposis and melanin spots of the oral mucosa, lips and digits; a syndrome of diagnostic significance. N Engl J Med. 1949;241:1031-1036. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 299] [Cited by in RCA: 283] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 4. | Nakayama H, Fujii M, Kimura A, Kajihara H. A solitary Peutz-Jeghers-type hamartomatous polyp of the rectum: report of a case and review of the literature. Jpn J Clin Oncol. 1996;26:273-276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 5. | Bott SJ, Hanks JB, Stone DD. Solitary hamartomatous polyp of the duodenum in the absence of familial polyposis. Am J Gastroenterol. 1986;81:993-994. [PubMed] |
| 6. | Naitoh H, Sumiyoshi Y, Kumashiro R, Inutsuka S, Fujita K, Yamamoto T, Murayama H. A solitary Peutz-Jeghers type hamartomatous polyp in the duodenum--a case report. Jpn J Surg. 1988;18:475-477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 7. | Tanaka H, Iida M, Kohrogi N, Matsui T, Yasunami Y, Yao T, Nakamura K, Fujishma M. Endoscopic removal of solitary hamartomatous polyps of the duodenum. Gastrointest Endosc. 1990;36:640-642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 8. | Ichiyoshi Y, Yao T, Nagasaki S, Sugimachi K. Solitary Peutz-Jeghers type polyp of the duodenum containing a focus of adenocarcinoma. Ital J Gastroenterol. 1996;28:95-97. [PubMed] |
| 9. | Oncel M, Remzi FH, Church JM, Goldblum JR, Zutshi M, Fazio VW. Course and follow-up of solitary Peutz-Jeghers polyps: a case series. Int J Colorectal Dis. 2003;18:33-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 42] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 10. | Kitaoka F, Shiogama T, Mizutani A, Tsurunaga Y, Fukui H, Higami Y, Shimokawa I, Taguchi T, Kanematsu T. A solitary Peutz-Jeghers-type hamartomatous polyp in the duodenum. A case report including results of mutation analysis. Digestion. 2004;69:79-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 11. | Itaba S, Namoto M, Somada S, Nakamura K, Kumashiro Y, Nakamura N, Yao T. Two cases of solitary Peutz-Jeghers-type hamartoma of the duodenum. Endoscopy. 2006;38 Suppl 2:E32-E33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 12. | Suzuki S, Hirasaki S, Ikeda F, Yumoto E, Yamane H, Matsubara M. Three cases of Solitary Peutz-Jeghers-type hamartomatous polyp in the duodenum. World J Gastroenterol. 2008;14:944-947. [PubMed] |
| 13. | Jamaludin AZ, Telisinghe PU, Yapp SK, Chong VH. Solitary duodenal hamartomatous polyp with malignant transformation: report of a case. Surg Today. 2009;39:527-532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 14. | Kantarcioglu M, Kilciler G, Turan I, Ercin CN, Karslioglu Y, Guvenc I, Polat Z, Bagci S. Solitary Peutz-Jeghers-type hamartomatous polyp as a cause of recurrent acute pancreatitis. Endoscopy. 2009;41 Suppl 2:E117-E118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 15. | Sekino Y, Inamori M, Hirai M, Suzuki K, Tsuzawa K, Akimoto K, Takahata A, Fujisawa N, Saito K, Tsunemi A. Solitary Peutz-Jeghers type hamartomatous polyps in the duodenum are not always associated with a low risk of cancer: two case reports. J Med Case Rep. 2011;5:240. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 16. | Solakoglu T, Akin E, Yavuz SH, Ersoy O. Endoscopic treatment of a solitary hamartomatous polyp in the intrahepatic biliary duct. Endoscopy. 2013;45 Suppl 2 UCTN:E356-E357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |