Published online Jul 14, 2015. doi: 10.3748/wjg.v21.i26.8208
Peer-review started: November 20, 2014
First decision: January 8, 2015
Revised: February 12, 2015
Accepted: April 3, 2015
Article in press: April 3, 2015
Published online: July 14, 2015
Processing time: 236 Days and 15.9 Hours
Recently, a new disease entity termed gastric adenocarcinoma of fundic gland type (GA-FG) was proposed. We treated five cases of GA-FG with endoscopic submucosal dissection. All tumors were small and located in the upper third of the stomach. Four tumors were macroscopically identified as 0-IIa and one was identified as 0-IIb. Narrow-band imaging with magnifying endoscopy showed an irregular microvascular pattern in 2 cases and a regular microvascular pattern in the remainder. All tumors arose from the deep layer of the lamina propria mucosae and showed submucosal invasion. Lymphatic invasion was seen only in one case, while no venous invasion was recognized. All tumors were positive for pepsinogen-I and MUC6 by immunohistochemistry. None showed p53 overexpression, and the labeling index of Ki-67 was low in all cases. All cases have been free from recurrence or metastasis. Herein, we discussed the clinicopathological features of GA-FG in comparison with past reports.
Core tip: Recently, a new disease entity termed gastric adenocarcinoma of fundic gland type (GA-FG) was proposed. We treated five cases that were diagnosed as GA-FG with endoscopic submucosal dissection. GA-FG has characteristic findings in endoscopic and pathological examinations. For accurate and early endoscopic diagnosis of GA-FG, careful endoscopic examination and detailed pathological evaluation are important. Herein, we discussed the clinicopathological features of GA-FG in comparison with past reports.
- Citation: Miyazawa M, Matsuda M, Yano M, Hara Y, Arihara F, Horita Y, Matsuda K, Sakai A, Noda Y. Gastric adenocarcinoma of fundic gland type: Five cases treated with endoscopic resection. World J Gastroenterol 2015; 21(26): 8208-8214
- URL: https://www.wjgnet.com/1007-9327/full/v21/i26/8208.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i26.8208
According to clinicopathological studies of gastric adenocarcinoma, it has been assumed that differentiated adenocarcinomas arise from intestinal metaplasia involving Helicobacter pylori (H. pylori) infection, and thus, has an intestinal phenotype while undifferentiated adenocarcinomas develop directly from the proper gastric mucosa without the process of intestinal metaplasia, and therefore, has a gastric phenotype. Recently, immunohistochemical staining for mucin has proven that some differentiated adenocarcinomas have a gastric phenotype[1,2]. Early differentiated adenocarcinomas with a gastric phenotype are thought to be difficult to diagnose by biopsy, which examines only a small amount of specimen, because of their mild histological atypism.
Regarding differentiated gastric adenocarcinomas with a gastric phenotype, Yao et al[3] in 2006 reported cases of extremely well-differentiated adenocarcinoma resembling gastric foveolar epithelium, mucous neck cells and pyloric glands. In 2007, Tsukamoto et al reported the first case of adenocarcinoma with differentiation to chief cells that comprise the fundic gland[4]. Furthermore in 2010, Ueyama et al[5] reported 10 cases that showed differentiation to chief cells, and proposed a new disease entity termed gastric adenocarcinoma of fundic gland type (GA-FG) based on their clinicopathological features. Since it is assumed that GA-FGs arise from normal gastric mucosa of the fundic gland region without intestinal metaplasia, the percentage of GA-FGs among all gastric adenocarcinomas is expected to increase along with decreasing prevalence of H. pylori infection.
Although the concept of GA-FG has been gradually spreading, quite a few cases may still be undiagnosed due to the difficulty in making a correct diagnosis. Since the current general consensus on treatment of early gastric adenocarcinomas is by endoscopic submucosal dissection (ESD), early endoscopic and pathological diagnosis of endoscopically resectable GA-FG is necessary. For accurate and early diagnosis, careful examination using narrow-band imaging with magnifying endoscopy (NBI-ME) in addition to conventional endoscopy and detailed pathological evaluation using immunohistochemical staining are extremely important. Pathological characteristics of GA-FG have occasionally been reported, but to the best of our knowledge, detailed reports on its endoscopic findings are not available. Herein, we examined and discussed the clinicopathological features, especially the endoscopic findings of GA-FG treated with ESD.
We studied the clinicopathological features of GA-FG treated with ESD at Toyama Prefectural Central Hospital from 2010 to 2013. We defined GA-FG as neoplastic lesions, which were composed of cells that mimic the fundic gland cells and were positive for pepsinogen-I (a marker of chief cells) by immunohistochemical staining. Lesion sites, macroscopic types and histopathological findings were described according to the Japanese classification of gastric carcinoma, 3rd English edition[6]. Before performing ESD, upper gastrointestinal endoscopy was conducted by employing NBI-ME in addition to conventional white light endoscopy. Endoscopic findings obtained by NBI-ME were described according to the VS classification system of Yao et al[7] While one biopsy specimen was obtained from a lesion for histological diagnosis, another four specimens were taken from the surrounding mucosa at a 5 mm distance from the margin of the lesion to verify non-malignancy and to assess the status of the background gastric mucosa.
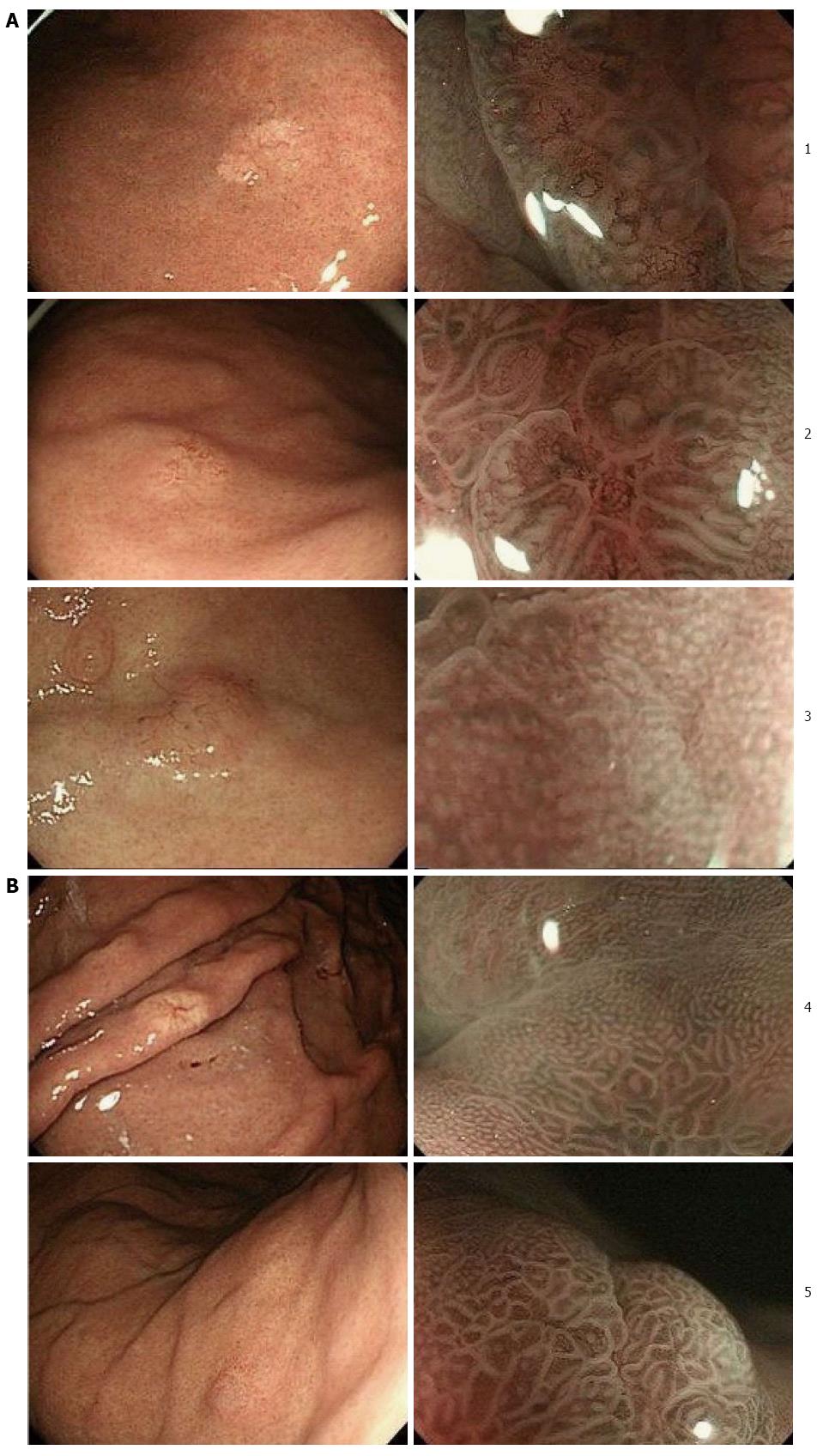
Of 506 early gastric adenocarcinomas that underwent ESD at our institution during a period from May 2010 to March 2013, 5 cases (0.98%) were GA-FG, including 3 males and 2 females with ages ranging from 67 to 78 years (average, 72.2 years). The characteristics of these 5 cases are summarized in Table 1. None of these cases had serum anti-H. pylori antibody. Only one case had antral atrophic gastritis while the remainder had no such lesion. Endoscopic findings of all cases are shown in Figure 1. All tumors were located in the upper third of the stomach. Four tumors were macroscopically identified as submucosal tumor (SMT)-like 0-IIa (superficial elevated type) and one was identified as 0-IIb (superficial flat type). They were 5 to 13 mm (average, 7.8 mm) in diameter and covered with normal colored or whitish vasodilated mucosa. NBI-ME showed an irregular microvascular pattern (MVP), which indicated nonuniformity and heterogeneity of microvessels, and an absent microsurface pattern (MSP), which indicated the lack of microstructure such as marginal crypt epithelium, on the small region of the tumor in 2 cases, while regular MVP and MSP were demonstrated in the remainder. Endoscopic examination prior to pathological diagnosis suspected GA-FG in 1 case and malignant lesions such as adenocarcinoma, SMT or carcinoid in 4 cases.
| Case 1 | Case 2 | Case 3 | Case 4 | Case 5 | |
| Age (yr)/sex | 78/Female | 74/Male | 67/Male | 72/Male | 70/Female |
| Serum anti-H. pylori antibody | Negative | Negative | Negative | Negative | Negative |
| Chronic gastritis | Antral gastritis | (-) | (-) | (-) | (-) |
| Location | Upper/Ant | Upper/Gre | Upper/Gre | Upper/Gre | Upper/Post |
| Size (mm) | 13 | 5 | 8 | 5 | 8 |
| Macroscopic feature | IIa | IIa | IIa | IIb | IIa |
| Microvascular pattern on NBI-ME | Irregular | Irregular | Regular | Regular | Regular |
| Endoscopic diagnosis | Adenocarcinoma | SMT | Carcinoid | GA-FG | Adenocarcinoma |
| Histological classification | tubular | tubular | tubular | tubular | tubular |
| Depth | SM (700 μm) | SM (80 μm) | SM (980 μm) | SM (110 μm) | SM (1230 μm) |
| Lymphatic/venous invasion | (+)/(-) | (-)/(-) | (-)/(-) | (-)/(-) | (-)/(-) |
| MUC2/MUC5AC/MUC6/Pepsinogen-I | (-)/(-)/(+)/(+) | (-)/(-)/(+)/(+) | (-)/(-)/(+)/(+) | (-)/(-)/(+)/(+) | (-)/(-)/(+)/(+) |
| p53/Ki-67 labeling index | (-)/Low | (-)/Low | (-)/Low | (-)/Low | (-)/Low |
ESD-resected specimens were subjected to immunohistochemical staining in addition to conventional hematoxylin and eosin staining. Immunohistochemical markers used were as follows: MUC5AC for foveolar cells, MUC6 for mucous neck cells or pyloric gland cells, MUC2 for goblet cells, and CD10 for intestinal brush border. The mucin phenotypes were assessed according to the expression of gastric type markers such as MUC5AC and MUC6 and intestinal type markers such as MUC2 and CD10. Furthermore, pepsinogen-I was also employed as a marker of differentiation to chief cells. When these markers were expressed in 10% or greater of the cytoplasm, they were considered as positive. Proliferative activity was assessed as a ratio of Ki-67 positive cells to 1000 tumor cells while p53 overexpression was defined as positive when the protein was expressed in 10% or greater of tumor cell nuclei.
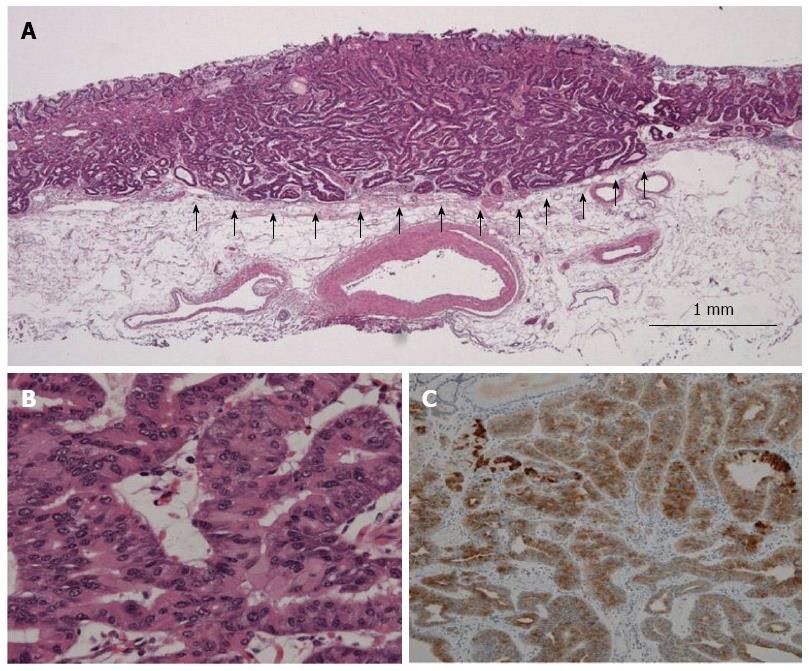
Pathological findings of typical GA-FG are shown in Figure 2 (case 1). All tumors showed well-differentiated tubular adenocarcinoma. The tumors arose from the deep layer of the lamina propria mucosae, and most of their surfaces were covered with non-atypical foveolar epithelium. All tumors showed submucosal invasion, and the depth of invasion ranged from 80 to 1230 μm (average, 620 μm). Lymphatic invasion was seen only in case 1, while no venous invasion was recognized. By immunohistochemical examination, it was found that in addition to pepsinogen-I positivity, all tumors showed diffuse positivity for MUC6. In contrast, MUC2 and MUC5AC were negative in all cases. The malignant potential of all cases was considered low as none showed overexpression of p53, and the labeling index of Ki-67 was low.
All cases could be followed up after ESD for a period from 9.7 to 27.7 mo. Of the three cases that showed submucosal invasion, the depth of which was greater than 500 μm, case 3 subsequently underwent additional fundusectomy, resulting in neither remnant adenocarcinoma nor lymph node metastasis. All cases have been free from recurrence or metastasis.
GA-FGs are characterized by the following: (1) they arise most commonly from normal gastric mucosa of the fundic gland region without intestinal metaplasia; (2) they are recognized as smooth elevated or depressed lesions; (3) they tend to invade the submucosal layer while they rarely show lymphatic and venous invasion; and (4) they show histologically mild atypism.
We reviewed previous reports on GA-FG published in the English literature or the Japanese literature with English abstract, and found that 30 domestic cases were reported until 2013[4,5,8-12], while Singhi et al[13] was the first to report 10 non-Japanese cases in 2012. Clinicopathological findings of the previous and the present cases are shown in Table 2. These cases were characterized by common occurring sites such as the upper gastric region, a tendency to take the form of SMT-like elevation and rare lymphatic and venous invasion despite frequent submucosal invasion. Most of the cases were treated with endoscopic resection, and there was no recurrence or metastasis except for a case that had local residual recurrence.
| Reports | [4] | [5,8] | [9] | [10] | [11] | [12] | [13] | Present cases |
| Number of patients | 1 | 25 (27 lesions) | 1 | 1 | 1 | 1 | 10 | 5 |
| Age (yr) | 82 | 67 (average) | 59 | 56 | 50s | 71 | 64.2 (average) | 72.2 (average) |
| Sex (Male:Female) | Female | 16:9 | Female | Male | Male | Female | 4:6 | 3:2 |
| Therapy | EMR | Operation: 3 | ESD | EAM | ESD | EMR | polypectomy | ESD |
| ESD or EMR: 7 | ||||||||
| ND: 15 | ||||||||
| Location (Upper:Middle:Lower) | Upper | 23:4:0 | Upper | Upper | Middle | Upper | 10:0:0 | 5:0:0 |
| Size (average, mm) | 16 | 12.2 (3-42) | 8 | 5 | 42 | ND | 4.3 | 7.8 (5-13) |
| Macroscopic feature (I:IIa:IIb:IIc:IIa + IIc) | I (protrude) | 1:14:2:8:2 | IIb | IIa | IIa + IIc | IIa | 10:0:0:0:0 | 0:4:1:0:0 |
| Depth (M:SM) | M | 6:21 | SM | SM | SM | SM | 10:00 | 0:05 |
| Lymphatic/venous | (-)/(-) | 2/1 | (-)/(-) | (-)/(-) | (-)/(-) | (-)/(-) | 0/0 | 1/0 |
| invasion | ||||||||
| Survival time (average, mo) | ND | 27 (10-70) | 17 | 12 | ND | 12 | 15 (6-39) | 10.6 (2-20) |
| Outcome | ND | NED: 10 | NED | NED | ND | NED | NED: 8 | NED:5 |
| ND: 15 | Persistence: 1 | |||||||
| ND: 1 |
Endoscopic findings of the present cases were similar to those of previous reports. In particular, the tumors were characteristically identified as whitish SMT-like elevations associated with vasodilation. The whitish coloring seemed to be produced by dimmed reddening of the mucosal surface due to reduced transparency of the submucosal vessels brought about by the tumor located in the deep layer of the mucosa. Vasodilation on the tumor surface is considered ascribable to displacement of the surface vessels by the tumor tissue. A possible reason for the macroscopic similarity to SMT is that the tumors arose from the deep layer of the gastric mucosa, growing laterally toward the lamina propria. If they had grown straight toward the mucosal surface, they would have been recognized as unsmooth tumors by endoscopy. However, since they were scarcely exposed on the mucosal surface, they were recognized as SMT-like elevations. From the above, careful endoscopic screening is very important for detecting slight differences between GA-FG and the surrounding mucosa even if there is no obvious irregularity in the mucosal surface.
In our study, NBI-ME was also performed in addition to conventional endoscopy. At present, observation of early gastric adenocarcinoma using NBI-ME is assessed based on the worldwide standard diagnostic system of the VS classification, which uses MVP and MSP as indices as proposed by Yao et al[7]. Only two of our cases showed a minimal irregular MVP and absent MSP under NBI-ME, which are consistent with the findings of gastric adenocarcinoma. In contrast, the other cases showed regular MVP and MSP probably because these tumors, which were located in the deep layer of the mucosa, hardly showed any inner abnormal microstructure through the thick mucosa.
Despite the detailed endoscopic examination, a definite diagnosis was finally made by histopathological examination. If GA-FG is suspected by endoscopy, a pathologist should be informed of such suspicion in order for immunohistochemical staining to be performed which may lead to a definite diagnosis. Even if not suspected, the lesion should be recognized as malignant that is distinct from a real SMT or a fundic gland polyp and biopsy should be performed. It is important to acquire a sufficient amount of tissue because the tumor location is commonly in the deep layer of the mucosa.
With regard to therapy, the majority of GA-FGs are thought to be indications for endoscopic resection. However, for lesions that show submucosal invasion deeper than 500 μm as in our cases, curative resection may not be possible according to Japanese gastric cancer treatment guidelines 2010 (ver.3)[14]. On the other hand, GA-FGs rarely demonstrate lymphatic and venous invasions despite deep submucosal invasion. Regarding therapeutic approaches following incomplete resection of GA-FG, which is relatively common among the elderly, it will be controversial in the future whether additional treatment such as for differentiated adenocarcinoma of intestinal phenotype should be administered or not.
In conclusion, we examined 5 cases of GA-FG and discussed their characteristics in comparison with previous reports. Even when atrophic mucosa due to H. pylori infection is not observed, detailed examination should be done under suspicion of GA-FG if a whitish SMT-like lesion with vasodilation on the surface is found in the upper third of the stomach. Whereas the consensus is for the use of conventional white light endoscopy to facilitate diagnosis, few reports on NBI-ME are available. It is therefore necessary to accumulate more endoscopic data to identify the characteristics of GA-FG. Furthermore, what remains to be done is to explore long-term outcomes of GA-FG such as the natural course, recurrence rate and prognosis after resection, and to elucidate the genetic aberrations involved in carcinogenesis.
Patients were 3 males and 2 females with ages ranging from 67 to 78 years.
All patients had no symptom and no specific physical signs.
The authors cannot make mention of differential diagnosis at this point.
None of five cases had serum anti-Helicobacter pylori (H. pylori) antibody.
Endoscopically, all tumors were located in the upper third of the stomach and macroscopically identified as superficial elevated type or flat type, which were 5 to 13 mm (average, 7.8 mm) in diameter and covered with normal colored or whitish vasodilated mucosa.
Pathologically, all tumors showed well-differentiated tubular adenocarcinoma, which arose from the deep layer of the lamina propria mucosae and showed submucosal invasion.
All tumors were treated with endoscopic submucosal dissection and no case has experienced recurrence or metastasis.
The authors reviewed previous reports and found 40 cases, whose clinicopathological findings were similar to the present cases.
Gastric adenocarcinoma of fundic gland type (GA-FG), which shows differentiation to chief cells and arises from normal gastric mucosa of the fundic gland region without intestinal metaplasia, was first proposed as a new disease entity in 2010.
Even when atrophic mucosa due to H. pylori infection is not observed endoscopically, detailed examination should be done under suspicion of GA-FG if a whitish SMT-like lesion with vasodilation findings on the surface is found in the upper third of the stomach.
This article shows detailed endoscopic and pathological findings of GA-FG for accurate diagnosis.
P- Reviewer: Grundmann O, Paydas S S- Editor: Qi Y L- Editor: A E- Editor: Ma S
| 1. | Egashira Y. [Mucin histochemical study of differentiated adenocarcinoma of stomach]. Nihon Shokakibyo Gakkai Zasshi. 1994;91:839-848. [PubMed] |
| 2. | Yao T, Kabashima A, Kouzuki T, Oya M, Tsuneyoshi M. The phenotypes of the gastric carcinoma - Evaluation by a new immunohistochemical method. Stomach Intest. 1999;34:477-485. |
| 3. | Yao T, Utsunomiya T, Oya M, Nishiyama K, Tsuneyoshi M. Extremely well-differentiated adenocarcinoma of the stomach: clinicopathological and immunohistochemical features. World J Gastroenterol. 2006;12:2510-2516. [PubMed] |
| 4. | Tsukamoto T, Yokoi T, Maruta S, Kitamura M, Yamamoto T, Ban H, Tatematsu M. Gastric adenocarcinoma with chief cell differentiation. Pathol Int. 2007;57:517-522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 67] [Article Influence: 3.7] [Reference Citation Analysis (1)] |
| 5. | Ueyama H, Yao T, Nakashima Y, Hirakawa K, Oshiro Y, Hirahashi M, Iwashita A, Watanabe S. Gastric adenocarcinoma of fundic gland type (chief cell predominant type): proposal for a new entity of gastric adenocarcinoma. Am J Surg Pathol. 2010;34:609-619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 161] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 6. | Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer. 2011;14:101-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2390] [Cited by in RCA: 2872] [Article Influence: 205.1] [Reference Citation Analysis (0)] |
| 7. | Yao K, Anagnostopoulos GK, Ragunath K. Magnifying endoscopy for diagnosing and delineating early gastric cancer. Endoscopy. 2009;41:462-467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 348] [Cited by in RCA: 337] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 8. | Yao T, Ueyama H, Kushima R, Nakashima Y, Hirakawa K, Ohshiro Y, Hirano H, Satake T, Shimizu S, Yamane T. New type of gastric carcinoma - Adenocarcinoma of the fundic gland type: its clinicopathological features and tumor development. Stomach Intest. 2010;45:1203-1211. |
| 9. | Miyaoka Y, Izumi D, Mikami H, Yasaki T, Morito Y, Imaoka H, Fujishiro H, Kouge N, Imaoka T, Onuma H. A case report of an extremely well differentiated gastric adenocarcinoma of the fundic gland type successfully treated with ESD. Gastroenterol Endosc. 2011;53:1778-1785. |
| 10. | Fukatsu H, Miyoshi H, Ishiki K, Tamura M, Yao T. Gastric adenocarcinoma of fundic gland type (chief cell predominant type) treated with endoscopic aspiration mucosectomy. Dig Endosc. 2011;23:244-246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 11. | Fujisawa T, Ueyama S, Ouchi S, Seki Y, Teranishi T, Hirano H, Hasuike N. Early gastric adenocarcinoma of the fundic gland type (chief cell predominant type) observed with magnifying endoscopy using narrow band imaging: report of a case. Gastroenterol Endosc. 2011;53:3769-3775. |
| 12. | Abe T, Nagai T, Fukunaga J, Okawara H, Nakashima H, Syutou M, Kajimoto N, Wake R, Oyama T, Yao T. Long-term follow-up of gastric adenocarcinoma with chief cell differentiation using upper gastrointestinal tract endoscopy. Intern Med. 2013;52:1585-1588. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 13. | Singhi AD, Lazenby AJ, Montgomery EA. Gastric adenocarcinoma with chief cell differentiation: a proposal for reclassification as oxyntic gland polyp/adenoma. Am J Surg Pathol. 2012;36:1030-1035. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 76] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 14. | Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2010 (ver. 3). Gastric Cancer. 2011;14:113-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1723] [Cited by in RCA: 1895] [Article Influence: 135.4] [Reference Citation Analysis (0)] |