Published online Jul 14, 2015. doi: 10.3748/wjg.v21.i26.8156
Peer-review started: January 8, 2015
First decision: January 22, 2015
Revised: February 3, 2015
Accepted: March 18, 2015
Article in press: March 19, 2015
Published online: July 14, 2015
Processing time: 187 Days and 13.7 Hours
AIM: To evaluate the efficacy and toxicity of stereotactic body radiotherapy using CyberKnife for locally advanced unresectable and metastatic pancreatic cancer.
METHODS: From June 2010 to May 2014, 25 patients with locally advanced unresectable and metastatic pancreatic cancer underwent stereotactic body radiotherapy. Nine patients presented with unresectable locally advanced disease and 16 had metastatic disease. Primary end-points of this study were overall survival, relief of abdominal pain, and toxicity.
RESULTS: Fourteen patients were treated with a total dose of 30-36 Gy in three fractions and the remainder with 40-48 Gy in four fractions. Median follow-up was 11 mo (range: 2-25 mo). The median survival duration calculated from the time of stereotactic body radiotherapy for the entire group, the locally advanced group, and the metastatic group was 9.0 mo, 13.5 mo, and 8.5 mo, respectively. Overall survival was 37% and 18% at one and two years, respectively. Abdominal pain relief was achieved within 2 wk of completing radiotherapy in the patients who received successful palliation (13 of 20 patients had significant pain). Five patients (20%) had grade 1 nausea, and one (4%) had grade 2 nausea. No acute grade 3+ toxicity was seen.
CONCLUSION: Stereotactic body radiotherapy using the CyberKnife system is a promising, noninvasive, palliative treatment with acceptable toxicity for locally advanced unresectable and metastatic pancreatic cancer.
Core tip: Locally advanced unresectable and metastatic pancreatic cancer is the most common presentation of pancreatic cancer. The available therapeutic option is chemotherapy or chemoradiotherapy. The low-dose radiation of conventional radiotherapy has unsatisfactory results for survival and local control, at a cost of increased hematologic toxicity. Doses > 54 Gy may be considered if clinically appropriate. Stereotactic body radiation therapy has become an important research topic to provide a higher biologically effective dose. We evaluated the efficacy and toxicity of stereotactic body radiation therapy using the CyberKnife system for patients with locally advanced unresectable and metastatic pancreatic cancer.
- Citation: Su TS, Liang P, Lu HZ, Liang JN, Liu JM, Zhou Y, Gao YC, Tang MY. Stereotactic body radiotherapy using CyberKnife for locally advanced unresectable and metastatic pancreatic cancer. World J Gastroenterol 2015; 21(26): 8156-8162
- URL: https://www.wjgnet.com/1007-9327/full/v21/i26/8156.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i26.8156
Pancreatic cancer is both an aggressive and prevalent malignancy. It is the fourth leading cause of cancer mortality in men and women in the United States[1]. In the Asia-Pacific region, the age-standardized incidence reached a plateau after 1985, while the incidence continued to rise due to the aging population in the region[2]. Only approximately 20% of patients are amenable to surgery at diagnosis. It is a highly aggressive entity with approximately 40% presenting with locally advanced but unresectable disease and an additional 40% presenting with metastatic disease[3]. Surgical resection remains the only curative therapeutic modality for early-stage pancreatic cancer. Despite improvements in surgical technique and patient selection, as well as adjuvant chemotherapy, the five-year survive rate remains low, ranging from 10% to 20%, following curative surgery[4-6]. In patients with locally unresectable pancreatic cancer, the only therapeutic option is chemoradiotherapy. The local control rate after chemoradiotherapy is still relatively low, ranging from 40% to 55%, with a median survival ranging from 5 mo to 14 mo[7-9]. The conventional radiation dose is usually between 45 and 54 Gy in 1.8-2.5-Gy fractions. These limited doses have a poor curative effect. Doses > 54 Gy may be considered if clinically appropriate[10-12]. Recently, stereotactic body radiotherapy (SBRT) has become an important research topic to provide a higher biologically effective dose. The conformity and rapid dose fall-off associated with SBRT offer the potential for dose escalation[13]. In this study, we analyzed the patients with locally advanced unresectable and metastatic pancreatic cancer who underwent SBRT.
Between June 2010 and May 2014, 25 patients with unresectable or metastatic pancreatic adenocarcinoma were included in this retrospective analysis. Ethical approval was given by the Medical Ethics Committee of Rui Kang Hospital, Guangxi, China. All patients gave written informed consent. Reasons for unresectability included the presence of metastatic disease and radiographic evidence of major vessel involvement, as determined by the surgeon and/or radiologist. Patients with metastatic disease who were treated with SBRT had distant disease that: (1) responded well to initial chemotherapy if the prognosis was that local disease potentially could lead to death or significant morbidity; or (2) the local tumor was causing symptoms of pain or obstruction. All patients’ hospital charts and irradiation documents were carefully reviewed.
Patients were immobilized in the supine position with arms over the head using a thermoplastic body mask and a styrofoam block provided abdominal compression to minimize internal organ motion (spontaneous or breath-induced). CT was performed with a slice thickness of 3 mm. The gross tumor volume was defined as the tumor visible on the CT scan, and in those with N1 disease, the nodes were not included in the target. The gross tumor volume was expanded by 1 or 2 mm to form the planning target volume (PTV). The dose-volume constraints for organs at risk were: duodenum, V 1 mL < 25 Gy; stomach and small bowel, V 1 mL < 25 Gy, and was strict with regard to keeping any 1 mL < 25 Gy; kidneys, 1/3 Vtot < 15 Gy; liver, total spared volume (Vtot - V 15 Gy) > 700 mL and V 15 Gy < 1/3 total volume; spinal cord, V 1 mL < 15 Gy, and strict with regard to keeping any 1 mL < 15 Gy. The radiosurgical plan was to deliver a dose of 30-36 Gy in three fractions or 40-48 Gy in four fractions. Plans were devised such that the prescription dose was the isodose line encompassing > 97% of the PTV. No more than 3% of the PTV was to receive < 93% of the prescription dose. For stereotactic localization, patients underwent a 4D-CT treatment simulation with the CyberKnife Robotic Radiosurgery System with the Xsight Spine Tracking System (Accuray Inc., Sunnyvale, CA, United States).
Patients were re-evaluated 1 mo after SBRT and then every 3 mo thereafter by the treating radiation oncologist. Clinical examination, determination of carbohydrate antigen 19-9 levels, and contrast-enhanced CT were performed at each step of follow-up. Acute and late toxicity was scored according to the NCI Common Terminology Criteria for Adverse Events version 3.0.
Overall survival (OS) was calculated from the date of SBRT to the date of progression and to the day of last follow-up or death using the Kaplan-Meier method. Acute toxicity was defined as that occurring within 90 d of SBRT, and late toxicity as that occurring thereafter. SPSS version 17.0 (SPSS Inc., Chicago, IL, United States) was used for statistical analysis. All enrolled patients were included in the statistical evaluation. The statistical methods of this study were reviewed by Zhen-Dong Yang from Rui Kang Hospital, Guangxi Traditional Chinese Medical University.
Twenty-five patients were treated in our hospital with SBRT for pancreatic cancer. The median age was 63 years (range: 44-80 years) and 72.0% (18/25) were male. All patients were considered to have unresectable/locally advanced (9/25; 36.0%) and metastatic (16/25; 64.0%) disease as determined by experienced pancreatic surgeons and/or radiologists. Patients were diagnosed with pancreatic cancer at clinical stages T3 (21/25; 84.0%) and T4 (4/25; 16.0%). The majority of patients were N0, but 48.0% (12/25) had N1 disease. Clinical characteristics of selected patients are described in Tables 1 and 2.
| Patient | Sex | T | N | M | CA19-9 | Dose/No. of fractions | Live/dead | Survival time (mo) | Toxicity |
| 1 | Female | 4 | 0 | 0 | Positive | 36 Gy/3 | Live | 4 | G1 |
| 2 | Male | 3 | 0 | 0 | Negative | 45 Gy/4 | Dead | 9 | |
| 3 | Male | 3 | 1 | 1 | Negative | 48 Gy/4 | Live | 2 | G1 |
| 4 | Male | 3 | 0 | 0 | Positive | 46 Gy/4 | Dead | 2 | |
| 5 | Male | 3 | 1 | 1 | Positive | 46 Gy/4 | Dead | 8 | |
| 6 | Male | 3 | 1 | 1 | Positive | 30 Gy/3 | Live | 8 | G1 |
| 7 | Female | 3 | 0 | 0 | Positive | 36 Gy/3 | Dead | 4 | |
| 8 | Female | 3 | 1 | 0 | Negative | 31.5 Gy/3 | Live | 17 | |
| 9 | Female | 3 | 1 | 1 | Negative | 33 Gy/3 | Dead | 5 | |
| 10 | Male | 3 | 0 | 1 | Positive | 36 Gy/3 | Dead | 9 | G1 |
| 11 | Male | 3 | 0 | 1 | Positive | 35 Gy/3 | Dead | 14 | |
| 12 | Female | 4 | 1 | 1 | Positive | 33 Gy/3 | Dead | 9 | |
| 13 | Female | 4 | 0 | 1 | Positive | 36 Gy/3 | Dead | 3 | G1 |
| 14 | Male | 3 | 0 | 0 | Positive | 36 Gy/3 | Live | 6 | |
| 15 | Male | 3 | 0 | 1 | Negative | 40 Gy/4 | Dead | 3 | |
| 16 | Female | 3 | 0 | 1 | Positive | 45 Gy/4 | Live | 15 | G1 |
| 17 | Male | 3 | 1 | 1 | Negative | 33 Gy/3 | Dead | 17 | |
| 18 | Male | 3 | 0 | 1 | Positive | 36 Gy/3 | Dead | 2 | G2 |
| 19 | Male | 3 | 1 | 1 | Positive | 36 Gy/3 | Dead | 2 | |
| 20 | Male | 3 | 1 | 0 | Negative | 42 Gy/4 | Dead | 3 | |
| 21 | Male | 3 | 0 | 0 | Negative | 33 Gy/3 | Live | 3 | G1 |
| 22 | Male | 4 | 1 | 1 | Positive | 40 Gy/4 | Live | 1 | |
| 23 | Male | 3 | 1 | 0 | Positive | 46 Gy/4 | Live | 25 | |
| 24 | Male | 3 | 1 | 1 | Negative | 48 Gy/4 | Dead | 9 | |
| 25 | Male | 3 | 0 | 1 | Positive | 40 Gy/4 | Live | 9 | G1 |
| Characteristic | n |
| Sex | |
| Male | 18 |
| Female | 7 |
| Age, yr | Median 63 (range: 44-80) |
| Stage1 | |
| T3 | 21 |
| T4 | 4 |
| N0 | 13 |
| N1 | 12 |
| M0 | 9 |
| M1 | 16 |
| Dose (Gy)/No. of fractions | |
| 30-36 Gy/3 | 14 |
| 40-48 Gy/4 | 11 |
| Carbohydrate antigen 19-9 | |
| Positive | 16 |
| Negative | 9 |
| Primary location of tumor | |
| Head of pancreas | 20 |
| Body or tail of pancreas | 5 |
Fourteen patients treated with SBRT received a dose of 30-36 Gy in three fractions and the remaining 11 received 40-48 Gy in four fractions. The mean target volume was 43.27 mL (range: 8.80-96.39 mL). The CyberKnife platform utilized 150-180 beams. Maximum spinal cord point dose was a mean 730 cGy (range: 390-1430 cGy), which was strictly maintained at 1 mL < 15 Gy. Maximum bowel point dose was a mean 3361 cGy (range: 2792-4018 cGy) for the PTV, which was strictly maintained at 1 mL < 25 Gy.
Two patients received neoadjuvant gemcitabine-based chemotherapy. Another two patients received adjuvant gemcitabine-based chemotherapy. The choice of chemotherapy was at the discretion of the medical oncologist. During SBRT, combined adjuvant medication was given, consisting of Chinese herbs and dexamethasone, vitamins, glutathione, and lansoprazole.
Twelve patients experienced grade 1 fatigue at 2 wk after SBRT, which required no treatment. Five patients (20%) had grade 1 nausea, and ondansetron was administered to one (1/25; 4%) patient with grade 2 nausea. None of these patients had persistent nausea after 1 mo. No acute grade 3+ toxicity was seen. Most toxicity was well tolerated.
According to the numerical rating scale scoring system, 20/25 (80%) patients experienced significant pain before SBRT. Abdominal pain relief was achieved within 2 wk of completing radiotherapy in the patients who received successful palliation. Ten patients achieved pain control after treatment, allowing suspension of analgesic administration. In three patients, analgesic dose was reduced by 50%, or the patients needed fewer analgesic drugs.
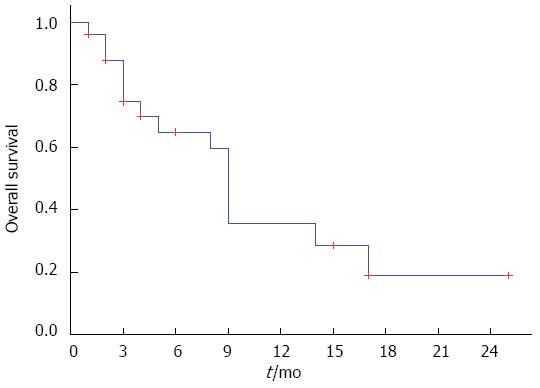
Survival data were available at a median follow-up of 11 mo (range: 2-25 mo). The median survival duration calculated from the time of SBRT for the entire group, the locally advanced group, and the metastatic group was 9.0 mo, 13.5 mo, and 8.5 mo, respectively. OS was 37% and 18% at one and two years, respectively (Figure 1).
Locally advanced unresectable and metastatic pancreatic cancer is the most common presentation of pancreatic cancer. There have been many clinical trials conducted to evaluate novel system regimens for advanced pancreatic cancer. Chemotherapy alone reduces the incidence of distant metastases in patients with localized disease, even though it may hardly improve local disease control. Gemcitabine monotherapy has conventionally been considered the standard regimen for advanced pancreatic cancer on the basis of phase III clinical trials. The median overall survival is limited to the range of 4.6 to 9.2 mo with gemcitabine treatment[12,13]. Among non-gemcitabine regimens, the most notable is FOLFRINOX. A phase III clinical trial showed that OS was significantly longer in the FOLFRINOX arm (11.1 mo vs 6.8 mo). Nevertheless, the FOLFRINOX regimen was at the cost of increased hematologic toxicity[13,14]. The only therapeutic option available is gemcitabine- or capecitabine-based chemoradiotherapy. The median survival ranges from 11.1 mo to 15.2 mo[12,15,16]. The local progression rate reported with conventional fractionation of radiotherapy is still relatively low, at 40%-55%[7-9]. In recent years, the unsatisfactory results of conventional radiotherapy led to several studies that investigated the efficacy and safety of SBRT. Recent encouraging results of SBRT for pancreatic cancer are shown in Table 3. Improvement of local disease control was relevant in these studies, with a success rate of 57%-94%. Median OS was 5.7-20.0 mo. Survival was extended for most of the patients. However, acute and late toxicity are still challenging. The rate of late gastroduodenal toxicity of grade 2 or higher was 4%-47% in several studies.
| Ref. | No. of patients | SBRT dose (Gy/No. of fractions) | Gemcitabine-based chemotherapy | LC (%) | PFS (mo) | OS (mo) | Toxicity (≥G2) (%) |
| Didolkar et al[17] | 85 | 15-30 Gy/3 | Sequential | 91.7 | - | 18.6 from diagnosis 8.6 from SBRT | 22 |
| Polistina et al[18] | 23 | 30 Gy/3 | Prior | 82.6 | 7.3 | 10.6 | None |
| Mahadevan et al[19] | 39 | 24-36 Gy/3 | Sequential | 85 | 15 | 20 from diagnosis | 9 |
| Schellenberg et al[20] | 16 | 25 Gy/1 | Sequential | 81 | 9 | 11.4 from diagnosis | 47 |
| Hoyer et al[21] | 22 | 45 Gy/3 | Sequential | 57 | 4.8 | 5.7 from diagnosis | 18 |
| Koong et al[22] | 15 | 15-25 Gy/1 | No | 77 | 2 | 11 from diagnosis | None |
| Chang et al[23] | 77 | 25 Gy/1 | Prior | 84 | - | 11.4 from diagnosis | 13 |
| Schellenberg et al[24] | 20 | 25 Gy/1 | Sequential | 94 | 9.2 | 11.8 from diagnosis | 20 |
| Rwigema et al[25] | 71 | 18-25 Gy/1 | No | 64.8 | - | 10.3 | 10 |
| Pollom et al[26] | 167 | 25-33 Gy/1-5 | Sequential or concurrent | - | - | 13.6 from diagnosis | 12.3 |
| Moningi et al[27] | 88 | 20-33 Gy/5 | Gemcitabine, cisplatin, FOLFIRINOX or paclitaxel | - | 9.8 | 18.4 from diagnosis | 5.7 G23.4 G3 |
| Gurka et al[28] | 38 | 25-30 Gy/5 | Gemcitabine, mFOLFOX or capecitabine | 79 | 9.2 | 14.3 from diagnosis | - |
| Present study | 25 | 30-36 Gy/3 or 42-48Gy/4 | 4 patients, Gemcitabine | - | - | M0 group 13.5 M1 group 8.5 from SBRT | 4 |
We investigated the outcomes in a series of patients with locally advanced unresectable and metastatic pancreatic cancer who underwent SBRT. Radiotherapy comprised 30-36 Gy in three fractions or 40-48 Gy in four fractions, and the priority was to evaluate the safety of the surrounding normal tissue. According to the standard equation, 30-36 Gy in three fractions has a biologically effective dose of 50-66 Gy, and 40-48 Gy in four fractions has a biologically effective dose of 68-88 Gy (assuming an α/β ratio of 10 for rapidly proliferating tumor cells and 3 for normal tissues). We found that the median OS was 9 mo. OS was 37% and 18% at one and two years, respectively. Palliative treatment with SBRT improved quality of life, especially palliation of pain, with acceptable toxicity. Our results support the use of palliative SBRT. The major advantages of this approach compared with conventional fractionated radiotherapy are: (1) more intensified treatment of the primary tumor; (2) increased patient convenience; and (3) minimal interference with the delivery of maximal systemic chemotherapy. We hypothesize that quality of life and OS benefit from local palliative SBRT for primary tumors, and large prospective clinical trials are warranted.
Locally advanced unresectable and metastatic pancreatic cancer is the most common presentation of pancreatic cancer. The available therapeutic option is chemotherapy or chemoradiotherapy. However, the low-dose radiation of conventional radiotherapy leads to unsatisfactory results for survival and local control, at a cost of increased hematologic toxicity. Doses > 54 Gy may be considered if clinically appropriate.
Stereotactic body radiotherapy (SBRT) with conformity and rapid dose fall-off has become an important research topic, to provide a higher biologically effective dose. It has been used to treat many cancers. The current research hotspot is to evaluate the efficacy and toxicity of SBRT for patients with locally advanced unresectable and metastatic pancreatic cancer.
This study presented outcomes in a series of patients with locally advanced unresectable and metastatic pancreatic cancer who underwent SBRT using the CyberKnife system. The radiotherapy plan was 30-36 Gy in three fractions or 40-48 Gy in four fractions. The dose-volume constraints for organs at risk were: duodenum, V 1 mL < 25 Gy; stomach and small bowel, V 1 mL < 25 Gy, and was strict with regard to keeping any 1 mL < 25 Gy; kidneys, 1/3 Vtot < 15 Gy; liver, total spared volume (Vtot - V 15 Gy) > 700 mL and V 15 Gy < 1/3 total volume; spinal cord, V 1 mL < 15 Gy, and strict with regard to keeping any 1 mL < 15 Gy. According to the standard equation, 30-36 Gy in three fractions has a relative biologic effectiveness of 50-66 Gy, and 40-48 Gy in four fractions has a biologically effective dose of 68-88 Gy (assuming an α/β ratio of 10 for rapidly proliferating tumor cells and 3 for normal tissues). The authors found that median overall survival was 9 mo. Overall survival was 37% and 18% at one and two years, respectively. Palliative treatment with SBRT was effective for pain relief (65%), with acceptable toxicity (grade 1: 20%, grade 2: 4%). These results support the use of palliative treatment with SBRT. The major advantages of this approach compared with conventional fractionated radiotherapy are: (1) more intensified treatment of the primary tumor; (2) increased patient convenience; and (3) minimal interference with the delivery of maximal systemic chemotherapy.
This study supports the use of palliative treatment with the CyberKnife for locally advanced unresectable and metastatic pancreatic cancer. It is remarkably effective in palliation of pain, with acceptable toxicity.
SBRT using the CyberKnife system is a promising noninvasive and palliative treatment with acceptable toxicity for locally advanced unresectable and metastatic pancreatic cancer.
This is an intriguing report on the experience with stereotactic body radiotherapy for locally advanced/metastatic pancreatic cancer, a topic of great interest for oncologists given the very difficult issue of local treatment/palliation in the setting of an aggressive histology with a high propensity to disseminate.
P- Reviewer: Ryoo JJ S- Editor: Qi Y L- Editor: AmEditor E- Editor: Zhang DN
| 1. | Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8406] [Cited by in RCA: 8970] [Article Influence: 690.0] [Reference Citation Analysis (0)] |
| 2. | Lin Y, Tamakoshi A, Wakai K, Kawamura T, Aoki R, Kojima M, Ohno Y. Descriptive epidemiology of pancreatic cancer in Japan. J Epidemiol. 1998;8:52-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 35] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 3. | Neoptolemos JP, Cunningham D, Friess H, Bassi C, Stocken DD, Tait DM, Dunn JA, Dervenis C, Lacaine F, Hickey H. Adjuvant therapy in pancreatic cancer: historical and current perspectives. Ann Oncol. 2003;14:675-692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 95] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 4. | Neoptolemos JP, Stocken DD, Bassi C, Ghaneh P, Cunningham D, Goldstein D, Padbury R, Moore MJ, Gallinger S, Mariette C. Adjuvant chemotherapy with fluorouracil plus folinic acid vs gemcitabine following pancreatic cancer resection: a randomized controlled trial. JAMA. 2010;304:1073-1081. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1144] [Cited by in RCA: 997] [Article Influence: 66.5] [Reference Citation Analysis (0)] |
| 5. | Oettle H, Post S, Neuhaus P, Gellert K, Langrehr J, Ridwelski K, Schramm H, Fahlke J, Zuelke C, Burkart C. Adjuvant chemotherapy with gemcitabine vs observation in patients undergoing curative-intent resection of pancreatic cancer: a randomized controlled trial. JAMA. 2007;297:267-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1779] [Cited by in RCA: 1763] [Article Influence: 97.9] [Reference Citation Analysis (0)] |
| 6. | Bilimoria KY, Bentrem DJ, Ko CY, Ritchey J, Stewart AK, Winchester DP, Talamonti MS. Validation of the 6th edition AJCC Pancreatic Cancer Staging System: report from the National Cancer Database. Cancer. 2007;110:738-744. [PubMed] |
| 7. | Small W, Berlin J, Freedman GM, Lawrence T, Talamonti MS, Mulcahy MF, Chakravarthy AB, Konski AA, Zalupski MM, Philip PA. Full-dose gemcitabine with concurrent radiation therapy in patients with nonmetastatic pancreatic cancer: a multicenter phase II trial. J Clin Oncol. 2008;26:942-947. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 121] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 8. | Shinchi H, Takao S, Noma H, Matsuo Y, Mataki Y, Mori S, Aikou T. Length and quality of survival after external-beam radiotherapy with concurrent continuous 5-fluorouracil infusion for locally unresectable pancreatic cancer. Int J Radiat Oncol Biol Phys. 2002;53:146-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 104] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 9. | Murphy JD, Adusumilli S, Griffith KA, Ray ME, Zalupski MM, Lawrence TS, Ben-Josef E. Full-dose gemcitabine and concurrent radiotherapy for unresectable pancreatic cancer. Int J Radiat Oncol Biol Phys. 2007;68:801-808. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 160] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 10. | Krishnan S, Rana V, Janjan NA, Varadhachary GR, Abbruzzese JL, Das P, Delclos ME, Gould MS, Evans DB, Wolff RA. Induction chemotherapy selects patients with locally advanced, unresectable pancreatic cancer for optimal benefit from consolidative chemoradiation therapy. Cancer. 2007;110:47-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 192] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 11. | Huguet F, Girard N, Guerche CS, Hennequin C, Mornex F, Azria D. Chemoradiotherapy in the management of locally advanced pancreatic carcinoma: a qualitative systematic review. J Clin Oncol. 2009;27:2269-2277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 185] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 12. | Loehrer PJ, Feng Y, Cardenes H, Wagner L, Brell JM, Cella D, Flynn P, Ramanathan RK, Crane CH, Alberts SR. Gemcitabine alone versus gemcitabine plus radiotherapy in patients with locally advanced pancreatic cancer: an Eastern Cooperative Oncology Group trial. J Clin Oncol. 2011;29:4105-4112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 578] [Cited by in RCA: 620] [Article Influence: 44.3] [Reference Citation Analysis (0)] |
| 13. | Conroy T, Paillot B, François E, Bugat R, Jacob JH, Stein U, Nasca S, Metges JP, Rixe O, Michel P. Irinotecan plus oxaliplatin and leucovorin-modulated fluorouracil in advanced pancreatic cancer--a Groupe Tumeurs Digestives of the Federation Nationale des Centres de Lutte Contre le Cancer study. J Clin Oncol. 2005;23:1228-1236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 165] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 14. | Conroy T, Desseigne F, Ychou M, Bouché O, Guimbaud R, Bécouarn Y, Adenis A, Raoul JL, Gourgou-Bourgade S, de la Fouchardière C. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364:1817-1825. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4838] [Cited by in RCA: 5628] [Article Influence: 402.0] [Reference Citation Analysis (1)] |
| 15. | Huguet F, André T, Hammel P, Artru P, Balosso J, Selle F, Deniaud-Alexandre E, Ruszniewski P, Touboul E, Labianca R. Impact of chemoradiotherapy after disease control with chemotherapy in locally advanced pancreatic adenocarcinoma in GERCOR phase II and III studies. J Clin Oncol. 2007;25:326-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 402] [Cited by in RCA: 369] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 16. | Mukherjee S, Hurt CN, Bridgewater J, Falk S, Cummins S, Wasan H, Crosby T, Jephcott C, Roy R, Radhakrishna G. Gemcitabine-based or capecitabine-based chemoradiotherapy for locally advanced pancreatic cancer (SCALOP): a multicentre, randomised, phase 2 trial. Lancet Oncol. 2013;14:317-326. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 253] [Cited by in RCA: 252] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 17. | Didolkar MS, Coleman CW, Brenner MJ, Chu KU, Olexa N, Stanwyck E, Yu A, Neerchal N, Rabinowitz S. Image-guided stereotactic radiosurgery for locally advanced pancreatic adenocarcinoma results of first 85 patients. J Gastrointest Surg. 2010;14:1547-1559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 85] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 18. | Polistina F, Costantin G, Casamassima F, Francescon P, Guglielmi R, Panizzoni G, Febbraro A, Ambrosino G. Unresectable locally advanced pancreatic cancer: a multimodal treatment using neoadjuvant chemoradiotherapy (gemcitabine plus stereotactic radiosurgery) and subsequent surgical exploration. Ann Surg Oncol. 2010;17:2092-2101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 128] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 19. | Mahadevan A, Miksad R, Goldstein M, Sullivan R, Bullock A, Buchbinder E, Pleskow D, Sawhney M, Kent T, Vollmer C. Induction gemcitabine and stereotactic body radiotherapy for locally advanced nonmetastatic pancreas cancer. Int J Radiat Oncol Biol Phys. 2011;81:e615-e622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 172] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 20. | Schellenberg D, Goodman KA, Lee F, Chang S, Kuo T, Ford JM, Fisher GA, Quon A, Desser TS, Norton J. Gemcitabine chemotherapy and single-fraction stereotactic body radiotherapy for locally advanced pancreatic cancer. Int J Radiat Oncol Biol Phys. 2008;72:678-686. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 243] [Cited by in RCA: 253] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 21. | Hoyer M, Roed H, Sengelov L, Traberg A, Ohlhuis L, Pedersen J, Nellemann H, Kiil Berthelsen A, Eberholst F, Engelholm SA. Phase-II study on stereotactic radiotherapy of locally advanced pancreatic carcinoma. Radiother Oncol. 2005;76:48-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 236] [Cited by in RCA: 262] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 22. | Koong AC, Le QT, Ho A, Fong B, Fisher G, Cho C, Ford J, Poen J, Gibbs IC, Mehta VK. Phase I study of stereotactic radiosurgery in patients with locally advanced pancreatic cancer. Int J Radiat Oncol Biol Phys. 2004;58:1017-1021. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 326] [Cited by in RCA: 315] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 23. | Chang DT, Schellenberg D, Shen J, Kim J, Goodman KA, Fisher GA, Ford JM, Desser T, Quon A, Koong AC. Stereotactic radiotherapy for unresectable adenocarcinoma of the pancreas. Cancer. 2009;115:665-672. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 283] [Cited by in RCA: 295] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 24. | Schellenberg D, Kim J, Christman-Skieller C, Chun CL, Columbo LA, Ford JM, Fisher GA, Kunz PL, Van Dam J, Quon A. Single-fraction stereotactic body radiation therapy and sequential gemcitabine for the treatment of locally advanced pancreatic cancer. Int J Radiat Oncol Biol Phys. 2011;81:181-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 190] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 25. | Rwigema JC, Parikh SD, Heron DE, Howell M, Zeh H, Moser AJ, Bahary N, Quinn A, Burton SA. Stereotactic body radiotherapy in the treatment of advanced adenocarcinoma of the pancreas. Am J Clin Oncol. 2011;34:63-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 123] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 26. | Pollom EL, Alagappan M, von Eyben R, Kunz PL, Fisher GA, Ford JA, Poultsides GA, Visser BC, Norton JA, Kamaya A. Single- versus multifraction stereotactic body radiation therapy for pancreatic adenocarcinoma: outcomes and toxicity. Int J Radiat Oncol Biol Phys. 2014;90:918-925. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 27. | Moningi S, Dholakia AS, Raman SP, Blackford A, Cameron JL, Le DT, De Jesus-Acosta AM, Hacker-Prietz A, Rosati LM, Assadi RK. The Role of Stereotactic Body Radiation Therapy for Pancreatic Cancer: A Single-Institution Experience. Ann Surg Oncol. 2015;22:2352-2358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 126] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 28. | Gurka MK, Kim C, He AR, Charabaty A, Haddad N, Turocy J, Johnson L, Jackson P, Weiner LM, Marshall JL. Stereotactic Body Radiation Therapy (SBRT) Combined With Chemotherapy for Unresected Pancreatic Adenocarcinoma. Am J Clin Oncol. 2014;Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 54] [Article Influence: 6.8] [Reference Citation Analysis (0)] |