Published online Jul 14, 2015. doi: 10.3748/wjg.v21.i26.8089
Peer-review started: November 30, 2014
First decision: January 8, 2015
Revised: February 4, 2015
Accepted: March 30, 2015
Article in press: March 31, 2015
Published online: July 14, 2015
Processing time: 225 Days and 16.7 Hours
AIM: To explore the mechanism of protection against acetaminophen-induced acute liver injury by Liuweiwuling tablets.
METHODS: Intraperitoneal injections of acetaminophen (250 mg/kg) were used to induce acute liver injury in male C57BL/6 mice. A total of 24 healthy mice were randomly assigned to two groups: an acute liver injury group (control group) and a Liuweiwuling tablet group. Mice were given Liuweiwuling tablets or a vehicle (PBS) orally prior to the administration of acetaminophen. Serum alanine aminotransferase (ALT) and aspartate aminotransaminase (AST) levels were measured at different time points within one week, and pathological examinations of liver tissues were performed 36 h after induction of acute liver injury. Serum inflammatory cytokines, such as high mobility group box protein B1 (HMGB1), tumor necrosis factor (TNF)-α and interleukin IL-1β, were detected using an ELISA method according to the manufacturer’s instructions. Hepatic morphological changes at 36 h were assessed by hematoxylin and eosin staining. Expression of proliferating cell nuclear antigen (PCNA) in liver tissue was determined by Western blot analysis. The mRNA levels of hepatocyte proliferation markers (PCNA, CyclinD1 and p21) were detected by real-time quantitative reverse transcription-polymerase chain reaction.
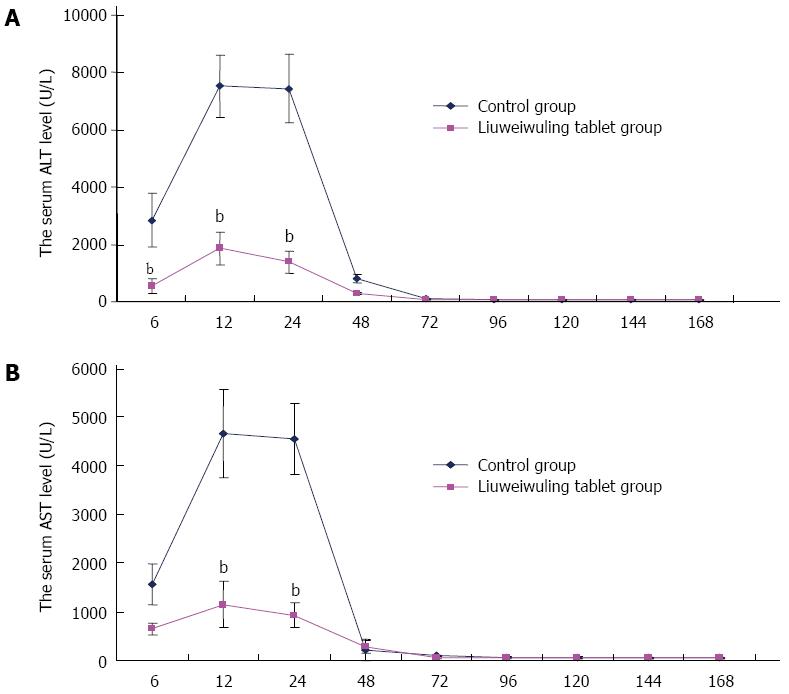
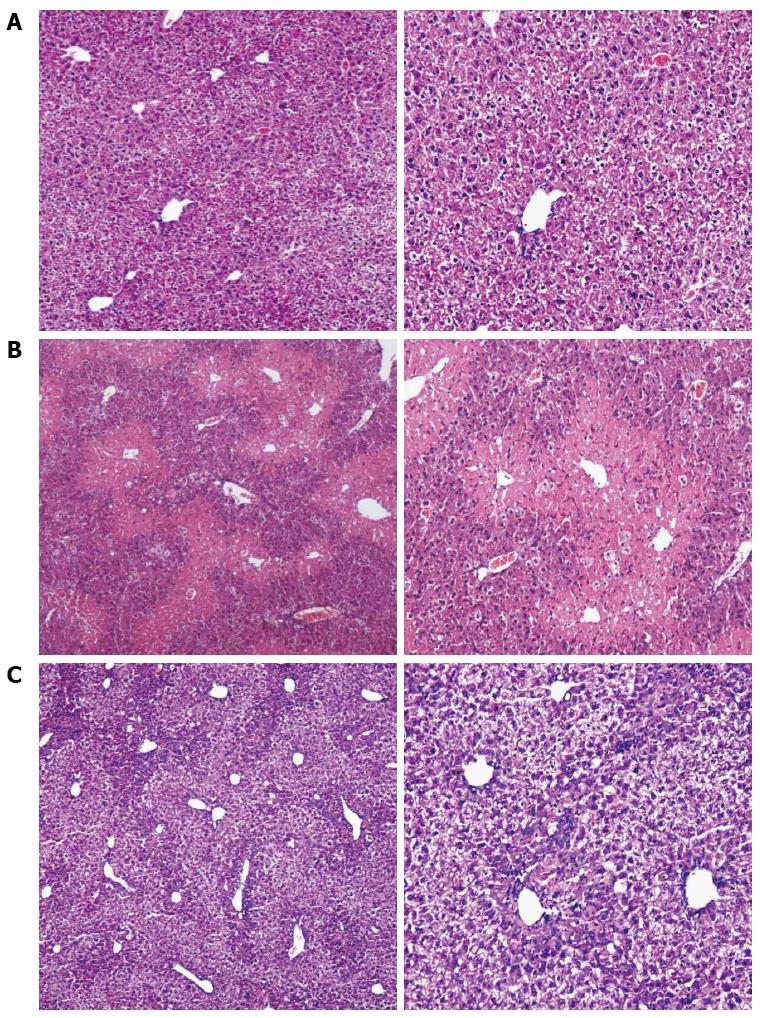
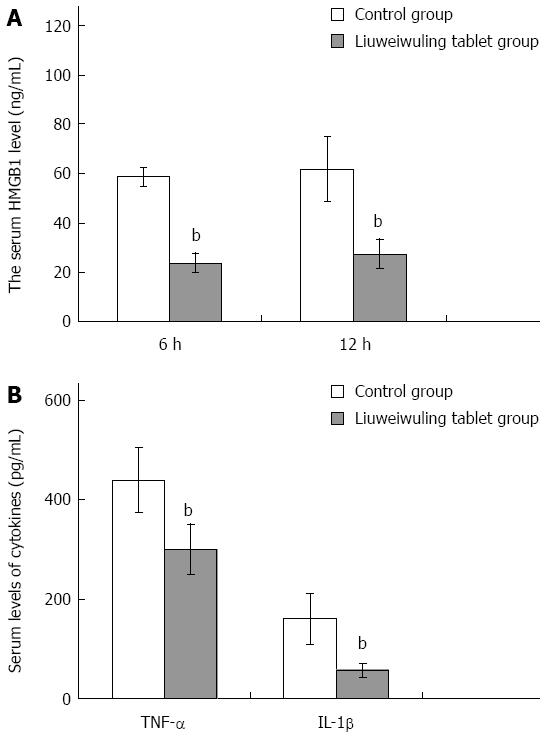
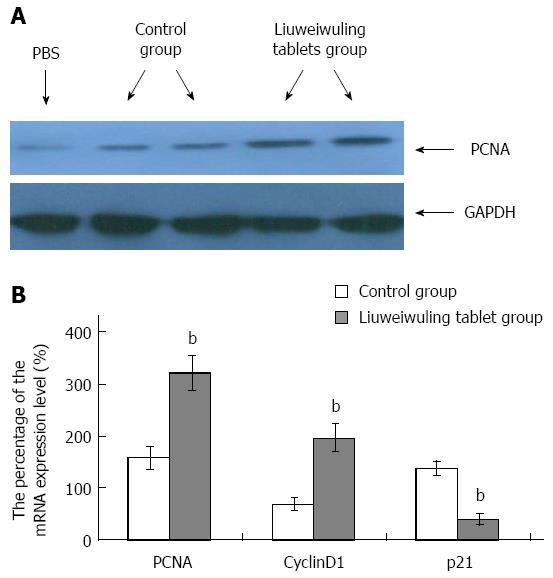
RESULTS: The levels of ALT/AST in the Liuweiwuling tablet group were decreased significantly at 6, 12 and 24 h compared to that of the control group (654.38 ± 120.87 vs 1566.17 ± 421.64, 1154.18 ± 477.72 vs 4654.84 ± 913.71 and 935.13 ± 252.34 vs 4553.75 ± 727.37, P < 0.01). Serum HMGB1 levels at 6 and 12 h for the Liuweiwuling tablet group were significantly lower than those of the control group (23.49 ± 3.89 vs 58.6 ± 3.65, 61.62 ± 13.07 vs 27.32 ± 5.97, P < 0.01). Furthermore, serum TNF-α and IL-1β levels at 12 h in the Liuweiwuling tablet group were also significantly lower than those of the control group (299.35 ± 50.61 vs 439.03 ± 63.59, 57.42 ± 12.98 vs 160.07 ± 49.87, P < 0.01). Centrilobular necrosis was evident in liver tissue of mice with acetaminophen-induced acute liver injury, but was almost abolished in the Liuweiwuling tablet group. The expression levels of PCNA and CyclinD1 were up-regulated in liver tissue in the Liuweiwuling tablet group (321.08 ± 32.87 vs 157.91 ± 21.52, 196.37 ± 25.39 vs 68.72 ± 11.27, P < 0.01); however, expression of p21 in liver tissue was down-regulated compared to that of the control group (40.26 ± 9.97 vs 138.24 ± 13.66, P < 0.01).
CONCLUSION: Liuweiwuling tablets can attenuate acute liver injury by decreasing inflammatory cytokine (HMGB1, TNF-α and IL-1β) levels and promoting liver regeneration.
Core tip: Clinical studies have shown that Liuweiwuling tablets are effective against a variety of liver injuries; however, its mechanism has not been established, especially for drug-induced liver injury. In this study, we found that Liuweiwuling tablets can attenuate acetaminophen-induced acute liver injury by decreasing inflammatory cytokine levels and promoting liver regeneration. Our results provide direct evidence for the effective therapy of liver damage with Liuweiwuling tablets and their value in clinical applications. Moreover, this is the first report showing that Liuweiwuling tablets can promote liver regeneration.
- Citation: Lei YC, Li W, Luo P. Liuweiwuling tablets attenuate acetaminophen-induced acute liver injury and promote liver regeneration in mice. World J Gastroenterol 2015; 21(26): 8089-8095
- URL: https://www.wjgnet.com/1007-9327/full/v21/i26/8089.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i26.8089
China’s ageing population is growing, and consequently, the medical demand is increasing. The irrational use of medicines and health products (including traditional Chinese medicine) and the incidence of drug-induced liver injury are also increasing. Therefore, it is important that better treatments are found to combat this disease. Clinical studies have demonstrated that Liuweiwuling tablets are effective against a variety of acute or chronic liver injuries that are caused by drugs, viruses and alcohol. Furthermore, they are also effective in preventing the progression of hepatic fibrosis and cirrhosis, and when combined with antivirals, may produce improved clinical effects[1-4]. However, the mechanism of the effect of Liuweiwuling tablets in the treatment of liver injury has not been established, especially for drug-induced liver injury. Research has shown that Liuweiwuling tablets can decrease alkaline phosphatase levels in mice with acetaminophen (APAP)-induced liver injury. The purpose of this study was to provide a better understanding of the mechanism of Liuweiwuling tablets in protecting hepatocytes from liver injury. By using an APAP-induced liver injury mouse model, we showed that Liuweiwuling tablets can protect hepatocytes from liver injury by decreasing the release of inflammatory cytokines, such as high mobility group box protein B1 (HMGB1), tumor necrosis factor (TNF)-α and interleukin IL-1β, and promoting liver regeneration.
Six- to eight-week-old male C57BL/6 mice (weighing 20 ± 0.5 g) were purchased from Hunan Slack King of Laboratory Animal Co. Ltd (Changsha, China). The mice were maintained in a specific pathogen-free facility under controlled laboratory conditions (23 °C, 12 h light/12 h dark cycle, 50% humidity, ad libitum access to food and water) for two weeks prior to experimentation and were treated humanely. All animal-related procedures were performed in the animal experiment center of Nanchang University and approved by the animal care and use committee of the Zhejiang Hospital. Animal breeding and processing were all in strict accordance with the laboratory animal breeding and user guide issued by the National Institutes of Health (NIH). Intragastric gavage was performed on conscious animals using straight gavage needles that were appropriate for the animal size (20 g body weight: 22 gauge, 1 inch length, 1.25 mm ball diameter). Reagents used included APAP (Sigma, United States), Trizol reagent (Invitrogen, United States) and rabbit anti-mouse PCNA polyclonal antibody (ABGENT, United States).
A total of 24 male C57BL/6 mice were subjected to 12 h of fasting, but were permitted to drink water before the trial commenced. The mice were randomly assigned to two groups, an acute liver injury group (control group) and a Liuweiwuling tablet group. Each group consisted of 12 mice. The acute liver injury group was administered APAP 250 mg/kg, by an intraperitoneal injection, and the Liuweiwuling tablet group was given Liuweiwuling tablets (10.0 g/kg, 2 times per day by lavage) three days before the intraperitoneal injections of APAP. The acute liver injury group was given an equivalent amount of PBS by lavage in a corresponding time frame.
Mice were anesthetized thoroughly with ether, and orbital blood was collected at 6, 12, 24, and 48 h and at one week after intraperitoneal injections of APAP. Serum was collected and stored at -80 °C. Some animals were sacrificed and their liver tissues used for reverse transcription-polymerase chain reaction (RT-PCR) and immunoblotting assays at 36 and 48 h. The specimens were fixed with neutral buffered 10% formalin and subjected to hematoxylin and eosin (HE) staining and immunohistochemical detection. All animals were euthanized by a barbiturate overdose (intravenous injection, 150 mg/kg pentobarbital sodium) for tissue collection.
Alanine aminotransferase (ALT) and aspartate aminotransaminase (AST) levels were measured with an automatic biochemical analyzer, while HMGB1, TNF-α and IL-1β were determined using an ELISA according to the manufacturer’s instructions. Results were calculated based on a standard curve.
Hepatic tissue mRNA expression levels were detected by quantitative RT-PCR among those mice with acute liver failure at 36 h. Total RNA extraction was conducted using Trizol reagent (Invitrogen, United States) in accordance with the instructions provided with the reagent. RT-PCR primer sequences were as follows: PCNA: forward, 5’-AGCCACATTGGAGATGCTGTAGCCGTATTCA-3’, reverse, 5’-AAGTTCCCATTGCCAAGCTCTCC-3’; CyclinD1: forward, 5’-GCTGCAAATGGAACTGCTTCTGGT-3’, reverse, 5’-TACCATGGAGGGTGGGTTGGAAAT-3’; GAPDH, forward, 5’-GTTGTCTCCTGCGACTTCA-3’, reverse, 5’-GGTGGTCCAGGGTTTCTTA-3’. A real-time quantitative PCR detection system (produced by Roche) was used to construct a standard curve. Results were standardized using GAPDH, and relative amounts were then calculated according to the 2ΔΔCT method[5].
Hepatic tissue (0.5 g) was ground to provide a 1 mL homogenate (including a protease inhibitor cocktail), which was diluted 20-fold with the RIPA efficient cracking liquid and then transferred to a nitrocellulose membrane after polyacrylamide gel electrophoresis. After blocking overnight with 10% BSA at 4 °C, the membrane was incubated with primary antibodies for 2 h at 37 °C, then with sheep anti-rabbit IgG antibody conjugated with horseradish peroxidase for 0.5 h at 37 °C. Finally, the target proteins were detected using a chemiluminescence method, and analysis of the grey levels was performed using the Quantity One software.
All data were statistically analyzed using the SPSS 18.0 software. Data are expressed as the mean ± SD, and the comparisons between the two groups were conducted by an independent sample t-test. A P-value less than 0.05 was considered significantly different.
Serum levels of ALT/AST in mice with APAP-induced acute liver injury began to increase at 6 h, reached a peak at 12 h and then recovered gradually at 48 h. The levels of ALT/AST in the Liuweiwuling tablets group were decreased significantly at 6, 12 and 24 h compared to those in the control group (P < 0.01) (Figure 1A and B).
Compared to the normal mice, the extent of the central necrosis of hepatic lobules in the acute liver injury group was greater, as a significant amount of necrosis was observed (Figure 2A and B), while less necrosis was observed in the liver tissue of the Liuweiwuling tablet group (Figure 2C).
Recently, HMGB1 has been shown to play an important role in the acute inflammation of the liver. Serum levels of HMGB1 in mice with APAP-induced acute liver injury began to increase at 6 h, reached a peak at 12 h and then declined at 24 h. Serum levels of HMGB1 at 6 and 12 h in the Liuweiwuling tablet group were all significantly lower than those in the control group (P < 0.01) (Figure 3A). In addition, the levels of TNF-α and IL-1β in the Liuweiwuling tablet group at 12 h were also significantly lower than those in the control group (P < 0.01) (Figure 3B).
To observe the influence of Liuweiwuling tablets on liver regeneration in mice with APAP-induced acute liver injury, immunoblotting assays were used to detect the expression of PCNA. The results demonstrated that the expression of PCNA in the Liuweiwuling tablet group was increased at 48 h compared to the control group (Figure 4A). The real-time quantitative RT-PCR detection of liver tissue mRNA expression levels showed that the levels of PCNA and CyclinD1 that promote liver cell regeneration in the Liuweiwuling tablet group were up-regulated 3.4-fold and 2.1-fold, respectively, while the level of p21 that inhibits liver cell regeneration was down-regulated 2.9-fold. These differences were significant when compared with the control group (P < 0.01) (Figure 4B).
The acute liver injury model produced by intraperitoneal injection of APAP in mice is a good simulation of drug-induced liver injury and is presently a mature model[6-8]. This study shows that male mice (C57BL/6) at the age of 6-8 wk and with weights of 20 ± 0.5 g are more ideal compared with female C57BL/6 and BALβ/c mice. Under the experimental conditions of this study, serum ALT/AST levels in mice with APAP-induced acute liver injury began to increase at 6 h, reached a peak at 12-24 h, and then recovered gradually at 48 h. HE staining at 36 h indicated necrosis in the central hepatic lobule in the acute liver injury group, which is in accordance with domestic and foreign research[9-11].
Recent studies indicate that HMGB1 plays a very important role in the inflammatory response of acute liver injury[12-16]. In this study, serum HMGB1 levels in mice with APAP-induced acute liver injury began to increase at 6 h, reached a peak at 12 h and then declined at 24 h. The serum HMGB1 levels at 6 and 12 h in the Liuweiwuling tablet group were all significantly lower than those of the control group. As observed with the HMGB1 levels, the levels of TNF-α, IL-1β and IL-6 in the Liuweiwuling tablet group were all significantly lower than those of the control group. The inflammatory factor HMGB1 can be released by stimulation of activated monocytes, macrophages, neutrophils and endothelial cells and can be passively released by necrotic cells[17-20]. HMGB1 can directly promote the release of inflammatory factors such as TNF-α and IL-1β. Furthermore, it can also promote the production of chemotactic factors that are released by peripheral blood mononuclear cells[21-23], increase the number of inflammatory cells and amplify the inflammatory response to aggravate liver damage. Recent studies indicate that TNF-α and IL-1β play an important role in APAP-induced acute liver injury[24-27]. Therapy with Liuweiwuling tablets inhibits the inflammatory response and liver tissue damage by decreasing the levels of HMGB1 and inflammatory factors TNF-α and IL-1β, leading to a decline of ALT/AST levels and an alleviation of liver inflammatory necrosis.
Modern pharmacological studies have shown that Schisandra chinensis, which is an ingredient in the Liuweiwuling tablet, is effective in reducing enzymes and in protecting the liver from chronic liver injury; fructus ligustri lucidi, forsythia and endives have a positive effect on reducing heat and removing toxicity for the liver; rhizoma zedoariae can desilt the extravasated blood and promote microcirculation; and ganoderma lucidum spore powder can enhance immunity and promote tissue repair. Therefore, we speculate that Liuweiwuling tablets can promote the regeneration of liver cells in acute liver injury. Previous studies have indicated that PCNA and CyclinD1 play important roles in liver cell regeneration; however, p21 inhibits liver cell regeneration[28-30]. This study shows that the expression of PCNA in the Liuweiwuling tablet group was increased at 48 h compared to a control group and that the levels of PCNA and CyclinD1 that promote liver cell regeneration in the Liuweiwuling tablet group were up-regulated 3.4-fold and 2.1-fold respectively, while the level of p21 that inhibits liver cell regeneration was down-regulated 2.9-fold. All of the above findings suggest that Liuweiwuling tablets can accelerate the regeneration of liver cells.
In conclusion, Liuweiwuling tablets can decrease the levels of ALT/AST and inflammatory factors (such as HMGB1, TNF-α and IL-1β) and promote liver regeneration. This may be one of the mechanisms involved in the treatment of acute liver damage. The above results regarding the effect of Liuweiwuling tablets on liver damage provide a new theoretical understanding and have important theoretical and application values.
Drug-induced liver injury is gradually increasing, and clinical studies have shown that Liuweiwuling tablets are effective against a variety of liver injuries caused by viruses, drugs and alcohol. However, the mechanism for the effectiveness of the treatment of liver injury has not been established, especially for drug-induced liver injury.
Existing research shows that Liuweiwuling tablets can decrease the alkaline phosphatase levels in mice with acetaminophen-induced liver injury.
Liuweiwuling tablets can attenuate acetaminophen (APAP)-induced acute liver injury by decreasing inflammatory cytokine levels and promoting liver regeneration. Moreover, this is the first report showing that Liuweiwuling tablets can promote liver regeneration.
The present results provide direct evidence for the effective therapy of liver damage with Liuweiwuling tablets and have value in clinical applications.
Previous studies have shown that Liuweiwuling tablets can be effective against a variety of liver injuries, but the mechanism is unknown. In this study, the authors found that Liuweiwuling tablets can decrease the extent of APAP-induced acute liver injury by decreasing the levels of inflammatory cytokines (high mobility group box protein B1, tumor necrosis factor-α and interleukin-1β) and by promoting liver cell regeneration. This is the first report that provides direct evidence for the effective therapy of liver damage with Liuweiwuling tablets and value in clinical applications.
P- Reviewer: Raff E, Zimmerman M S- Editor: Yu J L- Editor: Wang TQ E- Editor: Liu XM
| 1. | Xin SJ, Han J, Ding JB, Chen JJ, Wang RB, Li XH, Wang YD, Chen JM. Clinical study of Liuweiwuling tablet on patients with chronic hepatitis B. Zhongxiyi Jiehe Ganbing Zazhi. 2009;19:7-9. |
| 2. | Rong YH, Dong Z, Zhu B, You SL, Liu HL, Xin SJ. Therapeutic effect of Liuweiwuling tablet on alcoholic steato-hepatitis. Chuanranbing Xinxi. 2009;22:107-109. |
| 3. | Gao XJ, Li YL. Therapeutic effect of Liuweiwuling tablet on liver injury induced by anti-tuberculosis drugs. Zhonghua Ganzangbing Zazhi. 2012;4:9-11. |
| 4. | An J, Ni W, Qiao J. Therapeutic efficacy and quality of life investigation of traditional Chinese medicine-based therapy of chronic hepatitis B-related liver fibrosis. Zhonghua Ganzangbing Zazhi. 2014;22:30-32. [RCA] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 5. | Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149116] [Cited by in RCA: 133956] [Article Influence: 5581.5] [Reference Citation Analysis (1)] |
| 6. | Marques PE, Oliveira AG, Pereira RV, David BA, Gomides LF, Saraiva AM, Pires DA, Novaes JT, Patricio DO, Cisalpino D. Hepatic DNA deposition drives drug-induced liver injury and inflammation in mice. Hepatology. 2015;61:348-360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 140] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 7. | Lin Z, Wu F, Lin S, Pan X, Jin L, Lu T, Shi L, Wang Y, Xu A, Li X. Adiponectin protects against acetaminophen-induced mitochondrial dysfunction and acute liver injury by promoting autophagy in mice. J Hepatol. 2014;61:825-831. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 108] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 8. | Soeda J, Mouralidarane A, Ray S, Novelli M, Thomas S, Roskams T, Diehl AM, Oben JA. The β-adrenoceptor agonist isoproterenol rescues acetaminophen-injured livers through increasing progenitor numbers by Wnt in mice. Hepatology. 2014;60:1023-1034. [PubMed] |
| 9. | Singhal R, Ganey PE, Roth RA. Complement activation in acetaminophen-induced liver injury in mice. J Pharmacol Exp Ther. 2012;341:377-385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 34] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 10. | Saberi B, Ybanez MD, Johnson HS, Gaarde WA, Han D, Kaplowitz N. Protein kinase C (PKC) participates in acetaminophen hepatotoxicity through c-jun-N-terminal kinase (JNK)-dependent and -independent signaling pathways. Hepatology. 2014;59:1543-1554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 73] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 11. | Ni HM, Bockus A, Boggess N, Jaeschke H, Ding WX. Activation of autophagy protects against acetaminophen-induced hepatotoxicity. Hepatology. 2012;55:222-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 314] [Cited by in RCA: 355] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 12. | Erlandsson Harris H, Andersson U. Mini-review: The nuclear protein HMGB1 as a proinflammatory mediator. Eur J Immunol. 2004;34:1503-1512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 288] [Cited by in RCA: 303] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 13. | Zhou RR, Liu HB, Peng JP, Huang Y, Li N, Xiao MF, Wang H, Fan XG. High mobility group box chromosomal protein 1 in acute-on-chronic liver failure patients and mice with ConA-induced acute liver injury. Exp Mol Pathol. 2012;93:213-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 44] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 14. | Zhou RR, Zhao SS, Zou MX, Zhang P, Zhang BX, Dai XH, Li N, Liu HB, Wang H, Fan XG. HMGB1 cytoplasmic translocation in patients with acute liver failure. BMC Gastroenterol. 2011;11:21. [PubMed] |
| 15. | Wang LW, Wang LK, Chen H, Fan C, Li X, He CM, Gong ZJ. Ethyl pyruvate protects against experimental acute-on-chronic liver failure in rats. World J Gastroenterol. 2012;18:5709-5718. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 25] [Cited by in RCA: 23] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 16. | Craig DG, Lee P, Pryde EA, Masterton GS, Hayes PC, Simpson KJ. Circulating apoptotic and necrotic cell death markers in patients with acute liver injury. Liver Int. 2011;31:1127-1136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 71] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 17. | Tsung A, Sahai R, Tanaka H, Nakao A, Fink MP, Lotze MT, Yang H, Li J, Tracey KJ, Geller DA. The nuclear factor HMGB1 mediates hepatic injury after murine liver ischemia-reperfusion. J Exp Med. 2005;201:1135-1143. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 845] [Cited by in RCA: 939] [Article Influence: 47.0] [Reference Citation Analysis (0)] |
| 18. | Wang H, Bloom O, Zhang M, Vishnubhakat JM, Ombrellino M, Che J, Frazier A, Yang H, Ivanova S, Borovikova L. HMG-1 as a late mediator of endotoxin lethality in mice. Science. 1999;285:248-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2606] [Cited by in RCA: 2703] [Article Influence: 104.0] [Reference Citation Analysis (0)] |
| 19. | Scaffidi P, Misteli T, Bianchi ME. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature. 2002;418:191-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3079] [Cited by in RCA: 3285] [Article Influence: 142.8] [Reference Citation Analysis (0)] |
| 20. | Bonaldi T, Talamo F, Scaffidi P, Ferrera D, Porto A, Bachi A, Rubartelli A, Agresti A, Bianchi ME. Monocytic cells hyperacetylate chromatin protein HMGB1 to redirect it towards secretion. EMBO J. 2003;22:5551-5560. [PubMed] |
| 21. | Andersson U, Wang H, Palmblad K, Aveberger AC, Bloom O, Erlandsson-Harris H, Janson A, Kokkola R, Zhang M, Yang H. High mobility group 1 protein (HMG-1) stimulates proinflammatory cytokine synthesis in human monocytes. J Exp Med. 2000;192:565-570. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1100] [Cited by in RCA: 1173] [Article Influence: 46.9] [Reference Citation Analysis (0)] |
| 22. | Raucci A, Palumbo R, Bianchi ME. HMGB1: a signal of necrosis. Autoimmunity. 2007;40:285-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 142] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 23. | Krenkel O, Mossanen JC, Tacke F. Immune mechanisms in acetaminophen-induced acute liver failure. Hepatobiliary Surg Nutr. 2014;3:331-343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 108] [Reference Citation Analysis (0)] |
| 24. | Steinebrunner N, Mogler C, Vittas S, Hoyler B, Sandig C, Stremmel W, Eisenbach C. Pharmacologic cholinesterase inhibition improves survival in acetaminophen-induced acute liver failure in the mouse. BMC Gastroenterol. 2014;14:148. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 25. | Liu Z, Meng F, Li C, Zhou X, Zeng X, He Y, Mrsny RJ, Liu M, Hu X, Hu JF. Human umbilical cord mesenchymal stromal cells rescue mice from acetaminophen-induced acute liver failure. Cytotherapy. 2014;16:1207-1219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 55] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 26. | Leise MD, Poterucha JJ, Talwalkar JA. Drug-induced liver injury. Mayo Clin Proc. 2014;89:95-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 255] [Cited by in RCA: 278] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 27. | Hsu MK, Qiao L, Ho V, Zhang BH, Zhang H, Teoh N, Dent P, Farrell GC. Ethanol reduces p38 kinase activation and cyclin D1 protein expression after partial hepatectomy in rats. J Hepatol. 2006;44:375-382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 28. | Kawaguchi T, Kodama T, Hikita H, Tanaka S, Shigekawa M, Nawa T, Shimizu S, Li W, Miyagi T, Hiramatsu N. Carbamazepine promotes liver regeneration and survival in mice. J Hepatol. 2013;59:1239-1245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 29. | Toshima T, Shirabe K, Fukuhara T, Ikegami T, Yoshizumi T, Soejima Y, Ikeda T, Okano S, Maehara Y. Suppression of autophagy during liver regeneration impairs energy charge and hepatocyte senescence in mice. Hepatology. 2014;60:290-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 85] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 30. | Fan X, Jiang Y, Wang Y, Tan H, Zeng H, Wang Y, Chen P, Qu A, Gonzalez FJ, Huang M. Wuzhi tablet (Schisandra Sphenanthera extract) protects against acetaminophen-induced hepatotoxicity by inhibition of CYP-mediated bioactivation and regulation of NRF2-ARE and p53/p21 pathways. Drug Metab Dispos. 2014;42:1982-1990. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 52] [Article Influence: 4.7] [Reference Citation Analysis (0)] |