Published online Jul 14, 2015. doi: 10.3748/wjg.v21.i26.8081
Peer-review started: February 6, 2015
First decision: April 13, 2015
Revised: April 26, 2015
Accepted: May 20, 2015
Article in press: May 21, 2015
Published online: July 14, 2015
Processing time: 160 Days and 3.7 Hours
AIM: To evaluate preventative effects of ischemic preconditioning (IP) in a rat model of intestinal injury induced by ischemia-reperfusion (IR).
METHODS: Male Sprague-Dawley rats (250-300 g) were fasted for 24 h with free access to water prior to the operation. Eighteen rats were randomly divided into three experimental groups: S group (n = 6), rats were subjected to isolation of the superior mesenteric artery (SMA) for 40 min, then the abdomen was closed; IR group (n = 6), rats were subjected to clamping the SMA 40 min, and the abdomen was closed followed by a 4-h reperfusion; IP group (n = 6) rats underwent three cycles of 5 min ischemia and 5 min reperfusion, then clamping of the SMA for 40 min, then the abdomen was closed and a 4-h reperfusion followed. All animals were euthanized by barbiturate overdose (150 mg/kg pentobarbital sodium, i.v.) for tissue collection, and the SMA was isolated via median abdominal incision. Intestinal histologic injury was observed. Malondialdehyde (MDA), myeloperoxidase (MPO) and tumor necrosis factor (TNF)-α concentrations in intestinal tissue were measured. Intercellular adhesion molecule (ICAM)-1 and vascular cell adhesion molecule (VCAM)-1 expression, as well as nuclear factor (NF)-κB activity and expression in intestinal tissue were also determined.
RESULTS: Compared with the IR group, IP reduced IR-induced histologic injury of the intestine in rats (2.00 ± 0.71 vs 3.60 ± 0.84, P < 0.05). IP significantly inhibited the increase in MDA content (5.6 ± 0.15 μmol/L vs 6.84 ± 0.18 μmol/L, P < 0.01), MPO activity (0.13 ± 0.01 U/L vs 0.24 ± 0.01 U/L, P < 0.01), and TNF-α levels (7.79 ± 2.35 pg/mL vs 10.87 ± 2.48 pg/mL, P < 0.05) in the intestinal tissue of rats. IP also markedly ameliorated the increase in ICAM-1 (204.67 ± 53.27 vs 353.33 ± 45.19, P < 0.05) and VCAM-1 (256.67 ± 58.59 vs 377.33 ± 41.42, P < 0.05) protein expression in the intestinal tissues. Additionally, IP remarkably decreased NF-κB activity (0.48 ± 0.16 vs 0.76 ± 0.22, P < 0.05) and protein expression (320.23 ± 38.16 vs 520.76 ± 40.53, P < 0.01) in rat intestinal tissue.
CONCLUSION: IP may protect against IR-induced intestinal injury by attenuation of the neutrophil-endothelial adhesion cascade via reducing ICAM-1 and VCAM-1 expression and TNF-α-induced NF-κB signaling pathway activity.
Core tip: Although intestinal ischemic preconditioning (IP) triggers powerful protective effects, particularly in surgical ischemic operation and transplantation, mechanisms by which IP alleviates intestinal injury remain to be elucidated. The present study found that IP protects against intestinal ischemia-reperfusion-induced injury by reducing intercellular adhesion molecule-1 and vascular cell adhesion molecule-1 expression, and tumor necrosis factor-α-induced nuclear factor-κB signaling pathway activity. The results suggest that intestinal IP may be clinically useful in the future to treat patients with intestinal ischemia-reperfusion-induced injury.
- Citation: Ji YY, Wang ZD, Wang SF, Wang BT, Yang ZA, Zhou XR, Lei NN, Yue WN. Ischemic preconditioning ameliorates intestinal injury induced by ischemia-reperfusion in rats. World J Gastroenterol 2015; 21(26): 8081-8088
- URL: https://www.wjgnet.com/1007-9327/full/v21/i26/8081.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i26.8081
The intestine is more sensitive to the injury induced by ischemia-reperfusion (IR) than other internal organs[1]. Ischemia followed by reperfusion injury contributes to a complex series of events, including nitric oxide imbalance, neutrophil accumulation, cell apoptosis, and activation of inflammatory mediators, causing cellular and tissue damage[2,3]. Intestinal IR injury, which can been presented in several clinical settings such as intestinal obstruction, hemorrhagic shock, intestinal transplantation, and neonatal necrotizing enterocolitis, may result in local and remote organ damage including systemic inflammatory response syndromes and multiple organ dysfunction syndrome[4].
Various methods with protective effects, such as ischemic preconditioning (IP), pharmacologic treatment, and chemical agents, have been applied to decrease intestinal IR injury. IP refers to a phenomenon in which a tissue or organ is rendered resistant to the deleterious effects of prolonged IR by prior exposure to brief periods of vascular occlusion, displaying beneficial effects locally and systemically, reducing mucosal damage, neutrophil accumulation, cell apoptosis, and activation of inflammatory mediators. IP was first proposed as a possible therapeutic strategy in 1986 by Murry et al[5], who showed it reduced infarct size in rat heart. With respect to intestinal IP, Hotter et al[6] first reported the protection of IP from intestinal IR injury in 1996. Intestinal IP, repetitive brief periods of ischemia, provides a way of protecting intestinal tissue from damage inflicted by IR.
Intestinal IP has been widely investigated in recent years, ameliorating neutrophil sequestration, attenuating cell apoptosis, and decreasing inflammatory mediator release such as tumor necrosis factor (TNF)-α and interleukin-1[7-10]. However, the protective mechanisms of intestinal IP remain poorly understand. Therefore, the aim of this study was to determine the effects of IP in a rat model of intestinal IR by assessing histologic injury, oxygen-free radical injury [malondialdehyde (MDA)], neutrophil infiltration [myeloperoxidase (MPO)], inflammatory response (TNF-α), expression of endothelial cell adhesion molecules [intercellular adhesion molecule (ICAM)-1 and vascular cell adhesion molecule (VCAM)-1], and nuclear factor (NF)-κB activity and expression.
All animal experiments were carried out in accordance with the Animal Care Committee of Xi’an Jiaotong University, China. Eighteen male Sprague-Dawley rats (250-300 g) were purchased from the animal center of Xi’an Jiaotong University and housed under pathogen-free conditions. The animal protocol was designed to minimize pain or discomfort to the animals. The animals were acclimatized to laboratory conditions (23 °C, 12 h/12 h light/dark cycle, 50% humidity, ad libitum access to food and water) for 2 wk prior to experimentation. Animals were fasted 24 h and with free access to water prior to the operation. All animals were euthanized by barbiturate overdose (150 mg/kg pentobarbital sodium, i.v.) for tissue collection following isolation of the superior mesenteric artery (SMA) via median abdominal incision, as previously described[4].
The study was reviewed and approved by the Institutional Review Board of Xi’an Jiaotong University. Animals were randomly assigned to one of three groups: S group (n = 6), rats were subjected to isolation of the SMA for 40 min, then the abdomen was closed; IR group (n = 6), rats were subjected to clamping of the SMA for 40 min, and the abdomen was closed followed by a 4-h reperfusion; IP group (n = 6), rats underwent three cycles of 5 min ischemia and 5 min reperfusion, then clamping of the SMA for 40 min, then the abdomen was closed, followed by a 4-h reperfusion, as previously described[4].
Intestinal tissues (1.0-cm segments from 5 cm of the terminal ileum) were harvested, frozen immediately, and stored at -80 °C until assessment. A homogenate of 10% (w/v) was prepared. MDA level in intestinal tissues was determined by an MDA detection kit (Nanjing JianCheng Bioengineering Institute, Nanjing, China) following the colorimetric method provided by the manufacturer. The optical densities were read using a spectrophotometer at an absorbance of 532 nm. The results are expressed as μmol/L. Each sample was analyzed in duplicate and the results were averaged.
At the end of experiments, a 1.0-cm segment of intestinal tissue (from 5 cm of the terminal ileum) was harvested from each rat, frozen immediately, and stored at -80 °C until assessment. MPO, a mark of neutrophil accumulation and activation, was tested using a commercial assay kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, China). In brief, the intestinal tissues were homogenized in 50 mmol/L potassium phosphate buffer containing 0.5% hexadecyltrimethyl ammonium bromide (pH 6). The intestinal homogenates were centrifuged at 12500 g at 4 °C for 10 min. The supernatants were collected and reacted with 0.167 g/L o-dianisidine dihydrochloride, following 0.0005% H2O2 in 50 mmol/L phosphate buffer. Finally, the absorbance was determined spectrophotometrically at 460 nm.
A 1.0-cm segment intestinal tissue (from 5 cm of the terminal ileum) was harvested from each rat and frozen. Frozen tissues were homogenized and centrifuged at 1500 g for 15 min. Supernatants were transferred into fresh tubes for detection of TNF-α, determined using ELISA kits (R&D Systems, Minneapolis, MN, United States) according to manufacturer’s procedure. The absorbance was read at 450 nm by a microplate reader (Biotek Instruments, CA, United States). The results are expressed as pg/mL.
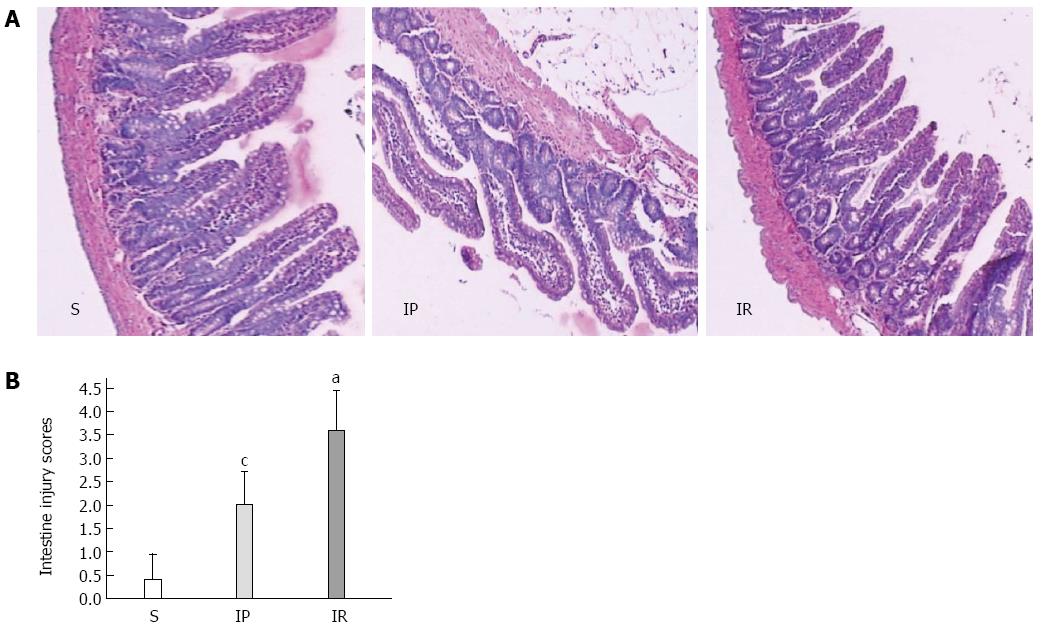
A 1.0-cm segment of intestinal tissue (from 5 cm of the terminal ileum) was harvested from each rat and fixed in 10% formaldehyde. The tissues were paraffin-embedded and then stained with hematoxylin and eosin for light microscopy. Intestinal mucosal damage was evaluated by three expert investigators blinded to the experimental groups using the criteria of Chiu et al[11]. A score scaled at 0 to 5 represents the severity: 0, normal mucosa villi; 1, development of subepithelial Gruenhagen’s space at the tip of villus; 2, extension of the subepithelial space with moderate epithelial lifting; 3, large epithelial lifting, possibly with a few denuded villi; 4, denuded villi with lamina propria and exposed capillaries; 5, disintegration of the lamina propria, ulceration, and hemorrhage.
NF-κB (p65) DNA-binding activity in the intestinal homogenate was tested using a commercial kit. The assay uses a 96-well plate coated with oligonucleotides containing the NF-κB-binding site. Activated NF-κB binds to the oligonucleotides and is tested by a specific antibody. The amount of active NF-κB was determined using a microplate reader (Biotek Instruments).
Protein samples (20 μg) were loaded onto SDS-PAGE gels and transferred onto a polyvinylidene difluoride membrane pretreated with 100% methanol (Bio-Rad Laboratories, Hercules, CA, United States). The membranes were blocked with 5% nonfat dry milk in Tris-buffered saline containing 0.1% Tween 20, and then rat monoclonal anti-ICAM-1 (1:500), VCAM-1 (1:500), and NF-κB (p65) (1:400) antibodies (Santa Cruz Biotechnology, Inc., Dallas, TX, United States) were added to the supernatant and the mixture was incubated on a rotating wheel at 4 °C overnight; β-actin (1:400) was used as a loading control. On the second day, membranes were washed with the same three times and incubated with a secondary antibody conjugated to horseradish peroxidase (1:2000; Santa Cruz Biotechnology, Inc.) for 1 h at room temperature, and the light signals were detected by X-ray film. Optical densities of the bands were scanned and quantified with the Syngene Gene Tools (Syngene Corp., Cambridge, United Kingdom). Three independent experiments were carried out to study protein expressions.
Data are expressed as mean ± SD, and were analyzed by analyses of variance followed by Tukey's post hoc tests (Prism 5; GraphPad Software, Inc., La Jolla, CA, United States). Differences were considered significant when P < 0.05.
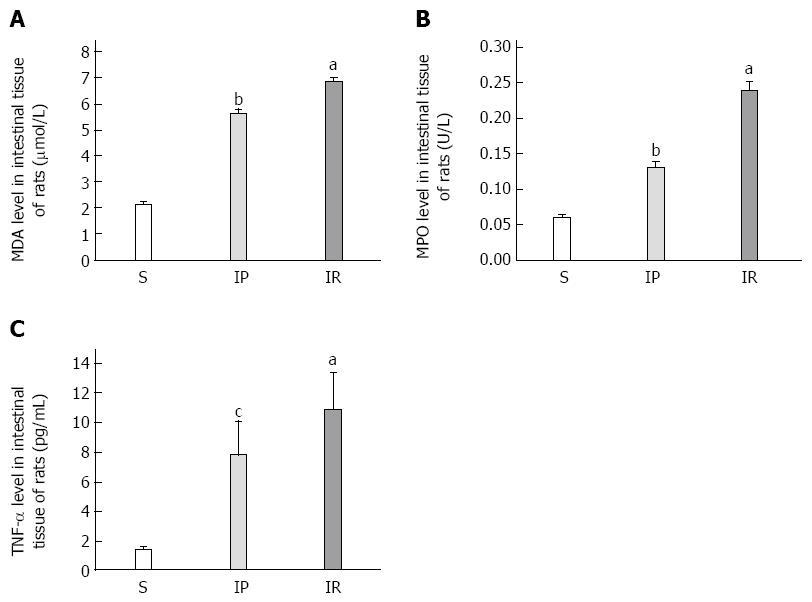
We examined whether IP attenuates intestinal tissue injury in rats. In this study, we characterized intestinal tissue injury using MDA levels tested 4 h after intestinal IP or IR. As shown in Figure 1A, intestinal tissue MDA levels in the IR group were significantly increased compared to the S group (6.84 ± 0.18 μmol/L vs 2.13 ± 0.11 μmol/L, P < 0.01). IP significantly decreased MDA levels compared to the IR group (5.6 ± 0.15 μmol/L vs 6.84 ± 0.18 μmol/L, P < 0.01).
We next evaluated whether IP decreases aggregation and adhesion of neutrophils in intestinal tissue. In this study, we tested intestinal MPO as marker of aggregation and adhesion of neutrophils. As shown in Figure 1B, MPO levels in the IR group were significantly increased compared to the S group (0.24 ± 0.01 U/L vs 0.06 ± 0.00 U/L, P < 0.01). IP significantly decreased MPO levels compared to the IR group (0.13 ± 0.01 U/L vs 0.24 ± 0.01 U/L, P < 0.01).
We then measured whether IP suppresses levels of proinflammatory molecules in intestinal tissue by measuring TNF-α. As shown in Figure 1C, intestinal tissue TNF-α levels in the IR group were significantly increased compared to the S group (10.87 ± 2.48 pg/mL vs 1.37 ± 0.21 pg/mL, P < 0.01). IP significantly decreased TNF-α levels compared to the IR group (7.79 ± 2.35 pg/mL vs 10.87 ± 2.48 pg/mL, P < 0.05).
We next investigated whether IP attenuates intestinal tissue injury assessed using the criteria of Chiu et al[11]. As shown in Figure 2, intestinal tissue injury in the IR group was significantly increased compared to the S group (3.60 ± 0.84 vs 0.40 ± 0.55, P < 0.01). IP significantly decreased intestinal tissue injury compared to the IR group (2.00 ± 0.71 vs 3.60 ± 0.84, P < 0.05).
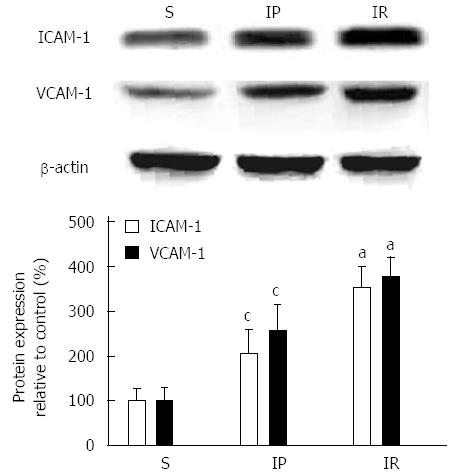
We next determined whether IP reduces the protein expression of intercellular adhesion molecules in intestinal tissue. As shown in Figure 3, ICAM-1 expression in the IR group was significantly increased compared to the S group (353.33 ± 45.19 vs 100.00 ± 25.00, P < 0.01). IP significantly decreased ICAM-1 protein expression compared to the IR group (204.67 ± 53.27 vs 353.33 ± 45.19, P < 0.05). Intestinal tissue VCAM-1 protein expression in the IR group was significantly increased compared to the S group (377.33 ± 41.42 vs 107.00 ± 15.72, P < 0.01). IP significantly decreased the expression of VCAM-1 compared to the IR group (256.67 ± 58.59 vs 377.33 ± 41.42, P < 0.05).
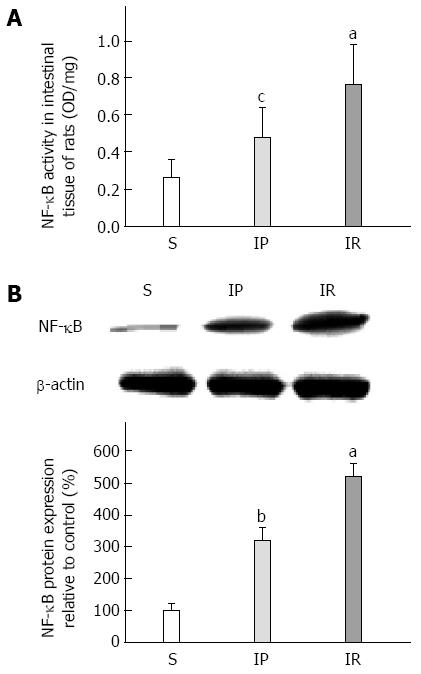
We next tested whether IP reduces the translocation of nuclear factors in intestinal tissue by measuring intestinal NF-κB activity and protein expression. As shown in Figure 4A, NF-κB activity in the IR group was significantly enhanced compared to the S group (0.76 ± 0.22 vs 0.26 ± 0.10, P < 0.01). IP dramatically decreased intestinal tissue NF-κB activity compared to the IR group (0.48 ± 0.16 vs 0.76 ± 0.22, P < 0.05). As shown in Figure 4B, intestinal tissue NF-κB protein expression in the IR group was significantly increased compared to the S group (320.23 ± 38.16 vs 100.03 ± 20.00, P < 0.01). IP dramatically decreased NF-κB expression compared to the IR group (320.23 ± 38.16 vs 520.76 ± 40.53, P < 0.01).
Prolonged IR rapidly causes substantial and irreversible intestinal tissue damage. Although restoration of blood flow to intestinal ischemic tissue is critical for tissue salvage, reperfusion also introduces inflammatory changes that exacerbate injury[12]. IP is a simple procedure conferring cytoprotection in critical organs that has clinical applications[13,14]. Although intestinal IP triggers powerful protective effects mechanisms by which it alleviates intestinal injury remain to be elucidated.
Hotter et al[6] demonstrated the protective benefits of IP against intestinal IR injury. IP also attenuates intestinal IR injury in a canine bowel transplantation model by decreasing oxidative stress[15]. Moreover, IP protects against intestinal IR injury via the mast cell degranulation-mediated release of mast cell carboxypeptidase A[1]. Furthermore, IP attenuates intestinal IR injury via actions in several signaling pathways, including suppression of heme oxygenase[16] and modulation of the arachidonic acid cascade[17].
Our previous study showed that intestinal IP attenuates the capacity of antioxygen-free radicals, inhibits the release of proinflammatory cytokines, and alleviates apoptosis in IR-caused lung injury in rats[4]. As serum MDA, MPO, and TNF-α levels in rats were tested, we further examined the levels of these proteins in intestinal tissue. The results presented here show that IP reduces intestinal injury induced by IR, observed as attenuated histologic injury and MDA, MPO, and TNF-α levels in the intestine, and suppressed expression of ICAM-1 and VCAM-1, along with reduced activity and expression of NF-κB activity.
Neutrophils are a critical component of the inflammatory response that characterizes intestinal IR[18,19]. Activated neutrophils, which infiltrate the intestine during IR, produce inflammatory mediators, including TNF-α, ICAM-1, and VCAM-1, in the development of intestinal IR by releasing neutrophil proteases and reactive oxygen species, and sequestering polymorphonuclear neutrophils (PMNs) into ischemic intestinal tissue[20-22].
Depletion or inhibition of PMNs decreases intestinal tissue injury in IR[23]. PMNs can aggregate together leading to a “no flow” phenomenon in ischemic tissue after reperfusion[10]. Moreover, PMNs could contribute to vasoconstriction and decreased blood flow via the release of potent cytotoxic cytokines[24]. In the present study, intestinal IR significantly induced infiltration of neutrophils or PMNs, as observed as and increased MPO level. Intestinal IP effectively decreased infiltration of neutrophils or PMNs, shown by decreased MPO levels. This suggests that IP attenuates infiltration of neutrophils or PMNs in intestinal IR induced injury.
TNF-α is a cell-signaling protein associated with systemic inflammation and is one of the cytokines that make up the acute-phase reaction. It is produced mainly by activated macrophages, neutrophils, and natural killer cells. The primary role of TNF-α is in the regulation of immune cells, and disruption of its production has been implicated in IR[25,26]. Moreover, TNF-α is a potent chemoattractant for neutrophils, and promotes the expression of adhesion molecules on endothelial cells, helping neutrophils migrate into tissue[27].
Intestinal injury induced by IR is thought to result from intestine-derived TNF-α. Moreover, TNF-α could upregulate neutrophil adhesion molecules in the intestine, particularly ICAM-1, which then plays a vital role in the influx of neutrophils in intestinal tissue[28,29]. In this study, intestinal IR induced TNF-α production, as observed as increased TNF-α levels, which were significantly reduced by intestinal IP.
Accumulating evidence demonstrates that leukocyte-endothelial adhesion is mediated by cell adhesion molecules on vascular endothelial cells and counterpart ligands on leukocytes, especially in IR[30,31]. ICAM-1 and VCAM-1 expressed on these cells are central for the adherence of neutrophils and monocytes to the endothelium[32]. Their expression is also induced by proinflammatory stimuli such as TNF-α. After intestinal IR, increased TNF-α levels in the intestinal tissue likely contributed to a mediator cascade, leading to neutrophil infiltration and the observed increases in ICAM-1 and VCAM-1 expression. Moreover, intestinal IP significantly decreased their expression. These data indicate that IP inhibits TNF-α production, and reduces ICAM-1 and VCAM-1 expression in an intestinal IR-induced acute inflammation reaction.
NF-κB is a critical transcription factor and is activated in intestinal IR, playing a key role in inflammatory responses[32]. Activation of NF-κB induces expression of multiple inflammation-related products, such as chemokines, cytokines, and adhesion molecules. Increased concentrations of these inflammatory proteins may result in intestinal injury. In general, NF-κB is composed of two polypeptides, a 50-kDa peptide (p50) and a 65-kDa peptide (p65). NF-κB is activated by a variety of signals associated with the etiology and pathophysiology of inflammation, including intracellular and/or extracellular stimuli such as TNF-α. Importantly, nuclear translocation of NF-κB (p65) allows it to bind to target promoter regions, including binding sites within the promoters for ICAM-1 and VCAM-1[33]. The data presented here indicate that intestinal IP significantly reduces TNF-α-induced activation and nuclear translocation of NF-κB as a molecular mechanism for protection against intestinal IR-induced injury.
The clinical application of IP involves a simple, safe, and tolerable procedure, with wide-ranging immunomodulatory effects. Recently, a randomized controlled trial showed that remote IP powerfully counteracts the injury to the intestinal mucosa caused by a period of ischemia and subsequent reperfusion during elective open infrarenal abdominal aortic aneurysm repair[13]. Although intestinal protection with IP is a reproducible and powerful phenomenon, it has not been readily translated to routine clinical use. Therefore, more clinical trials are needed to further investigate intestinal IP use during gastrointestinal surgeries.
Taken together, IP protects against intestinal IR-induced injury potentially by attenuating a neutrophil-endothelial adhesion cascade. The mechanism involves reducing ICAM-1 and VCAM-1 expression and TNF-α-induced NF-κB signaling pathway activity. The present study provides an insight into the cytoprotection of intestinal IP-reduced IR. These findings further support our contention that intestinal IP may be clinically useful in the future to treat patients at risk for intestinal IR-induced injury.
Intestinal ischemic preconditioning (IP) has been widely investigated in recent years. Prolonged ischemia-reperfusion (IR) induces morphologic changes in the intestine, attenuating cell apoptosis, ameliorating neutrophil sequestration, and decreasing inflammatory mediator release, such as of tumor necrosis factor (TNF)-α and interleukin-1. However, the protective mechanisms of intestinal IP remain poorly understand.
Although intestinal IP triggers a powerful protective effect, particularly in surgical ischemic operation and transplantation, mechanisms by which IP alleviates intestinal injury remain to be elucidated. The current research hotspot is to determine the effects of IP in a rat model of intestinal IR by analyzing data such as histologic injury, oxygen-free radical injury, neutrophil infiltration, levels of TNF-α, endothelial cell adhesion molecules expression, and nuclear factor (NF)-κB activity and expression.
IP protects against intestinal IR-induced injury potentially by attenuating the neutrophil-endothelial adhesion cascade via reducing expression of intercellular adhesion molecule-1 and vascular cell adhesion molecule-1 and TNF-α-induced NF-κB signaling pathway activity. The present study provides an insight into the cytoprotection of intestinal IP-reduced IR.
The study results suggest that intestinal IP may be clinically useful in the future to treat patients at risk for intestinal IR-induced injury.
IR is a process of ischemia followed by reperfusion injury, which contributes to a complex series of events, including nitric oxide imbalance, neutrophil accumulation, cell apoptosis, and activation of inflammatory mediators, thus causing cellular and tissue damage. IP refers to a phenomenon in which a tissue or organ is rendered resistant to these deleterious effects by prior exposure to brief periods of vascular occlusion.
Intestinal IP has been widely investigated in recent years, however, the protective mechanisms of intestinal IP remain poorly understand. The results of this well-designed study suggest that IP protects against IR-induced intestinal injury by attenuating of neutrophil-endothelial adhesion cascade via reducing expression of endothelial cell adhesion molecules (intercellular adhesion molecule-1 and vascular cell adhesion molecule-1) and TNF-α-induced NF-κB signaling pathway activity.
Animal care and use statement: The animal protocol was designed to minimize pain or discomfort to the animals. The animals were acclimatized to laboratory conditions (23 °C, 12h/12h light/dark cycle, 50% humidity, and
P- Reviewer: Qi Z S- Editor: Yu J L- Editor: AmEditor E- Editor: Liu XM
| 1. | Xing D, Zhang R, Li S, Huang P, Luo C, Hei Z, Xia Z, Gan X. Pivotal role of mast cell carboxypeptidase A in mediating protection against small intestinal ischemia-reperfusion injury in rats after ischemic preconditioning. J Surg Res. 2014;192:177-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 2. | Lv X, Wang ZM, Huang SD, Song SH, Wu FX, Yu WF. Emulsified isoflurane preconditioning reduces lung injury induced by hepatic ischemia/reperfusion in rats. Int J Med Sci. 2011;8:353-361. [PubMed] |
| 3. | Cho SS, Rudloff I, Berger PJ, Irwin MG, Nold MF, Cheng W, Nold-Petry CA. Remifentanil ameliorates intestinal ischemia-reperfusion injury. BMC Gastroenterol. 2013;13:69. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 40] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 4. | Wang Z, Ji Y, Wang S, Wang R, Li Z, Kang A, Xu H, Shi M, Zhao M. Protective effect of intestinal ischemic preconditioning on ischemia reperfusion-caused lung injury in rats. Inflammation. 2015;38:424-432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 5. | Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation. 1986;74:1124-1136. [PubMed] |
| 6. | Hotter G, Closa D, Prados M, Fernández-Cruz L, Prats N, Gelpí E, Roselló-Catafau J. Intestinal preconditioning is mediated by a transient increase in nitric oxide. Biochem Biophys Res Commun. 1996;222:27-32. [PubMed] |
| 7. | Stringa P, Romanin D, Lausada N, Machuca M, Raimondi JC, Cabanne A, Rumbo M, Gondolesi G. Ischemic preconditioning and tacrolimus pretreatment as strategies to attenuate intestinal ischemia-reperfusion injury in mice. Transplant Proc. 2013;45:2480-2485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 8. | Aban N, Cinel L, Tamer L, Aktas A, Aban M. Ischemic preconditioning reduces caspase-related intestinal apoptosis. Surg Today. 2005;35:228-234. [PubMed] |
| 9. | Cinel I, Avlan D, Cinel L, Polat G, Atici S, Mavioglu I, Serinol H, Aksoyek S, Oral U. Ischemic preconditioning reduces intestinal epithelial apoptosis in rats. Shock. 2003;19:588-592. [PubMed] |
| 10. | Mallick IH, Yang W, Winslet MC, Seifalian AM. Ischemia-reperfusion injury of the intestine and protective strategies against injury. Dig Dis Sci. 2004;49:1359-1377. [PubMed] |
| 11. | Chiu CJ, McArdle AH, Brown R, Scott HJ, Gurd FN. Intestinal mucosal lesion in low-flow states. I. A morphological, hemodynamic, and metabolic reappraisal. Arch Surg. 1970;101:478-483. [PubMed] |
| 12. | Wu MC, Brennan FH, Lynch JP, Mantovani S, Phipps S, Wetsel RA, Ruitenberg MJ, Taylor SM, Woodruff TM. The receptor for complement component C3a mediates protection from intestinal ischemia-reperfusion injuries by inhibiting neutrophil mobilization. Proc Natl Acad Sci USA. 2013;110:9439-9444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 124] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 13. | Li C, Li YS, Xu M, Wen SH, Yao X, Wu Y, Huang CY, Huang WQ, Liu KX. Limb remote ischemic preconditioning for intestinal and pulmonary protection during elective open infrarenal abdominal aortic aneurysm repair: a randomized controlled trial. Anesthesiology. 2013;118:842-852. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 94] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 14. | Li C, Xu M, Wu Y, Li YS, Huang WQ, Liu KX. Limb remote ischemic preconditioning attenuates lung injury after pulmonary resection under propofol-remifentanil anesthesia: a randomized controlled study. Anesthesiology. 2014;121:249-259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 74] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 15. | Ferencz A, Szántó Z, Kalmár-Nagy K, Horváth OP, Rõth E. Mitigation of oxidative injury by classic and delayed ischemic preconditioning prior to small bowel autotransplantation. Transplant Proc. 2004;36:286-288. [PubMed] |
| 16. | Mallick IH, Winslet MC, Seifalian AM. Ischemic preconditioning of small bowel mitigates the late phase of reperfusion injury: heme oxygenase mediates cytoprotection. Am J Surg. 2010;199:223-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 17. | Avgerinos ED, Kostopanagiotou G, Costopanagiotou C, Kopanakis N, Andreadou I, Lekka M, Nakos G, Smyrniotis V. Intestinal preconditioning ameliorates ischemia-reperfusion induced acute lung injury in rats: an experimental study. J Surg Res. 2010;160:294-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 18. | Grootjans J, Lenaerts K, Derikx JP, Matthijsen RA, de Bruïne AP, van Bijnen AA, van Dam RM, Dejong CH, Buurman WA. Human intestinal ischemia-reperfusion-induced inflammation characterized: experiences from a new translational model. Am J Pathol. 2010;176:2283-2291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 141] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 19. | Lu YZ, Wu CC, Huang YC, Huang CY, Yang CY, Lee TC, Chen CF, Yu LC. Neutrophil priming by hypoxic preconditioning protects against epithelial barrier damage and enteric bacterial translocation in intestinal ischemia/reperfusion. Lab Invest. 2012;92:783-796. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 42] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 20. | Gan X, Su G, Zhao W, Huang P, Luo G, Hei Z. The mechanism of sevoflurane preconditioning-induced protections against small intestinal ischemia reperfusion injury is independent of mast cell in rats. Mediators Inflamm. 2013;2013:378703. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 21. | Liu Y, Kalogeris T, Wang M, Zuidema MY, Wang Q, Dai H, Davis MJ, Hill MA, Korthuis RJ. Hydrogen sulfide preconditioning or neutrophil depletion attenuates ischemia-reperfusion-induced mitochondrial dysfunction in rat small intestine. Am J Physiol Gastrointest Liver Physiol. 2012;302:G44-G54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 47] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 22. | Xia G, Martin AE, Besner GE. Heparin-binding EGF-like growth factor downregulates expression of adhesion molecules and infiltration of inflammatory cells after intestinal ischemia/reperfusion injury. J Pediatr Surg. 2003;38:434-439. [PubMed] |
| 23. | Sun Z, Wang X, Lasson A, Böjesson A, Annborn M, Andersson R. Effects of inhibition of PAF, ICAM-1 and PECAM-1 on gut barrier failure caused by intestinal ischemia and reperfusion. Scand J Gastroenterol. 2001;36:55-65. [PubMed] |
| 24. | Sukhotnik I, Coran AG, Greenblatt R, Brod V, Mogilner J, Shiloni E, Shaoul R, Bitterman H. Effect of 100% oxygen on E-selectin expression, recruitment of neutrophils and enterocyte apoptosis following intestinal ischemia-reperfusion in a rat. Pediatr Surg Int. 2008;24:29-35. [PubMed] |
| 25. | Locksley RM, Killeen N, Lenardo MJ. The TNF and TNF receptor superfamilies: integrating mammalian biology. Cell. 2001;104:487-501. [PubMed] |
| 27. | Esposito E, Mazzon E, Muià C, Meli R, Sessa E, Cuzzocrea S. Splanchnic ischemia and reperfusion injury is reduced by genetic or pharmacological inhibition of TNF-alpha. J Leukoc Biol. 2007;81:1032-1043. [PubMed] |
| 28. | Olanders K, Sun Z, Börjesson A, Dib M, Andersson E, Lasson A, Ohlsson T, Andersson R. The effect of intestinal ischemia and reperfusion injury on ICAM-1 expression, endothelial barrier function, neutrophil tissue influx, and protease inhibitor levels in rats. Shock. 2002;18:86-92. [PubMed] |
| 29. | Spelman K, Aldag R, Hamman A, Kwasnik EM, Mahendra MA, Obasi TM, Morse J, Williams EJ. Traditional herbal remedies that influence cell adhesion molecule activity. Phytother Res. 2011;25:473-483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 30. | Xu H, Wang D, Peng C, Huang X, Ou M, Wang N, Wang P, Zhou L, Ye X. Rabbit sera containing compound danshen dripping pill attenuate leukocytes adhesion to TNF-alpha--activated human umbilical vein endothelial cells by suppressing endothelial ICAM-1 and VCAM-1 expression through NF-kappaB signaling pathway. J Cardiovasc Pharmacol. 2014;63:323-332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 31. | Hanusch C, Nowak K, Gill IS, Törlitz P, Rafat N, Mueller AM, Van Ackern KC, Yard B, Beck GC. Hypothermic preservation of lung allograft inhibits cytokine-induced chemoattractant-1, endothelial leucocyte adhesion molecule, vascular cell adhesion molecule-1 and intracellular adhesion molecule-1 expression. Clin Exp Immunol. 2007;149:364-371. [PubMed] |
| 32. | Zabot GP, Carvalhal GF, Marroni NP, Hartmann RM, da Silva VD, Fillmann HS. Glutamine prevents oxidative stress in a model of mesenteric ischemia and reperfusion. World J Gastroenterol. 2014;20:11406-11414. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 21] [Cited by in RCA: 30] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 33. | Kim SR, Bae YH, Bae SK, Choi KS, Yoon KH, Koo TH, Jang HO, Yun I, Kim KW, Kwon YG. Visfatin enhances ICAM-1 and VCAM-1 expression through ROS-dependent NF-kappaB activation in endothelial cells. Biochim Biophys Acta. 2008;1783:886-895. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 237] [Article Influence: 13.9] [Reference Citation Analysis (0)] |