Published online Jul 14, 2015. doi: 10.3748/wjg.v21.i26.8073
Peer-review started: December 21, 2014
First decision: January 22, 2015
Revised: February 11, 2015
Accepted: March 18, 2015
Article in press: March 19, 2015
Published online: July 14, 2015
Processing time: 206 Days and 19.2 Hours
AIM: To explore the anatomical feasibility of portacaval shunt using a magnetic compression technique (MCT) in cadavers.
METHODS: Computed tomography (CT) images of 30 portal hypertensive patients were obtained. The diameters of the portal vein (PV), the inferior vena cava (IVC), and distance between the two structures were measured. Similar measurements were performed on 20 adult corpses. The feasibility of portacaval shunt based on those measurements was analyzed. First stage of the extrahepatic portacaval shunt using MCT was performed on five cadavers. Specifically, the PV and IVC were exposed through an abdominal incision of the cadavers. The parent magnet was introduced from the femoral vein and was delivered into the IVC by an anchor wire and a 5F Cook catheter. The daughter magnet was introduced into the PV through the splenic vein using an interventional guide wire. When the daughter magnet met the parent magnet, they automatically clipped together and the first stage of the portacaval shunt was set up.
RESULTS: The average diameters of the PV and the IVC measured from the 30 CT image were 14.39 ± 2.36 mm and 18.59 ± 4.97 mm, respectively, and the maximum and minimum distances between the PV and the IVC were 9.79 ± 4.56 mm and 9.50 ± 4.79 mm, respectively. From 20 cadavers, the average diameters of the PV and the IVC were 14.48 ± 1.47 mm and 24.71 ± 2.64 mm, and the maximum and minimum distances between the PV and the IVC were 10.14 ± 1.70 mm and 8.93 ± 1.17 mm, respectively. The distances between the PV and the IVC from both the CT images and the cadavers were within the effective length of portacaval anastomosis using MCT (30.30 ± 4.19 mm). The PV and IVC are in close proximity to each other with no intervening tissues or structures in between. Simulated surgeries of the first stage using MCT on five cadavers was successfully performed.
CONCLUSION: Anatomically, extrahepatic portacaval shunt employing MCT is highly feasible in humans.
Core tip: The portacaval shunt using magnetic compression technique (MCT) was first established by our group in a canine model. Here, we assessed its feasibility in humans by analyzing computed tomography images and the anatomical parameters of cadavers. The average diameters of the portal vein (PV), the inferior vena cava (IVC) and the distances between the PV and the IVC were all within the parameters that allowed the setup of portacaval shunt using MCT. Finally, first stage simulation of the portacaval shunt was successfully set up in five cadavers. Our results indicated that the portacaval shunt is highly feasible in humans.
-
Citation: Yan XP, Liu WY, Ma J, Li JP, Lv Y. Extrahepatic portacaval shunt
via a magnetic compression technique: A cadaveric feasibility study. World J Gastroenterol 2015; 21(26): 8073-8080 - URL: https://www.wjgnet.com/1007-9327/full/v21/i26/8073.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i26.8073
Surgical shunting is an important treatment option for portal hypertension[1]. The traditional, hand-sewn surgical shunt currently used is time-consuming, highly technical and subject to postoperative complications. Side-to-side portacaval shunt has a high incidence of hepatic encephalopathy[2,3]. H-graft portacaval shunt, as a limited portacaval shunt, can effectively reduce the incidence of hepatic encephalopathy[4]. However, it is also technically challenging and patients who undergo the procedure must be put on long-term use of anticoagulants.
The magnetic compression technique (MCT) exploits the attraction between magnets to pull together an area of ischemic necrosis and its surrounding tissue to promote healing. The major advantages of the MCT are that it is simple, minimally invasive and reliable. Currently, MCT is applied in esophageal atresia[5,6], biliary stenosis[7-12], vascular anastomosis[13-19] and gastro-intestinal anastomosis[20-27]. Previously, we applied Roux-en-Y choledochojejunostomy incorporating MCT for obstructive jaundice in a canine model[28]. We also confirmed that magnetic rings could be used for rapid vascular reconstruction in a canine liver transplantation model[19].
We first reported the establishment of a portacaval shunt using MCT in dogs, the significant advantage of which is that no stent or any other foreign device was installed[29]. In the present study, we assessed some anatomical parameters that might affect the establishment of extrahepatic portacaval shunt using MCT in humans, and performed five preliminary cadaveric surgeries.
The entire study was carried out in strict accordance with protocols approved by the Xi’an Jiaotong University Biomedical Ethics Committee (Ethics Permit Number: 2014-0303). The cadavers were obtained from the Department of Anatomy, Health Science Center, Xi’an Jiaotong University, Shaan Xi Province, China. Written consent was obtained from the immediate family members of the deceased for educational and scientific research purposes. The format of the consent form is in accordance with the guidelines of the China Organ Donation Administrative Center.
The Xi’an Jiaotong University Biomedical Ethics Committee approved the use of the computed tomography (CT) data collected from patients. The project was explained to the patients. The privacy of the patients in this study was strictly protected and only age and CT data essential for the study were collected.
Twenty adult cadavers (14 male, 6 female) embalmed with formaldehyde were obtained. Cadavers of people with a history of abdominal surgery; abdominal tumor that might change the anatomical location of portal vein (PV) or inferior vena cava (IVC); diseases of PV or IVC system; prolonged storage after death, or improper handling that might interfere with the measurements were excluded.
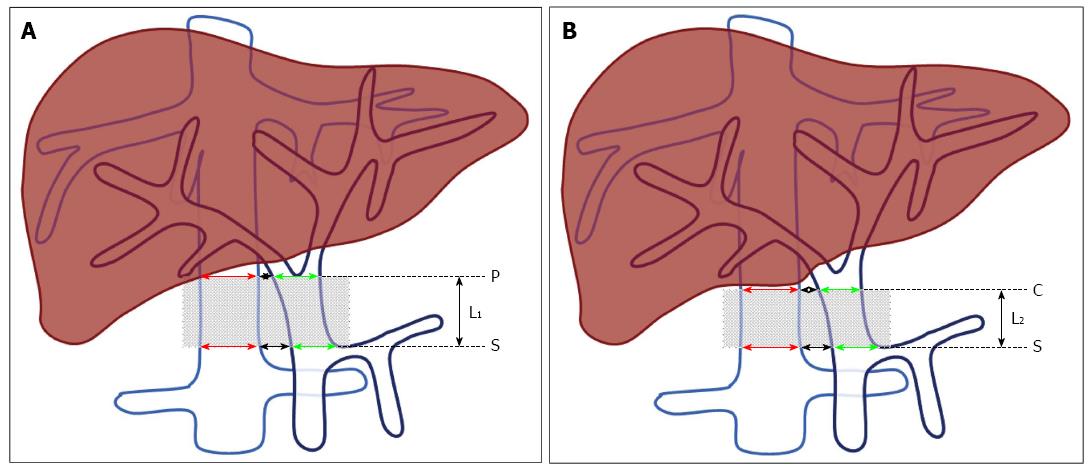
We first opened the abdomen of the cadaver and assessed the abdominal organs, and verified the absence of structural abnormalities or obvious lesions. We then incised the hepatoduodenal ligament, blunt dissected and observed the PV, IVC, common bile duct, and hepatic proper artery. The lower boundary of the effective area of portacaval anastomosis was defined as the plane of the splenic-mesenteric confluence (S, Figure 1). The upper boundary was defined as the plane of PV bifurcation (P, Figure 1A) or the inferior margin of the liver caudate lobe (C, Figure 1B). The effective length of the portacaval anastomosis was defined as the distance between S and P (L1, Figure 1A) or S and C (L2, Figure 1B). Twenty cadavers were measured.
From 2012 to 2013, 30 adult patients with portal hypertension underwent abdominal CT scans. All CT studies were performed with a 16-slice CT scanner (Somatom Sensation 16; Siemens, Germany). Patients with a history of abdominal surgery, portal venous system thrombosis, or other factors that might lead to distinct anatomical changes of the PV or IVC were excluded. The diameters of the PV and IVC at the position of the splenic-mesenteric confluence (S, Figure 1) and PV bifurcation (P, Figure 1A) or inferior margin of the liver caudate lobe (C, Figure 1B) were each measured as illustrated in Figure 1.
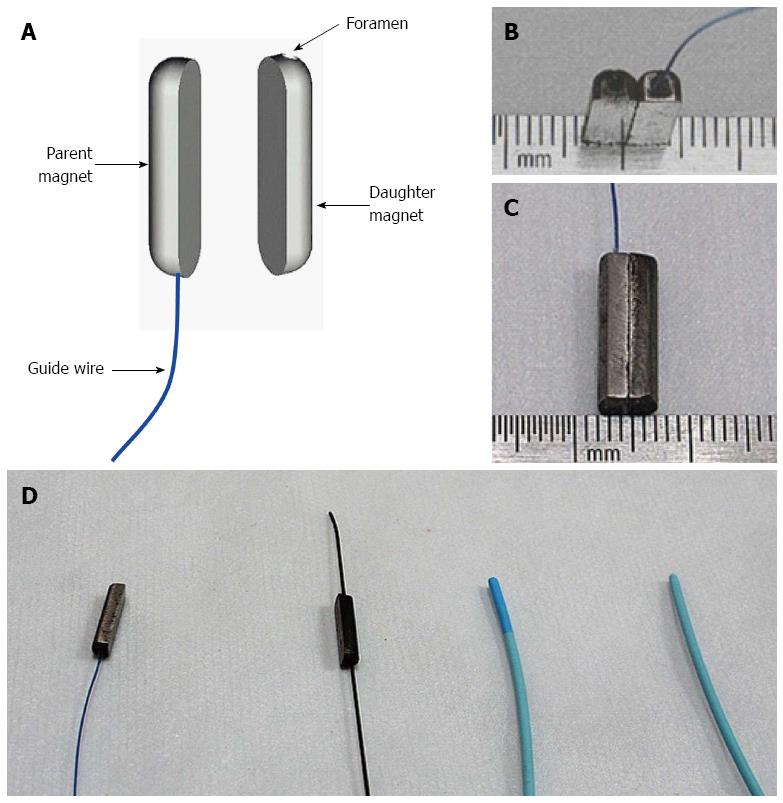
Two neodymium (Nd-Fe-B) permanent magnets (parent and daughter) were custom-manufactured for vascular experimental use (Northwest Institute for Nonferrous Metal Research, Xi’an, China; Figure 2). The parent magnet was a semi-cylinder with round ends, 15 mm L × 4 mm W × 3 mm H (Figure 2A-C). The parent magnet was attached to a special anchor wire, which was modified from a 2-0 prolene wire (Ethicon; Johnson and Johnson, Somerville, NJ, United States) by cutting off the suture needles from its ends. The daughter magnet, without wire, has the same dimensions as the parent magnet but with a long-axis paralleled foramen (diameter, 1.2 mm) inserted through the center.
The parent magnet was introduced from the femoral vein and delivered into the IVC by an anchor wire and a 5F Cook catheter (Cook, Bloomington, IN, United States). An interventional guide wire was inserted through the foramen of the daughter magnet. The daughter magnet was introduced into the PV through the splenic vein using an interventional guide wire (0.035 in; TERUMO, Japan) and a 5F Cook catheter (Cook; Figure 2D).
The force of the parent and daughter magnet was 6.8 N with 0-mm intermagnet separation and magnetic density 2400 G. The weights of the parent and daughter magnet were 0.80 g and 0.75 g, respectively.
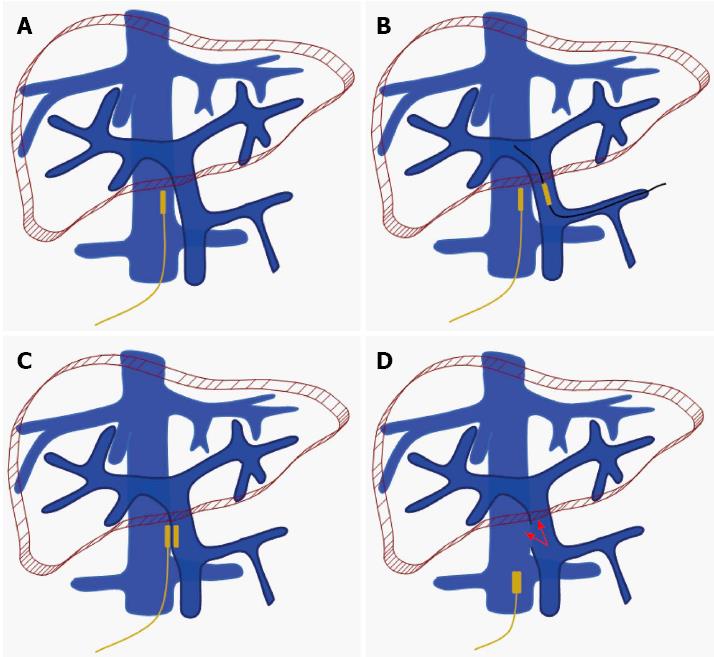
Shunt surgery was performed with reference to our previous procedure[29]. The PV and IVC were exposed through an abdominal incision, and the splenic vein and 15 mm of the right femoral vein were exposed. The prolene wire affixed to the parent magnet was inserted into a 5F Cook catheter, and the end of the prolene wire was tightly drawn to ensure that the parent magnet was fixed to the other end of the Cook catheter. An incision was made at the right femoral vein. Then, through the incision, the parent magnet was advanced to the target position on the IVC by the 5F Cook catheter.
A venotomy was performed on the splenic vein. An interventional guide wire was inserted through the hole of the daughter magnet. One end of the interventional guide wire was introduced into the PV through the incision on the splenic vein, and the other end was inserted into a 5F Cook catheter and extended the catheter. While the interventional guide wire was maintained in the PV, the daughter magnet, guided by the catheter, was moved to the target position of the PV through the incision of the splenic vein.
After the daughter magnet met the parent magnet, they automatically clipped together. The interventional guide wire and catheter were then withdrawn from the splenic vein and femoral vein. The first stage (steps 1-4) of the surgery was thus finished.
After a period of time (5-7 d in dogs), a RUPS-100 set with vascular sheath was navigated to the position of the magnet in the IVC through the femoral vein. Under X-ray guidance, the needle of the RUPS-100 set was advanced slowly and smoothly along the outline of the magnets and the magnets was detached from the vascular wall. The magnets were pulled out of the body by the wire fixed to the parent magnet. The portal-IVC shunt was set up (Figure 3).
In this study, only the first stage shunt procedures were performed on five cadavers.
The statistical methods of this study were reviewed by Qian Li from First Affiliated Hospital, Xi’an Jiaotong University. Data are expressed as mean ± SD. Gross anatomy and CT scan data were analyzed using an independent samples t-test. A P-value < 0.05 was considered statistically significant. Data were analyzed with SPSS 17.0 software.
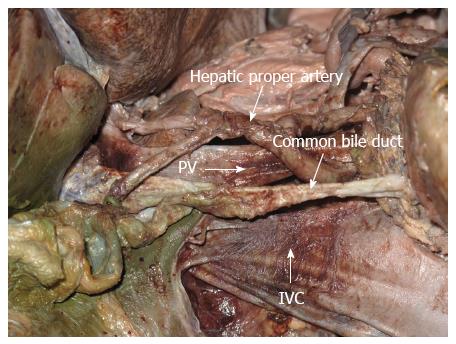
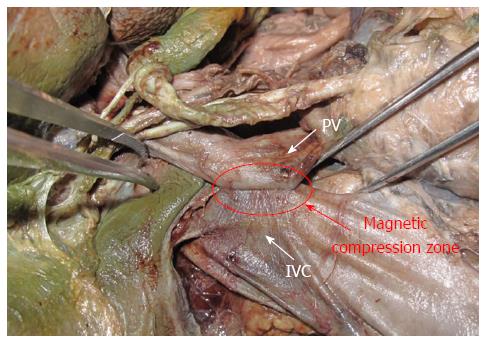
In this cadaveric study, the IVC was at the right-posterior position of the PV; the hepatoduodenal ligament, a small amount of connective tissue and the retroperitoneum lay between them. The hepatic proper artery was located at the left-inferior side of the PV and the common bile duct was right anterior to the PV (Figure 4). There was no important structure in the effective area of the portacaval anastomosis.
From 30 CT imaging measurements, the average diameters of the PV and the IVC in portal hypertensive patients were 14.39 ± 2.36 mm and 18.59 ± 4.97 mm, respectively (Table 1). The maximum and minimum distances between the PV and the IVC were 9.79 ± 4.56 mm and 9.50 ± 4.79 mm, respectively (Table 1).
| CT scan1 | Cadaver2 | P value3 | |
| Male, n | 17 | 14 | |
| Female, n | 13 | 6 | |
| Age, yr | 50.2 ± 11.0 | 53.9 ± 10.7 | |
| Diameter of PV, mm | 14.39 ± 2.36 | 14.48 ± 1.47 | 0.871 |
| Diameter of IVC, mm | 17.17 ± 5.94 | 24.71 ± 2.64 | 0.000 |
| Interval space 1, mm4 | 9.50 ± 4.79 | 10.14 ± 1.70 | 0.509 |
| Interval space 2, mm5 | 9.79 ± 4.56 | 8.93 ± 1.17 | 0.332 |
| Effective length, mm | - | 30.30 ± 4.19 | - |
From 20 cadavers, the average diameters of the PV and IVC were 14.48 ± 1.47 mm and 24.71 ± 2.64 mm, respectively (Table 1). The maximum and minimum distances between the PV and the IVC were 10.14 ± 1.70 mm and 8.93 ± 1.17 mm , respectively (Table 1). The distances between the PV and the IVC from both the CT image analysis and that of the cadavers are within the effective length of portacaval anastomosis using MCT (30.30 ± 4.19 mm).
The average diameters of the IVC determined from CT image measurements and those from the cadavers were significantly different (P = 0.000). The cross sections of the IVCs were highly variable on CT images: some were oval rather than round shaped. In such cases, we measured the length and width of the oval-shaped cross section and took the average as the value of its diameter. However, this measurement might not be accurate and could account for the deviation between CT and cadaveric measurements. Nevertheless, despite the discrepancy, the value of the IVC diameter measured from CT images was still within the parameters that allow the performance of the operation. There were no significant differences between the CT images and cadavers with regard to the diameters of the PV or the distances between the PV and the IVC (P > 0.005). This suggested that measurements taken from CT images are mostly reliable and close to the anatomical measurements.
The operation utilizing MCT was performed on five adult cadavers successfully, without any unexpected events. When the parent and daughter magnets each reached their target positions, they automatically joined and there was no damage to the proper hepatic artery, common bile duct, or other important structures because of the compression. More importantly, the magnetism was maintained at the satisfactory force and overcame the tension of the blood vessels (Figure 5).
Although we previously verified the reliability, feasibility, safety and efficiency of MCT in dogs[29], three essential issues remain to be resolved before it can be applied in humans. Firstly, whether there are intervening structures between the PV and the IVC that will be compressed when the parent and daughter magnets join. Secondly, whether the magnetic force between the parent and daughter magnets is strong enough to pull the two structures together. Thirdly, whether human anatomical characteristics allow this operation. The present study addressed all three of these key issues.
Zirinsky et al[30] first proposed the concept of a portacaval space, defining it as the gap between the PV and the IVC. Many studies have shown that the caudate lobe of the liver is generally in the upper part of the portacaval space, and the portacaval node, the portacaval vessels, and the cystic duct are in the lower part of the portacaval space[31,32]. Our anatomical analysis of 20 adult cadavers indicated that these structures cannot interfere with the portacaval anastomosis and would not be affected by the procedure. The hepatic PV lies left-inferior to the IVC; thus, when the parent and daughter magnets join, the right-posterior wall of the hepatic PV and the left-anterior wall of the IVC are compressed. Our autopsies showed that the proper hepatic artery and the bile duct are located left-inferior and right-inferior of the hepatic PV, respectively. However, the simulation procedure on the corpses confirmed that the magnets hardly compress these two structures.
One prerequisite for the procedure is that the distance between the PV and the IVC is within the working range of the magnets. According to our previous studies, the maximum distance that will allow attraction between the magnets is 3.5 cm. However, the ideal interval in experimental dogs was < 1.6 cm, because of the tension of the hepatic PV and the IVC vessel. The mean minimum distance between the hepatic PV and the IVC, based on measurements in the 20 adult corpses, was 0.96 cm ± 0.17 cm. Therefore, we assumed that the parent and daughter magnets are also able overcome the resistance of the blood vessels in the human body. We concluded that the magnetic attraction is sufficient to pull the PV and the IVC together.
In our previous study in dogs[29], the designed magnet was 12 mm in length and 2.5 mm in width. In humans, the diameter of the transjugular intrahepatic portosystemic shunt (TIPS) stent and prosthetic H-graft portacaval shunt is from 8 to 10 mm[1,4]. In our current study, the dimension of the magnet used was 15 mm × 4 mm × 3 mm. Our anatomical analysis revealed that the dimension of the hepatic PV and the IVC is sufficient to accommodate this size. Therefore, we suggest that the optimum length of a magnet used for a human portacaval shunt should be around 10 to 15 mm.
To better visualize the progress of the surgery, we opened the cadavers’ abdomen in this study. We reset the daughter magnet to the hepatic PV via the splenic vein. However, it is highly feasible clinically to implement minimally invasive surgery in humans by introducing the daughter magnet to the hepatic PV via the transjugular and transhepatic path.
Taken together, the results presented in the present study addressed the three essential issues regarding the feasibility of portacaval shunt with MCT in humans.
Based on our preliminary studies, the automatic assembly of the magnets was the critical problem. We emphasized the importance of a lack of previous abdominal surgeries, because postoperative adhesion might lead to extra resistance and impede attachment of the magnets. In the procedure, once the magnets had successfully clipped together, detachment was unavailable under interventional guidance; thus, it was crucial to hold still one magnet (the daughter one or the parent one) at the suitable compressed spot till the other one came for assembling. Also, during the withdrawal of the magnets, it required the assistance of a RUPS-100 needle to detach the device and the risk of puncturing IVC should be avoided.
A limitation of this study is that we only performed the first stage of the entire procedure. This is because the second stage requires the formation of copious fibrous connective tissue around the anastomosis, which is impossible to simulate in cadavers. In addition, the timing of the second surgery is crucial to the success of MCT - if there is not enough time remold, hemorrhage will be inevitable during the second surgery. On the other hand, if the second surgery is performed after a prolonged interval after the first, the magnets will become embedded in the adhesive tissues and will be difficult to remove. Based on our experience, 7 d after the first surgery is the optimum working window for the second surgery in dogs. The optimum timeframe for portacaval remolding in humans requires further study.
In conclusion, through preliminary trials on cadavers we confirmed the anatomical feasibility of implementing extrahepatic portacaval shunt using magnetic compression in humans. However, this pioneering technique requires further clinical validation.
Magnetic compression techniques (MCTs) have been applied in alimentary anastomosis, such as gastroenterostomy and bilioenterostomy, as well as in the end-to-end or side-to side vascular anastomosis. The authors originally established the side-to-side portacaval shunt using magnetic compression and interventional techniques in dogs. They analyzed the anatomical data and conducted the simulated operation on cadavers, aiming to demonstrate the preliminarily feasibility of this procedure in humans.
The MCT, which is easy, reliable, efficient and fast, has been intensively researched and applied in hollow organ anastomosis; however, no application in portacaval shunt establishment has been reported.
This study established an innovative side-to-side portacaval shunt combining magnetic compression and interventional techniques, and laid a foundation for the further clinical application by analyzing the anatomical relations and conducting a simulated procedure on corpses.
Utilizing the side-to-side portacaval shunt under magnetic compression and interventional techniques represents an alternative for the portal hypertension therapy, with the advantages such as minimal invasiveness, stable stoma and freedom from long-term implants.
Magnetic compression is a novel procedure utilizing a magnetic force to perform sutureless anastomosis in hollow organs. Combined with endoscopic or interventional techniques, some conventional laparotomies may be solved using this simplified minimal invasive procedure.
The research group pioneered the animal experiment of portacaval shunt using a MCT. Based on a previous study, they further tested its feasibility for human application. The study was well designed and conducted with great invention.
P- Reviewer: Ozen H S- Editor: Qi Y L- Editor: Stewart G E- Editor: Ma S
| 1. | Rosemurgy AS, Bloomston M, Clark WC, Thometz DP, Zervos EE. H-graft portacaval shunts versus TIPS: ten-year follow-up of a randomized trial with comparison to predicted survivals. Ann Surg. 2005;241:238-246. [PubMed] |
| 2. | Sakurabayashi S, Sezai S, Yamamoto Y, Hirano M, Oka H. Embolization of portal-systemic shunts in cirrhotic patients with chronic recurrent hepatic encephalopathy. Cardiovasc Intervent Radiol. 1997;20:120-124. [PubMed] |
| 3. | Tashiro H, Ide K, Amano H, Kobayashi T, Onoe T, Ishiyama K, Kuroda S, Tazawa H, Kono H, Aikata H. Surgical treatment for portosystemic encephalopathy in patients with liver cirrhosis: Occlusion of portosystemic shunt in combination with splenectomy. Hepatol Res. 2013;43:249-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 4. | Rosemurgy AS, Zervos EE, Bloomston M, Durkin AJ, Clark WC, Goff S. Post-shunt resource consumption favors small-diameter prosthetic H-graft portacaval shunt over TIPS for patients with poor hepatic reserve. Ann Surg. 2003;237:820-825; discussion 825-827. [PubMed] |
| 5. | Takamizawa S, Yamanouchi E, Muraji T, Nishijima E, Satoh S, Tsugawa J. MCRA of an anastomotic stenosis after esophagoesophagostomy for long gap esophageal atresia: a case report. J Pediatr Surg. 2007;42:769-772. [PubMed] |
| 6. | Zaritzky M, Ben R, Zylberg GI, Yampolsky B. Magnetic compression anastomosis as a nonsurgical treatment for esophageal atresia. Pediatr Radiol. 2009;39:945-949. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 60] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 7. | Muraoka N, Uematsu H, Yamanouchi E, Kinoshita K, Takeda T, Ihara N, Matsunami H, Itoh H. Yamanouchi magnetic compression anastomosis for bilioenteric anastomotic stricture after living-donor liver transplantation. J Vasc Interv Radiol. 2005;16:1263-1267. [PubMed] |
| 8. | Itoi T, Yamanouchi E, Ikeda T, Sofuni A, Kurihara T, Tsuchiya T, Tsuchida A, Kasuya K, Moriyasu F. Magnetic compression anastomosis: a novel technique for canalization of severe hilar bile duct strictures. Endoscopy. 2005;37:1248-1251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 9. | Okajima H, Kotera A, Takeichi T, Ueno M, Ishiko T, Hirota M, Asonuma K, Yamauchi E, Inomata Y. Magnet compression anastomosis for bile duct stenosis after duct-to-duct biliary reconstruction in living donor liver transplantation. Liver Transpl. 2005;11:473-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 44] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 10. | Matsuno N, Uchiyama M, Nakamura Y, Iwamoto H, Hama K, Ashizawa T, Nagao T, Yamanouchi E. A nonsuture anastomosis using magnetic compression for biliary stricture after living donor liver transplantation. Hepatogastroenterology. 2009;56:47-49. [PubMed] |
| 11. | Avaliani M, Chigogidze N, Nechipai A, Dolgushin B. Magnetic compression biliary-enteric anastomosis for palliation of obstructive jaundice: initial clinical results. J Vasc Interv Radiol. 2009;20:614-623. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 12. | Jang SI, Kim JH, Won JY, Lee KH, Kim HW, You JW, Itoi T, Lee D. Magnetic compression anastomosis is useful in biliary anastomotic strictures after living donor liver transplantation. Gastrointest Endosc. 2011;74:1040-1048. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 13. | Klima U, Falk V, Maringka M, Bargenda S, Badack S, Moritz A, Mohr F, Haverich A, Wimmer-Greinecker G. Magnetic vascular coupling for distal anastomosis in coronary artery bypass grafting: a multicenter trial. J Thorac Cardiovasc Surg. 2003;126:1568-1574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 14. | Klima U, Maringka M, Bagaev E, Kirschner S, Haverich A. Total magnetic vascular coupling for arterial revascularization. J Thorac Cardiovasc Surg. 2004;127:602-603. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 15. | Casselman FP, Meco M, Dom H, Foubert L, Van Praet F, Vanermen H. Multivessel distal sutureless off-pump coronary artery bypass grafting procedure using magnetic connectors. Ann Thorac Surg. 2004;78:e38-e40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 16. | Athanasiou T, Ashrafian H, Glenville B, Casula R. Coronary artery bypass with the use of a magnetic distal anastomotic device: surgical technique and preliminary experience. Heart Surg Forum. 2004;7:356-359. [PubMed] |
| 17. | Klima U, MacVaugh H, Bagaev E, Maringka M, Kirschner S, Beilner J, Haverich A. Magnetic Vascular Port in minimally invasive direct coronary artery bypass grafting. Circulation. 2004;110:II55-II60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 18. | Vicol C, Eifert S, Oberhoffer M, Boekstegers P, Knez A, Christ F, Reichart B. Early clinical results with a magnetic connector for distal coronary artery anastomoses. Ann Thorac Surg. 2005;79:1738-1742; discussion 1742-1743. [PubMed] |
| 19. | Shi Y, Lv Y, Wang B, Zhang Y, Jiang A, Li JH, Zhang XF, Li QY, Meng KW, Liu C. Novel magnetic rings for rapid vascular reconstruction in canine liver transplantation model. Transplant Proc. 2006;38:3070-3074. [PubMed] |
| 20. | Cope C. Evaluation of compression cholecystogastric and cholecystojejunal anastomoses in swine after peroral and surgical introduction of magnets. J Vasc Interv Radiol. 1995;6:546-552. [PubMed] |
| 21. | Cope C, Clark TW, Ginsberg G, Habecker P. Stent placement of gastroenteric anastomoses formed by magnetic compression. J Vasc Interv Radiol. 1999;10:1379-1386. [PubMed] |
| 22. | Cope C, Ginsberg GG. Long-term patency of experimental magnetic compression gastroenteric anastomoses achieved with covered stents. Gastrointest Endosc. 2001;53:780-784. [PubMed] |
| 23. | Chopita N, Vaillaverde A, Cope C, Bernedo A, Martinez H, Landoni N, Jmelnitzky A, Burgos H. Endoscopic gastroenteric anastomosis using magnets. Endoscopy. 2005;37:313-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 56] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 24. | van Hooft JE, Vleggaar FP, Le Moine O, Bizzotto A, Voermans RP, Costamagna G, Devière J, Siersema PD, Fockens P. Endoscopic magnetic gastroenteric anastomosis for palliation of malignant gastric outlet obstruction: a prospective multicenter study. Gastrointest Endosc. 2010;72:530-535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 25. | Myers C, Yellen B, Evans J, DeMaria E, Pryor A. Using external magnet guidance and endoscopically placed magnets to create suture-free gastro-enteral anastomoses. Surg Endosc. 2010;24:1104-1109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 26. | Pichakron KO, Jelin EB, Hirose S, Curran PF, Jamshidi R, Stephenson JT, Fechter R, Strange M, Harrison MR. Magnamosis II: Magnetic compression anastomosis for minimally invasive gastrojejunostomy and jejunojejunostomy. J Am Coll Surg. 2011;212:42-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 27. | Ryou M, Cantillon-Murphy P, Azagury D, Shaikh SN, Ha G, Greenwalt I, Ryan MB, Lang JH, Thompson CC. Smart Self-Assembling MagnetS for ENdoscopy (SAMSEN) for transoral endoscopic creation of immediate gastrojejunostomy (with video). Gastrointest Endosc. 2011;73:353-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 28. | Fan C, Yan XP, Liu SQ, Wang CB, Li JH, Yu L, Wu Z, Lv Y. Roux-en-Y choledochojejunostomy using novel magnetic compressive anastomats in canine model of obstructive jaundice. Hepatobiliary Pancreat Dis Int. 2012;11:81-88. [PubMed] |
| 29. | Yan X, Fan C, Ma J, Li J, Dong D, Wang H, Ma F, Zheng X, Lv Y. Portacaval shunt established in six dogs using magnetic compression technique. PLoS One. 2013;8:e76873. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 30. | Zirinsky K, Auh YH, Rubenstein WA, Kneeland JB, Whalen JP, Kazam E. The portacaval space: CT with MR correlation. Radiology. 1985;156:453-460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 34] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 31. | Ito K, Choji T, Fujita T, Kuramitsu T, Nakaki H, Kurokawa F, Fujita N, Nakanishi T. Imaging of the portacaval space. AJR Am J Roentgenol. 1993;161:329-334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 32. | McLoughlin RF, Rankin RN. Portacaval space anatomy: potential implications for percutaneous portacaval shunts. J Vasc Interv Radiol. 1996;7:761-767. [PubMed] |