Published online Jul 7, 2015. doi: 10.3748/wjg.v21.i25.7921
Peer-review started: December 5, 2014
First decision: December 26, 2014
Revised: January 11, 2015
Accepted: February 13, 2015
Article in press: February 13, 2015
Published online: July 7, 2015
Processing time: 216 Days and 18.2 Hours
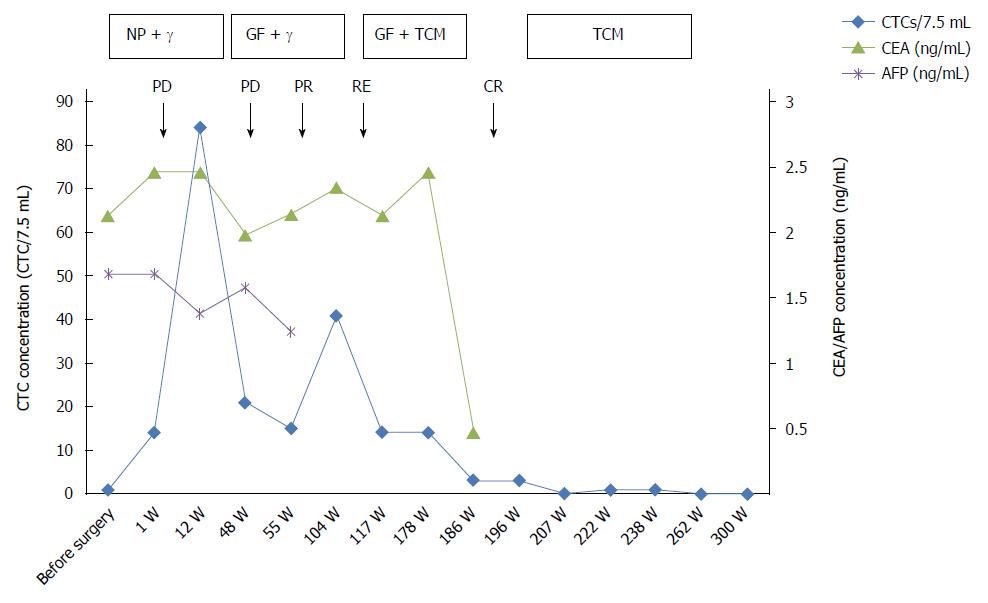
This study investigated whether changes in circulating tumor cell (CTC) numbers reflect tumor progression and treatment efficacy in esophageal squamous cell carcinoma (ESCC). A 47-year-old male patient with ESCC is presented in this case study. The patient was evaluated for a series of serum tumor markers and subjected to radiological examinations before and after surgery and during follow-up over the course of five years. In addition, the CTCs in 7.5 mL of peripheral blood were enriched by magnetic-activated cell sorting negative selection and identified by immunofluorescence staining. Serum tumor markers remained within normal ranges and were discordant with imaging scans during the follow-up. Initially, one CTC was detected in the peripheral blood sample, and 14 were observed seven days after the operation. After 12 wk, subcutaneous metastases and bone metastases occurred, and the number of CTCs increased to 84. After 48 wk, lung metastases were noted, and the CTC level was 21. At 104 wk, the number of CTCs was 14, and disease recurrence was detected by positron emission tomography-computed tomography. The CTC counts were in accord with the imaging studies at several time points. The additional information provided by CTC enumeration could thus facilitate monitoring of disease status and treatment efficacy and provide support for treatment decisions.
Core tip: We report a follow-up of a 47-year-old male patient with esophageal squamous cell carcinoma in this study. In addition to the conventional examination, a novel workflow was performed to detect circulating tumor cells (CTCs). We evaluated the relationship between CTC characteristics and other tests. The serum tumor markers were normal and thus did not appear to reflect changes in the disease, whereas the number of CTCs fluctuated with the disease progression and treatment and coincided with imaging studies performed during the follow-up. This case highlights CTCs as a useful diagnostic tool with potential applications during treatment.
- Citation: Qiao YY, Lin KX, Zhang Z, Zhang DJ, Shi CH, Xiong M, Qu XH, Zhao XH. Monitoring disease progression and treatment efficacy with circulating tumor cells in esophageal squamous cell carcinoma: A case report. World J Gastroenterol 2015; 21(25): 7921-7928
- URL: https://www.wjgnet.com/1007-9327/full/v21/i25/7921.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i25.7921
Esophageal carcinoma is a common malignancy, ranking sixth among global cancer-related deaths and thus representing a serious threat to human health. More than 90% of malignant esophageal tumors are esophageal squamous cell carcinomas (ESCCs), and the incidence of the disease has increased in recent decades. Due to the lack of early symptoms and specific diagnostic methods, ESCC is commonly diagnosed at an advanced stage and therefore has an extremely poor prognosis, with only 20%-30% survival at five years[1,2].
Tumor metastasis and recurrence are the major causes of death. Tumor lesions are mainly revealed by radiological examination and serum tumor markers. However, tumor lesions that are small in diameter are difficult to detect by imaging scans. Highly specific tumor markers are currently unavailable, especially for ESCC. Histopathology is the “gold standard” of tumor diagnosis. However, due to the limitations of specimen collection, real-time monitoring of tumor progression cannot be realized. Thus, it is critical to introduce new tumor-detection methods into clinical practice.
Studies have shown that circulating tumor cells (CTCs) are closely related to tumor metastasis and can be useful as a “window” to monitor disease prognosis both initially and after therapy[3]. The number and phenotype of CTCs can reflect the disease progression in real time and guide the treatment. Furthermore, CTCs can be easily obtained for series detection because the collection method is noninvasive and causes no trauma to patients.
However, owing to the rarity of CTCs in peripheral blood, their detection requires a combination of high specificity and sensitivity. Several CTC detection methods have been developed, including magnetic-activated cell sorting (MACS), polymerase chain reaction, and microfluidic chips[4-6]. In recent years, the immune magnetic bead enrichment method has been shown to efficiently separate cells of epithelial origin from blood samples. Two strategies for immunological isolation have emerged: positive enrichment and negative enrichment[7].
The negative selection strategy involves capturing cells of interest by depleting unwanted cells. During the metastatic cascade, tumor cells undergo epithelial-mesenchymal transition (EMT) and lose epithelial markers; therefore, positive enrichment may overlook CTCs. In the present study, we explored negative enrichment methods for obtaining CTCs. Negative enrichment has been confirmed as a promising approach for isolating CTCs[8,9].
Here, we report a series study of CTCs from an ESCC patient and analyze the relationship between the CTC enumeration and other examination results. CTC counts appear to provide a solid basis for disease monitoring and individualized treatment.
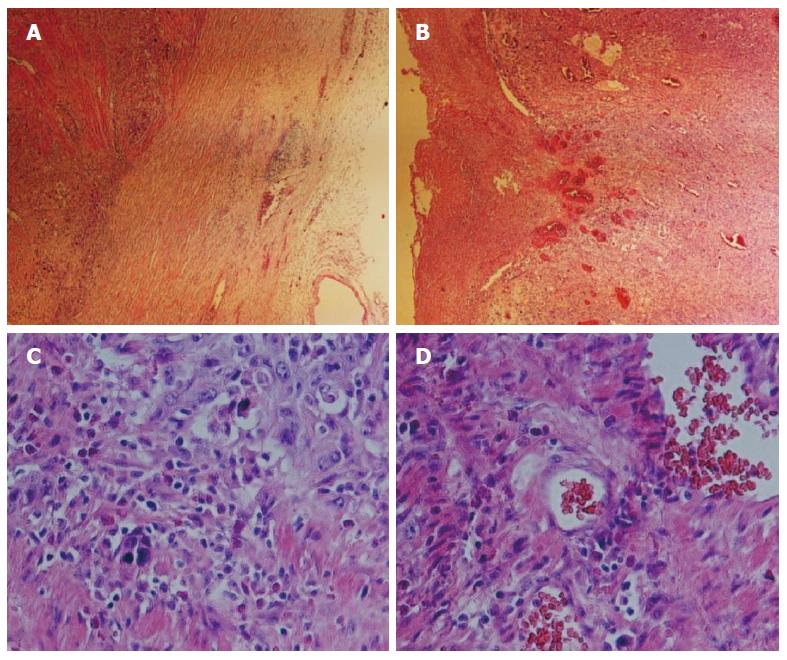
A 47-year-old male patient was referred for dysphagia in February 2009, and an esophageal barium meal and computerized tomography (CT) scan determined the presence of esophageal carcinoma. The diagnosis by gastroscopy and biopsy was poorly differentiated squamous cell carcinoma. The tumor lump was completely removed by surgical resection with mediastinal lymph node dissection. The final pathological result revealed a squamous cell carcinoma localized in the lower esophagus (Figure 1), 5.5 cm × 5.5 cm × 1.2 cm in size, with a stage classified as T3N2M; the carcinoma tissue invaded the entire esophageal wall. The patient presented lymph node metastases involving the paraesophageal lymph node (1/4), subcarinal lymph node (2/13), cardia lymph node (1/2), left gastric lymph node (2/5), and inferior pulmonary vein lymph node (1/1). He was treated with chemotherapy with an NP program (cisplatin + vinorelbine), followed by radiation therapy using DT4860cGy/27f/5w. After three months, when a metastasis was found in the bone, the patient received body γ-knife treatment using a boost dose in the mediastinal lymph nodes and abdominal lymph nodes, followed by completion of the second, third, and fourth cycles of chemotherapy, with the same treatment options as before (NP program). At 48 wk postoperation, a metastasis in the lower lobe of the right lung was identified, and a localized γ-knife at 4.8 Gy was employed for 10 cycles of treatment. The chemotherapy plan was then switched to the GF program (gemcitabine + fluorouracil + leucovorin) for three cycles (the fifth, sixth, and seventh cycles). Subsequently, the patient underwent traditional Chinese drug therapy and exhibited a stable condition. The follow-up process was conducted with informed consent by the patient, and the patient cooperated actively.
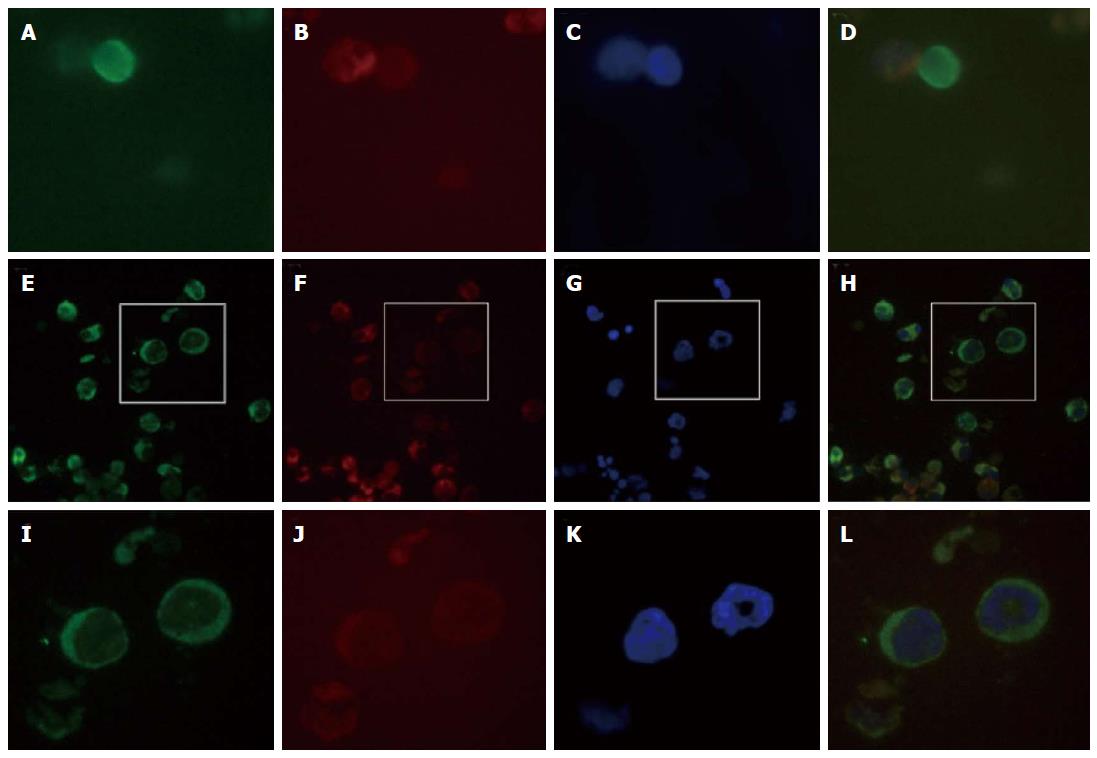
We performed CTC detection by MACS negative selection[6]. After the patient signed an informed consent form, 7.5 mL of peripheral blood was collected at multiple treatment points. The blood samples were treated with erythrocyte lysate buffer to remove red blood cells, and the rest of the cells were mixed with the appropriate amount of magnetic beads and incubated for 15 min. The cell suspensions were applied to LS columns (Miltenyi Biotec, Bergisch Gladbach, Germany), and the nucleated cells were collected under a strong magnetic field. The slide was incubated with fluorescent anti-CK8/18/19-FITC (1:100) and anti-CD45-PE (1:1000) (Miltenyi Biotec, Bergisch Gladbach, Germany) and mounted with 7 μL 4’,6-diamidino-2-phenylindole (DAPI); the cells were observed and counted under a microscope (Table 1 and Figure 2).
| Code | Time | Number of CTCs/7.5 mL | Results of the imaging study | Serum tumor marker levels | Disease progression | Treatment |
| Pre | Preoperative 2 d | 1 | CT revealed wall thickening of the mid-lower esophagus, with a high likelihood of esophageal carcinoma | Normal levels of CA125, CA15-3, CA19-9, CA242, AFP, CEA, HGH‚ PSA, f-PSA, β-HCG, NSE and FER | Preoperative diagnosis of poorly differentiated squamous cell carcinoma | Surgical treatment |
| Post 1 | Postoperative 1 wk | 14 | CEA 2.4 ng/mL; AFP 1.7 ng/mL | Tumor resection, lymph node dissection | Postoperative chemotherapy started | |
| Post 2 | Postoperative 12 wk | 84 | Bone imaging revealed an abnormal increase in salt metabolism in the 10th left front rib | Normal levels of CA125, CA15-3, CA19-9, CA242, AFP, CEA, HGH‚ PSA, f-PSA, β-HCG, NSE and FER | Subcutaneous metastasis and bone metastasis | Treatment by a systemic γ-knife with a boost dose and chemotherapy |
| Post 3 | Postoperative 48 wk | 21 | CT revealed occupying nodules in the lower right lobe, suggesting metastatic cancer | CEA 2.0 ng/mL; AFP 1.5 ng/mL | Right lung metastasis | Treatment with a localized γ-knife and chemotherapy |
| Post 4 | Postoperative 55 wk | 15 | CEA 2.2 ng/mL; AFP 1.2 ng/mL | Stable condition | Systemic chemotherapy | |
| Post 5 | Postoperative 104 wk | 41 | PET-CT revealed a high density of radionuclide in the esophageal residue, suggesting disease recurrence | Normal levels of CA125, CA15-3, CA19-9, CA242, AFP, CEA, HGH‚ PSA, f-PSA, β-HCG, NSE and FER | Recurrence | Systemic chemotherapy |
| Post 6 | Postoperative 117 wk | 14 | CEA 2.1 ng/mL | Disease in progression | Chemotherapy with Chinese medicine | |
| Post 7 | Postoperative 178 wk | 14 | CEA 2.4 ng/mL | Disease in progression | Chemotherapy with Chinese medicine | |
| Post 8 | Postoperative 186 wk | 5 | CEA 0.5 ng/mL | Condition improved | Chinese medicine | |
| Post 9 | Postoperative 196 wk | 3 | Stable condition | Adjuvant treatment with traditional Chinese medicine | ||
| Post 10 | Postoperative 207 wk | 0 | Stable condition | Adjuvant treatment with traditional Chinese medicine | ||
| Post 11 | Postoperative 222 wk | 1 | Stable condition | Adjuvant treatment with traditional Chinese medicine | ||
| Post 12 | Postoperative 238 wk | 1 (suspicious) | Normal levels of CA125, CA15-3, CA19-9, CA242, AFP, CEA, HGH‚ PSA, f-PSA, β-HCG, NSE and FER | Stable condition | Adjuvant treatment with traditional Chinese medicine | |
| Post 13 | Postoperative 262 wk | 0 | Stable condition | Adjuvant treatment with traditional Chinese medicine | ||
| Post 14 | Postoperative 300 wk | 0 | Stable condition | Adjuvant treatment with traditional Chinese medicine |
The Protein Chip System for Multi-tumor Marker Detection (S20010007, Huzhou Shu Kang Biological Technology Co., Ltd.) was used in this study. This protein biochip measures 12 tumor markers in the serum, including cancer antigen CA125, CA15-3, CA19-9, CA242, α-fetoprotein (AFP), carcinoembryonic antigen (CEA), human growth hormone (HGH)‚ prostate specific antigen (PSA), free-prostate specific antigen (f-PSA), β-human chorionic gonadotropin (β-HCG), neuron-specific enolase (NSE), and ferritin (FER). The serum tumor markers were evaluated with several replicates during the follow-up. The levels of these markers fluctuated slightly within normal ranges.
Under a fiber microscope, tissue abnormalities indicative of structural disorder and loss of normal polarization in the epithelial structure were observed. The cells were irregularly arranged, showing dense nuclear staining, a higher nucleus/cytoplasm ratio, and increased mitotic activity. The immunohistochemistry results revealed cytokeratin (CK) AE1/AE3 (+++), CK17 (++), p63 (++), p53 (++), epidermal growth factor receptor (EGFR) (+), CK7/20 (-) and CEA (-) in the tumor cells, with a Ki67 index of approximately 70%. At every patient visit, CT/positron emission tomography-CT (PET-CT), MRI, or a bone scan was performed at the physician’s discretion.
Examination results (pre- and postoperative, as well as five-year follow-up) were collected. The follow-up occurred after every treatment or recurrence for a period of five years. The changes in CTC numbers were nearly consistent with the imaging results, thereby possibly reflecting the disease progression and treatment efficacy, as shown in Table 1.
Esophageal carcinoma has a poor prognosis and high malignancy, with 20%-30% five-year survival rate[2]. Effective diagnosis and treatment can prolong the survival of patients with malignant tumors[10]. To date, p53, squamous cell carcinoma (SCC), p5-Ab, CEA, and cytokeratin 19 fragments (CYFRA21-1) are used for esophageal carcinoma, but their sensitivity is as low as 11%-40%[11]. The patient in this study successively underwent CEA, AFP and other serum tumor marker quantitative tests initially, after therapy and treatments or metastases points during five years of follow-up. The test results were continuously in normal range, suggesting that CEA and AFP levels did not reflect changes in his disease status. However, the SCC and CYFRA21-1 test was not performed at our hospital, and we unfortunately did not obtain these data.
Imaging scans have great significance for diagnosis of esophageal carcinoma. Our study showed that CTC changes were consistent with imaging results. CTCs may provide additional tests for metastatic cancer to supplement the current methods[12]. CTC are used to predict survival in several metastatic cancers and the studies investigated that increased number of CTCs suggested a high risk of disease progression and a poor prognosis[13-18].
However, few studies have yet reported CTCs in ESCC patients. In a study of certain digestive tumors, the presence of two or more CTCs was significantly correlated with peritoneal dissemination of gastric or colorectal cancer and pleural dissemination of esophageal cancer[19]. We used negative selection method to obtain CTCs and analyzd by immunocytochemistry. In Figure 2, CTCs were nucleated and elliptical or elongated with a singular or clustered appearance and larger than 10 μm, expressed cytokeratin, lacked CD45, and were positive for DAPI staining. Some CTCs exhibited morphologically apoptotic features due to chemotherapy or radiotherapy.
Our group’s previous studies found CTCs (using a cutoff value of 5) to be a significant prognostic factor. The results were confirmed in this case study (Table 1). Initially, one CTC was detected in the peripheral blood of the patient. The Multi-tumor Marker Detection was normal. Seven days after surgery, 14 CTCs presented. Two possible reasons were considered: first, the patient had lymph node metastases, and because some of the lymph nodes were around the gastric artery, they could not be excised completely; second, the operation procedure may promote tumor micrometastases. One study reported that CTCs number in breast cancer patients was increased by 85% during the third to fourth postoperative days and may even rise to 1000 times the original level in some cases[20]. CEA and AFP were normal at this time point, 2.4 ng/mL and 1.7 ng/mL, respectively. The immunohistochemistry revealed cytokeratin (CK) AE1/AE3 (+++), CK17 (++), p63 (++), p53 (++), EGFR(+), CK7/20 (-), and CEA (-) in the tumor cells, with a Ki67 index of approximately 70%. These indicated that the tumor cells were cytokeratin-positive and actively proliferating and confirmed that we could obtain CTCs based on cytokeratin markers. After chemotherapy, three months (12 wk) postoperation, bone and subcutaneous metastases were found through imaging, and CTCs rose to 84 per 7.5 mL of peripheral blood.CEA and AFP remained within normal ranges. After an initial response, three additional rounds of chemotherapy were performed. After one year (48 wk, a lung metastasis was found by CT scan, and the number of CTCs was 21 per 7.5 mL of peripheral blood. CEA and AFP were still normal. Concurrently, the patient attempted to use traditional Chinese medicine (TCM) in addition to the chemotherapy. One part of the medicine was Astragalus. Many studies have shown that Astragalus membranaceus polysaccharide can promote antitumor activity via improving the immune responses of the host organism[21].
After 104 wk, a PET-CT examination revealed a relapse, and CTCs was increased to 41 per 7.5 mL, concordant with the PET-CT results. After integrative anti-tumor therapy with TCM, the patient was stable. CTCs after 186 wk decreased to three from 14 at 117 wk, and the patient had no abnormalities by radiographic examination. The patient is currently in a stable condition. The CTC counts and the treatment and test results are summarized in Figure 3. The CTCs were increased postoperatively, and the CTC concentration was maintained at a high level after radiotherapy and chemotherapy. Bone and lung metastasis relapses occurred during this period. With the integrative therapy, the CTC number dropped, and the patient attained a stable status. During the follow-up, CTC enumeration could effectively complement imaging studies, especially for patients with normal serum markers. CTC also indirectly reflects the reactivity of the metastatic cancer cells to a chemotherapeutic drug[22]. Most importantly, the CTC test is noninvasive and allows successive detection of CTCs to monitor tumor metastasis or recurrence. As a real-time dynamic screening method, CTC technology will be greatly improved and widely applied in cancer diagnosis and treatment.
A 47-year-old male patient was referred for dysphagia in February 2009. An esophageal barium meal and computerized tomography (CT) scan determined the presence of esophageal carcinoma.
The patient was diagnosed with poorly differentiated squamous cell carcinoma by imaging and biopsy. The authors conducted a 5 year follow-up of patients, to evaluate the therapeutic effect and disease progression.
Serum tumor markers, imaging and circulating tumor cells (CTCs) detection were used to monitor disease tumor progression and treatment efficacy of the patient.
Serum tumor markers of the patient maintained among normal range. The number of circulating tumor cells in patients fluctuated with the disease and treatment response and coincident with the imaging diagnosis.
For this cases, CT scan and esophageal barium meal showed squamous cell carcinoma in the in the lower esophagus. Twelve weeks after operation, the patient performed Bone imaging.
The immunohistochemistry results revealed cytokeratin (CK) AE1/AE3 (+++), CK17 (++), p63 (++), p53 (++), epidermal growth factor receptor (+), CK7/20 (-), and carcinoembryonic antigen (-) in the tumor cells, with a Ki67 index of approximately 70%.
The patient received tumor resection and lymph node dissection. Further treatments including radiotherapy, chemotherapy and traditional Chinese medicine were performed.
Very few cases of circulating tumor cells in esophageal squamous cell carcinoma (ESCC) patients have been reported in the literature.
CTCs detections of the ESCC patient were reported in this case and analyzed the consistence with the disease progression. With more researches, CTCs are expected to provide support to monitor the disease status and treatment efficacy.
This manuscript is overall interesting, but some revision is needed.
P- Reviewer: Biramijamal F, Diakowska D, Hsu PK S- Editor: Yu J L- Editor: A E- Editor: Liu XM
| 1. | Alemar J, Schuur ER. Progress in using circulating tumor cell information to improve metastatic breast cancer therapy. J Oncol. 2013;2013:702732. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 2. | Stein HJ, Feith M, Bruecher BL, Naehrig J, Sarbia M, Siewert JR. Early esophageal cancer: pattern of lymphatic spread and prognostic factors for long-term survival after surgical resection. Ann Surg. 2005;242:566-573; discussion 573-575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 323] [Cited by in RCA: 322] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 3. | Friedlander TW, Ngo VT, Dong H, Premasekharan G, Weinberg V, Doty S, Zhao Q, Gilbert EG, Ryan CJ, Chen WT. Detection and characterization of invasive circulating tumor cells derived from men with metastatic castration-resistant prostate cancer. Int J Cancer. 2014;134:2284-2293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 101] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 4. | Pearl ML, Zhao Q, Yang J, Dong H, Tulley S, Zhang Q, Golightly M, Zucker S, Chen WT. Prognostic analysis of invasive circulating tumor cells (iCTCs) in epithelial ovarian cancer. Gynecol Oncol. 2014;134:581-590. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 76] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 5. | Yu Y, Xu G, Cao J, Jin S, Man Y, Shang L. Combination of four gene markers to detect circulating tumor cells in the peripheral blood of patients with advanced lung adenocarcinoma using real-time PCR. Oncol Lett. 2013;5:1400-1406. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 6. | Ren C, He P, Zhang J, Zheng Z, Qiao Y, Zhao X. Malignant characteristics of circulating tumor cells and corresponding primary tumor in a patient with esophageal squamous cell carcinoma before and after surgery. Cancer Biol Ther. 2011;11:633-638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 7. | Hosokawa M, Kenmotsu H, Koh Y, Yoshino T, Yoshikawa T, Naito T, Takahashi T, Murakami H, Nakamura Y, Tsuya A. Size-based isolation of circulating tumor cells in lung cancer patients using a microcavity array system. PLoS One. 2013;8:e67466. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 125] [Cited by in RCA: 130] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 8. | Liu Z, Fusi A, Klopocki E, Schmittel A, Tinhofer I, Nonnenmacher A, Keilholz U. Negative enrichment by immunomagnetic nanobeads for unbiased characterization of circulating tumor cells from peripheral blood of cancer patients. J Transl Med. 2011;9:70. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 139] [Cited by in RCA: 142] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 9. | Joshi P, Jacobs B, Derakhshan A, Moore LR, Elson P, Triozzi PL, Borden E, Zborowski M. Enrichment of circulating melanoma cells (CMCs) using negative selection from patients with metastatic melanoma. Oncotarget. 2014;5:2450-2461. [PubMed] |
| 10. | Shimada H, Takeda A, Arima M, Okazumi S, Matsubara H, Nabeya Y, Funami Y, Hayashi H, Gunji Y, Suzuki T. Serum p53 antibody is a useful tumor marker in superficial esophageal squamous cell carcinoma. Cancer. 2000;89:1677-1683. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 138] [Reference Citation Analysis (0)] |
| 11. | Maheswaran S, Sequist LV, Nagrath S, Ulkus L, Brannigan B, Collura CV, Inserra E, Diederichs S, Iafrate AJ, Bell DW. Detection of mutations in EGFR in circulating lung-cancer cells. N Engl J Med. 2008;359:366-377. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1363] [Cited by in RCA: 1318] [Article Influence: 77.5] [Reference Citation Analysis (0)] |
| 12. | Yu M, Stott S, Toner M, Maheswaran S, Haber DA. Circulating tumor cells: approaches to isolation and characterization. J Cell Biol. 2011;192:373-382. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 820] [Cited by in RCA: 809] [Article Influence: 57.8] [Reference Citation Analysis (0)] |
| 13. | Li C, Zhao Z, Liu R. Comment on Han L et al.: prognostic value of circulating tumor cells in patients with pancreatic cancer: a meta-analysis. Tumour Biol. 2014;35:8353-8354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 14. | de Bono JS, Scher HI, Montgomery RB, Parker C, Miller MC, Tissing H, Doyle GV, Terstappen LW, Pienta KJ, Raghavan D. Circulating tumor cells predict survival benefit from treatment in metastatic castration-resistant prostate cancer. Clin Cancer Res. 2008;14:6302-6309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1786] [Cited by in RCA: 1701] [Article Influence: 100.1] [Reference Citation Analysis (0)] |
| 15. | Sun YF, Yang XR, Zhou J, Qiu SJ, Fan J, Xu Y. Circulating tumor cells: advances in detection methods, biological issues, and clinical relevance. J Cancer Res Clin Oncol. 2011;137:1151-1173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 128] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 16. | Klinac D, Gray ES, Freeman JB, Reid A, Bowyer S, Millward M, Ziman M. Monitoring changes in circulating tumour cells as a prognostic indicator of overall survival and treatment response in patients with metastatic melanoma. BMC Cancer. 2014;14:423. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 45] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 17. | Nolé F, Munzone E, Zorzino L, Minchella I, Salvatici M, Botteri E, Medici M, Verri E, Adamoli L, Rotmensz N. Variation of circulating tumor cell levels during treatment of metastatic breast cancer: prognostic and therapeutic implications. Ann Oncol. 2008;19:891-897. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 127] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 18. | Camara O, Kavallaris A, Nöschel H, Rengsberger M, Jörke C, Pachmann K. Seeding of epithelial cells into circulation during surgery for breast cancer: the fate of malignant and benign mobilized cells. World J Surg Oncol. 2006;4:67. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 71] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 19. | Hiraiwa K, Takeuchi H, Hasegawa H, Saikawa Y, Suda K, Ando T, Kumagai K, Irino T, Yoshikawa T, Matsuda S. Clinical significance of circulating tumor cells in blood from patients with gastrointestinal cancers. Ann Surg Oncol. 2008;15:3092-3100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 150] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 20. | Kularatne BY, Lorigan P, Browne S, Suvarna SK, Smith MO, Lawry J. Monitoring tumour cells in the peripheral blood of small cell lung cancer patients. Cytometry. 2002;50:160-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 48] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 21. | Yang B, Xiao B, Sun T. Antitumor and immunomodulatory activity of Astragalus membranaceus polysaccharides in H22 tumor-bearing mice. Int J Biol Macromol. 2013;62:287-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 161] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 22. | Lucci A, Hall CS, Lodhi AK, Bhattacharyya A, Anderson AE, Xiao L, Bedrosian I, Kuerer HM, Krishnamurthy S. Circulating tumour cells in non-metastatic breast cancer: a prospective study. Lancet Oncol. 2012;13:688-695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 385] [Cited by in RCA: 393] [Article Influence: 30.2] [Reference Citation Analysis (0)] |