Published online Jul 7, 2015. doi: 10.3748/wjg.v21.i25.7842
Peer-review started: January 2, 2015
First decision: January 22, 2015
Revised: February 9, 2015
Accepted: March 27, 2015
Article in press: March 27, 2015
Published online: July 7, 2015
Processing time: 188 Days and 18.7 Hours
AIM: To investigate hepatitis B virus (HBV) prevalence in the general population in China.
METHODS: A total of 148931 individuals were investigated by multistage random sampling in Eastern China. Data were collected on demographics and hepatitis B vaccination history, and serum was tested for hepatitis B surface antigen (HBsAg) by ELISA.
RESULTS: A total of 11469 participants (7.70%, 95%CI: 7.57%-7.84%) were positive for HBsAg. HBsAg prevalence was 0.77% among children < 5 years old but increased progressively from adolescents (1.40%-2.55%) to adults (5.69%-11.22%). A decrease in HBsAg prevalence was strongly associated with vaccination and familial history of HBV among both children and adult groups. Meanwhile, HBsAg risk in adults was associated with invasive testing and sharing needles. The HBV immunization rate among participants aged < 20 years was 93.30% (95%CI: 93.01%-93.58%). Significant difference in HBsAg prevalence appeared between vaccinated and unvaccinated participants (3.59% vs 10.22%).
CONCLUSION: Although the national goal of HBsAg prevalence < 1% among children < 5 years old has been reached, immunization programs should be maintained to prevent resurgence.
Core tip: A total of 148931 individuals were investigated in Eastern China to evaluate the impact of hepatitis B vaccination since 1992. 7.70% were positive for hepatitis B surface antigen (HBsAg) which has not achieved the national goal for the whole population in decreasing the prevalence of HBsAg to < 7%. Prevalence was 0.77% among children aged < 5 years and the rate of hepatitis B virus immunization among teenagers aged < 20 years was 93.30%, which have reached the national goals of < 1% and > 90%, respectively.
- Citation: Huang P, Zhu LG, Zhu YF, Yue M, Su J, Zhu FC, Yang HT, Zhang Y, Shen HB, Yu RB, Zhai XJ, Peng ZH. Seroepidemiology of hepatitis B virus infection and impact of vaccination. World J Gastroenterol 2015; 21(25): 7842-7850
- URL: https://www.wjgnet.com/1007-9327/full/v21/i25/7842.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i25.7842
Due to its global prevalence and potential for adverse consequences such as cirrhosis and hepatocellular carcinoma, chronic hepatitis B virus (HBV) infection has become a serious public health problem[1,2]. The WHO estimates there are approximately 2 billion people infected by HBV worldwide, and 240 million of them have chronic infection. Each year an estimated 600000 people die from liver diseases caused by HBV infection[3]. The distribution of HBV infection is uneven, and sub-Saharan Africa, Asia, and the Pacific Islands, where HBV is most commonly spread from mother to child at birth or from person to person in early childhood, have the most significant burden. In China, a highly endemic area, the national prevalence for hepatitis B surface antigen (HBsAg) positivity is 7.20%[2,4].
The Centers for Disease Control and Prevention (CDC) reported that the incidence of newly acquired HBV infection in China has declined steadily since the 1990s by virtue of a number of public health interventions, such as the screening of pregnant women and the vaccination of infants and adolescents, the promotion of safe injection practices, and the development of health education in general[4,5]. Yet, HBV infection prevalence is still high among Chinese high-risk populations such as drug users (ranging from 8.8% to 51.6%) and men who have sex with men (MSM) (ranging from 6.5% to 10.3%)[6-10]. Moreover, few studies have evaluated the impact of the hepatitis B vaccination program since 1992 in a large general population, and studies on the relationship between socioeconomic status or familial history and the HBV carrier state are scant.
In this study, we planned a community-based epidemiological and serological study to identify simultaneously the prevalence of HBsAg among all age groups, to determine the potential risk factors for infection, and to evaluate the impact of the hepatitis B vaccination program in Eastern China. These findings are hoped eventually to guide the development, adaptation, and evaluation of prevention strategies for HBV.
This study was approved by the Institutional Review Board of Nanjing Medical University (Nanjing, China). All participants provided written informed consent to be interviewed. Planning for this study was started in January 2011 and data analysis was completed in December 2012. All field work was conducted from September 2011 to July 2012.
The target population was local residents of all age groups living in Jiangsu provinces, Eastern China (Supplemental Figure 1). Participants were selected as representative of the population of Eastern China, in that there was no statistically significant difference between participants in this study and the whole of Eastern China in demographics, the situation of the population or economic conditions. All local residents who had resided in Jiangsu for > 6 mo at the time of the survey were selected. A list of residents was obtained from the Residents’ Committee.
The sample size was calculated as following:
n = p(1 - p)/(d/za)2 = za2×p(1 - p)/d2
where p means the estimated prevalence rate, d represented power and was calculated by 0.1 ×p and zα was the statistics of α. For this study, α was set 0.05. Considering the variance of hepatitis C virus prevalence in different age groups in the survey (0.5% for age < 5 years, 1.5% for age 5-19 years, and 8% for age > 19 years), the desired sample size was 110466, and included 79600 people age < 5 years, 26266 people age 5-19 years, and 4600 people age > 19 years.
To represent the population of the whole province, a multistage sampling method was applied. First, the whole province was divided into three major groups (south, middle and north) determined by regional distinctions in earnings, education level, awareness of health care among the inhabitants, and health care system. One county was randomly selected from each major regional group: Zhangjiagang, Danyang and Taixing were chosen to represent south, middle and north sites, respectively. There were 887 communities in these three counties, and 20 communities were randomly chosen in each county. Finally, all local residents from those 60 communities were targeted for participation in this study by cluster sampling.
Before the survey was administered, community doctors issued a notice of physical examination to each household in the list of residents. The letter introduced the survey objective, examination items, and matters needing attention. Local residents willing to participate arrived at the survey spots in the community hospital at the appointed time. In each community hospital, there was a survey location and a team of 20-25 trained staff, including physicians, nurses, and community doctors, to carry out the investigation. A structured questionnaire was used by trained staff to ask basic information such as name, gender, birth date, education, occupation, marital status, smoking, drinking, history of hypertension and diabetes, immunization and familial history of HBV, and possible risk factors. All investigations were conducted through face to face interviews. Information on children under 15 years old was provided by their parents. Educational level was defined by academic certificate. Persons who had frequent contact with the public, such as an employee in a hotel, restaurant, barbershop, and market, were defined as public service workers. Children under 17 years old established their immunization status from the record of the immunization certificate kept by the parents. If they had no information about hepatitis B immunization, it was necessary to review the village doctor’s registry; if these sources were not available, the status was recorded as unvaccinated. In cases in which adults had no immunization records, immunization information for adults was from memory recall (vaccinated, unvaccinated, or unknown).
Blood samples collected from each study participant included 5 mL for the population aged > 2 years and 2 mL for children aged ≤ 2 years. Blood samples were collected without anticoagulant and were separated by centrifugation at 1800 ×g for 10 min and room temperature. Serum samples were stored at -70 °C. These procedures were completed within 6 h of sample collection. All samples were collected and frozen according to standardized procedures and tested in a central laboratory. HBsAg was detected by EILSA (Kehua Bio-Engineering Co. Ltd., Shanghai, China). Each reaction plate included two negative controls, three positive controls, and one blank control. More than 10% of the samples were randomly selected for repeated assays, and the results were 97% concordant.
Differences in demographic characteristics between HBsAg-positive and -negative groups were calculated using the Student t test or one-way analysis of variance (for continuous variables) and the χ2 test (for categorical variables). The associations of potential risk factors with HBV infection risk were estimated by computing the ORs and their 95%CIs from both univariate and multivariate logistic regression analyses. The Cochran-Armitage test was used for trend analysis. All statistical analyses were performed with SAS 9.1.3 software (SAS Institute, Cary, NC, United States), and P < 0.05 in a two-sided test was considered statistically significant.
The characteristics of the study population are shown in Table 1. Among the 148931 participants, 0.79% were aged 1-4 years, 18.83% aged 5-19 years, and 80.38% ≥ 20 years. The male to female ratio was 0.82:1. Among those aged ≥ 20 years, 88.88% were married, 57.27% were illiterate or had a primary school diploma, 33.72% were agricultural workers, and 72.80% and 79.75% never smoked and drank, respectively. The proportion of those adults without a history of hypertension and diabetes were 82.97% and 97.49%, respectively. In all age groups, subjects with a familial history of HBV and those reporting any hepatitis B vaccination were 8.23% and 34.28%.
| Variables | Value | |
| Age (yr) | 1-4 | 1175 (0.79) |
| 5-19 | 28046 (18.83) | |
| ≥ 20 | 119710 (80.38) | |
| Sex | Male | 67319 (45.20) |
| Female | 81612 (54.80) | |
| Marital status (≥ 20 yr) | Unmarried | 6614 (5.53) |
| Married | 106395 (88.88) | |
| Divorced | 6264 (5.23) | |
| Unknown | 437 (0.37) | |
| Education (≥ 20 yr) | Illiterate or primary school | 68552 (57.27) |
| Middle school | 43460 (36.31) | |
| College school | 7097 (5.93) | |
| Unknown | 599 (0.50) | |
| Occupation (≥ 20 yr) | Agricultural worker | 40363 (33.72) |
| Worker | 40530 (33.86) | |
| Student | 1248 (1.04) | |
| Civil servant | 5129 (4.28) | |
| Public service worker | 5222 (4.36) | |
| Unemployed | 21763 (18.18) | |
| Others | 4883 (4.08) | |
| Unknown | 572 (0.48) | |
| Smoking status (≥ 20 yr) | Never | 87144 (72.80) |
| Current | 27024 (22.57) | |
| Past | 2731 (2.28) | |
| Unknown | 2811 (2.35) | |
| Drinking status (≥ 20 yr) | Never | 95470 (79.75) |
| Frequently | 5900 (4.93) | |
| Every day | 2132 (1.78) | |
| Unknown | 16208 (13.54) | |
| BMI (kg/m2) (≥ 20 yr) | < 18.5 | 4238 (3.54) |
| 18.5-24 | 59117 (49.38) | |
| 24-28 | 41559 (34.72) | |
| ≥ 28 | 14796 (12.36) | |
| History of hypertension (≥ 20 yr) | No | 99327 (82.97) |
| Yes | 20383 (17.03) | |
| History of diabetes (≥ 20 yr) | No | 116701 (97.49) |
| Yes | 3009 (2.51) | |
| Familial history of HBV | No | 132997 (91.06) |
| Yes | 12016 (8.23) | |
| Unknown | 1034 (0.71) | |
| Immunization history of HBV | No | 75868 (51.77) |
| Yes | 50233 (34.28) | |
| Unknown | 20444 (13.95) |
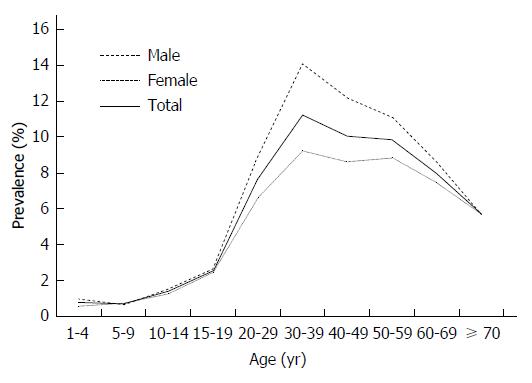
Among the 148931 participants studied, 11469 were HBsAg positive, while the overall prevalence of HBsAg in this study population was 7.70% (95%CI: 7.57%-7.84%). There was a great variation in HBsAg positivity among different age and gender groups. The prevalence was low in children (0.70%-0.77%), but increased progressively from adolescents (1.40%-2.55%) to adults aged ≥ 20 years (5.69%-11.22%) (Figure 1). Meanwhile, men had a higher prevalence of HBV infection than women in all age strata except the 5-9 years age group.
The prevalence of HBsAg stratified by other demographic and selected variables in this study population is described in Table 2. Significant differences were observed in marital status, education level, occupation, immunization and familial history of HBV about the prevalence of HBsAg. Among participants aged ≥ 20 years, the HBsAg prevalence among married participants (9.41%, 95%CI: 9.24%-9.59%) was higher than among the unmarried (7.36%, 95%CI: 6.75%-8.02%) or divorced (7.28%, 95%CI: 6.65%-7.95%) participants. HBsAg prevalence increased with decreasing education level. Participants who were illiterate or had primary school diplomas had the highest HBsAg prevalence (9.82%, 95%CI: 9.60%-10.05%), followed by middle school (8.35%, 95%CI: 8.09%-8.62%) and college school groups (8.05%, 95%CI: 7.42%-8.70%). HBsAg prevalence was significantly lower among students (6.81%, 95%CI: 5.48%-8.35%) and unemployed groups (6.74%, 95%CI: 6.41%-7.08%). The prevalence of HBsAg was higher in participants who had no history of hepatitis B vaccine, while it was lower in participants without a family history of HBV (P < 0.05 for both comparisons).
| Variables | HBsAg negative (n = 137462) | HBsAg positive (n = 11469) | Prevalence (%, 95%CI) | P value |
| Marital status (≥ 20 yr) | < 0.001 | |||
| Unmarried | 6127 | 487 | 7.36 (6.75-8.02) | |
| Married | 96383 | 10012 | 9.41 (9.24-9.59) | |
| Divorced | 5808 | 456 | 7.28 (6.65-7.95) | |
| Education (≥ 20 yr) | < 0.001 | |||
| Illiterate or primary school | 61819 | 6733 | 9.82 (9.60-10.05) | |
| Middle school | 39830 | 3630 | 8.35 (8.09-8.62) | |
| College school | 6526 | 571 | 8.05 (7.42-8.70) | |
| Occupation (≥ 20 yr) | < 0.001 | |||
| Agricultural worker | 36186 | 4177 | 10.35 (10.05-10.65) | |
| Worker | 36717 | 3813 | 9.41 (9.13-9.70) | |
| Student | 1163 | 85 | 6.81 (5.48-8.35) | |
| Civil servant | 4672 | 457 | 8.91 (8.14-9.72) | |
| Public service worker | 4749 | 473 | 9.06 (8.29-9.87) | |
| Unemployed | 20297 | 1466 | 6.74 (6.41-7.08) | |
| Others | 4411 | 472 | 9.67 (8.85-10.53) | |
| Immunization history of HBV | < 0.001 | |||
| No | 68111 | 7757 | 10.22 (10.01-10.44) | |
| Yes | 48430 | 1803 | 3.59 (3.43-3.76) | |
| Familial history of HBV | ||||
| No | 123472 | 9525 | 7.16 (7.02-7.30) | < 0.001 |
| Yes | 10319 | 1697 | 14.12 (13.50-14.76) |
The frequency of various risk factors associated with HBV infection and the calculated crude OR estimated by univariate and multivariate analysis are shown in Tables 3 and 4. Among the population aged 1-19 years, age, sex, hepatitis B immunization, and familial history of HBV were correlated with HBsAg positivity. However, after adjusting for those variables, older age (OR = 4.12, 95%CI: 1.93-8.75 for 15-19 years vs 1-4 years group) and familial history of HBV (OR = 2.0, 95%CI: 1.88-2.13) were associated with an increased risk of HBV infection. Participants with a history of immunization had a 29% decrease in the risk of HBV infection.
| Variables | uaj. OR (95%CI) | P value | aj. OR (95%CI) | P value |
| Age group (yr) | ||||
| 5-9 vs 1-4 | 0.91 (0.45-1.86) | 0.805 | 1.14 (0.52-2.54) | 0.740 |
| 10-14 vs 1-4 | 1.84 (0.93-3.63) | 0.078 | 1.18 (0.86-3.21) | 0.095 |
| 15-19 vs 1-4 | 3.39 (1.74-6.60) | < 0.001 | 4.12 (1.93-8.75) | < 0.001 |
| Sex (female vs male) | 0.89 (0.74-1.07) | 0.218 | - | - |
| Immunization history of HBV (yes vs no) | 0.50 (0.35-0.70) | < 0.001 | 0.71 (0.49-0.98) | 0.028 |
| Familial history of HBV (yes vs no) | 2.52 (1.83-3.49) | < 0.001 | 2.00 (1.88-2.13) | < 0.001 |
| Variables | uaj. OR (95%CI) | P value | aj. OR (95%CI) | P value |
| Age group (yr) | ||||
| 30-39 vs 20-29 | 1.52 (1.41-1.65) | < 0.001 | 1.18 (1.08-1.30) | < 0.001 |
| 40-49 vs 20-29 | 1.35 (1.25-1.45) | < 0.001 | 0.97 (0.88-1.06) | 0.494 |
| 50-59 vs 20-29 | 1.32 (1.22-1.42) | < 0.001 | 0.87 (0.79-1.02) | 0.108 |
| 60-69 vs 20-29 | 1.04 (0.96-1.13) | 0.339 | 0.84 (0.57-1.02) | 0.212 |
| ≥ 70 vs 20-29 | 0.92 (0.85-1.10) | 0.962 | 0.81 (0.69-1.02) | 0.478 |
| Sex (female vs male) | 0.74 (0.71-0.77) | < 0.001 | 0.72 (0.69-0.75) | < 0.001 |
| Familial history of HBV (yes vs no) | 1.90 (1.80-2.01) | < 0.001 | 1.93 (1.83-2.05) | < 0.001 |
| Immunization history of HBV (yes vs no) | 0.56 (0.53-0.59) | < 0.001 | 0.52 (0.49-0.56) | < 0.001 |
| Hospitalization (yes vs no) | 0.95 (0.92-1.04) | 0.227 | - | - |
| Surgery (yes vs no) | 0.92 (0.85-1.03) | 0.312 | - | - |
| Blood transfusion (yes vs no) | 1.00 (0.88-1.13) | 0.966 | - | - |
| Blood donation (yes vs no) | 0.88 (0.75-1.12) | 0.215 | - | - |
| Invasive testing (yes vs no) | 1.44 (1.36-1.53) | < 0.001 | 1.18 (1.11-1.26) | < 0.001 |
| Hemodialysis (yes vs no) | 0.56 (0.20-1.53) | 0.255 | - | - |
| Acupuncture (yes vs no) | 0.94 (0.87-1.09) | 0.612 | - | - |
| Dental therapy (yes vs no) | 0.88 (0.82-1.01) | 0.061 | - | - |
| Tattoos (yes vs no) | 1.24 (0.84-1.82) | 0.280 | - | - |
| Piercing (yes vs no) | 0.97 (0.92-1.03) | 0.388 | - | - |
| Sharing needles (yes vs no) | 1.40 (1.09-1.80) | 0.008 | 1.44 (1.12-1.86) | 0.005 |
| Sharing razors (yes vs no) | 0.90 (0.81-1.02) | 0.478 | - | - |
| Sharing toothbrush (yes vs no) | 1.04 (0.76-1.41) | 0.814 | - | - |
Among the population aged ≥ 20 years, univariate logistic regression showed that age, sex, hepatitis B immunization, familial history of HBV, invasive testing, and sharing needles were correlated with HBsAg positivity. After adjusting for those variables, older age (OR = 1.18, 95%CI: 1.08-1.30 for 30-39 years vs 20-29 years group), familial history of HBV (OR = 1.93, 95%CI: 1.83-2.05), invasive testing (OR = 1.18, 95%CI: 1.11-1.26), and sharing needles (OR = 1.44, 95%CI: 1.12-1.86) were associated with an increased risk of HBV infection. However, female gender and a history of HBV immunization had a significant decrease in the risk of HBV infection (OR = 0.72, 0.52 for gender and immunization, respectively).
Among the 148931 participants studied, 12016 (8.28%, 95%CI: 8.14%-8.43%) had a familial history of HBV. When different classes of relatives were analyzed separately, HBV infection risks showed a statistically significant increase for mothers, fathers, spouses, offspring and siblings (Table 5). In particular, mothers and siblings who were HBsAg seropositive significantly increased risk by 25.7% and 21.0%, respectively. We evaluated the combined effects by adding up the number of families with HBsAg seropositivity. The ORs for risk of HBV infection increased concomitantly with the number of HBsAg-seropositive family members (Table 5). Participants with 3-6 family members who were HBsAg seropositive were significantly associated with HBV infection (OR = 9.82, 95%CI: 4.80-20.11), compared with participants without a familial history of HBV.
| Variables | HBsAg negative | HBsAg positive | OR (95%CI) | P value |
| Mother | 1031 | 317 | 3.57 (3.12-4.08) | < 0.001 |
| Father | 2488 | 432 | 1.95 (1.75-2.17) | < 0.001 |
| Spouse (≥ 20 yr) | 3363 | 531 | 1.02 (1.00-1.04) | 0.041 |
| Offspring (≥ 20 yr) | 1817 | 283 | 1.61 (1.41-1.83) | < 0.001 |
| Siblings | 1059 | 353 | 3.10 (2.73-3.51) | < 0.001 |
| Other families | 1061 | 130 | 1.57 (1.30-1.89) | < 0.001 |
| Combined familial history of HBV and HBV infection | ||||
| 0 | 123472 (92.29) | 9525 (84.88) | 1 | - |
| 1 | 9838 (7.36) | 1562 (13.95) | 1.93 (1.82-2.05) | < 0.001 |
| 2 | 462 (0.34) | 121 (1.05) | 3.19 (2.60-3.92) | < 0.001 |
| 3-6 | 19 (0.01) | 14 (0.12) | 9.82 (4.80-20.11) | < 0.001 |
| Trend | < 0.0011 | |||
| 0 | 123472 (92.29) | 9525 (84.88) | 1 | - |
| 1-6 | 10319 (7.71) | 1697 (15.12) | 2.00 (1.89-2.12) | < 0.001 |
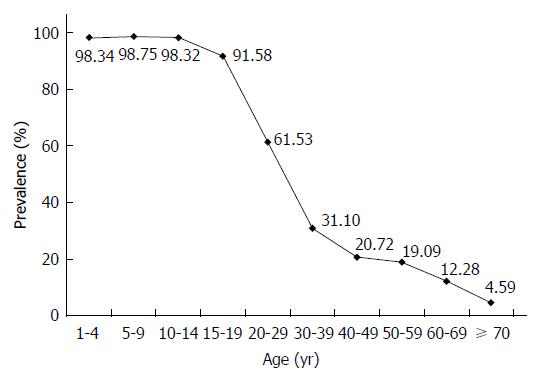
Among the 126101 participants effectively surveyed about hepatitis B immunization, 50233 (39.84%, 95%CI: 35.57%-40.11%) had a history of HBV immunization. There was a significant decreasing trend in vaccination prevalence associated with increasing age (Figure 2). There was great variation in immunization prevalence among different age groups, ranging from 98.75% to 4.59%. The rate among children aged 1-19 years born after the hepatitis B vaccine was recommended for routine childhood immunization was 93.30% (95%CI: 93.01%-93.58%). Among all hepatitis-B-vaccinated persons in this study, the main methods of immunization were planned immunization and collective organization vaccination (44.84% and 41.10%, respectively). The main reason for lack of immunization was that participants had little information about hepatitis B vaccination (80.60%).
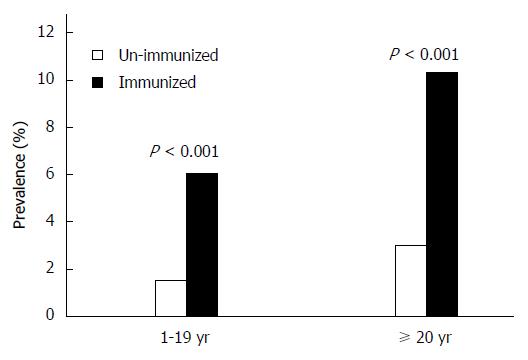
The prevalence of HBsAg among vaccinated persons was only 3.59% (95%CI: 3.56%-3.90%), compared to 10.22% (95%CI: 10.15%-11.63%) among unvaccinated persons (P < 0.001). This difference was proportionally greater in adult groups (Figure 3).
We report here a community-based epidemiological study of HBV infection in a large sample from an Eastern Chinese population. Unlike the previous population-based epidemiological studies that were mainly from Europe and North America, the prevalence of HBV infection and characterization of prevailing hepatitis B immunization in the same population provide new insight into the contribution of HBV to the causation of liver disease in China, and this knowledge may also be relevant to other countries; particularly those with similar levels of endemicity[11-16]. Earlier studies of HBV prevalence mostly focused on high-risk populations such as drug users, paid blood donors, and MSM in China and other countries[6-10,17,18]. However, owing to low numbers of children and elderly in these groups investigated, their results cannot be extrapolated to the general population.
Without a strict standard of sampling procedures, prior community-based studies on HBV prevalence in Eastern China could not eliminate bias. Compared with these prior studies, this study involved a greater number of participants[19-21]. Moreover, the present investigation used a multistage sampling procedure. Zhangjiagang, Danyang and Taixing were chosen to represent southern, middle and northern sites of Jiangsu province, Eastern China, respectively. This aimed to eliminate bias from local social, economic or cultural factors that might be associated with the prevalence of HBV. Twenty communities were randomly chosen in each county. Finally, all local residents in those 60 communities were targeted for participation by cluster sampling. Therefore, the data in this study with a rigorous standard of sampling procedures and a large sample size represents the general population in Jiangsu effectively.
The prevalence of HBsAg (7.70%, 95%CI: 7.57%- 7.84%) in the present survey was close to that of the whole country (7.20%) and southeast area (7.90%)[4,19]. The age-specific prevalence of HBV in the study was low in children (0.70%-0.77%) but increased progressively from adolescents (1.40%-2.55%) to adults aged ≥ 20 years (5.69%-11.22%). This suggests a cumulative increase of incidence of infection, probably as a result of the sporadic transmission of infection persisting in the community through the years. In this survey, men had a higher prevalence of HBV infection than women. This may be because drug users are more likely to be male, which may lead the prevalence among males to be higher, or because cultural factors induce women to focus on their health status and pay more attention to personal hygiene in China, which may effectively decrease the risk of HBV infection.
Our multivariate analysis revealed that among adults aged ≥ 20 years, invasive testing and sharing needles increased the risk of HBV infection. One reason might be that the general population in that period had less education on health care, and a lack of knowledge about modes of viral transmission also increased the risk of infection. Another reason might be the scarcity of systematically trained physicians. Without high standards for medical training, most medical staff may not be able to understand HBV and sterilization procedures well. However, the most influential factor associated with HBV was a familial history of HBV and hepatitis B vaccination in both children and adults groups. One of the reasons for this might be mother-to-child vertical transmission, which is the main route of chronic HBV infection in China[22]. Meanwhile, studies also indicate that chronic infection with HBV may closely related to the transmission of HBV between siblings during childhood in certain areas[23,24].
After the hepatitis B vaccine was recommended for routine infant immunizations in 1992, and especially after it was fully integrated into routine infant immunizations in 2002, newborn children had less HBV infection and lower HBsAg prevalence[25]. The decline of HBsAg will eventually decrease the prevalence of HBsAg and the future incidence of cirrhosis and hepatocellular carcinoma[3,26,27]. One cohort study has estimated that on a global scale, the vaccine has prevented 16-20 million HBV carriers and 2.8-3.5 million future HBV infections and deaths[28]. Most of the unimmunized population that we studied lacked knowledge about the hepatitis B vaccine. Therefore, health education of the general older population is important and may prove useful in preventive interventions in China.
In a large country like China, having population-based data on HBV prevalence will be informative. At present, China has successfully decreased the number of HBsAg carriers and reached the national goal of reducing HBsAg prevalence to < 1% among children under 5 years, and the hepatitis B immunization rate to > 90% among teenagers, < 20 years after integrating hepatitis B vaccine into routine immunization programs. However, the national goal for the whole population of decreasing the prevalence of HBsAg to < 7% has not yet been achieved. Therefore, free immunization of infants should be maintained, expanded vaccination is needed for adults, especially, in those aged ≥ 30 years, and health education should be further strengthened to limit the spread of HBV infection.
We thank the students who have worked on the study, including Ying-Ying Zhu, Qing Wang, Hui Zheng, Yuan-Yuan Zhang, Yin Xu, Wen-Zhe Ma. We also thank the staff from the Centers for Disease Control and Prevention of Jiangsu province for organization of the field investigation. This research would not have been possible without the consent and help of the participants.
Hepatitis B virus (HBV) poses a serious global health problem because of its adverse clinical outcomes, such as cirrhosis and hepatocellular carcinoma. The WHO estimates there are approximately 2 billion people infected by HBV worldwide, and 240 million of them have chronic infection. In China, a highly endemic area, the national prevalence for hepatitis B surface antigen (HBsAg) positivity is 7.20%.
Many studies have suggested that the incidence of newly acquired HBV infection in China has declined steadily since the 1990s by virtue of a number of public health interventions, such as the screening of pregnant women and the vaccination of infants and adolescents, the promotion of safe injection practices, and the development of health education in general. Yet HBV infection prevalence is still high among Chinese high-risk populations such as drug users and men who have sex with men.
The present study aimed to evaluate the impact of the hepatitis B vaccination program since 1992 in a large general population, and to clarify the relationship between socioeconomic status or familial history and the HBV carrier state.
7.70% were positive for HBsAg which has not achieved the national goal for the whole population in decreasing the prevalence of HBsAg to < 7%. The prevalence was just 0.77% among children aged < 5 years and the rate of HBV immunization among teenagers < 20 years was 93.30%, which have reached the national goal of < 1% and > 90%, respectively.
It is important to know impact of the hepatitis B vaccination program. The study is innovative in nature. The original study conducted on large groups of participants is very valuable.
P- Reviewer: Ferenci P, Kanda T, Singh S, Utama A S- Editor: Ma YJ L- Editor: Kerr C E- Editor: Ma S
| 1. | Lavanchy D. Chronic viral hepatitis as a public health issue in the world. Best Pract Res Clin Gastroenterol. 2008;22:991-1008. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 74] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 2. | El-Serag HB. Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology. 2012;142:1264-1273.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2183] [Cited by in RCA: 2493] [Article Influence: 191.8] [Reference Citation Analysis (1)] |
| 3. | Ott JJ, Stevens GA, Groeger J, Wiersma ST. Global epidemiology of hepatitis B virus infection: new estimates of age-specific HBsAg seroprevalence and endemicity. Vaccine. 2012;30:2212-2219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1217] [Cited by in RCA: 1328] [Article Influence: 102.2] [Reference Citation Analysis (0)] |
| 4. | Liang X, Bi S, Yang W, Wang L, Cui G, Cui F, Zhang Y, Liu J, Gong X, Chen Y. Reprint of: Epidemiological serosurvey of Hepatitis B in China--declining HBV prevalence due to Hepatitis B vaccination. Vaccine. 2013;31 Suppl 9:J21-J28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 116] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 5. | Siddiqui MR, Gay N, Edmunds WJ, Ramsay M. Economic evaluation of infant and adolescent hepatitis B vaccination in the UK. Vaccine. 2011;29:466-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 33] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 6. | Zhou YH, Liu FL, Yao ZH, Duo L, Li H, Sun Y, Zheng YT. Comparison of HIV-, HBV-, HCV- and co-infection prevalence between Chinese and Burmese intravenous drug users of the China-Myanmar border region. PLoS One. 2011;6:e16349. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 41] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 7. | Nelson PK, Mathers BM, Cowie B, Hagan H, Des Jarlais D, Horyniak D, Degenhardt L. Global epidemiology of hepatitis B and hepatitis C in people who inject drugs: results of systematic reviews. Lancet. 2011;378:571-583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 949] [Cited by in RCA: 972] [Article Influence: 69.4] [Reference Citation Analysis (0)] |
| 8. | Ruan Y, Liang S, Zhu J, Li X, Pan SW, Liu Q, Song B, Wang Q, Xing H, Shao Y. Evaluation of harm reduction programs on seroincidence of HIV, hepatitis B and C, and syphilis among intravenous drug users in southwest China. Sex Transm Dis. 2013;40:323-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 32] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 9. | Ruan Y, Luo F, Jia Y, Li X, Li Q, Liang H, Zhang X, Li D, Shi W, Freeman JM. Risk factors for syphilis and prevalence of HIV, hepatitis B and C among men who have sex with men in Beijing, China: implications for HIV prevention. AIDS Behav. 2009;13:663-670. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 80] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 10. | Ma X, Zhang Q, He X, Sun W, Yue H, Chen S, Raymond HF, Li Y, Xu M, Du H. Trends in prevalence of HIV, syphilis, hepatitis C, hepatitis B, and sexual risk behavior among men who have sex with men. Results of 3 consecutive respondent-driven sampling surveys in Beijing, 2004 through 2006. J Acquir Immune Defic Syndr. 2007;45:581-587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 176] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 11. | Kim WR, Benson JT, Therneau TM, Torgerson HA, Yawn BP, Melton LJ. Changing epidemiology of hepatitis B in a U.S. community. Hepatology. 2004;39:811-816. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 57] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 12. | Mitchell AE, Colvin HM, Palmer Beasley R. Institute of Medicine recommendations for the prevention and control of hepatitis B and C. Hepatology. 2010;51:729-733. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 157] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 13. | Sheikh MY, Atla PR, Raoufi R, Sadiq H, Sadler PC. Prevalence of hepatitis B infection among young and unsuspecting Hmong blood donors in the Central California Valley. J Community Health. 2012;37:181-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 14. | Chen CJ, Wang LY, Yu MW. Epidemiology of hepatitis B virus infection in the Asia-Pacific region. J Gastroenterol Hepatol. 2000;15 Suppl:E3-E6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 176] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 15. | Rein DB, Lesesne SB, O’Fallon A, Weinbaum CM. Prevalence of hepatitis B surface antigen among refugees entering the United States between 2006 and 2008. Hepatology. 2010;51:431-434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 16. | Brouard C, Gautier A, Saboni L, Jestin C, Semaille C, Beltzer N. Hepatitis B knowledge, perceptions and practices in the French general population: the room for improvement. BMC Public Health. 2013;13:576. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 36] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 17. | Zou S, Stramer SL, Notari EP, Kuhns MC, Krysztof D, Musavi F, Fang CT, Dodd RY. Current incidence and residual risk of hepatitis B infection among blood donors in the United States. Transfusion. 2009;49:1609-1620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 93] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 18. | Gough E, Kempf MC, Graham L, Manzanero M, Hook EW, Bartolucci A, Chamot E. HIV and hepatitis B and C incidence rates in US correctional populations and high risk groups: a systematic review and meta-analysis. BMC Public Health. 2010;10:777. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 81] [Cited by in RCA: 78] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 19. | Luo Z, Xie Y, Deng M, Zhou X, Ruan B. Prevalence of hepatitis B in the southeast of China: a population-based study with a large sample size. Eur J Gastroenterol Hepatol. 2011;23:695-700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 45] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 20. | Li X, Zheng Y, Liau A, Cai B, Ye D, Huang F, Sheng X, Ge F, Xuan L, Li S. Hepatitis B virus infections and risk factors among the general population in Anhui Province, China: an epidemiological study. BMC Public Health. 2012;12:272. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 33] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 21. | Wong VW, Wong GL, Chu WC, Chim AM, Ong A, Yeung DK, Yiu KK, Chu SH, Chan HY, Woo J. Hepatitis B virus infection and fatty liver in the general population. J Hepatol. 2012;56:533-540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 194] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 22. | Lu FM, Zhuang H. Management of hepatitis B in China. Chin Med J (Engl). 2009;122:3-4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 23. | Ucmak H, Faruk Kokoglu O, Celik M, Ergun UG. Intra-familial spread of hepatitis B virus infection in eastern Turkey. Epidemiol Infect. 2007;135:1338-1343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 24. | Chakravarty R, Chowdhury A, Chaudhuri S, Santra A, Neogi M, Rajendran K, Panda CK, Chakravarty M. Hepatitis B infection in Eastern Indian families: need for screening of adult siblings and mothers of adult index cases. Public Health. 2005;119:647-654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 25. | Liang X, Bi S, Yang W, Wang L, Cui G, Cui F, Zhang Y, Liu J, Gong X, Chen Y. Evaluation of the impact of hepatitis B vaccination among children born during 1992-2005 in China. J Infect Dis. 2009;200:39-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 230] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 26. | Chang MH, Chen CJ, Lai MS, Hsu HM, Wu TC, Kong MS, Liang DC, Shau WY, Chen DS. Universal hepatitis B vaccination in Taiwan and the incidence of hepatocellular carcinoma in children. Taiwan Childhood Hepatoma Study Group. N Engl J Med. 1997;336:1855-1859. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1328] [Cited by in RCA: 1194] [Article Influence: 42.6] [Reference Citation Analysis (0)] |
| 27. | Lu SQ, McGhee SM, Xie X, Cheng J, Fielding R. Economic evaluation of universal newborn hepatitis B vaccination in China. Vaccine. 2013;31:1864-1869. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 36] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 28. | Goldstein ST, Zhou F, Hadler SC, Bell BP, Mast EE, Margolis HS. A mathematical model to estimate global hepatitis B disease burden and vaccination impact. Int J Epidemiol. 2005;34:1329-1339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 494] [Cited by in RCA: 477] [Article Influence: 23.9] [Reference Citation Analysis (0)] |