Published online Jul 7, 2015. doi: 10.3748/wjg.v21.i25.7824
Peer-review started: December 18, 2014
First decision: January 22, 2015
Revised: February 20, 2015
Accepted: April 17, 2015
Article in press: April 17, 2015
Published online: July 7, 2015
Processing time: 206 Days and 0.2 Hours
AIM: To investigate gadolinium-ethoxybenzyl-diethylenetriamine-pentaacetic acid (Gd-EOB-DTPA)-enhanced magnetic resonance imaging (MRI) of intraductal papillary mucinous neoplasms of the bile duct (IPMN-B).
METHODS: The imaging findings of five cases of IPMN-B which were pathologically confirmed at our hospital between March 2012 and May 2013 were retrospectively analyzed. Three of these cases were diagnosed by duodenal endoscopy and biopsy pathology, and two cases were diagnosed by surgical pathology. All five patients underwent enhanced and non-enhanced computed tomography (CT), magnetic resonance cholangiopancreatography, and Gd-EOB-DTPA-enhanced MRI; one case underwent both Gd-EOB-DTPA-enhanced MRI and positron emission tomography-CT. The clinical data and imaging results for these cases were compared and are presented.
RESULTS: Conventional imaging showed diffuse dilatation of bile ducts and multiple intraductal polypoid and papillary neoplasms or serrated changes along the bile ducts. In two cases, Gd-EOB-DTPA-enhanced MRI revealed dilated biliary ducts and intraductal tumors, as well as filling defects caused by mucin in the dilated bile ducts in the hepatobiliary phase. Gd-EOB-DTPA-enhanced MRI in one case clearly showed a low-signal tumor in the hepatobiliary phase, similar to what was seen by positron emission tomography-CT. In two patients, routine inspection was unable to discern whether the lesions were inflammation or tumors. However, Gd-EOB-DTPA-enhanced MRI revealed a pattern of gradual enhancement during the hepatobiliary phase, and the signal intensity of the lesions was lower than the surrounding liver parenchyma, suggesting tissue inflammation in both cases, which were confirmed by surgical pathology.
CONCLUSION: Gd-EOB-DTPA-enhanced MRI reveals the intraductal mucin component of IPMN-B in some cases and the extent of tumor infiltration beyond the bile ducts in invasive cases.
Core tip: Gadolinium-ethoxybenzyl-diethylenetriamine-pentaacetic acid (Gd-EOB-DTPA)-enhanced magnetic resonance imaging (MRI) can be used to demonstrate the filling defects due to mucin secreted by intraductal papillary mucinous neoplasms of the bile duct (IPMN-B) and to display the extent of tumor infiltration beyond bile ducts in cases with invasive IPMN-B. It also has the potential to differentiate tumor tissue from inflammatory lesions. Therefore, Gd-EOB-DTPA-enhanced MRI may improve the clinical management of IPMN-B.
- Citation: Ying SH, Teng XD, Wang ZM, Wang QD, Zhao YL, Chen F, Xiao WB. Gd-EOB-DTPA-enhanced magnetic resonance imaging for bile duct intraductal papillary mucinous neoplasms. World J Gastroenterol 2015; 21(25): 7824-7833
- URL: https://www.wjgnet.com/1007-9327/full/v21/i25/7824.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i25.7824
Intraductal papillary mucinous neoplasms of the bile duct (IPMN-B) are a subtype of intraductal papillary neoplasms of the bile duct with macroscopically visible mucin secretion[1-7]. The clinical manifestations, histopathologic features, and immunohistochemical and biologic behaviors of this bile duct subtype are similar to IPMNs of the pancreas[1-6]. IPMN-B originates from biliary epithelial cells, and can be pathologically defined as papillary adenoma, papillomatosis, carcinoma in situ, or invasive adenocarcinoma. Imaging findings of IPMN-B include: papillary or polypoid growth of the tumor along the bile duct or serrated inner lining of the bile duct; expansive and significant dilation of the bile duct upstream and downstream of the tumor; or aneurysmal dilation of the bile duct at the site of the tumor[8-10]. However, confirmation of a diagnosis requires the use of endoscopic retrograde cholangiopancreatography (ERCP) to determine the presence of mucin secreted by the tumor[4]. In addition, malignant IPMN-Bs can invade the liver and form a mass, although conventional ultrasonography (US), computed tomography (CT), and dynamic enhancement magnetic resonance scans cannot define the scope of tumor invasion, or distinguish the accompanying inflammation from the tumor[11,12].
Gadolinium-ethoxybenzyl-diethylenetriamine pentaacetic acid (Gd-EOB-DTPA) is a double-specific contrast agent that provides enhancement effects similar to Gd-DTPA in dynamic enhanced scans. With Gd-EOB-DTPA, 50% of the contrast injection is absorbed by hepatocytes and drained via the bile duct in the hepatobiliary phase. Although this contrast agent has been used for the diagnosis of hepatogenic tumor lesions[11], only two reports (four cases) describe the use of Gd-EOB-DTPA-enhanced magnetic resonance imaging (MRI) for the diagnosis of IPMN-B[13,14]. In those reports, Gd-EOB-DTPA-enhanced MRI not only revealed the dilated bile duct and the enhanced tumor tissues within it, but also confirmed that the filling defect of the bile duct at the hepatobiliary phase was mucus secreted by the tumors[13,14], thus demonstrating its unique value for tumor diagnosis. The present study describes the application of Gd-EOB-DTPA-enhanced MRI in five cases of IPMN-B. In addition to displaying the features of IPMN-Bs, this method reveals the extent of invasion into the extrahepatic bile duct in malignant cases. Furthermore, Gd-EOB-DTPA-enhanced MRI can discern tumor tissue from surrounding inflammation, which has not previously been reported.
Five cases of IPMN-B in our hospital during the period from March 2012 to May 2013 were retrospectively analyzed (Table 1). Two cases were confirmed by surgical pathology. Three cases underwent thick-needle biopsy, and 3-5 pieces of cord-like tissue were obtained for each case; diagnoses were confirmed by two senior pathologists.
| No. | Sex | Age (yr) | History and symptoms | Laboratory findings (normal range) | Imaging | ||||||
| US | CT + C | Gd-DTPA MRI | MRCP | ERCP | PET-CT | Gd-EOB-DTPA-enhanced MRI | |||||
| 1 | Female | 59 | Recurrent upper abdominal pain with nausea and vomiting for 6 mo | No significant abnormalities | A | A | NA | A | A | NA | A |
| 2 | Male | 52 | Physical examination revealed liver tumor | CEA: 7.5 ng/mL (0.0-8.0) | A | A | NA | A | A | NA | A |
| 3 | Female | 72 | Jaundice | CEA: 9.8 ng/mL | A | A | NA | A | A | NA | A |
| CA199: 715.1 U/mL (0.0-37.0) | |||||||||||
| Total bilirubin: 611 mmol/L (0-21) | |||||||||||
| 4 | Male | 66 | Jaundice | CEA: 44.0 ng/mL | A | A | NA | A | NA | NA | A |
| CA199: > 12000 U/mL | |||||||||||
| Total bilirubin: 424 mmol/L | |||||||||||
| 5 | Male | 56 | 8-yr history of liver contusion; liver pain for 6 mo | CA199: 217.3 U/mL | A | A | A | A | NA | A | A |
| CRP: 34.5 mg/L (0.0-8.0) | |||||||||||
CT images were acquired by a 16-slice CT scanner (Aquilion; Toshiba, Tokyo, Japan) using the following scan parameters: 120 kV; 250 mA; reconstruction thickness, 5 mm; layer spacing, 5 mm. CT scans were obtained before and after intravenous administration of 80-100 mL contrast agent (300 mg I/mL Ultravist; Bayer Healthcare, Berlin, Germany) at a speed of 3.0 mL/s. CT scan delays after the injection of contrast agent were 25-30 s, 65 s, and 120 s for the arterial, portal venous, and delayed phases, respectively.
MRI was performed using a 3.0 T scanner (Signa HDxt 3.0 T; GE Healthcare, Little Chalfont, United Kingdom) with a corresponding eight-channel phase-array abdomen coil system. MRI sequences included axial T2- and diffusion-weighted imaging, magnetic resonance cholangiopancreatography (MRCP) and fat suppression T1-weighted three-dimensional liver acquisition with volume acceleration (LAVA)-enhanced imaging. The parameters for MRI sequences used in this study are listed in Table 2.
| Sequence | TR (ms) | TE (ms) | Section thickness (mm) | Gap(mm) | Matrix size(pixels) | Flip angle(degrees) | Field of view (cm) |
| T2WI | 6000-10000 | 91 | 6 | 2 | 320 × 224 | 90 | 40 × 32 |
| DWI (b = 1000) | 8000 | 65 | 6 | 2 | 128 × 128 | 90 | 40 × 32 |
| MRCP | 6000-10000 | 800 | 2.4 | 0 | 384 × 224 | 90 | 40 × 32 |
| Gd-DTPA-enhanced MRI | 2.84 | 1.34 | 5 | 0 | 384 × 256 | 10 | 40 × 32 |
| Gd-EOB-DTPA-enhanced MRI | 2.84 | 1.34 | 5 | 0 | 384 × 256 | 10 | 40 × 32 |
The LAVA-enhanced MRI was conducted via bolus i.v. injection of Gd-DPTA (Magnevist; Schering, Berlin, Germany) at 0.1 mmol/kg body weight and a speed of 2 mL/s. Pre-contrast and dynamic contrast-enhanced multi-phase MRI scans were acquired. The MRI scan delays were 25-30 s, 85 s, and 180 s for the arterial, portal venous, and delayed phases, respectively. The same sequence and parameters were used for dynamic Gd-EOB-DPTA-enhanced MRI following a bolus i.v. injection of EOB (Primovist; Bayer Healthcare) at 0.025 mmol/kg body weight and a speed of 2 mL/s. Fat suppression T1-weighted three-dimensional LAVA was performed 20 min after contrast injection and the images of the initial hepatobiliary phase were obtained, and repeated after 42-52 min for the delayed scans of the hepatobiliary phase. The image of the hepatobiliary phase was visualized with maximum intensity projections or multiple planar reconstructions to observe the filling condition of the intra- and extrahepatic bile ducts[11,12,15].
All US studies were performed using a color ultrasound (Sequoia 512; Siemens Medical Solutions, Munich, Germany) with a 1.0- to 4.0-MHz convex probe. One patient received a whole-body PET/CT scan performed on an integrated PET/CT scanner (Biograph 16; Siemens Medical Solutions) after the injection of 350 MBq 18F-FDG.
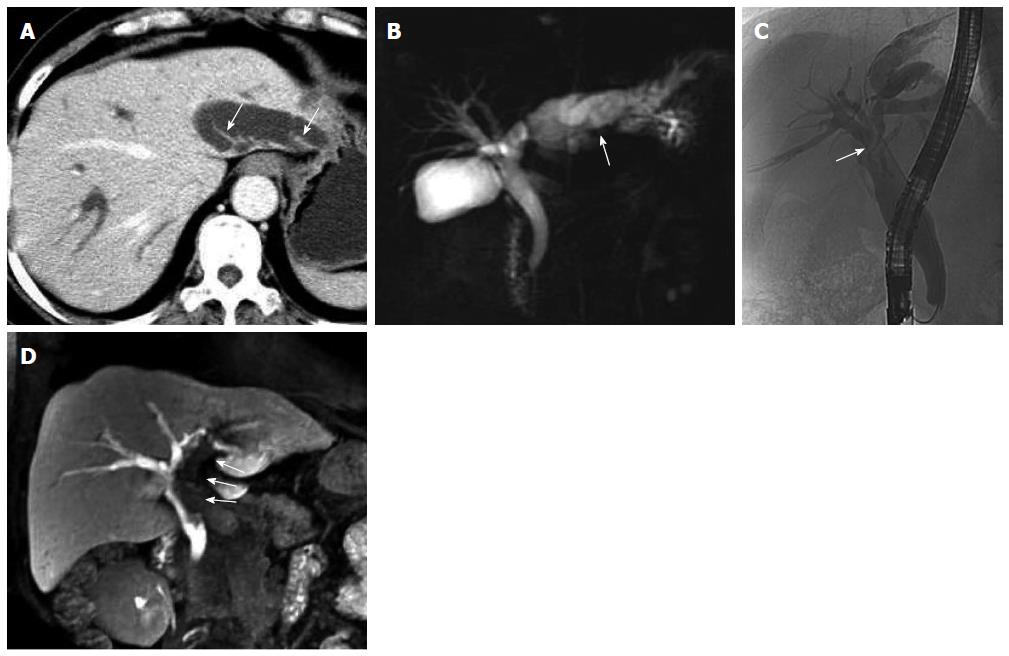
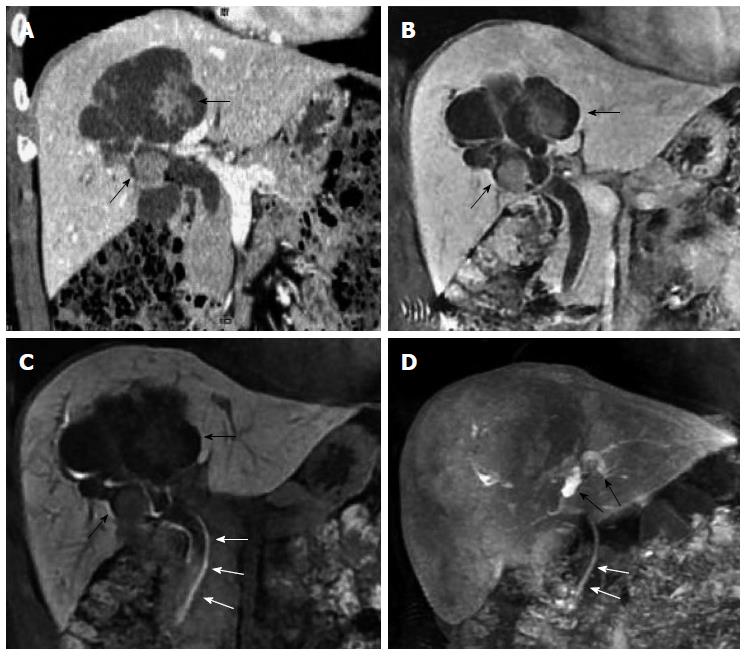
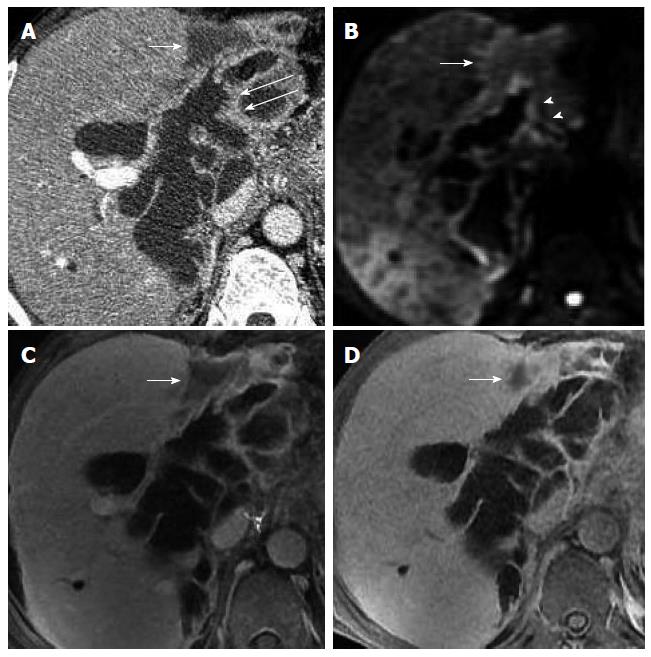
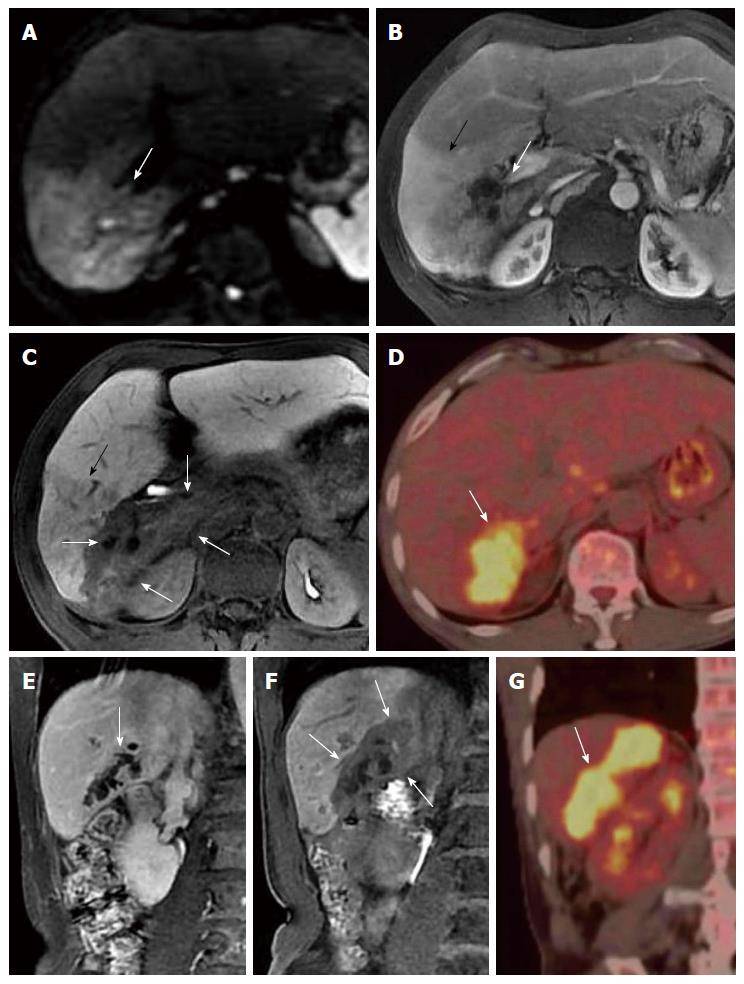
US, CT/MRI, and MRCP depicted extensive intra- and extrahepatic bile duct dilatation in cases 1-4, including upstream and downstream of the tumor and at the segmental bile duct (Figures 1, 2, 3 and 4). In contrast-enhanced CT and MRI examinations, multiple polypoid or papillary tumors which were distributed along the bile ducts showed mild to medium enhancement. In case 2, partial right intrahepatic bile ducts were seen as aneurysm-like dilatations (Figure 2). In case 4, a slightly higher-signal nodule was also seen at the edge of the dilated left intrahepatic bile ducts in T2- and diffusion-weighted imaging, which was enhanced slightly during all three phases of the contrast-enhanced CT scan (Figure 4). In this case, the tumor lesions were considered to penetrate into the liver via the bile duct wall. In case 5, CT/MRI scans depicted right hepatic atrophy surrounding the right dilated intrahepatic bile duct (Figure 5). Enhanced CT and Gd-DPTA-enhanced MRI in both the arterial and portal venous phases depicted marked, although ill-defined, patchy enhancement around the dilated bile duct, which was isodense in the delayed phase. Positron emission tomography (PET)-CT in case 5 revealed a mass of 6.5 cm × 6.0 cm with high uptake of fluorodeoxyglucose (FDG) around the dilated bile duct in the posterior segment of the liver (the maximum standard uptake value was 6.5), indicating a malignant tumor (Figure 5). Therefore, the tumor in this patient was considered to be an invasive IPMN-B.
Dilated bile ducts and tumors on the bile duct walls in the five cases were also observed with plain MRI and dynamic triple-enhanced EOB scans. In addition, some specific signs were seen in ≥ 20 min delayed scans, namely the hepatobiliary phase. Cases 1 and 2 showed irregular columnar filling defects, and the edge of the contrast reagent appeared in a cupped or half-ring distribution (Figures 1 and 2).
Cholangiectasis was apparent in cases 3 and 4, and a high signal of contrast filling was not seen in the dilated bile ducts in the hepatobiliary phase (Figures 3 and 4). Total bilirubin levels were markedly elevated in these two cases (Table 1). Routine inspection showed an abnormal signal and low-strengthened focal area in the edge of the left intrahepatic bile duct in case 4, and the edge of the lesion was enhanced in the 20-min delayed EOB-enhanced MRI. In the 45-min delayed scan, most of the area showed isointensity, with the exception of the low signal in the lesion center, which is a pattern of gradual enhancement (Figure 4). These findings suggest decreased hepatic uptake of contrast agent, indicative of inflammation. No signs of tumor invasion were seen in the EOB-enhanced scans of cases 1-4.
Although there was no high signal of contrast filling in the dilated bile duct on the inferior right liver in case 5, the 20-min delayed scan showed a well-defined massive (6.5 cm × 6.0 cm) lesion with low-signal intensity around the dilated bile duct, indicating a lack of EOB uptake in the adjacent region of the liver (Figure 5). This was in accordance with the tumor extent seen on PET-CT. The signal in the liver parenchyma in front of the tumor was slightly lower than the normal liver tissue, but higher than the mass tissue in the hepatobiliary phase of EOB-enhanced MRI. A low signal vessel with normal direction can be seen within it, suggesting that this area is not tumor tissue, but inflamed liver tissue with reduced uptake ability for the contrast agent (Figure 5).
Cases 1-3 underwent duodenal endoscopy to drain mucus from the duodenal papilla within 3 d. In case 4, EOB-enhanced MRI showed tumor distribution in the left and right hepatic ducts, but no signs of extra-biliary tumor invasion, therefore, biliary tumor enucleation and T tube drainage were performed. During the procedure, the dilated bile duct was filled with mucus, and multiple nodular or papillary tumors were observed in the bile duct.
Microscopic examination of the biopsies from cases 1-4 showed cuboidal or columnar epithelial cells around the fibers and vessels growing within the bile duct, with no tumor invasion beyond the bile duct. Immunohistochemical results were: mucin (MUC)1-, MUC2+, MUC5AC+, MUC6-, and p53-.
Based on the findings from imaging, endoscopy, and biopsy or surgical pathology, cases 1 and 3 were pathologically diagnosed as papillary adenoma, and cases 2 and 4, which showed obvious tumor nuclear atypia in the focal lesions, were diagnosed as papillary adenoma accompanied by focal cancer. In case 4, lesions beyond the left dilated bile duct were removed, and the pathology showed infiltration of inflammatory cells without tumor cells, thus, these were diagnosed as inflammatory lesions. Lesions in cases 1 and 2 were confined to the left or right lobe, suggesting lobe resection, although the patients refused surgery. Case 3 involved the left and right intrahepatic bile ducts and lobe resection could not be performed. Hence, cases 1-3 underwent endoscopic nasobiliary drainage and/or percutaneous transhepatic cholangial drainage to alleviate the symptoms.
Case 5 underwent a right hepatic resection. The EOB-enhanced MRI indicated that the anterior right liver was inflamed, so the middle hepatic vein was completely retained. During surgery, a mass (6.5 cm × 6.5 cm × 6.0 cm) was seen in the posterior right liver, showing extra-biliary infiltration with dilated bile ducts filling with mucus. Postoperative pathology showed cuboidal or columnar epithelial cells in the tumor tissues growing around the fibers and vessels inside the bile duct. The tumor cells had obvious nuclear atypia, which invaded outward to the bile duct wall. Immunohistochemistry showed that the cells were MUC1+, MUC2+, MUC5AC+, MUC6-, and p53+, confirming the diagnosis of infiltrating intraductal papillary mucinous adenocarcinoma of the bile duct. The right anterior liver parenchyma showed interstitial fibrous tissue hyperplasia with chronic inflammatory cell infiltration, which was diagnosed as chronic inflammation with fibrous tissue hyperplasia.
Surgery is the preferred treatment for IPMN-B. Partial hepatectomy can be performed for localized tumors, whereas bilateral bile duct involvement may require liver transplantation, duodenal papilla dissection, endoscopic nasobiliary drainage, and/or percutaneous transhepatic cholangial drainage[16-19]. Biliary tumor enucleation can be performed for tumors that do not invade into the bile duct[6,20-23]. Thus, accurate preoperative diagnosis, with determination of the extent of tumor involvement, is crucial for treatment decisions.
Reports by Lim et al[8-10] describe the typical imaging findings of IPMN-B. The disease can be clearly diagnosed when imaging findings are indicative of IPMN-B, and the presence of mucin within the dilated bile duct is confirmed. Although duodenal endoscopy, ERCP, biliary tract endoscopy, and endoscopic US can be used to clearly identify the tumor and mucus[13,24-27], these are invasive examinations, the success of which is associated with the proficiency of the operator, and are accompanied by a risk of pancreatitis. Moreover, it is difficult to evaluate upstream of the bile duct obstruction and extra-biliary conditions with these methods[28-30].
Takanami et al[13] and Oki et al[14] used EOB-enhanced MRI to study four patients with IPMN-B, and found that Gd-EOB-DTPA-enhanced MRI shows the dilated bile duct and tumor, but also the bile duct filling defects caused by mucin retention. Therefore, Gd-EOB-DTPA-enhanced MRI is an alternative to ERCP for the diagnosis of IPMN-B. Indeed, the performance of EOB-enhanced MRI in cases 1 and 2 in the present study is in agreement with the previous reports[13,14]. Although biliary sludge and stones can also appear as filling defects in EOB-enhanced MRI, they show a low signal in T2-weighted images that can be differentiated from mucus, as shown in cases 1 and 2. Although the cystic appearance of the IPMN-B in case 2 is similar to cystadenoma or cystadenocarcinoma, these cystic lesions are not connected with the bile duct. Moreover, contrast agent was seen in both the cystic lesion and the bile duct in case 2 during the hepatobiliary phase of Gd-EOB-DTPA-enhanced MRI.
A large amount of mucin in the bile duct was confirmed during ERCP/operation in cases 3 and 4, which resulted in marked bile duct dilatation and obvious impaired liver function (indicated by elevated total bilirubin levels). Furthermore, liver cell uptake and secretion of EOB into the bile duct was obviously decreased, with none observed in the hepatobiliary phase in the 42-52-min delayed scan. Mucin within the bile duct can be distinguished from bile based on the similarity with signals in the downstream bile duct, lobe, or segmental bile duct where the tumor is not located.
Case 4 showed a long T2-signal nodule with hypo-enhancement at the edge of the left intrahepatic bile duct, which was considered to be focal. However, EOB-enhanced imaging during the hepatobiliary phase showed gradual enhancement of the lesion area, consistent with chronic inflammation. Therefore, the patient underwent biliary tumor enucleation and T tube drainage, a procedure that could accelerate the disease if the tumor had invaded tissue outside of the bile duct. Therefore, when multifocal IPMN-B occurs in intrahepatic bile ducts, EOB-enhanced MRI in the hepatobiliary phase can be used to select the appropriate treatment.
Importantly, case 5 describes imaging results from an invasive IPMN-B, which cannot be discerned with conventional-enhanced CT or Gd-DTPA-enhanced MRI[1,6,16]. With similar cases, it is difficult to judge the extent of tumor invasion into the extrahepatic bile duct by routine inspection, as demonstrated in this patient. The extent of the infringement was clear by PET-CT examination, and the FDG uptake suggested that the tumor was malignant, although it is difficult to distinguish this tumor type from cholangiocarcinoma and other malignant liver tumors. Takanami et al[31] also reported PET-CT manifestations of invasive IPMN-B, observed as high intake of FDG. Despite its advantage, PET-CT should be used very carefully due to the relatively high cost and risk of radioactive damage to patients. Case 5 further demonstrates that normal liver cells uptake Gd-EOB-DTPA, whereas tumor cells do not, thus more clearly defining the extent of liver invasion. Moreover, case 5 shows that the signal intensity of the right anterior liver in the hepatobiliary phase is intermediate between normal liver parenchyma and tumor, representing the nature of inflammatory tissue with decreased liver function (EOB uptake). In this case, the tumor, inflammation, and normal liver tissues were clearly demarcated, thus we decided to retain the middle hepatic vein during the right liver resection, and were confident in the negative cut edge of the tumor.
In conclusion, patients with suspected IPMN-B based on imaging findings would benefit from EOB-enhanced MRI for determination of the presence of mucus and extent of extra-biliary invasion.
Reports and awareness of intraductal papillary mucinous neoplasm of the bile duct (IPMN-B) are increasing. However, difficulties remain with noninvasive methods for diagnosis and determining the extent of the tumor.
This study reports the use of a novel imaging technique, gadolinium-ethoxybenzyl-diethylenetriamine-pentaacetic acid (Gd-EOB-DTPA)-enhanced magnetic resonance imaging (MRI), for diagnosing IPMN-B.
This study shows that Gd-EOB-DTPA-enhanced MRI can reveal bile duct filling defects due to mucin, which is highly suggestive of a diagnosis for IPMN-B. Compared with positron emission tomography-computed tomography, Gd-EOB-DTPA-enhanced MRI can be used to differentiate inflammatory lesions from tumor tissue. Therefore, Gd-EOB-DTPA-enhanced MRI facilitates accurate diagnosis and proper management of IPMN-B.
EOB-enhanced MRI is a useful, noninvasive method to detect mucus and assess the extent of extra-biliary invasion in patients whose imaging findings indicate IPMN-B.
Gd-EOB-DTPA is a double-specific contrast agent that is absorbed by hepatocytes and drained via the bile duct in the hepatobiliary phase, and can be used to detect filling defects due to mucus. It can also be used to distinguish between tumor cells, which do not take up the contrast agent, and inflammatory cells, which show reduced signal intensity compared to normal liver tissue.
This is an interesting case series of patients with IPMN-B, in which Gd-EOB-DTPA-enhanced MRI was applied for better evaluation of the lesion and differentiation between tumor invasion and inflammation.
P- Reviewer: dos Santos JS, Li YY, Mansour-Ghanaei F, Kaiser GM, Kumar A, Schofield JB S- Editor: Ma YJ L- Editor: Webster JR E- Editor: Ma S
| 1. | Zen Y, Fujii T, Itatsu K, Nakamura K, Minato H, Kasashima S, Kurumaya H, Katayanagi K, Kawashima A, Masuda S. Biliary papillary tumors share pathological features with intraductal papillary mucinous neoplasm of the pancreas. Hepatology. 2006;44:1333-1343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 288] [Cited by in RCA: 282] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 2. | Ohtsuka M, Kimura F, Shimizu H, Yoshidome H, Kato A, Yoshitomi H, Furukawa K, Takeuchi D, Takayashiki T, Suda K. Similarities and differences between intraductal papillary tumors of the bile duct with and without macroscopically visible mucin secretion. Am J Surg Pathol. 2011;35:512-521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 87] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 3. | Nakanuma Y. A novel approach to biliary tract pathology based on similarities to pancreatic counterparts: is the biliary tract an incomplete pancreas? Pathol Int. 2010;60:419-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 127] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 4. | Yeh TS, Tseng JH, Chen TC, Liu NJ, Chiu CT, Jan YY, Chen MF. Characterization of intrahepatic cholangiocarcinoma of the intraductal growth-type and its precursor lesions. Hepatology. 2005;42:657-664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 67] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 5. | D’souza MA, Isaksson B, Löhr M, Enochsson L, Swahn F, Lundell L, Arnelo U. The clinicopathological spectrum and management of intraductal papillary mucinous neoplasm of the bile duct (IPMN-B). Scand J Gastroenterol. 2013;48:473-479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 6. | Rocha FG, Lee H, Katabi N, DeMatteo RP, Fong Y, D’Angelica MI, Allen PJ, Klimstra DS, Jarnagin WR. Intraductal papillary neoplasm of the bile duct: a biliary equivalent to intraductal papillary mucinous neoplasm of the pancreas? Hepatology. 2012;56:1352-1360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 181] [Article Influence: 13.9] [Reference Citation Analysis (2)] |
| 7. | Klibansky DA, Reid-Lombardo KM, Gordon SR, Gardner TB. The clinical relevance of the increasing incidence of intraductal papillary mucinous neoplasm. Clin Gastroenterol Hepatol. 2012;10:555-558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 126] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 8. | Lim JH, Yoon KH, Kim SH, Kim HY, Lim HK, Song SY, Nam KJ. Intraductal papillary mucinous tumor of the bile ducts. Radiographics. 2004;24:53-66; discussion 66-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 88] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 9. | Lim JH, Jang KT, Choi D. Biliary intraductal papillary-mucinous neoplasm manifesting only as dilatation of the hepatic lobar or segmental bile ducts: imaging features in six patients. AJR Am J Roentgenol. 2008;191:778-782. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 33] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 10. | Lim JH, Jang KT, Rhim H, Kim YS, Lee KT, Choi SH. Biliary cystic intraductal papillary mucinous tumor and cystadenoma/cystadenocarcinoma: differentiation by CT. Abdom Imaging. 2007;32:644-651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 40] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 11. | Van Beers BE, Pastor CM, Hussain HK. Primovist, Eovist: what to expect? J Hepatol. 2012;57:421-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 292] [Cited by in RCA: 321] [Article Influence: 24.7] [Reference Citation Analysis (0)] |
| 12. | Seale MK, Catalano OA, Saini S, Hahn PF, Sahani DV. Hepatobiliary-specific MR contrast agents: role in imaging the liver and biliary tree. Radiographics. 2009;29:1725-1748. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 299] [Cited by in RCA: 289] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 13. | Takanami K, Yamada T, Tsuda M, Takase K, Ishida K, Nakamura Y, Kanno A, Shimosegawa T, Unno M, Takahashi S. Intraductal papillary mucininous neoplasm of the bile ducts: multimodality assessment with pathologic correlation. Abdom Imaging. 2011;36:447-456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 58] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 14. | Oki H, Hayashida Y, Namimoto T, Aoki T, Korogi Y, Yamashita Y. Usefulness of gadolinium-ethoxybenzyl-diethylenetriamine pentaacetic acid-enhanced magnetic resonance cholangiography for detecting mucin retention in bile ducts: a rare intraductal papillary mucinous neoplasm of the bile duct. Jpn J Radiol. 2011;29:590-594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 15. | Lee NK, Kim S, Lee JW, Lee SH, Kang DH, Kim GH, Seo HI. Biliary MR imaging with Gd-EOB-DTPA and its clinical applications. Radiographics. 2009;29:1707-1724. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 161] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 16. | Budzynska A, Hartleb M, Nowakowska-Dulawa E, Krol R, Remiszewski P, Mazurkiewicz M. Simultaneous liver mucinous cystic and intraductal papillary mucinous neoplasms of the bile duct: a case report. World J Gastroenterol. 2014;20:4102-4105. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 15] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 17. | Minagawa N, Sato N, Mori Y, Tamura T, Higure A, Yamaguchi K. A comparison between intraductal papillary neoplasms of the biliary tract (BT-IPMNs) and intraductal papillary mucinous neoplasms of the pancreas (P-IPMNs) reveals distinct clinical manifestations and outcomes. Eur J Surg Oncol. 2013;39:554-558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 48] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 18. | Kato H, Tabata M, Azumi Y, Osawa I, Kishiwada M, Hamada T, Mizuno S, Usui M, Sakurai H, Isaji S. Proposal for a morphological classification of intraductal papillary neoplasm of the bile duct (IPN-B). J Hepatobiliary Pancreat Sci. 2013;20:165-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 19. | Wan XS, Xu YY, Qian JY, Yang XB, Wang AQ, He L, Zhao HT, Sang XT. Intraductal papillary neoplasm of the bile duct. World J Gastroenterol. 2013;19:8595-8604. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 108] [Cited by in RCA: 104] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 20. | Yeh TS, Tseng JH, Chiu CT, Liu NJ, Chen TC, Jan YY, Chen MF. Cholangiographic spectrum of intraductal papillary mucinous neoplasm of the bile ducts. Ann Surg. 2006;244:248-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 84] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 21. | Paik KY, Heo JS, Choi SH, Choi DW. Intraductal papillary neoplasm of the bile ducts: the clinical features and surgical outcome of 25 cases. J Surg Oncol. 2008;97:508-512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 46] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 22. | Carlos RC, Branam JD, Dong Q, Hussain HK, Francis IR. Biliary imaging with Gd-EOB-DTPA: is a 20-minute delay sufficient? Acad Radiol. 2002;9:1322-1325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 45] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 23. | Lee NK, Kim S, Lee JW, Lee SH, Kang DH, Kim DU, Kim GH, Seo HI. MR appearance of normal and abnormal bile: correlation with imaging and endoscopic finding. Eur J Radiol. 2010;76:211-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 24. | Kim KM, Lee JK, Shin JU, Lee KH, Lee KT, Sung JY, Jang KT, Heo JS, Choi SH, Choi DW. Clinicopathologic features of intraductal papillary neoplasm of the bile duct according to histologic subtype. Am J Gastroenterol. 2012;107:118-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 80] [Article Influence: 6.2] [Reference Citation Analysis (1)] |
| 25. | Lee NK, Kim S, Kim HS, Jeon TY, Kim GH, Kim DU, Park do Y, Kim TU, Kang DH. Spectrum of mucin-producing neoplastic conditions of the abdomen and pelvis: cross-sectional imaging evaluation. World J Gastroenterol. 2011;17:4757-4771. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 50] [Cited by in RCA: 46] [Article Influence: 3.3] [Reference Citation Analysis (1)] |
| 26. | Xu J, Sato Y, Harada K, Yoneda N, Ueda T, Kawashima A, Akishiooi Y. Intraductal papillary neoplasm of the bile duct in liver cirrhosis with hepatocellular carcinoma. World J Gastroenterol. 2011;17:1923-1926. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 2] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 27. | Ito K, Fujita N, Kanno A, Matsubayashi H, Okaniwa S, Nakahara K, Suzuki K, Enohara R. Risk factors for post-ERCP pancreatitis in high risk patients who have undergone prophylactic pancreatic duct stenting: a multicenter retrospective study. Intern Med. 2011;50:2927-2932. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 36] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 28. | Sohn WJ, Jo S. A huge intraductal papillary mucinous carcinoma of the bile duct treated by right trisectionectomy with caudate lobectomy. World J Surg Oncol. 2009;7:93. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 29. | Yamashita Y, Fukuzawa K, Taketomi A, Aishima S, Yoshizumi T, Uchiyama H, Tsujita E, Harimoto N, Harada N, Wakasugi K. Mucin-hypersecreting bile duct neoplasm characterized by clinicopathological resemblance to intraductal papillary mucinous neoplasm (IPMN) of the pancreas. World J Surg Oncol. 2007;5:98. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 30. | Güllüoglu MG, Ozden I, Poyanli A, Cevikbas U, Ariogul O. Intraductal growth-type mucin-producing peripheral cholangiocarcinoma associated with biliary papillomatosis. Ann Diagn Pathol. 2007;11:34-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 31. | Takanami K, Hiraide T, Kaneta T, Hayashi H, Unno M, Fujishima F, Fukuda H, Yamada S, Takahashi S. FDG PET/CT findings in malignant intraductal papillary mucinous neoplasm of the bile ducts. Clin Nucl Med. 2010;35:83-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 1.4] [Reference Citation Analysis (0)] |