Published online Jul 7, 2015. doi: 10.3748/wjg.v21.i25.7718
Peer-review started: December 9, 2014
First decision: January 22, 2015
Revised: February 25, 2015
Accepted: April 9, 2015
Article in press: April 9, 2015
Published online: July 7, 2015
Processing time: 211 Days and 4.4 Hours
AIM: To evaluate the effect of an extract of Geranium schiedeanum (Gs) as a hepatoprotective agent against ethanol (EtOH)-induced toxicity in rats.
METHODS: Male Wistar rats weighing 200-230 g were subjected to a 70% partial hepatectomy (PH); they were then divided into three groups (groups 1-3). During the experiment, animals in group 1 drank only water. The other two groups (2-3) drank an aqueous solution of EtOH (40%, v/v). Additionally, rats in group 3 received a Gs extract daily at a dose of 300 mg/kg body weight intragastically. Subsequently, to identify markers of liver damage in serum, alanine aminotransferase, aspartate aminotransferase, albumin and bilirubin were measured by colorimetric methods. Glucose, triglyceride and cholesterol concentrations were also determined. In addition, oxidative damage was estimated by measuring lipid peroxidation [using thiobarbituric-acid reactive substances (TBARS)] in both plasma and the liver and by measuring the total concentration of antioxidants in serum and the total antioxidant capacity in the liver. In addition, a liver mass gain assessment, total DNA analysis and a morpho-histological analysis of the liver from animals in all three groups were performed and compared. Finally, the number of deaths observed in the three groups was analyzed.
RESULTS: Administration of the Geranium shiedeanum extract significantly reduced the unfavorable effect of ethanol on liver regeneration (restitution liver mass: PH-EtOH group 60.68% vs PH-Gs-EtOH group 69.22%). This finding was congruent with the reduced levels of hepatic enzymes and the sustained or increased levels of albumin and decreased bilirubin in serum. The extract also modified the metabolic processes that regulate glucose and lipid levels, as observed from the serum measurements. Lower antioxidant levels and the liver damage induced by EtOH administration appeared to be mitigated by the extract, as observed from the TBARs (PH-EtOH group 200.14 mmol/mg vs PH-Gs-EtOH group 54.20 mmol/mg; P < 0.05), total status of antioxidants (PH-EtOH group 1.43 mmol/L vs PH-Gs-EtOH group 1.99 mmol/L; P < 0.05), total antioxidant capacity values, liver mass gain and total DNA determination (PH-EtOH group 4.80 mg/g vs PH-Gs-EtOH 9.10 mg/g; P < 0.05). Overall, these processes could be related to decreased mortality in these treated animals.
CONCLUSION: The administered extract showed a hepatoprotective effect, limiting the EtOH-induced hepatotoxic effects. This effect can be related to modulating oxido-reduction processes.
Core tip: The geranium is an alternative preventive agent to protect the liver from diverse substances that cause cellular damage, such as ethanol (EtOH). In this paper, according to the phytochemical studies, administering geranium and its compounds, primarily tannins, provided evidence of potentially being protective against liver damage caused by EtOH.
-
Citation: Madrigal-Santillán E, Bautista M, Gayosso-De-Lucio JA, Reyes-Rosales Y, Posadas-Mondragón A, Morales-González &, Soriano-Ursúa MA, García-Machorro J, Madrigal-Bujaidar E, Álvarez-González I, Morales-González JA. Hepatoprotective effect of
Geranium schiedeanum against ethanol toxicity during liver regeneration. World J Gastroenterol 2015; 21(25): 7718-7729 - URL: https://www.wjgnet.com/1007-9327/full/v21/i25/7718.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i25.7718
When there is a lesion in the liver, hepatic regeneration normally presents via a complex process that can be stimulated by diverse processes. Experimentally, the most utilized hepatic lesion model is one that is surgically induced through a partial hepatectomy (PH), which restores the liver function and hepatic mass[1-5]. Through this procedure, the anatomical (80%) and functional restitution of the regenerating liver occurs approximately 8 d after the PH. Thus, it is a good model for studying the liver regenerative process under physiological and pathological conditions[1,6,7].
Because the liver is the main organ that metabolizes ethanol (EtOH), it suffers the most important harmful effects due to both the molecule and the products of its metabolism, including acetaldehyde and free radicals, which significantly contribute to alcohol-related liver disease[8-10]. Various studies have been conducted to identify the adverse effects of EtOH administration on the physiology of this organ and during the liver regeneration process. Alterations in oxidative stress (OS), hepatic metabolism and histological changes, among others[10-14] have been identified, with OS being the main component in the physiopathology of alcohol-related liver disease.
Conversely, the use of herbal treatments is increasingly being used to treat diverse pathologies, including hepatopathies. There are reports of the hepatoprotective effects of diverse plants and natural extracts against agents that induce the production of free radicals, such as EtOH. The bioactivity of these extracts has been shown to be directly related to the sequestering capacity of free radicals[15-22], a property that situates them as an excellent antioxidant.
One order of plants that contain substances with potential antioxidant properties and can therefore function as a hepatoprotector is the geranium (Geraniales)[23,24]. Studies are lacking that demonstrate the capacity of this order. Among the compounds that have been attracting attention to these plants are the hydrolyzable tannins, which contain the dehydrohexahydroxydephenyl group, such as geraniin (Figure 1A). These tannins can be isolated as condensed derivatives with acetone and, from some geraniums (Family: Geraniaceae) such as acetonyl-geraniin (Figure 1B), compounds that exhibit different characteristics from their precursors under biological conditions[25].
Within the genus Geranium, 423 accepted species are distributed into the following three subgenera: Erodioidea, Geranium, and Robertium. To date, eight different species have been classified in the state of Hidalgo, Mexico[26], and only three have been studied so far. Phytochemical studies on these species indicate the invariable presence of geraniin[23,24]. From this compound, adducts have been described by condensation with ascorbic acid or acetone[27]; however, these acetone condensates are known to be much more stable under pH conditions with solute concentrations similar to those of plasma; thus, they can be adequate alternatives for pharmacological studies[25,28]. Currently, tannins are well known for their antioxidant properties[27,29]. Tannin-protein complexes in the gastrointestinal tract provide persistent antioxidant activity, yielding the hypothesis that studying an additional genus could reveal a novel, useful hepatoprotective agent to prevent alcohol-related liver damage.
In this study, we evaluated the hepatoprotective effect of the acetone-water extract of Geranium schiedeanum (Gs) on the toxicity induced by EtOH on partial post-hepatectomy liver regeneration in rats.
We utilized male Wistar rats with an initial body weight (BW) of 200-230 g that were obtained from the Escuela Superior de Medicina (ESM) Bioterium of the Instituto Politécnico Nacional. The rats were housed in cages in the Bioterium (ESM). They were maintained at a temperature of 22 °C with 12-h/12-h light-dark cycles and received standard rat-pellet food (Purina de México, S.A.) and water ad libitum prior to the treatments. After 14 d of adaptation, the procedure was initiated. The protocol and the experimental procedures were conducted according to the Mexican Official Norm for the use and care of laboratory animals (NOM-062-ZOO-1999, México)[30].
We obtained the extract as previously described[21]. In brief, 1 kg of the dried and ground aerial parts of Geranium shiedeanum were extracted by maceration over 7 d with 20 L acetone-water (at a ratio of 7:3) and were concentrated by reduced pressure until obtaining a volume of 3 L, which was extracted with CHCl3, yielding obtaining 12.75 g of F-CHCl3 and 105 of F-Ac). Twenty grams of F-Ac was submitted to chromatography in a column with Sephadex LH-20, utilizing mixtures of H2O-MeOH (1:0; 9:1; 4:1; 7:3; 3:2; 1:1; 2:3; 3:7; 1:4; 1:9, and 0:1) with 300 mL in each one (Gayosso-de-Lucio et al[21] 2014). The fractions were grouped based on their chromatographic profiles using thin-layer chromatography (TLC), and subsequent chromatographies (silica gel and C-18) achieved identification of the following four majority components: ellagic acid, gallic acid, 3-O-a-L arabinofuranoside-7-O-a-L-ramnopyranoside of Kaempferol, and geranium acetonitrile. Notably, the latter represents approximately 40% of the F-Ac; thus, we suggest that it is the active compound[28].
Surgical removal of two-thirds of the liver using a technique known as a PH was performed according to the procedure described by Higgins and Anderson[1]. The surgical procedures were performed between 08:00 and 10:00 am under light anesthesia with ethyl ether. As a surgical control, we utilized sham rats on which we only carried out laparotomy, without removing the hepatic mass.
After the surgical procedure, the rats were housed individually. They were grouped (n = 5-6, for each experimental group) in the following manner: (1) Control group (sham); (2) group with PH; (3) group with PH plus intragastric (ig) administration of EtOH (PH-EtOH); (4) group receiving a hepatectomy, the Gs extract and EtOH (PH-Gs-EtOH); and (5) group receiving a PH and the Gs extract (PH-Gs). The rats in all groups received food and water throughout the treatment period.
EtOH-treated animals received an ig dose of 1.5 g/kg BW (an EtOH solution at 40% in isotonic saline solution), equivalent to blood alcohol values between 75 and 150 mg/dL, which have been reported to be capable of inhibiting the liver regenerative process, as reported previously[7,31,32]. The geranium extract dose was 300 mg/kg BW ig, as reported previously[21]. All treatments (EtOH solution and geranium extract) were administered daily for 7 d.
On day 8, the animals were sacrificed by decapitation after being previously anesthetized with pentobarbital sodium (40 mg/kg BW). Blood samples were obtained and centrifuged in a clinical centrifuge to obtain the sera, which were frozen at -70 °C for later use. The liver was isolated, weighed, rapidly placed in cold phosphate-buffered saline solution (PBS) solution with a phosphate tampon, pH 7.5), and washed to completely eliminate the blood. The liver was placed in 9 volumes of cold buffer (sucrose 0.25 mol/L, TRIS 10 mmol/L, EGTA 0.3 mmol/L, and bovine serum albumin 0.2%, pH 7.4). The liver was homogenized using a homogenizer with a piston-type driver with a Teflon tip. The homogenate was divided into aliquots and frozen at -70 °C until later use. The total concentration of protein of the homogenate was determined by the method of Lowry[33], utilizing bovine serum albumin (BSA) solution as a standard.
Liver regeneration was determined by calculating the liver restitution weight and determining the total DNA concentration. After the rats were killed, the liver of each animal was resected and washed as previously described. To estimate the percentage of restitution of the hepatic mass, we employed a previously reported procedure[34]. For this, we proceeded as follows: the resected liver was weighed, and that weight was divided by 0.7 to obtain the estimate of the initial weight (pre-PH) of each liver. The percentage of restituted hepatic mass was calculated using the following formula: remnant liver weight/initial liver × 100[34]. The results of each group were utilized to calculate the average restitution of hepatic mass in each corresponding group. The DNA concentration was determined in liver samples according to the technique of Labarca and Paigen[35] as modified by Ramírez-Farías et al[6].
Hepatic samples from each group were used for the light microscopy. Samples were fixed with formaldehyde (10% in isotonic solution), embedded in wax, and stained with hematoxylin-eosin. Biopsy specimens were coded and read blindly without knowledge of the other data by independent observers at two different laboratories (J.A.M.G and J.B.R.). The criteria used to analyze the morphological abnormalities were the same as those reported by Morales-González et al[10] as follows: fatty infiltration (+, mild; ++, moderate; +++, severe; and ++++, very severe); inflammation (+, zonal localization, focal inflammatory cells; ++, moderate, not restricted to one zone of the acinus; +++, diffuse); and hepatocellular disorganization (+, isolated foci in zone 3 of the liver acinus; ++, more widespread; and +++, definitively diffused in the hepatic acini).
The activities of serum alanine aminotransferase [ALT; expansion coefficient (EC) 2.6.1.2] and aspartate aminotransferase (AST, EC 2.6.1.1) were measured colorimetrically using diagnostic kits (Spinreact de México, SA de CV), following the manufacturer’s instructions; the results are reported in units/L.
Serum concentrations of glucose, triacylglycerides, cholesterol, bilirubin, and albumin were determined by spectrophotometric techniques using diagnostic kits (Spinreact de México, SA de CV), following the instructions provided by the manufacture; the results are reported in mg/dL, except for albumin, which is reported in g/dL.
The total antioxidant status (TAS) was determined utilizing the Randox Kit (Randox Laboratories Ltd., United Kingdom) and is reported in mmol/L.
The total antioxidant capacity (TAC) was determined using a BioAssay Systems DTAC-100 (CA, United States), and the result is reported in μmol/mg (Trolox).
We determined thiobarbituric acid reactive substances (TBARS) using the DTBA-100 Assay Kit (BioAssay Systems, CA, United States), following the manufacturer’s instructions and reporting results in μmol/mg of protein.
Enzymes in the samples of liver homogenate were determined according to standard techniques described previously[6,10,36-38]. We determined the specific activity of the following enzymes: ALT (EC 2.6.1.2) and AST (EC 2.6.1.1). The result is expressed as μmol/min per milligram of protein.
The results were analyzed using Sigma Plot ver. 12.3 statistical program software. The results are expressed as the mean ± SE, as required. We carried out a statistical analysis using Student’s t-test and/or analysis of variance (ANOVA). We considered differences among the groups to be statistically significant when P < 0.05.
The PH-Gs group did not show differences compared with the PH group in terms of any study indicator, which demonstrated that the Gs extract does not exert a toxic effect, in agreement with the previously reported results[21].
Survival as well as changes in liver regeneration indicators and DNA concentrations in hepatic tissue for each study group are depicted in Table 1. The PH-EtOH group presented a significant diminution in hepatic indicators (Table 1) compared with the PH group. Similarly, we observed that the PH-EtOH group resulted in a 30% mortality rate compared with the PH group (P < 0.05). In contrast, administration of the Gs extract (PH-Gs-EtOH) significantly diminished mortality (P < 0.05) compared with the PH-EtOH group.
| Group | Mortality (%) | Resected liver mass (g) | Final liver weight (g) | Restitution of liver mass (%) | mgDNA/g liver |
| Sham | - | - | 9.410 ± 0.47 | 100 | 4.40 ± 0.35 |
| PH | 0 | 6.185 ± 0.30 | 6.705 ± 0.29 | 75.89 ± 3.5 | 9.20 ± 0.20ª |
| PH-EtOH | 33ce | 6.374 ± 0.29 | 5.525 ± 0.27ª,c,e | 60.68 ± 2.8ce | 4.80 ± 0.15ce |
| PH-Gs-EtOH | 0 | 6.797 ± 0.33 | 6.721 ± 0.31 | 69.22 ± 1.4 | 9.10 ± 0.27ª |
With respect to the weight gain of the liver, the experimental group treated with EtOH in combination with the Gs extract (PH-Gs-EtOH) demonstrated a restored weight gain in the liver, obtaining values comparable to those of the PH group; the difference between the groups was 6.67%. Conversely, the PH-EtOH group had an increase of only 60.68% in restoring hepatic mass with the latter significantly lower than the pH group (75.89%; P < 0.05). Additionally, we observed that treatment with EtOH significantly diminished the concentration of DNA compared with the PH group (4.80 mg DNA/g vs 9.20 mg DNA/g, P < 0.05). In contrast, the PH-Gs-EtOH group showed a value of 9.10 mgDNA/g.
In Table 2, we can observe the effect exerted by Gs on histological changes during liver regeneration and ethanol administration. A significant increase in the parameters of fatty change and inflammation in the PH-EtOH group was observed compared with the PH group. In the PH-Gs-EtOH group, however, administering geranium significantly diminished the increases in fatty change and inflammatory histological parameters caused by ethanol. In addition, the geranium extract moderately increased hepatocellular disorganization.
| Group | Fatty change | Inflammation | Hepatocellular disorganization |
| Sham | 0 | 0 | 0 |
| PH | + | 0 | 0 |
| PH-EtOH | ++/+++ | ++/+++ | 0 |
| PH-Gs-EtOH | + | + | +/++ |
Table 3 illustrates the concentrations of serum metabolites whose metabolism primarily occurred in the liver on 8 d in all of the experimental groups. In the pH group, the glucose levels exhibited similar levels to those of the sham group 8 d post-surgery. In contrast, the PH-EtOH group presented a decrease in serum glucose levels compared with those of the sham (120 mg/dL; P < 0.05) and the PH (112 mg/dL; P < 0.05) groups. In contrast, the PH-Gs-EtOH group had normalized glucose levels, which differed from the PH-EtOH group (124 mg/dL vs 86 mg/dL, P < 0.05).
The concentration of serum cholesterol in the PH-EtOH group (34 mg/dL) was statistically significant compared with those obtained in the sham (57 mg/dL; P < 0.05) and the PH (41 mg/dL; P < 0.05) groups. Conversely, the concentration of serum cholesterol in the PH-Gs-EtOH group (55 mg/dL) was found to be similar to the sham group (57 mg/dL). Independently to the experimental group, the concentration of triacylglycerides was found to be lower than that of the sham group (Table 3).
The results of the metabolic integrity of the liver are presented in Table 4. Similar concentrations of bilirubin and albumin in serum were present in the sham group and the PH group. In contrast, in the PH-EtOH group, a significant decrease in the serum concentration of albumin (2.72 g/dL vs 3.03 g/dL, P < 0.05) and an increase in bilirubin (0.15 mg/dL vs 0.07 mg/dL, P < 0.05) occurred compared with the PH group. However, in the PH-Gs-EtOH group, we found that the albumin concentration increased to 3.0 g/dL but that the bilirubin value decreased to 0.11 mg/dL.
The effect of the Gs extract was evaluated through determining the activity of ALT and AST because these enzymes classically reflect liver function that is dependent on morphofunctional integrity.
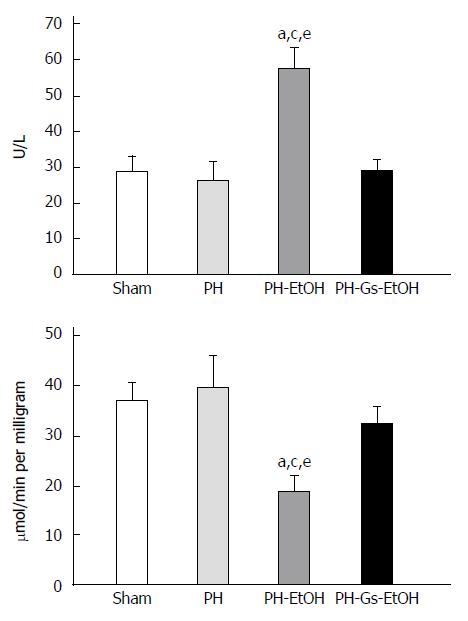
Figure 2 depicts the AST activity in serum (upper panel) and the liver (lower panel) in the diverse experimental groups. Whereas the AST activity was not different in the PH group compared with the sham group, alcohol administration in rats with PH induced a significant increase in AST activity in serum on day 7 post-surgery (Figure 2, upper panel; P < 0.05). In addition, AST activity in the liver presented the following behavior (Figure 2, lower panel): the PH-EtOH group showed a decrease in AST activity compared with the sham (50%; P < 0.05) and the PH (53%; P < 0.05) groups. In contrast, the PH-Gs-EtOH group presented levels similar to those in the sham group.
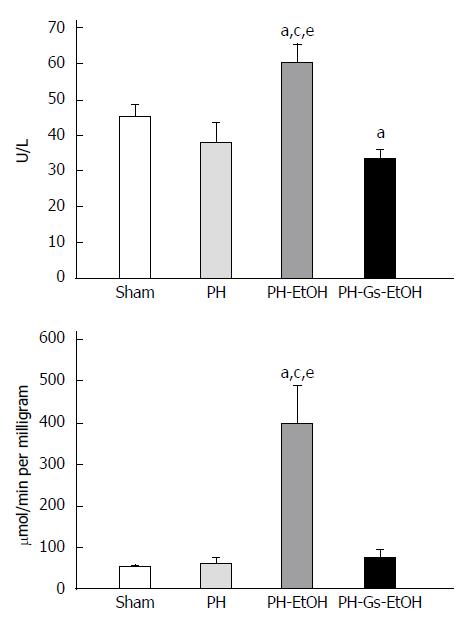
Figure 3 illustrates the ALT activity in serum (upper panel) and in the liver (lower panel) in the experimental groups. In both cases, there was a significant increase in ALT activity in the PH-EtOH group compared with the sham and PH groups. In serum (upper panel), the activity of the enzyme in the sham group was 45 U/L, whereas a decrease was observed in the PH group (37.5 U/L) and an increase was observed in the PH-EtOH group (60 U/L; P < 0.05). ALT activity in the liver (lower panel) in the sham group was 53.23 μmol/min/mg without a significant difference in the PH group (60.08 μmol/min per milligram); conversely, the PH-EtOH group reported a significant increase compared with the two prior groups (399.75 μmol/min per milligram; P < 0.05).
In contrast, rats in the PH-Gs-EtOH group exhibited a significant decrease in serum ALT levels, reaching values comparable to those reported for the sham group. When comparing the ALT levels of this group with those of the group administered EtOH, we observed the following findings: serum (upper panel), PH-EtOH group 60 U/L vs PH-Gs-EtOH group 33 U/L; P < 0.05; liver (lower panel), PH-EtOH group 399.75 μmol/min per milligram vs PH-Gs-EtOH group 75.20 μmol/min per milligram; P < 0.05.
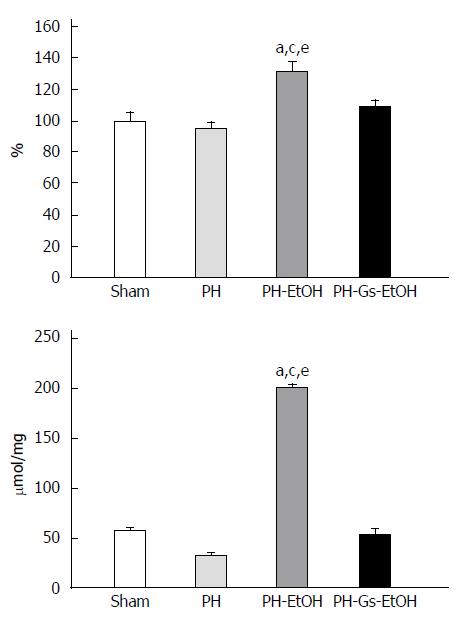
To evaluate the damage produced by reactive oxygen species (ROS), we determined the TBARS concentration in the liver and serum of animals treated with EtOH and the Gs extract. Regarding the TBARS concentrations in serum (Figure 4, upper panel), the PH-EtOH group presented a significant increase of 32% relative to the sham group. In contrast, when the Gs extract was administered to EtOH-intoxicated rats, there were no differences between the serum TBARS concentrations compared with those observed in the PH and sham groups. The hepatic concentrations of TBARS in the different study groups are presented in Figure 4 (lower panel). As observed in the figure, there was an increase in TBARS in the PH-EtOH group of 200.14 mmol/mg, which was 3.5 and 6.1 times greater in comparison with the sham group (56.07 mmol/mg) and the PH group (32.38 mmol/mg), respectively. In contrast, the PH-Gs-EtOH group demonstrated a decrease in the hepatic concentration of TBARS (54.20 mmol/mg); this result was significant compared with the PH-EtOH group (54.20 mmol/mg vs 200.14 mmol/mg, P < 0.05).
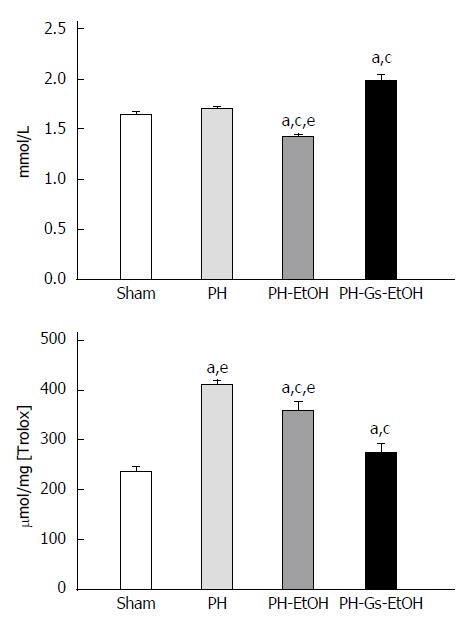
In Figure 5, the levels of TAS are shown as quantified in serum (upper panel) and TAC as determined in the liver (lower panel).
The level of TAS diminished significantly due to the administration of EtOH (1.43 mmol/L) compared with the sham group (1.65 mmol/L; P < 0.05) and the PH group (1.7 mmol/L; P < 0.05). Administration of the Gs extract resulted in a concentration of 1.99 mmol/L in the PH-Gs-EtOH group; this finding was significant compared with the sham group, as can be observed in Figure 5 (upper panel).
In Figure 5 (lower panel), the levels of TAC determined in the liver are illustrated. The regenerative process increased the TAC levels, as observed in the PH group compared with the sham group as follows: 409 μmol/mg[Trolox] vs 237 μmol/mg[Trolox], respectively. Conversely, EtOH administration diminished the TAC levels in the PH-EtOH group (360 μmol/mg[Trolox]) compared with the PH group. Finally, in the PH-Gs-EtOH group, we found a values of 275 μmol/mg[Trolox].
Chronic degenerative diseases are increasingly exhibiting an increase in morbidity and mortality. Among these pathologies, cirrhosis of the liver and liver cancer have been found to represent a public health problem in Mexico and worldwide[39]. One of the diverse causal agents of these diseases is chronic consumption of alcohol; according to the World Health Organization, alcohol consumption has increased in recent years[40,41]. A good model for studying the mechanisms of damage due to EtOH and the hepatoprotective effect of diverse agents is liver regeneration induced by PH in rats[1-3]. Liver regeneration is a highly regulated process in which diverse molecular changes and metabolic adjustments intervene, which have been characterized by an increase in DNA synthesis and cellular proliferation[42]. Acute EtOH administration negatively alters PH-induced liver regeneration, resulting in a decrease in cellular regeneration parameters in the liver remnant[10,43]. However, a 1-1.5-g/kg dose of EtOH has been shown to strongly inhibit thymidine kinase and thymidylate synthase enzymatic activity in the remnant liver of rats subjected to PH[10,44]. These enzymes are closely related to the synthesis of DNA. Due to the high morbidity and mortality represented by hepatic cirrhosis, studying therapeutic agents that can prevent or limit the damage caused by EtOH to the liver is necessary. A relatively new field consists of using phytochemicals that protect the liver from damage caused by EtOH. In a recent study, we reported the chemical composition and protective effects that are possessed by the Gs extract on damage caused by thioacetamide to the liver[21]. Therefore, in this work, we evaluated the use of the Gs extract as an alternative in liver lesions utilizing the liver regeneration model in rats, seeking to form part of the pioneering reports aiming to show the potential relevance of using phytochemical extracts to treat hepatopathies.
Weight gain correlates with DNA concentration, both of which are cellular proliferation parameters (Table 1). The increase in DNA concentration is due to the activity of thymidine kinase and thymidylate synthase enzyme, which increase in the early phase of PH-induced liver regeneration[10,45]. One of the mechanisms of damage by EtOH to the hepatocyte, which inhibits the proliferative process of this organ, is the production of free radicals[6,46-48]; this inhibition is reverted by the use of diverse antioxidant agents such as vitamins[6,47,48]. The results demonstrate that in animals with only PH, the DNA concentration is twice as high as that of the sham group (Table 1). The concentration of DNA diminished in EtOH-treated PH rats, which correlates with a gain in liver weight; however, in the PH-Gs-EtOH group, weight gain and DNA concentrations were restored to levels similar to the PH group. This result and the previous studies support the possible protective effect of antioxidants on liver regeneration because of their capacity to eliminate the free radicals formed by EtOH metabolism; however, there are differences in the protective capacity of each antioxidant. Thus, it is noteworthy that there are differences in their physicochemical properties, dose, administration route, and mechanism of action; thus, it is necessary to conduct more studies that are targeted at finding the best protective agents. Our data demonstrate the protective effect of Gs extract on inhibiting the effects of EtOH on liver regeneration, which has not been previously reported, to the best of our knowledge.
Morphologically, on 8 d after PH, cellular changes were no longer observed. The administration of ethanol during liver regeneration resulted in the presence of fat drops and moderate-to-severe grade inflammation (Table 2). However, when the Gs extract was administered, the accumulations of fat drops and inflammation were similar to those in the group in which only PH was performed. Additionally, structural changes in hepatocellular disorganization occurred because of the Gs extract administration. These changes were associated with weight gain of the remnant liver and the concentration of DNA in the hepatocytes (Table 1) and were in direct correlation with the proliferative process, which agrees with the previously reported results[10,14].
A good indicator of the harmful effect of EtOH on hepatocyte function during liver regeneration are the serum metabolites, such as glucose, triacylglycerides, and cholesterol, which directly regulate this organ.
We previously demonstrated that the regenerative process of the liver in itself results in a decrease in serum concentrations of glucose and cholesterol and an elevation in triacylglyceride levels that return to their basal levels after completing the regenerative process[10,46]. However, a high dose of EtOH (5 g/kg) administered immediately after PH has been shown to increase serum levels of glucose and triacylglycerides[38]. In contrast, administering a low dose of EtOH (1.5 g/kg) at different post-PH liver regeneration time points causes a decrease in serum concentration of these metabolites[10,46]. Some reports[10,34] have demonstrated that EtOH consumption increases lipid deposits in the liver and diminishes protein synthesis; thus, this finding can be associated with a decrease in serum cholesterol in the EtOH-treated rats compared with the sham group. Orrego et al[34] also suggested a reduction in cholesterol transport through the organ due to the decrease in the proteins required for transport. Our results demonstrate that treatment with EtOH (1.5 g/kg) for 7 d caused a decrease in serum concentrations of the three metabolites (glucose, cholesterol, and triacylglycerides) to levels lower than those of the sham group. This decrease in triacylglyceride concentration was correlated with a decrease in liver regeneration (liver weight and DNA concentration gain) because the increase in serum triacylglyceride levels was found to be a consistent characteristic of hepatic proliferation advancement, which has been attributed to liver permeability for the lipids[49]. In our study, administration of the Gs extract normalized the serum concentrations of these metabolites (Table 3), which is in agreement with findings by Nakanishi et al[50], who compared the protective effect of three compounds (geraniin, ellagic acid, and gallic acid) on liver damage caused by carbon tetrachloride, D-galactosamine, and thioacetamide. Three hepatotoxic compounds were found to increase serum levels of triacylglycerides and cholesterol, but pre-treatment with geraniin and ellagic acid avoided this increase, instead exhibiting a protective effect against the cellular damage in the liver caused by carbon tetrachloride, D-galactosamine, and thioacetamide. Similarly, it was reported that the compounds contained in the Gs extract were gallic acid, ellagic acid, and acetonyl-geraniin, and a flavonoid, 3-Oα-L-arabinofuranoside-7-O-β-D-ramnoside of kaempferol, which possesses a protective effect according to previous results[21].
EtOH has been thought to be an important cause of damage to hepatic cells. The hepatic metabolites (albumin and bilirubin) are indicators of good liver functioning and can be utilized to identify the protective effect of the Gs extract. Serum concentrations of albumin and bilirubin and AST and ALT enzymatic activities are the most sensitive evidence of diagnosing hepatic diseases[51]. In this study, we observed that rats treated with EtOH presented with a decrease in serum albumin concentration and an increase in bilirubin concentration (Table 4), which is in agreement with the results reported previously by our group[10,46]. These studies demonstrate a hepatotoxic effect of EtOH; the decrease in serum albumin is possibly directly related to a decrease in ATP generated in the liver; this effect is due to EtOH consumption and not to the PH, in which the level of this protein is similar to the control group[10]. In our study, we observed that in the PH-Gs-EtOH group, serum concentrations of albumin and bilirubin were normalized (Table 4). The latter is most likely due to the protective effect of the Gs extract on liver damage caused by EtOH during liver regeneration, which, in turn, is most likely due to the antioxidant effect of the extract’s components. Various reports have demonstrated the protective effect of antioxidants (e.g., Vitamin C, Vitamin E, glycine, geraniin, and ellagic acid) on the diverse toxic, free radical-generating agents that cause damage to the liver, including the following agents: EtOH, carbon tetrachloride, D-galactosamine, and thioacetamide[10,46,50,52]. Among the biological results, we found a normalization of albumin and bilirubin levels in serum. In our work, we found a protective effect in the liver of the Gs extract, which is similar to the findings reported for other antioxidants, because of the components of the extract, most likely the acetonyl-geraniin component.
The release of AST, lactic dehydrogenase, ALT, glutamate dehydrogenase (GDH), and ornithine transcarbamylase enzymes by the hepatocyte and the increase in their serum enzymatic activity post-PH of 70% has been reported previously[6,10,37,46,53]. The increase of enzymatic activity in serum during liver regeneration has been interpreted in the following two ways: first, as a consequence of a necrotic event in the liver and second, as an increase in the permeability of the cellular and mitochondrial membrane.
In previous studies, alcohol administration in early stages of liver regeneration in rats with PH has been shown to diminish serum activity of these enzymes, and this activity was not related to liver necrosis but with the selective release of these enzymes during liver regeneration[10]. This activity could be a mechanism of interorgan signaling, which depends on the dose and the EtOH administration route and on the liver regeneration stage being studied. In fact, the increase in serum activities of some enzymes post-PH could be due to a release from the damaged cells or from cells with alterations in permeability, resulting in greater synthesis and enzyme release[10].
The enzymatic rise in serum in late stages of liver regeneration when EtOH was administered daily for 1 wk can most likely be attributed to damage to the structural integrity of the liver cells. As a result, these enzymes are found in the cytoplasm and are released into the circulation after cellular damage; EtOH could possibly damage other organelles such as the mitochondria, causing their enzymes [GDH, AST, and malate dehydrogenase (MDH)] to be released into the blood. This finding indicates that this xenobiotic causes damage to the plasma membrane as well as to the mitochondrial membrane[6,37,46,54]. In the PH-Gs-EtOH group, we observed a decrease in the activity of AST and ALT enzymes in serum, suggesting a possible capacity of the extract to preserve the normal structure of the liver (Figures 2 and 3).
Our results correlate with previous reports by various authors. Gayosso-De-Lucio et al[21] reported that Gs pre-treatment for 3 d resulted in a decrease in serum AST and ALT enzymatic activity after elevation by thioacetamide administration, concluding that this extract protects against liver damage caused by thioacetamide. Nakanishi et al[50] compared the protective effect of the three compounds (geraniin, ellagic acid, and gallic acid) on the damage caused to the liver by carbon tetrachloride, D-galactosamine, and thioacetamide; they found that geraniin and ellagic acid significantly decreased serum levels of both enzymes, which had been increased by exposure to these hepatotoxic agents. Both studies were conducted when the liver was not regenerating; however, our study was performed when this process was present, induced by PH, and when EtOH and the extract were administered at the same time.
Some mechanisms by which EtOH inhibits liver regeneration are known to be due to the increase of free radicals produced by EtOH metabolism, causing cellular damage as well as altering liver functions[55]. Our results suggest that the Gs extract eliminates free radicals and consequently eliminates the inhibiting effect of EtOH on liver regeneration, thereby diminishing damage in the cellular membrane and consequently lowering the concentration of lipid peroxidation in serum as well as in the liver, as can be observed in Figure 4, which correlates with findings reported by various authors[21,50,52].
Alterations in TAS, together with an important increase in the concentration of TBARS, are characteristic of OS[56]. As noted, our results indicate that OS was caused by the administration of EtOH, similar to the findings reported by our group[6]. Some investigators have used these to understand other types of xenobiotics, such as paracetamol and thioacetamide[21,57].
The administration of xenobiotics (EtOH, paracetamol, thioacetamide) during PH-induced liver regeneration promotes an increase in antioxidant mechanisms as a system of defense in an attack of the ROS that are produced during the OS generated by these hepatotoxins[20]. The decrease in TAS (serum) and increase in TAC (liver) found in our study (Figure 5) agree with previous studies, indicating that EtOH favors OS in this model, as demonstrated by the high TBARS levels found (Figure 4). The increase in the TBARS concentration indicates severe damage in cellular membranes throughout the organism, in experimental models with laboratory animals, and with alcohol-related liver disease. Ingesting antioxidant supplements has been shown to significantly diminish the TBARS levels in serum and restore antioxidant mechanisms to their normal levels in the liver[6,46]. Our results suggest that the GS extract increases the antioxidant defenses (TAS and TAC), importantly diminishing OS (TBARS), thus improving liver function (e.g., albumin, bilirubin, and triacylglycerides) and the liver’s proliferative capacity (weight and DNA gain).
The biological importance of this work consists of contributing some advances in the mechanisms of action implied in the hepatoprotective effect of Gs against the toxic action of alcohol. We found that Gs possesses an antioxidant effect in vitro that is most likely also expressed in vivo, as proposed for other species[58], which indicates the need to conduct more studies to confirm this effect. In addition, to our knowledge, there are no biological or pharmacological reports about its use. Our results also constitute, to our knowledge, the first report of the protective effect of Gs (most likely through the acetonyl-geraniin component) on damage caused by EtOH on liver regeneration. This study contributes new knowledge on a potential therapeutic alternative to treat alcohol-related liver damage and is relevant to public health because this damage constitutes one of the main causes of morbidity and mortality worldwide.
In summary, we have addressed the protective effect shown by the geranium extract on the damage caused by ethanol on liver regeneration. However, knowing the cellular or molecular mechanisms of action of the phytochemicals is important and will be an interesting area for future studies. Various reports have demonstrated that the polyphenol compounds act to activate the nuclear factor erythroid-2-related factor 2 (Nrf-2) transcription factor and/or directly as free radical scavengers. Thus, molecular and cellular studies will be needed to study the specific effect of the components of Gs, such as acetonyl-geraniin, on the regulation of the Keap1-Nrf2-ARE pathway and the principal antioxidant enzymes and their gene expression, amount of protein and specific activity.
Hepatopathies associated with consuming alcohol comprise a group of diseases of interest due to their great social impact. Plant extracts have been poorly applied as a protective agent against the damage caused by alcohol consumption. This article describes the hepatoprotective effect of an extract of Geranium schiedeanum (Gs) on a model of regenerative liver.
Studying the effect of natural products on limiting the damaging effect of alcohol consumption and the mechanisms for this protective effect is currently a hot topic.
This article presents an innovative approach for evaluating the hepatoprotective effects of plant extracts. This approach includes biochemical markers of metabolism, antioxidative performance and damage in serum and the liver and observations on the mortality of the studied animals.
The results presented in this article strongly suggest the use of geranium extract as a hepatoprotective agent. Moreover, this application can be extended to other processes in which limiting oxidative phenomena can be beneficial.
Partial hepatectomy is an operation consisting of removing the median and left lateral lobes of the liver. Liver regeneration after partial hepatectomy in the rat has been widely employed as an experimental model to study mammalian cell proliferation.
The authors investigated the protective effect of a Gs extract (exGs) in regenerative livers of rats receiving ethanol as hepatotoxin. For this purpose, they measured alanine aminotransferase, aspartate aminotransferase, albumin, bilirubin and some markers of carbohydrates and lipid metabolism in plasma as well as a biochemical, particularly antioxidant profile and histological consequences in the liver. Rats treated chronically with ethanol and exGs showed a significant decrease in mortality, oxidative stress and biochemical parameters of liver damage compared with rats that were not treated with exGs. In addition, the treatment with exGs was associated with an increase in liver regeneration.
P- Reviewer: Boscá L, Morales-Ruiz M, Perez MJ, Yu DY S- Editor: Ma YJ L- Editor: Wang TQ E- Editor: Ma S
| 1. | Higgins GM, Anderson RM. Experimental pathology of the liver. I. Restoration of the liver of the white rat following partial surgical removal. Arc Pathol. 1931;12:186-202. |
| 2. | Fausto N. Liver regeneration: from laboratory to clinic. Liver Transpl. 2001;7:835-844. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 104] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 3. | Michalopoulos GK, DeFrances MC. Liver regeneration. Science. 1997;276:60-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2649] [Cited by in RCA: 2468] [Article Influence: 88.1] [Reference Citation Analysis (0)] |
| 4. | Kountouras J, Boura P, Lygidakis NJ. Liver regeneration after hepatectomy. Hepatogastroenterology. 2001;48:556-562. [PubMed] |
| 5. | Haga S, Morita N, Irani K, Fujiyoshi M, Ogino T, Ozawa T, Ozaki M. p66(Shc) has a pivotal function in impaired liver regeneration in aged mice by a redox-dependent mechanism. Lab Invest. 2010;90:1718-1726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 6. | Ramírez-Farías C, Madrigal-Santillán E, Gutiérrez-Salinas J, Rodríguez-Sánchez N, Martínez-Cruz M, Valle-Jones I, Gramlich-Martínez I, Hernández-Ceruelos A, Morales-Gonzaléz JA. Protective effect of some vitamins against the toxic action of ethanol on liver regeneration induced by partial hepatectomy in rats. World J Gastroenterol. 2008;14:899-907. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 25] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 7. | Morales-González JA, Gutiérrez-Salinas J, Hernández-Muñoz R. Pharmacokinetics of the ethanol bioavailability in the regenerating rat liver induced by partial hepatectomy. Alcohol Clin Exp Res. 1998;22:1557-1563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 0.6] [Reference Citation Analysis (1)] |
| 8. | Maher JJ. Exploring alcohol’s effects on liver function. Alcohol Health Res World. 1997;21:5-12. [PubMed] |
| 9. | Halliwell B, Gutteridge JM. The antioxidants of human extracellular fluids. Arch Biochem Biophys. 1990;280:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 907] [Cited by in RCA: 856] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 10. | Morales-González JA, Gutiérrez-Salinas J, Yáñez L, Villagómez-Rico C, Badillo-Romero J, Hernández-Muñoz R. Morphological and biochemical effects of a low ethanol dose on rat liver regeneration: role of route and timing of administration. Dig Dis Sci. 1999;44:1963-1974. [PubMed] |
| 11. | Thurman RG, Bradford BU, Iimuro Y, Frankenberg MV, Knecht KT, Connor HD, Adachi Y, Wall C, Arteel GE, Raleigh JA. Mechanisms of alcohol-induced hepatotoxicity: studies in rats. Front Biosci. 1999;4:e42-e46. [PubMed] |
| 12. | Gupta S, Pandey R, Katyal R, Aggarwal HK, Aggarwal RP, Aggarwal SK. Lipid peroxide levels and antioxidant status in alcoholic liver disease. Indian J Clin Biochem. 2005;20:67-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 13. | Wu D, Cederbaum AI. Alcohol, oxidative stress, and free radical damage. Alcohol Res Health. 2003;27:277-284. [PubMed] |
| 14. | Morales-González JA, Jiménez-García LF, Guitérrez-Salinas J, Sepúlveda J, Leija-Salas A, Hernández-Muñoz R. Effects of ethanol administration on hepatocellular ultrastructure of regenerating liver induced by partial hepatectomy. Dig Dis Sci. 2001;46:360-369. [PubMed] |
| 15. | Saleem TS, Chetty CM, Ramkanth S, Rajan VS, Kumar KM, Gauthaman K. Hepatoprotective herbs-a review. Int J Res Pharm Sci. 2010;1:1-5. |
| 16. | Adewusi EA, Afolayan AJ. A review of natural products with hepatoprotective activity. J Med Plant Res. 2010;4:1318-1334. [DOI] [Full Text] |
| 17. | Kono H, Arteel GE, Rusyn I, Sies H, Thurman RG. Ebselen prevents early alcohol-induced liver injury in rats. Free Radic Biol Med. 2001;30:403-411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 96] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 18. | Wheeler MD, Nakagami M, Bradford BU, Uesugi T, Mason RP, Connor HD, Dikalova A, Kadiiska M, Thurman RG. Overexpression of manganese superoxide dismutase prevents alcohol-induced liver injury in the rat. J Biol Chem. 2001;276:36664-36672. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 138] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 19. | Rosengren RJ. Catechins and the treatment of breast cancer: possible utility and mechanistic targets. IDrugs. 2003;6:1073-1078. [PubMed] |
| 20. | Vargas-Mendoza N, Madrigal-Santillán E, Morales-González A, Esquivel-Soto J, Esquivel-Chirino C, García-Luna Y González-Rubio M, Gayosso-de-Lucio JA, Morales-González JA. Hepatoprotective effect of silymarin. World J Hepatol. 2014;6:144-149. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 294] [Cited by in RCA: 242] [Article Influence: 22.0] [Reference Citation Analysis (4)] |
| 21. | Gayosso-De-Lucio J, Bautista M, Velazquez-González C, De la O Arciniega M, Morales-González JA, Benedí J. Chemical composition and hepatotoxic effect of Geranium schiedeanum in a thioacetamide-induced liver injury model. Pharmacogn Mag. 2014;10:S574-S580. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 22. | Madrigal-Santillán E, Madrigal-Bujaidar E, Álvarez-González I, Sumaya-Martínez MT, Gutiérrez-Salinas J, Bautista M, Morales-González Á, García-Luna y González-Rubio M, Aguilar-Faisal JL, Morales-González JA. Review of natural products with hepatoprotective effects. World J Gastroenterol. 2014;20:14787-14804. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 284] [Cited by in RCA: 225] [Article Influence: 20.5] [Reference Citation Analysis (4)] |
| 23. | Gayosso-De-Lucio JA, Torres-Valencia JM, Cerda-García-Rojas CM, Joseph-Nathan P. Ellagitannins from Geranium potentillaefolium and G. bellum. Nat Prod Commun. 2010;5:531-534. [PubMed] |
| 24. | Gayosso-De-Lucio J, Torres-Valencia M, Rojo-Domínguez A, Nájera-Peña H, Aguirre-López B, Salas-Pacheco J, Avitia-Domínguez C, Téllez-Valencia A. Selective inactivation of triosephosphate isomerase from Trypanosoma cruzi by brevifolin carboxylate derivatives isolated from Geranium bellum Rose. Bioorg Med Chem Lett. 2009;19:5936-5939. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 25. | Tanaka T, Fijisaki H, Nonaka G, Nishioka I. Tannins and Related Compounds CXVIII. Structures, Preparation, High-Performance Liquid Chromatography and some reactions of Dehydroellagitaniin-Acetone Condensates. Chem Pharm Bull. 1992;40:2937-2944. |
| 26. | Pérez-Escandón BE, Villavicencio MA, Ramirez-Aguirre A. Lista Floristica del Estado de Hidalgo Recopilación Bibliografica. 1st ed. Pachuca, Hidalgo, México: UAEH 1998; . |
| 27. | Okuda T, Ito H. Tannins of Constant Structure in Medicinal and Food Plants - Hydrolyzable Tannins and Polyphenols Related to Tannins. Molecules. 2011;16:2191-2217. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 156] [Cited by in RCA: 148] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 28. | Bautista M, Gayosso de Lúcio JA, Vargas-Mendoza N, Velázquez-González C, De la O Arciniega M, Almaguer-Vargas G. Geranium Species as Antioxidants. 1st ed. Croatia: Morales-Gonzalez, J.A. Ed 2013; 113-129. [DOI] [Full Text] |
| 29. | Gülçin I, Huyut Z, Elmastas M, Aboul-Enein HY. Radical scavenging and antioxidant activity of tannic acid. Arab J Chem. 2010;3:43-53. [RCA] [DOI] [Full Text] [Cited by in Crossref: 482] [Cited by in RCA: 520] [Article Influence: 34.7] [Reference Citation Analysis (0)] |
| 30. | NORMA Oficial Mexicana NOM-062-ZOO-1999, Especificaciones técnicas para la producción, cuidado y uso de los animales de laboratorio. Available from: http://www.fmvz.unam.mx/fmvz/principal/archivos/062ZOO.PDF. |
| 31. | DeCarli LM, Lieber CS. Fatty liver in the rat after prolonged intake of ethanol with a nutritionally adequate new liquid diet. J Nutr. 1967;91:331-336. [PubMed] |
| 32. | Gill K, France C, Amit Z. Voluntary ethanol consumption in rats: an examination of blood/brain ethanol levels and behavior. Alcohol Clin Exp Res. 1986;10:457-462. [PubMed] |
| 33. | Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265-275. [PubMed] |
| 34. | Orrego H, Crossley IR, Saldivia V, Medline A, Varghese G, Israel Y. Long-term ethanol administration and short- and long-term liver regeneration after partial hepatectomy. J Lab Clin Med. 1981;97:221-230. [PubMed] |
| 35. | Labarca C, Paigen K. A simple, rapid, and sensitive DNA assay procedure. Anal Biochem. 1980;102:344-352. [PubMed] |
| 36. | Bautista M, Andres D, Cascales M, Morales-González JA, Sánchez-Reus MI, Madrigal-Santillán E, Valadez-Vega C, Fregoso-Aguilar T, Mendoza-Pérez JA, Gutiérrez-Salinas J. Role of Kupffer cells in thioacetamide-induced cell cycle dysfunction. Molecules. 2011;16:8319-8331. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 37. | Morales-González JA, Gutiérrez-Salinas J, Piña E. Release of mitochondrial rather than cytosolic enzymes during liver regeneration in ethanol-intoxicated rats. Arch Med Res. 2004;35:263-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 38. | Gutiérrez-Salinas J, Aranda-Fraustro A, Paredes-Díaz R, Hernández-Muñoz R. Sucrose administration to partially hepatectomized rats: a possible model to study ethanol-induced inhibition of liver regeneration. Int J Biochem Cell Biol. 1996;28:1007-1016. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 39. | Galicia-Moreno M, Gutiérrez-Reyes G. The role of oxidative stress in the development of alcoholic liver disease. Rev Gastroenterol Mex. 2014;79:135-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 58] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 40. | Global Information System on Alcohol and Health (GISAH). Accessed on 24-10-2014. Available from: http://www.who.int/gho/alcohol/en/. |
| 41. | Mathurin P, Deltenre P. Effect of binge drinking on the liver: an alarming public health issue? Gut. 2009;58:613-617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 95] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 42. | Michalopoulos GK. Liver regeneration: molecular mechanisms of growth control. FASEB J. 1990;4:176-187. [PubMed] |
| 43. | Frank WO, Rayyes AN, Washington A, Holt PR. Effect of acute ethanol administration upon hepatic regeneration. J Lab Clin Med. 1979;93:402-413. [PubMed] |
| 44. | Yoshida Y, Komatsu M, Ozeki A, Nango R, Tsukamoto I. Ethanol represses thymidylate synthase and thymidine kinase at mRNA level in regenerating rat liver after partial hepatectomy. Biochim Biophys Acta. 1997;1336:180-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 45. | Tsukamoto I, Taniguchi Y, Miyoshi M, Kojo S. Purification and characterization of thymidine kinase from regenerating rat liver. Biochim Biophys Acta. 1991;1079:348-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 46. | Parra-Vizuet J, Camacho-Luis A, Madrigal-Santillan E, Bautista M, Esquivel-Soto J, Esquivel-Chirino C, García-Luna M, Mendoza-Pérez JA, Chanona-Pérez J, Morales-González JA. Hepatoprotective effects of glycine and vitamin E, during the early phase of liver regeneration in the rat. African J Pharm Pharmacol. 2009;8:384-390. |
| 47. | Crabb DW, Pinairs J, Hasanadka R, Fang M, Leo MA, Lieber CS, Tsukamoto H, Motomura K, Miyahara T, Ohata M. Alcohol and retinoids. Alcohol Clin Exp Res. 2001;25:207S-217S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 19] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 48. | Portari GV, Jordão Júnior AA, Meirelles MS, Marchini JS, Vannucchi H. Effect of beta-carotene supplementation on rats submitted to chronic ethanol ingestion. Drug Chem Toxicol. 2003;26:191-198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 49. | Morsiani E, Mazzoni M, Aleotti A, Gorini P, Ricci D. Increased sinusoidal wall permeability and liver fatty change after two-thirds hepatectomy: an ultrastructural study in the rat. Hepatology. 1995;21:539-544. [PubMed] |
| 50. | Nakanishi Y, Orita M, Okuda T, Abe H. Effects of geraniin on the liver in rats I - effects of geraniin compared to ellagic acid, and gallic acido n hepatic injuries induced by CCl4, D-galactosamine, and thioacetamide. Nat Med. 1998;52:396-403. |
| 51. | Kumar Rajagopal S, Manickam P, Periyasamy V, Namasivayam N. Activity of Cassia auriculata leaf extract in rats with alcoholic liver injury. J Nutr Biochem. 2003;14:452-458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 64] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 52. | Nakanishi Y, Kubo M, Okuda T, Orita M, Abe H. Hematological effect of geraniin in rat. Nat Med. 1998;52:179-183. |
| 53. | Greco M, Moro L, Pellecchia G, Di Pede S, Guerrieri F. Release of matrix proteins from mitochondria to cytosol during the prereplicative phase of liver regeneration. FEBS Lett. 1998;427:179-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 54. | He S, Rehman H, Shi Y, Krishnasamy Y, Lemasters JJ, Schnellmann RG, Zhong Z. Suramin decreases injury and improves regeneration of ethanol-induced steatotic partial liver grafts. J Pharmacol Exp Ther. 2013;344:417-425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 55. | Diehl AM. Recent events in alcoholic liver disease V. effects of ethanol on liver regeneration. Am J Physiol Gastrointest Liver Physiol. 2005;288:G1-G6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 35] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 56. | Nielsen F, Mikkelsen BB, Nielsen JB, Andersen HR, Grandjean P. Plasma malondialdehyde as biomarker for oxidative stress: reference interval and effects of life-style factors. Clin Chem. 1997;43:1209-1214. [PubMed] |
| 57. | Olaleye MT, Amobonye AE, Komolafe K, Akinmoladun AC. Protective effects of Parinari curatellifolia flavonoids against acetaminophen-induced hepatic necrosis in rats. Saudi J Biol Sci. 2014;21:486-492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 37] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 58. | Ito H. Metabolites of the ellagitannin geraniin and their antioxidant activities. Planta Med. 2011;77:1110-1115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 83] [Article Influence: 5.9] [Reference Citation Analysis (0)] |