Published online Jun 28, 2015. doi: 10.3748/wjg.v21.i24.7571
Peer-review started: December 24, 2014
First decision: January 23, 2015
Revised: February 14, 2015
Accepted: April 9, 2015
Article in press: April 9, 2015
Published online: June 28, 2015
Processing time: 188 Days and 7.5 Hours
AIM: To identify the most effective treatment of duodenal stump fistula (DSF) after gastrectomy for gastric cancer.
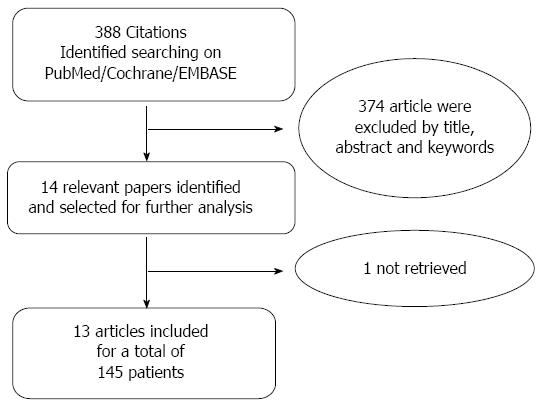
METHODS: A systematic review of the literature was performed. PubMed, EMBASE, Cochrane Library, CILEA Archive, BMJ Clinical Evidence and UpToDate databases were analyzed. Three hundred eighty-eight manuscripts were retrieved and analyzed and thirteen studies published between 1988 and 2014 were finally selected according to the inclusion criteria, for a total of 145 cases of DSF, which represented our group of study. Only patients with DSF after gastrectomy for malignancy were selected. Data about patients’ characteristics, type of treatment, short and long-term outcomes were extracted and analyzed.
RESULTS: In the 13 studies different types of treatment were proposed: conservative approach, surgical approach, percutaneous approach and endoscopic approach (3 cases). The overall mortality rate was 11.7% for the entire cohort. The more frequent complications were sepsis, abscesses, peritonitis, bleeding, pneumonia and multi-organ failure. Conservative approach was performed in 6 studies for a total of 79 patients, in patients with stable general condition, often associated with percutaneous approach. A complete resolution of the leakage was achieved in 92.3% of these patients, with a healing time ranging from 17 to 71 d. Surgical approach included duodenostomy, duodeno-jejunostomy, pancreatoduodenectomy and the use of rectus muscle flap. In-hospital stay of patients who underwent relaparotomy ranged from 1 to 1035 d. The percutaneous approach included drainage of abscesses or duodenostomy (32 cases) and percutaneous biliary diversion (13 cases). The median healing time in this group was 43 d.
CONCLUSION: Conservative approach is the treatment of choice, eventually associated with percutaneus drainage. Surgical approach should be reserved for severe cases or when conservative approaches fail.
Core tip: To our knowledge, this is the first review available in the literature focusing on the duodenal stump fistula following resection for gastric cancer. Previous review have been published concerning this topic but not limited to oncologic patients. Furthermore, in this review, a more recent time period is analyzed, increasing reliability of the conclusions of the manuscript considering advancement in technologies and treatment strategies.
- Citation: Aurello P, Sirimarco D, Magistri P, Petrucciani N, Berardi G, Amato S, Gasparrini M, D’Angelo F, Nigri G, Ramacciato G. Management of duodenal stump fistula after gastrectomy for gastric cancer: Systematic review. World J Gastroenterol 2015; 21(24): 7571-7576
- URL: https://www.wjgnet.com/1007-9327/full/v21/i24/7571.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i24.7571
Gastric cancer is still one of the most frequent malignancies in Europe. In United States the estimated new cases in 2010 were 21000 (12730 male and 8270 female) with 10570 estimated deaths (6350 male and 4220 female)[1,2]. A total or subtotal gastrectomy with R0 margins remains the standard of care for gastric cancer[3,4]. Despite this, in low-volume centers gastrectomy still remains a challenging procedure with a notable morbidity rate (33%-43%) and mortality rate (7%-12%)[5,6]. Duodenal stump fistula (DSF) represents an infrequent but severe complication after total or subtotal gastrectomy for gastric cancer, with incidence of 3% and mortality rate ranging from 7% to 67%[7]. Several factors were identified as possible cause of DSF, such as local hematoma, inflammation, intra-operative inadequate closure of the duodenal stump, incorrect drain position, devascularization, post-operative distension of the duodenum and R1-R2 resections[7,8]. Abscess, complete anastomotic dehiscence, peritonitis, wound infections and sepsis often affect patients with DSF. Cholecystitis, pancreatitis, pneumonia, malnutrition, fluid and electrolyte disorders may also occur[7,9]. However, despite the importance of this kind of complication, there are no reports indicating the correct management of this life-threatening condition in patients with gastric cancer. In fact, in 2013 Babu et al[10] reported the current status of the management of duodenal fistula, but they did not distinguish between neoplastic and non-neoplastic disease. They considered all the cases of duodenal fistula and a longer time frame (from 1948 to 2011)[10], while we included in our work only cases of fistula after gastrectomy for gastric cancer from 1988 to December 2014. Therefore, we decided to review the literature from the last twenty-six years, in order to identify the attitude of surgeons in treating duodenal stump leakage after gastrectomy for gastric cancer, and to assess the most effective treatment.
The research was systematically performed on PubMed, EMBASE and Cochrane Library databases CILEA Archive, BMJ Clinical Evidence and UpToDate databases by entering the strings “duodenal stump fistula AND treatment ” or “duodenal stump leakage AND treatment” or “duodenal stump leak AND treatment” or “duodenal stump fistula AND management ” or “duodenal stump leakage AND management” or “duodenal stump leak AND management” or “duodenal fistula AND gastrectomy AND treatment” or “duodenal fistula AND gastrectomy AND management”. The research was limited to articles written in English and published between January 1988 and December 2014. A total of 388 manuscripts were retrieved. Title, abstract section, and keywords were screened in order to select studies for further assessment. Articles including randomized clinical trials (RCTs), controlled clinical trials, cohort studies, case-control studies, case series and case reports regarding total and subtotal gastrectomies for gastric cancer and post-operative duodenal stump leakage management, as well as endoscopic and percutaneous procedures case series and case reports, were considered eligible to be included in the study. Studies reporting pediatric patients and non-surgery related fistulae were not included, as well as studies not reporting enough information on the outcomes (Figure 1). Only patients with gastric cancer where included in our review, even when the case series included both benign and malignant disease[7,11-13].
The two works by Cozzaglio et al[7,8] included in our review refer to different institutions, different cohorts of patients and different study intervals, therefore we decided to include them both.
Only studies published between 1988 and 2014 that reported the results of the management of duodenal stump leakage after gastrectomy for malignancy were considered. We excluded the studies reporting data about gastrojejunal anastomosis leakage and other form of duodenal fistula. According to the aforementioned criteria, thirteen studies were considered eligible, for a total of 145 patients (Table 1).
| Ref. (year) | Country | Period | Patients, n | Study | Healing time in days (mean) | Conservative treatment | Surgical treatment | Endoscopic treatment | Percutaneous transhepatic biliary diversion | Percutaneus treatment (drainage of abscesses or duodenostomy) | Results | Complications |
| Garden et al[15] 1988 | United Kingdom | 1979-1985 | 13 | RS | 17-71(35) | 12 | 1 | 0 | 0 | 9 | 12 spontaneous closure 1 need surgical | nr |
| Bloch et al[19] 1989 | France | 1989 | 1 | Case | 5 wk | 0 | 0 | 0 | 0 | 1 | Solved | 0 |
| Kyzer et al[12]1997 | Israel | 1991-1994 | 2 | RS | nr | 1 | 1 | 0 | 0 | 0 | The pt. Surgically treated dead for sepsis | 1pt. dead |
| Wong et al[11] 2000 | China | 1993-1997 | 1 | Case | 2 | 1 | 0 | 1 | 0 | 0 | Closure in 2 day | 0 |
| Oh et al[14] 2009 | South Korea | 1987-2004 | 5 | RS | 10-28(18) | 0 | 5 | 0 | 0 | 0 | Solved | |
| Lee et al[20] 2009 | South Korea | 2009 | 1 | Case | 17 | 0 | 0 | 1 | 0 | 0 | Solved | |
| Cozzaglio et al[7] 2010 | Italy | 1991-2006 | 68 | MT/RS | 1-1035(median 19) | 51 | 27 | NR | 4 | 17 | Non surgical Solved in25 day (7-65); surgical 58d. (1-1035) | 51 (75%), 11 (16.2%) pts. Dead |
| Cozzaglio et al[8] 2011 | Italy | 2005-2010 | 6 | PR | 20-604(median 43) | 0 | 0 | 0 | 6 | 0 | 3 Solved | 3pts. dead (50%) |
| Curcio et al[13] 2012 | Italy | 2012 | 1 | Case | 60 | 0 | 0 | 1 | 0 | 0 | Closure in 2 months | |
| Blouhos et al[22] 2013 | Greece | 2013 | 1 | Case | 45 | 0 | 1 | 0 | 0 | 0 | Solved | |
| Orsenigo et al[23] 2014 | Italy | 1987-2012 | 32 | RS | 31.2 ± 19.7 (mean conservative, PTBD and drainage), 45.2 ± 57.4 (mean surgical) | 11 | 13 | 0 | 3 | 5 | Conservative:11/11 solvedPTBD 3/3 solvedDrainage 5/5 solvedSurgical:10/13 solved3/13 death | 1 pt. Bleeding2 pts. Septic shock |
| Kim et al[24] 2014 | South Korea | 2002-2012 | 13 | RS | 14-134 (57.3 surgical), 8-18 (11.7 mean conservative) | 3 | 10 | 0 | 0 | 0 | Conservative: 3/3 solvedSurgical 8/10 solved2/10 death | 2 pts. dead |
| Vasiliadis et al[25] 2014 | Greece | 2014 | 1 | case | 17 | 0 | 1 | 0 | 0 | 0 | 1 surgical solved in 17 days | |
| Tot. (1988-2014), n (%) | 1979-2014 | 145 | 1-1035 | 79 (54.5) | 59 (40.7) | 3 (2.1) | 13 (9.0) | 32 (22.1) |
The overall mortality rate was 11.7%. Complications included sepsis, abscesses, peritonitis, bleeding, pneumonia and MOF, as showed in Table 1.
Different treatments were grouped into four main categories: conservative approach (79 cases), surgical approach (59 cases), endoscopic approach (3 cases) and percutaneous approach (45 cases).
The surgical approach included duodenostomy, duodeno-jejunostomy, pancreatoduodenectomy and the use of rectus muscle flap[7] and was used generally in patients with more severe conditions. We identified 8 studies that reported this approach and presented a total of 59 cases. In-hospital stay of patients who underwent relaparotomy for DSF range from 1 to 1035 days (median 19)[7,14].
The conservative approach was used in 6 studies including 79 patients and was considered as the first approach to treat DSF, in patients with stable general condition (without sepsis, bleeding or abscesses); it was often associated with percutaneous treatment. This approach consisted in administration of drugs, such as somatostatin or octreotide, together with the positioning of a nasogastric tube[7,11,12,15]. According to the reported data, a complete resolution of the leakage was achieved in 92.3% of patients treated conservatively, with an healing time interval ranging from 17 to 71 d (mean 35)[15].
The percutaneous approach should be further divided in drainage of abscesses or duodenostomy (32 cases) and percutaneous biliary diversion (13 cases). Percutaneous approach was often associated with conservative treatment and consisted in abscess drainage, biliary drainage, biliary diversion or fistula closure with cyanoacrylate[7,16]. The median healing time in this group was 43 d (range: 20-604 d)[8]. Our review identified only 3 studies that reported data about endoscopic treatment, for a total of 3 patients. Mean healing time after endoscopic treatment ranged from 2 d to 2 mo[11,13]. No data about nutritional status were reported regarding these 3 patients. The first paper, by Curcio and colleagues, reported a case of DSF with wide orefice (2 cm) surrounded by hyperemic mucosa treated using tulip bundle technique and injection of 4 mL of fibrin glue in submucosa[13,17,18]. Lee described a small fistula, treated with clipping, healed in 17 d[13]. Wong reported a case of DSF treated with debridement and fibrin glue closure solved in 2 d without complication.
In this manuscript we systematically reviewed the literature from the last twenty-six years to identify the most appropriate treatment for DSF after gastrectomy for gastric cancer.
As a matter of fact, in their work Cozzaglio and colleagues reported that patients have been assigned to percutaneous drainage if they had a high daily DSF output (median 500 mL, range 300-1000 mL) or if a previous conservative treatment was unsuccessful (parenteral and enteral nutrition, antibiotics, antifungals, octreotide and percutaneous drainage of abdominal abscesses); also patients with severe general conditions who couldn’t undergo a re-laparotomy[8] were treated by percutaneous approach. Percutaneous drainage is a useful treatment not only for the fistula itself but also for the prevention of infections; it is important to consider continuous local irrigation and suction from the tube in order to improve drainage efficacy and avoid drain’s closure.
Furthermore, Cozzaglio et al[8] suggests performing a biliary diversion to reduce the output of DSF in patients with severe clinical conditions, unfit for a conservative treatment or invasive approaches, such as relaparotomy, and when other approaches failed (output reduced from a median of 500 mL/d to 100 mL/d, P = 0.02)[8]. Garden et al[15] reported 12 cases of spontaneous closure of DSF with conservative approach, associated in 9 cases with a percutaneous drainage of the abscesses. Only 1 case needed relaparotomy and surgical stump closure[15]. Bloch et al[19] treated one patient with a percutaneous approach (elective intubation of fistula) and obtained a complete resolution in 5 wk. Kyzer et al[12] reported a case of DSF approached conservatively with complete resolution, and a case of a patient who underwent relaparotomy who eventually died due to sepsis. Unfortunately they did not clarify why this patient needed a relaparotomy. Wong et al[11] report a novel endoscopic approach. In that case they inspected the fistula tract under direct vision and they closed the tract with gelatin sponge and fibrin glue after irrigation and drainage of abscess, reporting rapid and complete resolution of the DSF. In their work Oh et al[14] reported 5 cases of DSF: patients were septic so they were treated with relaparotomy and primary closure, apparently without complications. However, there aren’t any further data available on the postoperative outcomes of those patients.
Endoscopic approach was presented by Lee et al[20] for a small perforation of duodenal stump after gastrectomy with Billroth II reconstruction. They treated DSF using endoscopic clipping; after 17 d complete healing was confirmed by a gastrografin study. In this article they suggest that the success of this approach depended on rapid clip deployment and early detection of fistula[20,21].
Cozzaglio et al[7] in 2010 showed in a large study that conservative approach should be the treatment of choice, while surgical approach should be considered only when other approaches fail or when DSF is associated with severe complications, i.e., bleeding or sepsis. Curcio et al[13] report a case of endoscopic closure of a wide DSF. The endoscopic approach was adopted after conservative treatment and percutaneous drainage failed: DSF resolved in 2 mo. Blouhos et al[22] reports the case of a patient with evidence of DSF (bile-stained fluid in drainage) in which was performed a relaparotomy on POD 1°, due to bleeding complications. A duodenostomy, biliary diversion and closure of duodenal leak was performed with complete resolution of bleeding and DSF. However, the reported results demonstrate that conservative approach provides the highest rate of success, which is higher than 92%[7,15]. Endoscopic approach seems promising, but needs further studies since only 3 cases are reported in literature. As described above, surgical approach demonstrates a high morbidity rate, therefore all the authors agree that relaparotomy should be performed only for patients with severe complications (sepsis, active bleeding or fistula with involvement of surrounding organs[7]) or when other approaches fail[14]. Orsenigo in 2014 presented a retrospective study of 32 patients affected by DSF from 1987 through 2012, 19 patients were treated with non-surgical approaches, such as conservative treatment (11 pts.), biliary diversion (3 pts.), and percutaneous drainage (5 pts.). All those patients received also treatment with octreotide or somatostatine, and nutritional support, either parenteral, enteral or by mouth. The mean healing time for patients in non-surgical group was 31.2 d. Surgical treatment can be divided in: direct stump closure (4 patients), stump resection and closure (6 patients), external duodenal drainage (2 patients) and surgical abdominal drainage (1 patient), all associated with treatment with drugs (octreotide or somatostatine), TPN or EN. In this group, after stump resection and closure, 2 patients died for septic shock and 1 patient died for bleeding. However, DSF resolved in 45.2 d (on average) in 10 patients[23]. In 2014 Kim et al[24] published a retrospective study on the risks factors associated with DSF. They reviewed the data of 2970 patients who underwent gastric surgery for cancer. Patients who underwent Billroth-I procedure, palliative procedures, gastric wedge resection and patients who experienced complications other than DSF were excluded from the analysis. Finally, 1195 patients were included in their study. DFS occurred in 13 patients, and was treated with surgical approach in 10 cases and with conservative approach in 3 cases. Two patients died after relaparotomy, one for sepsis and one for MOF. On the other hand, DSF healing was observed in all the 3 patients treated with conservative approach in a mean of 11.7 d.
Vasiliadis et al[25] reported in 2014 a case of a duodenal stump fistula treated with re-stapling of the duodenum and retrograde decompressing tube duodenostomy. The treatment was successful and the DFS healed in 17 d.
There are some biases to elucidate. Table 1 clearly shows that the number of reported procedures is higher than the number of patients, which is due to the frequent association between conservative and endoscopic or percutaneous treatment, therefore some patients were considered and reported in two or more groups.
Moreover, when those approaches failed or in case of sepsis or bleeding, a further surgical correction was required. Some patients treated with surgical approach were in poor general conditions or septic or had an active bleeding. Therefore, there is a patient-selection bias among the surgical treatment group affecting mortality and in-hospital rate, that appear higher in this group.
Patient nutritional status is an important factor for the healing of DSF. Enteral or parenteral nutrition may influence the closure of DSF, improving patient nutritional status. Parenteral nutrition is the most commonly used artificial nutrition, whereas enteral nutrition was used only by a few authors, and often associated with parenteral nutrition. Only a few data about DSF are present in the literature and role of artificial nutrition should be further clarified[7,26-28].
An interesting experience is presented in the literature concerning growth hormone treatment for DSF; despite this, the article was not included in the review because of the chinese language limitation (exclusion criteria of our systematic review). We do believe that future research could be developed in order to understand the role of this alternative treatment for the disease[29].
Babu et al[10] recently reviewed the literature to identify the most appropriate treatment for DSF. They suggest that it would be advisable to adopt a conservative policy of “wait and watch” for 4-6 wk. These results are consistent with our findings, although they included in their work both neoplastic and non-neoplastic diseases. Moreover, even if they considered studies from a wider range of years, they noticed the same patient-selection biases that we previously underlined.
Conservative approach is the treatment of choice and should be attempted for at least 4-6 wk, unless the clinical conditions require reoperation. Maintaining or positioning a nasogastric tube and maintaining a percutaneous drainage can be associated with conservative treatment. The positioning of percutaneous drainage should be considered in case of abdominal abscesses. Percutaneous biliary diversion can be used to reduce leakage and daily output in patients with severe generals condition (that are unfit for surgery), persistent DSF or high daily DSF output. Surgical approach should be considered for severe cases like bleeding, sepsis or leak in adjacent organs, or when conservative and percutaneous approaches fail (in cases in which the general conditions permit). Endoscopic approach showed good results but in our analysis only 3 patients underwent this procedure, so more studies are needed to clarify the efficacy of this approach.
Gastric cancer is still one of the most frequent malignancies in Europe. A total or subtotal gastrectomy with R0 margins remains the standard of care for gastric cancer. Despite this, in low-volume centers gastrectomy still remains a challenging procedure with a notable morbidity rate and mortality rate. Duodenal stump fistula (DSF) represents an infrequent but severe complication after total or subtotal gastrectomy for gastric cancer, with incidence of 3% and mortality rate ranging from 7% to 67%.
Babu et al reported the current status of the management of duodenal fistula, but they did not distinguish between neoplastic and non-neoplastic disease.
Cholecystitis, pancreatitis, pneumonia, malnutrition, fluid and electrolyte disorders may also occur. However, despite the importance of this kind of complication, there are no reports indicating the correct management of this life-threatening condition in patients with gastric cancer. The authors included their work only cases of fistula after gastrectomy for gastric cancer from 1988 to December 2014. Therefore, the authors decided to review the literature from the last twenty-six years, in order to identify the attitude of surgeons in treating duodenal stump leakage after gastrectomy for gastric cancer, and to assess the most effective treatment.
This is a well written manuscript regarding the review of management for duodenal stump leakage after surgery for gastric cancer. However, the case number enrolled seems to be not enough. She suggest that the authors might use the keyword as anastomosis leakage instead of duodenal stump leakage while searching the databases.
P- Reviewer: Fang WL, Karakoyun R, Shao QS S- Editor: Yu J L- Editor: A E- Editor: Zhang DN
| 1. | Inghelmann R, Grande E, Francisci S, Verdecchia A, Micheli A, Baili P, Capocaccia R, De Angelis R. Regional estimates of stomach cancer burden in Italy. Tumori. 2007;93:367-373. [PubMed] |
| 2. | Aurello P, Bellagamba R, Rossi Del Monte S, D’Angelo F, Nigri G, Cicchini C, Ravaioli M, Ramacciato G. Apoptosis and microvessel density in gastric cancer: correlation with tumor stage and prognosis. Am Surg. 2009;75:1183-1188. [PubMed] |
| 3. | Aurello P, Magistri P, Nigri G, Petrucciani N, Novi L, Antolino L, D’Angelo F, Ramacciato G. Surgical management of microscopic positive resection margin after gastrectomy for gastric cancer: a systematic review of gastric R1 management. Anticancer Res. 2014;34:6283-6288. [PubMed] |
| 4. | Martin RC, Jaques DP, Brennan MF, Karpeh M. Achieving RO resection for locally advanced gastric cancer: is it worth the risk of multiorgan resection? J Am Coll Surg. 2002;194:568-577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 104] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 5. | Pedrazzani C, Marrelli D, Rampone B, De Stefano A, Corso G, Fotia G, Pinto E, Roviello F. Postoperative complications and functional results after subtotal gastrectomy with Billroth II reconstruction for primary gastric cancer. Dig Dis Sci. 2007;52:1757-1763. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 36] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 6. | McCulloch P, Ward J, Tekkis PP. Mortality and morbidity in gastro-oesophageal cancer surgery: initial results of ASCOT multicentre prospective cohort study. BMJ. 2003;327:1192-1197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 265] [Cited by in RCA: 270] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 7. | Cozzaglio L, Coladonato M, Biffi R, Coniglio A, Corso V, Dionigi P, Gianotti L, Mazzaferro V, Morgagni P, Rosa F. Duodenal fistula after elective gastrectomy for malignant disease: an italian retrospective multicenter study. J Gastrointest Surg. 2010;14:805-811. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 45] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 8. | Cozzaglio L, Cimino M, Mauri G, Ardito A, Pedicini V, Poretti D, Brambilla G, Sacchi M, Melis A, Doci R. Percutaneous transhepatic biliary drainage and occlusion balloon in the management of duodenal stump fistula. J Gastrointest Surg. 2011;15:1977-1981. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 33] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 9. | Rossi JA, Sollenberger LL, Rege RV, Glenn J, Joehl RJ. External duodenal fistula. Causes, complications, and treatment. Arch Surg. 1986;121:908-912. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 10. | Babu BI, Finch JG. Current status in the multidisciplinary management of duodenal fistula. Surgeon. 2013;11:158-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 11. | Wong SK, Lam YH, Lau JY, Lee DW, Chan AC, Chung SC. Diagnostic and therapeutic fistuloscopy: an adjuvant management in postoperative fistulas and abscesses after upper gastrointestinal surgery. Endoscopy. 2000;32:311-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 12. | Kyzer S, Binyamini Y, Melki Y, Ohana G, Koren R, Chaimoff C, Wolloch Y. Comparative study of the early postoperative course and complications in patients undergoing Billroth I and Billroth II gastrectomy. World J Surg. 1997;21:763-76; discussion 767. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 13. | Curcio G, Badas R, Miraglia R, Barresi L, Tarantino I, Traina M. Duodenal stump fistula following Roux-en-Y gastrectomy, treated with single-balloon enteroscopy using the tulip bundle technique and fibrin glue injection. Endoscopy. 2012;44 Suppl 2 UCTN:E364-E365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 14. | Oh SJ, Choi WB, Song J, Hyung WJ, Choi SH, Noh SH. Complications requiring reoperation after gastrectomy for gastric cancer: 17 years experience in a single institute. J Gastrointest Surg. 2009;13:239-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 66] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 15. | Garden OJ, Dykes EH, Carter DC. Surgical and nutritional management of postoperative duodenal fistulas. Dig Dis Sci. 1988;33:30-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 16. | Zarzour JG, Christein JD, Drelichman ER, Oser RF, Hawn MT. Percutaneous transhepatic duodenal diversion for the management of duodenal fistulae. J Gastrointest Surg. 2008;12:1103-1109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 18] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 17. | Curcio G, Traina M, Panarello G, Barresi L, Tarantino I, Arcadipane A, Gridelli B. Refractory gastric ulcer bleeding treated with new endoloop/clips technique. Dig Endosc. 2011;23:203-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 18. | Mocciaro F, Curcio G, Tarantino I, Barresi L, Spada M, Petri SL, Traina M. Tulip bundle technique and fibrin glue injection: unusual treatment of colonic perforation. World J Gastroenterol. 2011;17:1088-1090. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 19. | Bloch P, Gompel H. Treatment of postoperative duodenal fistulae by transparietal abdominal endoscopic intubation. Surg Endosc. 1989;3:167-169. [PubMed] |
| 20. | Lee JY, Ryu KW, Cho SJ, Kim CG, Choi IJ, Kim MJ, Lee JS, Kim HB, Lee JH, Kim YW. Endoscopic clipping of duodenal stump leakage after Billroth II gastrectomy in gastric cancer patient. J Surg Oncol. 2009;100:80-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 21. | Devereaux CE, Binmoeller KF. Endoclip: closing the surgical gap. Gastrointest Endosc. 1999;50:440-442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 24] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 22. | Blouhos K, Boulas KA, Konstantinidou A, Salpigktidis II, Katsaouni SP, Ioannidis K, Hatzigeorgiadis A. Early rupture of an ultralow duodenal stump after extended surgery for gastric cancer with duodenal invasion managed by tube duodenostomy and cholangiostomy. Case Rep Surg. 2013;2013:430295. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 23. | Orsenigo E, Bissolati M, Socci C, Chiari D, Muffatti F, Nifosi J, Staudacher C. Duodenal stump fistula after gastric surgery for malignancies: a retrospective analysis of risk factors in a single centre experience. Gastric Cancer. 2014;17:733-744. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 44] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 24. | Kim KH, Kim MC, Jung GJ. Risk factors for duodenal stump leakage after gastrectomy for gastric cancer and management technique of stump leakage. Hepatogastroenterology. 2014;61:1446-1453. [PubMed] |
| 25. | Vasiliadis K, Fortounis K, Kokarhidas A, Papavasiliou C, Nimer AA, Stratilati S, Makridis C. Delayed duodenal stump blow-out following total gastrectomy for cancer: Heightened awareness for the continued presence of the surgical past in the present is the key to a successful duodenal stump disruption management. A case report. Int J Surg Case Rep. 2014;5:1229-1233. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 26. | Braga M, Ljungqvist O, Soeters P, Fearon K, Weimann A, Bozzetti F. ESPEN Guidelines on Parenteral Nutrition: surgery. Clin Nutr. 2009;28:378-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 443] [Cited by in RCA: 394] [Article Influence: 24.6] [Reference Citation Analysis (0)] |
| 27. | Sandler JT, Deitel M. Management of duodenal fistulas. Can J Surg. 1981;24:124-125. [PubMed] |
| 28. | Weimann A, Braga M, Harsanyi L, Laviano A, Ljungqvist O, Soeters P, Jauch KW, Kemen M, Hiesmayr JM, Horbach T. ESPEN Guidelines on Enteral Nutrition: Surgery including organ transplantation. Clin Nutr. 2006;25:224-244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 736] [Cited by in RCA: 659] [Article Influence: 34.7] [Reference Citation Analysis (0)] |
| 29. | Tang Y, Li R, Chen L, Wu X. [Nutritional support of duodenal stump leakage after gastrectomy for gastric carcinoma]. Zhonghua Wei Chang Wai Ke Zazhi. 2008;11:47-49. [PubMed] |