Published online Jun 28, 2015. doi: 10.3748/wjg.v21.i24.7545
Peer-review started: January 24, 2015
First decision: March 10, 2015
Revised: March 31, 2015
Accepted: May 7, 2015
Article in press: May 21, 2015
Published online: June 28, 2015
Processing time: 157 Days and 21.1 Hours
AIM: To assess the evolution of duodenal lymphocytosis (DL), a condition characterized by increased intraepithelial lymphocytes (IELs), over 2 years of follow-up.
METHODS: Consecutive patients undergoing upper endoscopy/histology for abdominal pain, diarrhea, weight loss, weakness or other extraintestinal features compatible with celiac disease (CD) were included. Evaluation of IELs infiltrate in duodenal biopsy samples was carried out by CD3-immunohistochemistry and expressed as number of positive cells/100 enterocytes. Diagnostic agreement on the IELs count was tested by calculating the weighted k coefficient. All patients underwent serological detection of autoantibodies associated with CD: IgG and IgA anti-tissue transglutaminase and endomysium. Each patient underwent further investigations to clarify the origin of DL at baseline and/or in the course of 2 years of follow-up every six months. Autoimmune thyroiditis, intestinal infections, parasitic diseases, bacterial intestinal overgrowth, hypolactasia and wheat allergy were detected. Colonoscopy and enteric magnetic resonance imaging were performed when necessary. Risk factors affecting the final diagnosis were detected by multinomial logistic regression and expressed as OR.
RESULTS: Eighty-five patients (16 males, 69 females, aged 34.1 ± 12.5 years) were followed up for a mean period of 21.7 ± 11.7 mo. At baseline, endoscopy/duodenal biopsy, CD3 immunohistochemistry revealed: > 25 IELs/100 enterocytes in 22 subjects, 15-25 IELs in 37 and < 15 IELs in 26. They all had negative serum anti-transglutaminase and anti-endomysium, whilst 5 showed IgG anti-gliadin positivity. In the course of follow-up, 23 developed CD seropositivity and gluten sensitivity (GS) was identified in 19. Other diagnoses were: 5 Helicobacter pylori infections, 4 jejunal Crohn’s disease, 1 lymphocytic colitis and 1 systemic sclerosis. The disease in the remaining 32 patients was classified as irritable bowel syndrome because of the lack of diagnostic evidence. At multivariate analysis, the evolution towards CD was associated with an IELs infiltrate > 25 (OR = 1640.4) or 15-25 (OR = 16.95), human leukocyte antigen (HLA) DQ2/8 (OR = 140.85) or DQA1*0501 (OR = 15.36), diarrhea (OR = 5.56) and weakness (OR = 11.57). GS was associated with IELs 15-25 (OR = 28.59), autoimmune thyroiditis (OR = 87.63), folate deficiency (OR = 48.53) and diarrhea (OR = 54.87).
CONCLUSION: DL may have a multifactorial origin but the IELs infiltrate and HLA are strong predictive factors for CD development and a clinical diagnosis of GS.
Core tip: Duodenal lymphocytosis may pose a diagnostic challenge since it could be associated with different pathological conditions, which may or may not be related to gluten. In the present study, we demonstrated that during 2 years of follow-up of 85 patients with duodenal lymphocytosis, 27% developed celiac disease and 22% had a clinical diagnosis of gluten sensitivity; the remaining patients were affected by non-gluten-related conditions. At multivariate analysis, the haplotype DQ2/8 and the density of the intraepithelial CD3 lymphocyte infiltrate were the best predictors of the future development of gluten-related disorders.
- Citation: Losurdo G, Piscitelli D, Giangaspero A, Principi M, Buffelli F, Giorgio F, Montenegro L, Sorrentino C, Amoruso A, Ierardi E, Di Leo A. Evolution of nonspecific duodenal lymphocytosis over 2 years of follow-up. World J Gastroenterol 2015; 21(24): 7545-7552
- URL: https://www.wjgnet.com/1007-9327/full/v21/i24/7545.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i24.7545
Duodenal lymphocytosis (DL) is a pathological condition characterized by the presence of lymphocytes in the epithelial layer of the duodenum with a normal villous architecture[1]. These particular lymphocytes are called “intraepithelial lymphocytes” (IELs) and are T cells which express the CD3 molecule on their membranes. IELs are involved in many functions; in particular, they play a pivotal role in surveillance and activation of the immune response against food antigens[2]. Owing to this peculiar function, a dysregulation of their task has been invoked as the most important pathogenic mechanism in the development of celiac disease (CD)[3]. Indeed, a large infiltration of IELs in the duodenum is a hallmark and key finding in the pathological diagnosis of this disorder. According to the Marsh-Oberhuber classification, the presence of more than 25 IELs per 100 enterocytes, the so-called Marsh stage 1, is the most widely employed cutoff for diagnosing CD[4]. However, CD is not the only cause of DL; other gluten-related conditions, such as gluten sensitivity (GS) or wheat allergy may be characterized by a similar microscopic duodenal pattern[5]. On the other hand, many other non-gluten-related conditions may be linked to DL, for example autoimmune disorders (systemic lupus erythematosus, systemic sclerosis, vasculitides), drugs (aspirin, olmesartan), Helicobacter pylori (H. pylori) gastritis, intestinal bacterial/viral infections or parasitic infestations, Crohn’s disease, small intestinal bacterial overgrowth (SIBO) and congenital/acquired immune deficiencies[6]. Moreover, in children, a condition of DL may not in fact be pathological[7]. Based on the above-reported evidence, DL is a nonspecific condition and therefore follow-up including multiple investigations is often needed to make a clear diagnosis.
Many studies have investigated the prevalence of CD in DL. In the Mayo Clinic pediatric series of 1290 duodenal biopsy samples, only 56 showed DL and 16% of these patients had CD[8]. Aziz et al[9] found a prevalence of about 22% of CD in DL and demonstrated that the main predictors of CD were serological positivity of anti-tissue transglutaminase (anti-tTG) and anti-endomysium antibodies (EMA) and the compatibility of the HLA status. The highest prevalence of gluten-related enteropathy in DL was reported in a Spanish series and was 43%[10].
The clinical pattern of subjects with DL is often similar to that of CD. VandeVoort showed that the prevalence of diarrhea, weight loss and abdominal pain are almost the same when comparing CD and non-celiac DL, whereas symptoms suggesting systemic or organic disorders, such as anemia or skin diseases, are more common in CD[11]. Likewise, Zanini et al[12] showed that apart from a common clinical background marked by abdominal discomfort and diarrhea, patients with DL related to CD had a higher prevalence of extraintestinal manifestations than non-gluten-related DL.
On these bases, we report the results of 2 years of follow-up of a series of patients with DL, with the aim of elucidating how their evolution towards gluten or non-gluten-related conditions may be influenced by clinical, serological, genetic and histopathological factors.
Consecutive patients enrolled in the period August 2008 - August 2013 complaining of abdominal pain, diarrhea, weight loss, weakness or other extraintestinal features compatible with CD were considered for this study. At baseline, all subjects had undergone upper endoscopy and biopsy sampling of the stomach and duodenum for histological evaluation. Only those with histological evidence of DL (i.e., the presence of lymphocytes in the epithelial layer of the duodenum with a normal villous architecture)[1] were enrolled for the follow-up. Oral consent for the treatment and management of biological samples was obtained by phone interview. Thereafter, they were referred to the outpatient clinic of our unit for 6 monthly investigations. This study was conducted in conformity with the Declaration of Helsinki illustrating the ethical principles for medical research involving human subjects[13].
According to the guidelines, four biopsy specimens were collected, two from the bulb and two from the distal duodenum[14]. Biopsies were mounted on fiber-free paper to aid orientation and then embedded in formalin. Initial evaluation of the sample was performed by hematoxylin-eosin staining. Immunohistochemistry of CD3 lymphocytes for the IELs count was performed using monoclonal murine antibody (Novocastra Leica Biosystems, Newcastle Ltd, United Kingdom), according to the manufacturer’s instructions. In all subjects, IELs were counted in a field containing at least 1000 enterocytes and expressed as number/100 enterocytes. The count was confined to the epithelial layer and performed by two blinded observers (DP and FB). Three groups were selected according to the IELs count: < 15 IELs/100 enterocytes, 15 to 25 IELs/100 enterocytes and > 25 IELs/100 enterocytes. Diagnostic agreement on the IELs count was tested by calculating the weighted k coefficient interpreted in accordance with the Landis and Koch benchmarks, with a value of more than 0.8 indicating excellent agreement. Collection and processing was performed according to BRISQ recommendations[15].
At baseline, all patients underwent serological detection of autoantibodies associated with CD: IgG and IgA anti-tissue transglutaminase (tTG) and endomysium (EMA) (Bio-Rad Laboratories, Inc, Segrate - MI - Italy) for CD diagnosis. In addition, IgA and IgG anti-native gliadin (AGA) were tested for suspected GS. Blood samples were taken for assays of a full blood count, folate, vitamin B12, serum protein electrophoresis with immunoglobulin subclasses and human leukocyte antigen (HLA) genotyping.
Follow-up included a large series of investigations. Autoimmune thyroiditis was detected by assaying anti-thyroid antibodies and thyroid hormones (TSH, fT3 and fT4). Stool samples were used to detect intestinal infection and parasitic diseases (in particular Giardia lamblia) and to assess fecal occult blood FOBT (Hemoccult, Beckman Coulter, Cassina de’ Pecchi, MI - Italy) and fecal calprotectin (CAL Detect®, Sofar SpA, Trezzano Rosa - MI - Italy). If one of the last two tests was positive, colonoscopy with histology and/or enteric magnetic resonance imaging were performed. Despite a negative histology at baseline, H. pylori infection was again checked in all patients by urea breath test and stool antigen test and, if positive, confirmed by a further endoscopic and histological examination of four biopsy samples, two taken from the body and two from the antrum. Glucose and lactose breath tests were carried out to exclude SIBO and hypolactasia, respectively. Finally, wheat allergy was tested by skin prick test and radioallergosorbent test (RAST).
The above-reported laboratory tests were repeated every 6 mo. Colonoscopy and enteric magnetic resonance imaging were repeated after one year, if necessary.
At the end of the follow-up period, diagnoses were classified in to three groups: CD, GS or non-gluten-related conditions.
Comparisons between the data obtained in our groups of patients were performed by χ2 test for trend for the analysis of percentages or proportions. Significance was indicated by P < 0.05.
Multinomial logistic regression was employed to evaluate the risk factors that could have influenced the final diagnosis. We considered the three possible diagnostic outcomes (CD, GS and non-gluten-related disorders) as dependent variables and all the other clinical and laboratory data listed above as independent variables. For the independent variables found to be statistically significant, OR and 95%CI were calculated. All statistical tests were 2-tailed and performed at the 5% level of significance. The statistical analysis was performed using the software SPSS Statistics for Windows, Version 20.0. Armonk, NY: IBM Corp.
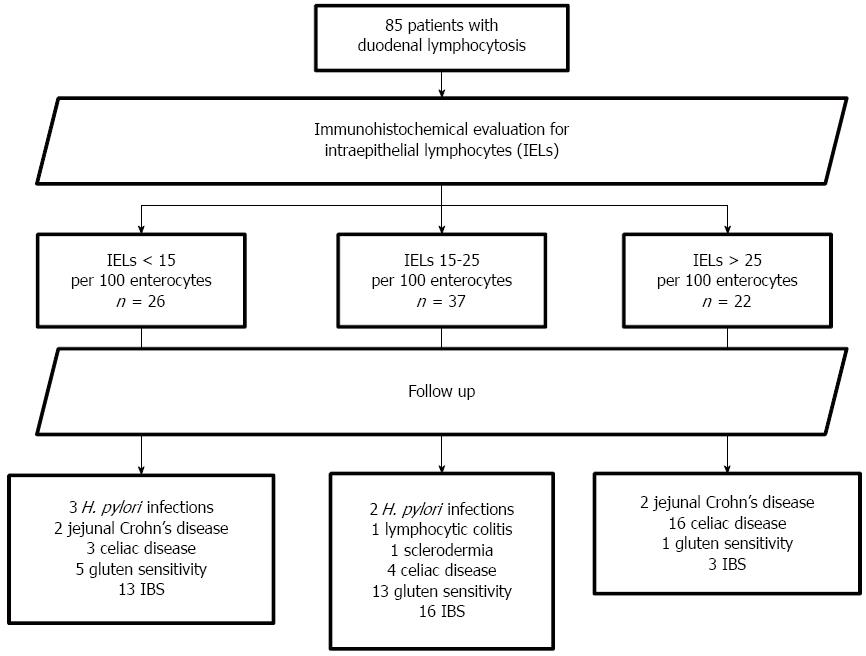
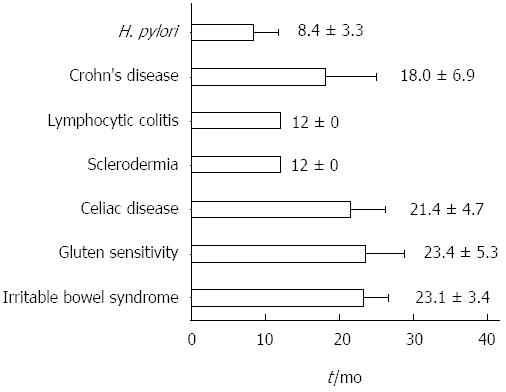
Eighty-five patients (16 males, 69 females, aged 34.1 ± 12.5 years, range 16-64, median 32) were eligible according to the inclusion criteria. The mean follow-up was 21.7 ± 11.7 mo, range 12-67, median 18. The overall review of the follow-up is reported in Figure 1. In Figure 2, the mean time needed to achieve the final diagnosis is reported separately for each disease. CD, GS, irritable bowel syndrome and Crohn’s disease required the longest follow-up period, namely 21.4 ± 4.7, 23.4 ± 5.3, 23.1 ± 3.4 and 18.0 ± 6.9 mo, respectively. Additionally, Table 1 summarizes the diagnosed diseases associated with DL in the course of the follow-up period, as well as the disease distribution in relation to the density of the IELs infiltrate.
| Diagnosed disease | IELs < 15 | IELs 15-25 | IELs > 25 | Total (n) | P value |
| (n) | (n) | (n) | |||
| Helicobacter pylori infection | 3 | 2 | 0 | 5 | 0.23 (NS) |
| Jejunal Crohn’s disease | 2 | 0 | 2 | 4 | 0.19 (NS) |
| Lymphocytic colitis | 0 | 1 | 0 | 1 | 0.53 (NS) |
| Scleroderma | 0 | 1 | 0 | 1 | 0.53 (NS) |
| Celiac disease | 3 | 4 | 16 | 23 | < 0.0001 |
| Gluten sensitivity | 5 | 13 | 1 | 19 | 0.02 |
| Irritable bowel syndrome | 13 | 16 | 3 | 32 | 0.01 |
| Total (n) | 26 | 37 | 22 | 85 |
After the baseline immunohistochemical evaluation, 22 patients had > 25 IELs, 37 had 15-25 IELs and 26 had < 15 IELs/100 enterocytes. Analysis of agreement about the IELs calculation between the observers yielded k = 0.87 (95%CI: 0.76-0.98). Enrolled patients were all negative at baseline for anti-tTG and EMA antibodies, 5 were positive for IgG anti-gliadin. Wheat allergy was not present in any patient. At the end of the follow-up, 23 (27.1%) had developed seropositivity for both anti-tTG and EMA and a second histological assessment showed a clear histological picture of CD (2 patients Marsh 3c, 6 Marsh 3a, 9 Marsh 2 and 6 Marsh 1). In these subjects, the diagnosis of CD was then confirmed by the histological and serological improvement induced by a gluten-free diet (GFD).
Five patients appeared to be positive for H. pylori infection in the course of the follow-up. They all had an IEL infiltrate < 25/100 enterocytes. After sequential regimen assumption, the urea breath test confirmed the successful eradication in all.
In four subjects, positivity for both FOBT and fecal calprotectin prompted the execution of colonoscopy and enteric magnetic resonance, revealing a jejunal localization of Crohn’s disease.
In one subject suffering from watery diarrhea, colonoscopy and histology demonstrated lymphocytic colitis. Another subject was diagnosed with systemic sclerosis at the rheumatology unit of our hospital. None of the enrolled patients were taking drugs that could induce DL. Breath tests did not demonstrate SIBO or lactose malabsorption in any patient.
In the remaining 51 subjects, a 4 mo GFD was proposed in order to achieve a clinical diagnosis of GS. Only 19 patients reported an improvement of symptoms after a personal interview and so they were classified as GS. Five of them were positive for IgG-AGA at baseline. The other 32 patients did not report any beneficial effect of a GFD and the final diagnosis made was irritable bowel syndrome.
Finally, after a mean follow-up of 2 years, 23 patients developed CD (27.1%) and 19 had GS (22.3%), while the remaining 43 (50.6%) suffered from non-gluten-related disorders (32 functional and 11 organic). The main clinical and laboratory features of the three groups of diagnoses are shown in Table 2.
| CD | GS | Non-gluten related disorders | P value | |
| n = 23 | n = 19 | n = 43 | ||
| Familiarity for CD | 13.0% | 10.5% | 0% | 0.02 |
| IgG - anti-gliadin antibodies | 0% | 10.5% | 0% | NS |
| Autoimmune thyroiditis | 21.7% | 21.0% | 12.7% | NS |
| Iron deficiency anemia | 21.7% | 10.5% | 17.0% | NS |
| Folate deficiency | 8.7% | 26.3% | 10.3% | NS |
| DQ 2-8 | 82.6% | 68.4% | 38.2% | 0.001 |
| DQA1*0501 heterozygosis | 17.3% | 21.1% | 10.6% | NS |
| Weight loss | 60.8% | 42.1% | 21.3% | 0.0008 |
| Abdominal pain | 82.6% | 94.7% | 57.4% | 0.001 |
| Diarrhea | 65.2% | 42.1% | 17.1% | 0.0001 |
| Weakness | 43.5% | 42.1% | 14.9% | 0.006 |
| Headache | 0% | 42.1% | 2.3% | NS |
At univariate analysis, the HLA DQ2/8 haplotypes were significantly associated with gluten-related disorders (P = 0.001). Among the clinical features, we found a positive association of gluten-related disorders with weight loss (P = 0.0008), diarrhea (P = 0.0001), weakness (P = 0.006) and abdominal pain (P = 0.001).
At multivariate analysis, the evolution towards CD was associated with an IELs infiltrate >25 (OR = 1640.4; 95%CI: 104.7-2341.1; P = 0.004), IELs 15-25 (OR = 16.95; 95%CI: 4.9-61.2; P = 0.01), HLA DQ2/8 (OR = 140.85; 95%CI: 4.9-678.84; P = 0.012), HLA DQA1*0501 heterozygosis (OR = 15.36; 95%CI: 2.11-47.32; P = 0.02), diarrhea (OR = 5.56; 95%CI: 3.55-12.45; P = 0.02) and weakness (OR = 11.57; 95%CI: 6.61-18.34; P = 0.017). The GS diagnosis was associated with IELs 15-25 (OR = 28.59; 95%CI: 10.12-57.26; P = 0.009), autoimmune thyroiditis (OR = 87.63; 95%CI: 28.16-129.93; P = 0.017), folate deficiency (OR = 48.53; 95%CI: 26.73-76.12; P = 0.01) and diarrhea (OR = 54.87; 95%CI: 46.12-71.36; P = 0.008). Finally, a comparison between univariate and multivariate analyses is reported in Table 3.
| CD | GS | |||
| Univariate | Multivariate | Univariate | Multivariate | |
| Age | NS | NS | NS | NS |
| Sex | NS | NS | NS | NS |
| IELs count | -IELs > 25 | -IELs > 25 | -IELs > 25 NS | -IELs > 25 NS |
| OR = 30.5; 95%CI: 6.994-132.8; P < 0.0001 | OR = 1640.4; 95%CI: 104.7-2341.1; P = 0.004 | -IELs 15-25 | -IELs 15-25 | |
| -IELs 15-25 | -IELs 15-25 | |||
| OR = 2.5 95%CI: 1.16-3.95; P = 0.02 | OR = 16.95; 95%CI: 4.9-61.2; P = 0.01 | OR = 8.76; 95%CI: 4.31-21.45; P = 0.01 | OR = 28.59; 95%CI: 10.12-57.26; P = 0.009 | |
| Familiarity for CD | OR = 18.48; 95%CI: 2.161-1016; P = 0.007 | NS | NS | NS |
| IgG - anti-gliadin antibodies | NS | NS | NS | NS |
| Autoimmune thyroiditis | NS | NS | NS | OR = 87.63; 95%CI: 28.16-129.93; P = 0.017 |
| Iron deficiency anemia | NS | NS | NS | NS |
| Folate deficiency | NS | NS | OR = 3.48; 95%CI: 1.78-12.32; P = 0.04 | OR = 48.53; 95%CI: 26.73-76.12; P = 0.01 |
| DQ 2-8 | OR = 11.58; 95%CI: 3.399-39.43; P < 0.0001 | OR = 140.85; 95%CI: 4.9-678.84; P = 0.012 | NS | NS |
| DQA1*0501 heterozygosis | NS | OR = 15.36; 95%CI: 2.11-47.32; P = 0.02 | NS | NS |
| Weight loss | NS | NS | NS | NS |
| Abdominal pain | NS | NS | NS | NS |
| Diarrhea | OR = 10.45; 95%CI: 3.221-33.88; P < 0.0001 | OR = 5.56; 95%CI: 3.55-12.45; P = 0.02 | OR = 5.571; 95%CI: 1.567-19.80; P = 0.01 | OR = 54.87; 95%CI: 46.12-71.36; P = 0.008 |
| Weakness | OR = 5.0; 95%CI: 1.519-16.46; P = 0.01 | OR = 11.57; 95%CI: 6.61-18.34; P = 0.017 | NS | NS |
| Headache | NS | NS | NS | NS |
DL is a common condition which has often been associated with gluten-related disorders. In a study by Shmidt et al[16], a constantly increasing trend of DL and a 20.9% prevalence rate of CD were demonstrated in the period between 2000-2010. A similar value (27.1%) was observed in the present study when patients with DL were followed up for 2 years. By contrast, the prevalence of a GS diagnosis in DL was tested in a study by Kakar et al[17] and estimated to be 9.3%. We found more than twice this value (22.3%) in our 2 years of follow-up experience. An explanation for this discrepancy could be the period of the previous study (2003, when the awareness of this novel entity was not very widespread) as well as the lack of objective markers of this condition. Moreover, our report demonstrates that the range of disorders associated with DL is extremely wide, although gastrointestinal functional disorders[18] are preponderant (37.6% in our study), in agreement with other reports in literature[19,20].
A first notable finding of our study is that the IELs count may be the best predictor for the development of CD and GS. People with less than 15 IELs/100 enterocytes are less likely to develop gluten-related conditions, confirming the utility of the immunohistochemical evaluation of IELs recommended as an essential diagnostic tool for CD[21]. Additionally, the presence of a mild IELs infiltrate was found to be associated with GS, consistent with the current definition of GS[22]. However, as shown in the present study, the IELs count alone is unable to diagnose CD since DL is a heterogeneous disorder for which many investigations are needed to gain a correct interpretation. In this regard, immunohistochemical evaluation of an IEL subtype, i.e., the one expressing the γδ T cell receptor (IEL-γδ) has been proposed as an alternative tool, with excellent results and a better power in discriminating CD from other non-gluten-related conditions[23]. Future studies are needed in order to establish whether IEL-γδ detection may have a true predictive and diagnostic role in DL.
The second significant result is the direct correlation between CD and HLA status. HLA is the locus of genes encoding for proteins on the cell surface responsible for regulation of the immune system in humans. HLA haplotype has already been demonstrated to be one of the best predictors at birth of a future onset of CD[24]. DQ2 and DQ8 are haplotypes deriving from the combination between two alleles[25], one of which may be DQA1*0501. Although several epidemiological studies exploring the relationship between DQ2/8 and CD have reported concordant results[26], the role played by DQA1*0501 heterozygosity remains unclear. To the best of our knowledge, this study is the first to have found a clear association of this last HLA haplotype with the risk of CD.
Unexpectedly, multivariate analysis demonstrated that folate deficiency was associated with GS more than CD. This finding may be explained by the fact that low folate is a common event in early malabsorption conditions[27], including GS, and it may be found even in patients with mild duodenal damage, as shown by a recent study[28]. Autoimmune thyroiditis is frequently associated with CD and many reports have confirmed this finding even in atypical, latent or seronegative CD[29,30]. Therefore, the association with GS in our series could be explained by the relationship of autoimmune thyroiditis with the overall spectrum of gluten-related conditions rather than a single disorder.
Familiarity for CD was not a predictive factor for CD development in DL at multivariate analysis, while univariate analysis demonstrated a good correlation of familiarity with both CD and GS compared to non-gluten-related conditions. In this regard, analysis of the same parameters by χ2 test underlined some differences in statistical significance compared to multivariate analysis.
Surprisingly, five patients were positive for H. pylori, even if at first examination this infection was not detected and only further histology and UBT revealed this condition. This finding could be explained by the problem of “missed”H. pylori infections due to sampling errors that are frequently classified as H. pylori-negative gastritis[31].
In conclusion, DL is a variable condition that includes disorders that are and are not related to gluten. Novel expert consensus reports have suggested including this condition under the more specific term of “microscopic enteritis” for the purposes of underlining the importance of this often underestimated condition[32]. The possibility that in some cases DL may evolve to CD seems to be secondary to genetic and histopathological factors. In these cases, conditions such as latent CD[33,34] or seronegative CD[35-37] could be hypothesized but the best management of DL nowadays remains watchful waiting with targeted examinations[32].
Duodenal lymphocytosis (DL) is a non-specific condition characterized by the presence of intraepithelial CD3 lymphocytes. It has often been associated with gluten-related disorders.
We followed up a series of patients with DL for 2 years with the aim of evaluating the evolution of the disorder and final diagnosis.
After a mean follow-up of 2 years, 23 patients developed celiac disease (27.1%) and 19 had gluten sensitivity (22.3%), while the remaining 43 (50.6%) suffered from non-gluten-related disorders: 32 functional (irritable bowel syndrome) and 11 organic conditions (5 Helicobacter pylori infections, 4 jejunal Crohn’s disease, 1 lymphocytic colitis and 1 systemic sclerosis). Thus, watchful follow-up is needed in such conditions to reach a firm diagnosis.
At multivariate analysis, the evolution towards celiac disease was associated with the intraepithelial lymphocytes (IELs) infiltrate > 25 (OR = 1640.4) or 15-25 (OR = 16.95), HLA DQ2/8 (OR = 140.85) or DQA1*0501 (OR = 15.36), diarrhea (OR = 5.56) and weakness (OR = 11.57). Gluten sensitivity was associated with IELs 15-25 (OR = 28.59), autoimmune thyroiditis (OR = 87.63), folate deficiency (OR = 48.53) and diarrhea (OR = 54.87). These variables could therefore be useful to support clinical diagnosis in suspected cases.
A study concerning the evolution of non-specific duodenal lymphocytosis in a 2 year follow-up period is a difficult task to accomplish. The authors did tell us that follow-up patients of DL with IELs in addition to other diagnostic tests or factors can differentiate between CD, gluten sensitivity or non-gluten-related disorders somehow. Although the study results are not definite, they are informative. The article is worth reading for gastroenterologists.
P- Reviewer: Lee CL, Li JF, Shimada S S- Editor: Ma YJ L- Editor: Roemmele A E- Editor: Liu XM
| 1. | Hammer ST, Greenson JK. The clinical significance of duodenal lymphocytosis with normal villus architecture. Arch Pathol Lab Med. 2013;137:1216-1219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 38] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 3. | Gujral N, Freeman HJ, Thomson AB. Celiac disease: prevalence, diagnosis, pathogenesis and treatment. World J Gastroenterol. 2012;18:6036-6059. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 412] [Cited by in RCA: 414] [Article Influence: 31.8] [Reference Citation Analysis (6)] |
| 4. | Oberhuber G, Granditsch G, Vogelsang H. The histopathology of coeliac disease: time for a standardized report scheme for pathologists. Eur J Gastroenterol Hepatol. 1999;11:1185-1194. [PubMed] |
| 5. | Sapone A, Bai JC, Ciacci C, Dolinsek J, Green PH, Hadjivassiliou M, Kaukinen K, Rostami K, Sanders DS, Schumann M. Spectrum of gluten-related disorders: consensus on new nomenclature and classification. BMC Med. 2012;10:13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 809] [Cited by in RCA: 676] [Article Influence: 52.0] [Reference Citation Analysis (0)] |
| 6. | Dickson BC, Streutker CJ, Chetty R. Coeliac disease: an update for pathologists. J Clin Pathol. 2006;59:1008-1016. [PubMed] |
| 7. | Guz-Mark A, Zevit N, Morgenstern S, Shamir R. Duodenal intraepithelial lymphocytosis is common in children without coeliac disease, and is not meaningfully influenced by Helicobacter pylori infection. Aliment Pharmacol Ther. 2014;39:1314-1320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 8. | Shmidt E, Smyrk TC, Faubion WA, Oxentenko AS. Duodenal intraepithelial lymphocytosis with normal villous architecture in pediatric patients: Mayo Clinic experience, 2000-2009. J Pediatr Gastroenterol Nutr. 2013;56:51-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 26] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 9. | Aziz I, Key T, Goodwin JG, Sanders DS. Predictors for Celiac Disease in Adult Cases of Duodenal Intraepithelial Lymphocytosis. J Clin Gastroenterol. 2015;49:477-482. [PubMed] |
| 10. | Rosinach M, Esteve M, González C, Temiño R, Mariné M, Monzón H, Sainz E, Loras C, Espinós JC, Forné M. Lymphocytic duodenosis: aetiology and long-term response to specific treatment. Dig Liver Dis. 2012;44:643-648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 11. | Vande Voort JL, Murray JA, Lahr BD, Van Dyke CT, Kroning CM, Moore SB, Wu TT. Lymphocytic duodenosis and the spectrum of celiac disease. Am J Gastroenterol. 2009;104:142-148. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 62] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 12. | Zanini B, Lanzarotto F, Villanacci V, Carabellese N, Ricci C, Lanzini A. Clinical expression of lymphocytic duodenosis in “mild enteropathy” celiac disease and in functional gastrointestinal syndromes. Scand J Gastroenterol. 2014;49:794-800. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 13. | WMA Declaration of Helsinki - Ethical Principles for Medical Research Involving Human Subjects. 2013. Available from: http://www.wma.net/en/30publications/10policies/b3/. |
| 14. | Ludvigsson JF, Bai JC, Biagi F, Card TR, Ciacci C, Ciclitira PJ, Green PH, Hadjivassiliou M, Holdoway A, van Heel DA. Diagnosis and management of adult coeliac disease: guidelines from the British Society of Gastroenterology. Gut. 2014;63:1210-1228. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 689] [Cited by in RCA: 782] [Article Influence: 71.1] [Reference Citation Analysis (2)] |
| 15. | Moore HM, Kelly AB, Jewell SD, McShane LM, Clark DP, Greenspan R, Hayes DF, Hainaut P, Kim P, Mansfield EA. Biospecimen reporting for improved study quality (BRISQ). Cancer Cytopathol. 2011;119:92-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 146] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 16. | Shmidt E, Smyrk TC, Boswell CL, Enders FT, Oxentenko AS. Increasing duodenal intraepithelial lymphocytosis found at upper endoscopy: time trends and associations. Gastrointest Endosc. 2014;80:105-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 31] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 17. | Kakar S, Nehra V, Murray JA, Dayharsh GA, Burgart LJ. Significance of intraepithelial lymphocytosis in small bowel biopsy samples with normal mucosal architecture. Am J Gastroenterol. 2003;98:2027-2033. [PubMed] |
| 18. | Jadallah KA, Khader YS. Celiac disease in patients with presumed irritable bowel syndrome: a case-finding study. World J Gastroenterol. 2009;15:5321-5325. [PubMed] |
| 19. | Arévalo F, Aragon V, Montes P, Guzmán E, Monge E. [Increase of intraepithelial lymphocytes in patients with irritable bowel syndrome]. Rev Gastroenterol Peru. 2011;31:315-318. [PubMed] |
| 20. | Sundin J, Rangel I, Kumawat AK, Hultgren-Hörnquist E, Brummer RJ. Aberrant mucosal lymphocyte number and subsets in the colon of post-infectious irritable bowel syndrome patients. Scand J Gastroenterol. 2014;49:1068-1075. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 47] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 21. | Villanacci V, Ceppa P, Tavani E, Vindigni C, Volta U. Coeliac disease: the histology report. Dig Liver Dis. 2011;43 Suppl 4:S385-S395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 74] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 22. | Catassi C, Bai JC, Bonaz B, Bouma G, Calabrò A, Carroccio A, Castillejo G, Ciacci C, Cristofori F, Dolinsek J. Non-Celiac Gluten sensitivity: the new frontier of gluten related disorders. Nutrients. 2013;5:3839-3853. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 363] [Cited by in RCA: 314] [Article Influence: 26.2] [Reference Citation Analysis (0)] |
| 23. | Lonardi S, Villanacci V, Lorenzi L, Lanzini A, Lanzarotto F, Carabellese N, Volta U, Facchetti F. Anti-TCR gamma antibody in celiac disease: the value of count on formalin-fixed paraffin-embedded biopsies. Virchows Arch. 2013;463:409-413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 24. | Liu E, Lee HS, Aronsson CA, Hagopian WA, Koletzko S, Rewers MJ, Eisenbarth GS, Bingley PJ, Bonifacio E, Simell V. Risk of pediatric celiac disease according to HLA haplotype and country. N Engl J Med. 2014;371:42-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 253] [Cited by in RCA: 250] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 25. | Megiorni F, Pizzuti A. HLA-DQA1 and HLA-DQB1 in Celiac disease predisposition: practical implications of the HLA molecular typing. J Biomed Sci. 2012;19:88. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 126] [Cited by in RCA: 153] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 26. | Rostami-Nejad M, Romanos J, Rostami K, Ganji A, Ehsani-Ardakani MJ, Bakhshipour AR, Zojaji H, Mohebbi SR, Zali MR, Wijmenga C. Allele and haplotype frequencies for HLA-DQ in Iranian celiac disease patients. World J Gastroenterol. 2014;20:6302-6308. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 33] [Cited by in RCA: 45] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 27. | Wierdsma NJ, van Bokhorst-de van der Schueren MA, Berkenpas M, Mulder CJ, van Bodegraven AA. Vitamin and mineral deficiencies are highly prevalent in newly diagnosed celiac disease patients. Nutrients. 2013;5:3975-3992. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 147] [Cited by in RCA: 173] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 28. | Zanini B, Caselani F, Magni A, Turini D, Ferraresi A, Lanzarotto F, Villanacci V, Carabellese N, Ricci C, Lanzini A. Celiac disease with mild enteropathy is not mild disease. Clin Gastroenterol Hepatol. 2013;11:253-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 65] [Article Influence: 5.4] [Reference Citation Analysis (1)] |
| 29. | Virili C, Bassotti G, Santaguida MG, Iuorio R, Del Duca SC, Mercuri V, Picarelli A, Gargiulo P, Gargano L, Centanni M. Atypical celiac disease as cause of increased need for thyroxine: a systematic study. J Clin Endocrinol Metab. 2012;97:E419-E422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 83] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 30. | Metso S, Hyytiä-Ilmonen H, Kaukinen K, Huhtala H, Jaatinen P, Salmi J, Taurio J, Collin P. Gluten-free diet and autoimmune thyroiditis in patients with celiac disease. A prospective controlled study. Scand J Gastroenterol. 2012;47:43-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 48] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 31. | Losurdo G, Principi M, Di Leo A, Ierardi E. Letter: Helicobacter-negative gastritis--a distinct condition? Aliment Pharmacol Ther. 2015;41:597-598. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 32. | Rostami K, Aldulaimi D, Holmes G, Johnson MW, Robert M, Srivastava A, Fléjou JF, Sanders DS, Volta U, Derakhshan MH. Microscopic enteritis: Bucharest consensus. World J Gastroenterol. 2015;21:2593-2604. [PubMed] |
| 33. | Rostami Nejad M, Hogg-Kollars S, Ishaq S, Rostami K. Subclinical celiac disease and gluten sensitivity. Gastroenterol Hepatol Bed Bench. 2011;4:102-108. [PubMed] |
| 34. | Kaukinen K, Collin P, Mäki M. Latent coeliac disease or coeliac disease beyond villous atrophy? Gut. 2007;56:1339-1340. [PubMed] |
| 35. | Abrams JA, Diamond B, Rotterdam H, Green PH. Seronegative celiac disease: increased prevalence with lesser degrees of villous atrophy. Dig Dis Sci. 2004;49:546-550. [PubMed] |
| 36. | Montenegro L, Piscitelli D, Giorgio F, Covelli C, Fiore MG, Losurdo G, Iannone A, Ierardi E, Di Leo A, Principi M. Reversal of IgM deficiency following a gluten-free diet in seronegative celiac disease. World J Gastroenterol. 2014;20:17686-17689. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 9] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 37. | Ierardi E, Losurdo G, Piscitelli D, Giorgio F, Sorrentino C, Principi M, Montenegro L, Amoruso A, Di Leo A. Seronegative celiac disease: Where is the specific setting? Gastroenterol Hepatol Bed Bench. 2015;8:110-116. |