Published online Jun 28, 2015. doi: 10.3748/wjg.v21.i24.7468
Peer-review started: January 7, 2015
First decision: January 22, 2015
Revised: February 26, 2015
Accepted: April 3, 2015
Article in press: April 3, 2015
Published online: June 28, 2015
Processing time: 173 Days and 18 Hours
AIM: To explore the effect of grape seed proanthocyanidin (GSP) in liver ischemia/reperfusion (IR) injury and alleviation of endoplasmic reticulum stress.
METHODS: Male Sprague-Dawley rats (220-250 g) were divided into three groups, namely, sham, IR, and GSP groups (n = 8 each). A liver IR (70%) model was established and reperfused for 6 h. Prior to reperfusion, the GSP group was administered with GSP (100 mg/kg) for 15 d, and liver histology was then investigated. Serum aminotransferase and inflammatory mediators coupled with superoxide dismutase and methane dicarboxylic aldehyde were detected. Western blot was conducted to analyze the expression of glucose-regulated protein 78, CCAAT/enhancer-binding protein homologous protein, activating transcription factor-4, inositol-requiring enzyme-1, procaspase-12, and nuclear factor-κb. Apoptotic cells were detected by TUNEL staining.
RESULTS: The serum aminotransferase, apoptotic cells, and Suzuki scores decreased in the GSP group compared with the IR group (Ps < 0.05). The methane dicarboxylic aldehyde level was decreased in the GSP group, but the superoxide dismutase level was reversed (Ps < 0.05). Similarly, GSP downregulated the proinflammatory factors and upregulated the levels of anti-inflammatory factors (Ps < 0.05). Western blot data showed that GSP increased glucose-regulated protein 78 expression and suppressed expression of CCAAT/enhancer-binding protein homologous protein, activating transcription factor-4, inositol-requiring enzyme-1, procaspase-12, and nuclear factor-κb compared with the IR group.
CONCLUSION: GSP possesses antioxidative, anti-inflammatory, and antiapoptotic effects by relieving endoplasmic reticulum stress through regulation of related signaling pathways to protect the liver against IR injury.
Core tip: Liver ischemia/reperfusion (IR) injury induces endoplasmic reticulum (ER) stress. Numerous studies show that excessive ER stress aggravates IR injury. Grape seed proanthocyanidin (GSP) is an effective protector against IR injury. However, the detailed protective mechanisms remain unclear. Therefore, this study explored the effect of GSP in the liver for protection against IR injury and the alleviation of ER stress. The results indicate that GSP possesses antioxidative, anti-inflammatory, and antiapoptotic effects by relieving ER stress in the liver through regulation of related signaling pathways.
- Citation: Xu ZC, Yin J, Zhou B, Liu YT, Yu Y, Li GQ. Grape seed proanthocyanidin protects liver against ischemia/reperfusion injury by attenuating endoplasmic reticulum stress. World J Gastroenterol 2015; 21(24): 7468-7477
- URL: https://www.wjgnet.com/1007-9327/full/v21/i24/7468.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i24.7468
Ischemia/reperfusion (IR) injury of the liver can occur in several clinical settings, such as hepatic trauma, resection of large intrahepatic tumors, and liver transplantation[1], resulting in cell death and tissue destruction[2]. Increasing evidence has shown that both proinflammatory cytokines and reactive oxygen species (ROS) are key mediators of liver IR injury[3]. However, the exact mechanisms regarding endoplasmic reticulum (ER) stress on IR has not been clearly elucidated.
The ER is a key organelle in eukaryotic cells, where lipid synthesis, protein folding (into tertiary and quaternary structures), and protein maturation occur. The ER senses and responds to homeostatic changes with various stimuli, such as ischemia, hypoxia, elevated protein synthesis, and Ca2+ overload[4]. The ER protein folding capacity is reduced under stress, leading to accumulation of unfolded proteins. A major response to ER stress is the activation of glucose-regulated protein 78 (GRP78) through dissociation from its transmembrane receptor, which allows subsequent regulation of the levels of accumulated, unfolded proteins[5]. Slight and moderate ER stress can protect cells from death, but severe ER stress induces caspase-12-dependent cell apoptosis[6]. When severe ER stress occurs, activating transcription factor (ATF)-4 can increase the expression of CCAAT/enhancer-binding protein homologous protein (CHOP), promoting ER stress response through numerous mechanisms, and CHOP promotes oxidative stress inflammation and apoptosis[7]. Inositol-requiring enzyme (IRE)-1 is an important protein relative to ER stress and is vital to the occurrence of inflammation induced by ER stress and simultaneous activation of nuclear factor (NF)-κb[8]. Numerous studies show that ER stress plays a critical role in a variety of processes[9], and is also important in the occurrence of IR damage[10]. Data have suggested that attenuating ER stress-induced apoptosis can protect the brain against IR injury[11]. Thus, ER stress is closely related to IR injury.
Proanthocyanidins are highly bioavailable and provide a significantly greater protection against damage from oxidative stress than vitamin C, vitamin E, or β-carotene[12]. ER stress intensifies various types of damage, leading to inflammation, oxidative stress injury, and abnormal cell apoptosis. Thus, the botanical ingredients previously mentioned can effectively inhibit the occurrence of injury[13] induced by ER stress. Grape seed proanthocyanidin (GSP) is abundant in phenolic compounds and exerts antibacterial, antiviral, anticarcinogenic, antimutagenic, anti-inflammatory, antiallergic, and vasodilatory effects[14]. Various animal studies have also shown its antiapoptotic effect[15]. Several natural botanical ingredients have been reported to effectively alleviate the injury owing to their benefits on anti-inflammatory, antioxidant, and other pharmacologic properties, but the relevant mechanism remains unclear. Therefore, this study aimed to investigate whether regulation of ER stress is one of the mechanisms by which GSP protects the liver against IR.
Male Sprague-Dawley rats weighing 220-250 g obtained from Beijing Vital River Experimental Animal Technology Co. Ltd (SCXK2010-0001) were maintained at room temperature with a 12 h light/dark cycle. The rats were allowed to move freely, and food and water were available ad libitum. All procedures involving animals were reviewed and approved by the Institutional Animal Care and Use Committee of the Nanjing Medical University (IACUC protocol number: NJMU08-092).
The rats were randomly divided into three groups: sham, IR, and GSP groups (n = 8 each). GSP (purity > 95%; Tianjin Jianfeng Natural Product R&D Co., Ltd., Tianjin, China) was dissolved in distilled water and administered to the GSP group (a daily dose of 100 mg/kg)[16] by oral gavage for 15 d prior to surgery. The sham and IR groups received equal amounts of saline (0.9%) for 15 d. None of the animals died during the procedure.
Surgery was performed on rats after a 10-h abrosia. Under the intraperitoneal injection of hydrate (10%, 3 mL/kg) anesthesia, a midline laparotomy was made using minimal dissection. Hepatic ischemia (70%) was induced for 60 min by clamping the portal vein, hepatic artery, and bile duct of the left and median, and the rats were then reperfused for 6 h after the surgery. The rats were anesthetized, and tissue blood samples were collected. Parts of the hepatic tissue samples were stored at -80 °C for Western blot and reverse transcription (RT)-PCR analyses. Other parts of the hepatic tissue samples were placed in formaldehyde (10%) for histologic evaluation[17] and TUNEL staining[18]. Blood samples were collected from the rats’ vena cavae using a Bioclean injector (5 mL) and centrifuged at 3000 r/min for 10 min to obtain the serum, which was stored at -80 °C until further examination.
Serum aspartate aminotransferase (AST) and alanine aminotransferase (ALT), the indices of hepatocyte damage, were determined spectrophotometrically using an automated analyzer in the clinical biochemical room of the First Affiliated Hospital of Nanjing Medical University.
For light microscopic detections, hepatic tissue specimens were fixed in 10% formaldehyde, dehydrated in alcohol series, cleared in toluene, and embedded in paraffin. Paraffin sections (5 μm) were stained with hematoxylin and eosin and examined under a photomicroscope (Olympus BX51; Olympus Corp., Tokyo, Japan). Sections were scored from 0 to 4 for sinusoidal congestion, vacuolization of hepatocyte cytoplasm, and parenchymal necrosis as described by Suzuki et al[19] (Table 1).
| Score | Congestion | Vacuolization | Necrosis |
| 0 | None | None | None |
| 1 | Minimal | Minimal | Single cell necrosis |
| 2 | Mild | Mild | < 30% |
| 3 | Moderate | Moderate | 30%–60% |
| 4 | Severe | Severe | > 60% |
Serum levels of interleukin (IL)-6, tumor necrosis factor (TNF)-α, IL-10, and transforming growth factor (TGF)-β1 were detected using ELISA according to the manufacturer’s protocols (DL goat anti-rat TNF-α enzyme-linked immunosorbent assay (ELISA) kit, ADL goat anti-rat IL-6 ELISA kit, ADL goat anti-rat IL-10 ELISA kit, and ADL goat anti-rat TGF-β1 ELISA kit) and are expressed as pg/mL.
Quantitative analysis of the mRNA expression of IL-6, TNF-α, IL-10, and TGF-β1 was performed by RT-PCR using 96-well optical reaction plates in the ABI Prism 7500 System (Applied Biosystems of Thermo Fisher Scientific, Waltham, MA, United States). The primers used in the current study (Table 2) were selected from the PubMed database. The measurements of each sample were performed in triplicate. The real-time PCR data were analyzed using the relative gene expression (i.e., ∆∆CT) method. In brief, the data are presented as the fold change in gene expression normalized to the endogenous reference gene (GAPDH) and relative to a calibrator.
| Gene | Sequence |
| Interleukin-6 | F: 5'-CCCTGCGTTTCTCTGCAAAC-3' |
| R: 3'-TTTCAGGGTGGAAGGCAGAC-5' | |
| Tumor necrosis factor-α | F: 5'-CATCCGTTCTCTACCCAGCC-3' |
| R: 3'-AATTCTGAGCCCGGAGTTGG-5' | |
| Interleukin-10 | F: 5'-CCTCTGGATACAGCTGCGAC-3' |
| R: 3'-GTAGATGCCGGGTGGTTCAA-5' | |
| Tumor growth factor-β1 | F: 5'-AGGGCTACCATGCCAACTTC-3' |
| R: 3'-CCACGTAGTAGACGATGGGC-5' |
The serum superoxide dismutase (SOD) activity and the methane dicarboxylic aldehyde (MDA) concentration were determined using assay kits (Nanjing Jiancheng Corp., China) following the manufacturer’s recommendations. The amount of MDA was measured by reaction with thiobarbituric acid at 532 nm with a Perkin Elmer Lambda 20 spectrophotometer (Norwalk, CT, United States). The values were calculated using the molar extinction coefficient of chromophore (1.56 × 10 mol/L per cm). The SOD assay was conducted using a modified pyrogallol autoxidation method, and the activity was measured at 420 nm[20].
The anesthetized animals were perfused with formaldehyde, and the livers were removed and processed as previously described[21]. The KlenowFragEL DNA Fragmentation Detection Kit (EMD Chemicals, Gibbstown, NJ, United States) was used to detect the DNA fragmentation characteristic of apoptosis in formalin-fixed, paraffin-embedded liver sections[22]. Results were scored semi-quantitatively by averaging the number of TUNEL+ apoptotic cells/microscopic field at 200× magnification. Ten fields were evaluated per tissue sample.
Proteins (40 μg/sample) from frozen liver samples were subjected to 12% SDS-polyacrylamide gel electrophoresis and transferred to nitrocellulose membranes (Bio-Rad Laboratories Inc., Hercules, CA, United States). Anti-GRP78, anti-CHOP, anti-ATF-4, anti-IRE-1, anti-procaspase-12, anti-NF-κb, and anti-GAPDH were obtained from Abcam (Cambridge, United Kingdom). The relative quantities of proteins were determined by a densitometer and expressed in absorbance units.
Data analysis was carried out using GraphPad Prism 5.0 (GraphPad Software Inc. La Jolla, CA, United States). All data are expressed as mean ± SE. Differences between groups were statistically analyzed by analysis of variance and Dunnett’s test for unpaired data when appropriate. A P < 0.05 was considered statistically significant.
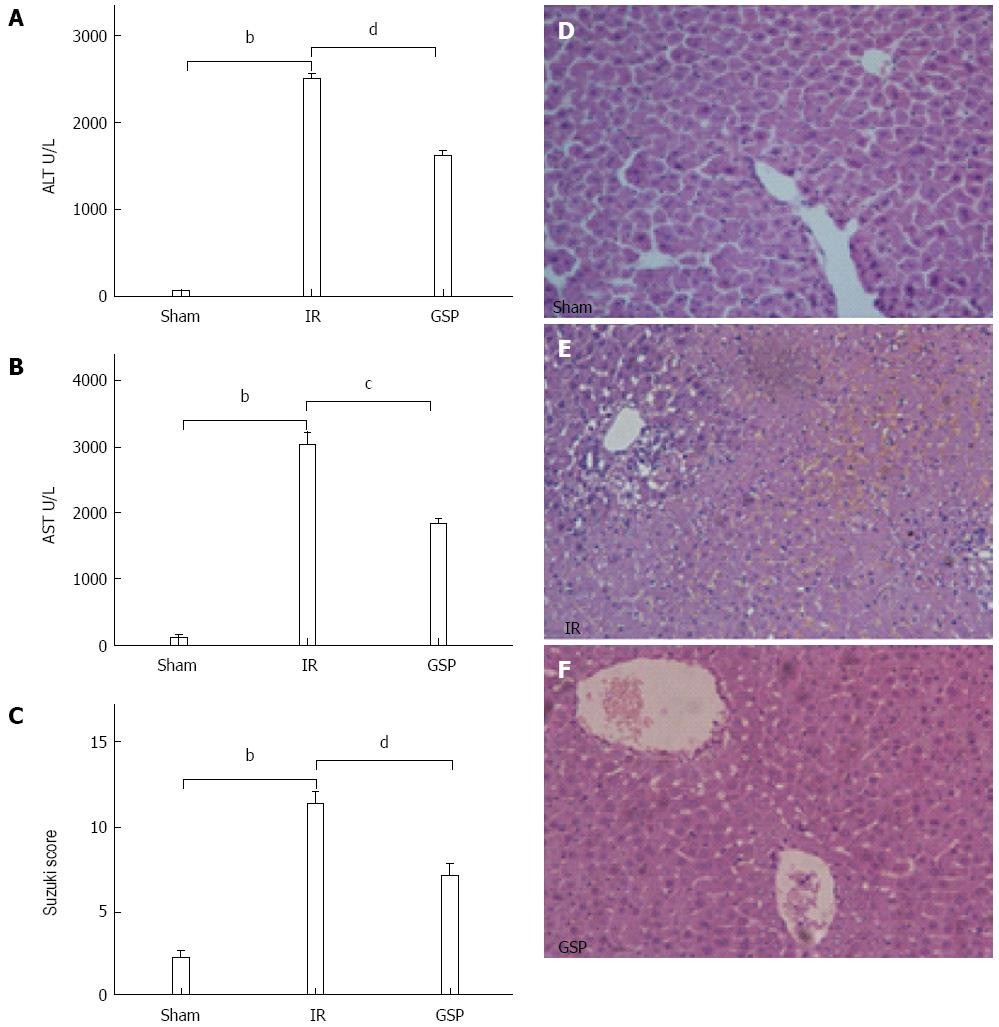
Serum aminotransferase level is an important indicator of liver injury, which significantly increases after IR and peaks 6 h after reperfusion[20]. Figure 1 shows that the detected serum ALT (Figure 1A) and AST (Figure 1B) levels were significantly increased in the IR group compared with the sham group (Ps < 0.05). Interestingly, GSP treatment significantly decreased these levels compared to the IR group (Ps < 0.05).
Whether GSP treatment could alter the liver pathology after IR was also examined. After reperfusion, the pathologic features of IR liver tissue displayed severe lobular distortion with widespread necrosis, apparent edema, hemorrhage, and neutrophil infiltration in the IR group (Figure 1E). However, the GSP treatment considerably relieved the aforementioned pathologic changes. Mild architectural damage characterized by interstitial edema and less neutrophil infiltration was observed (Figure 1F). A significantly higher Suzuki score was observed in the IR group, which was attenuated with GSP treatment (Ps < 0.05) (Figure 1C).
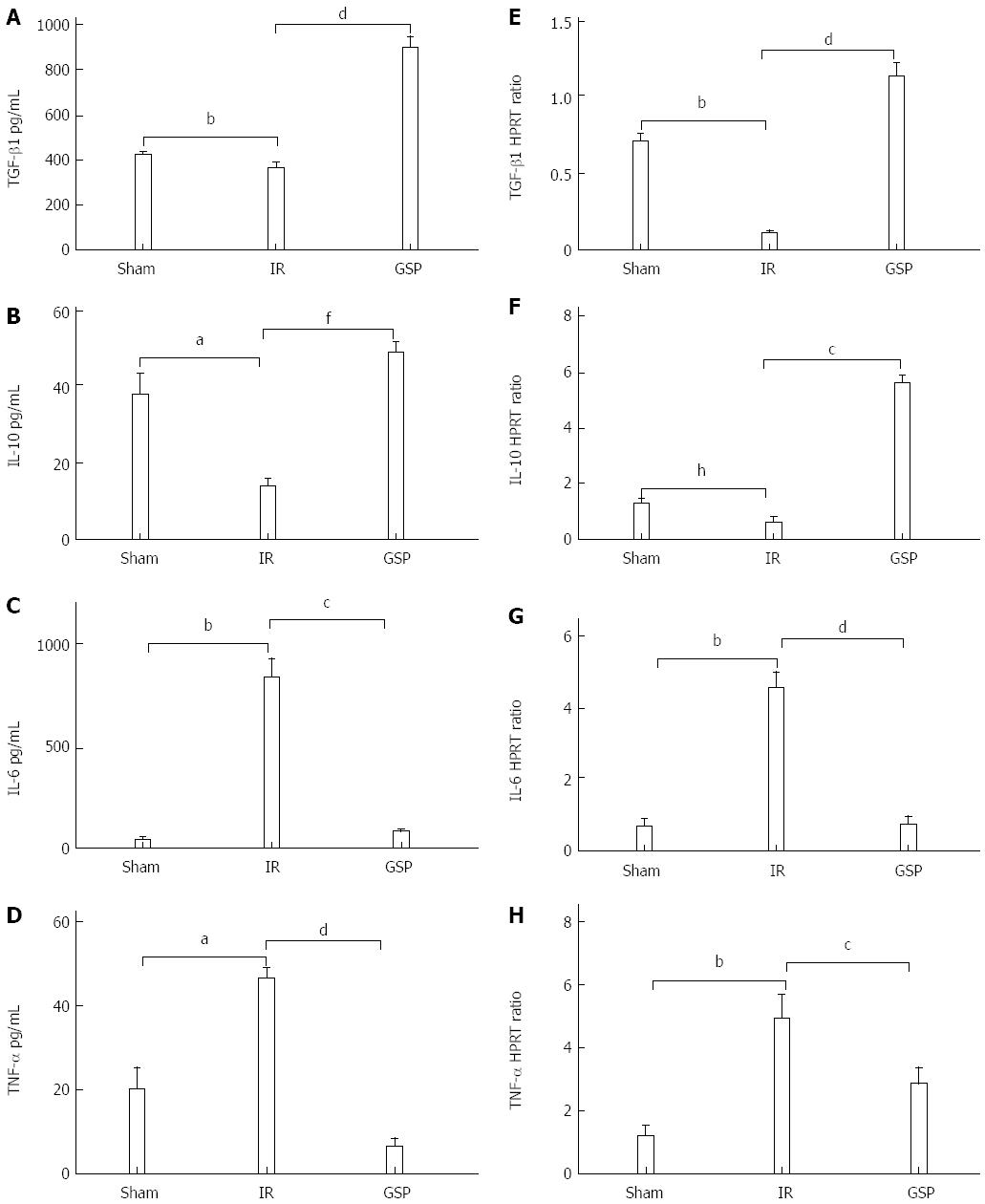
To detect the effect of GSP on the involvement of inflammatory cytokines in liver IR, the serum concentrations (by ELISA) and mRNA expression levels (by RT-PCR) of TGF-β1, IL-10, TNF-α, and IL-6 were measured. Compared with the IR group, the serum levels of TGF-β1 and IL-10 were significantly increased in the GSP group (Ps < 0.05) (Figure 2A and B). GSP treatment significantly reduced the secretion of TNF-α and IL-6 (Ps < 0.05) (Figure 2C and D). To further analyze whether the increase in serum cytokines was caused by intrahepatic production, the expression of hepatic cytokines was measured by RT-PCR, which showed a similar trend of change in mRNA expression. GSP treatment reversed the decrease in the anti-inflammatory cytokines TGF-β1 (Figure 2E) and IL-10 (Figure 2F) and increased the proinflammatory cytokines IL-6 (Figure 2G) and TNF-α (Figure 2H) induced by IR.
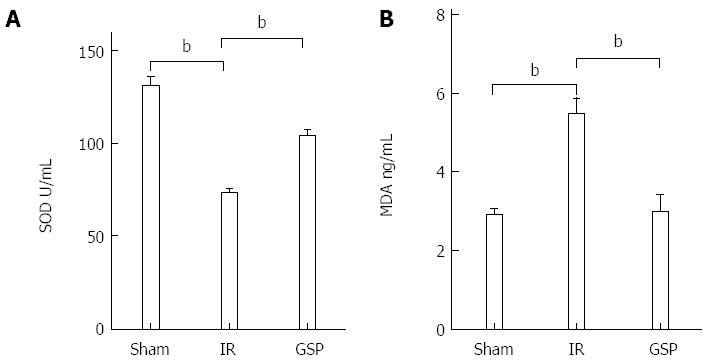
SOD and MDA are two indices of oxidative stress. The SOD level was decreased in the IR group compared with the sham group, but the SOD level in the GSP group was significantly increased compared with the IR group (all P < 0.01) (Figure 3A). However, the MDA concentration was significantly increased in the IR group, which was significantly attenuated by GSP (all P < 0.01) (Figure 3B).
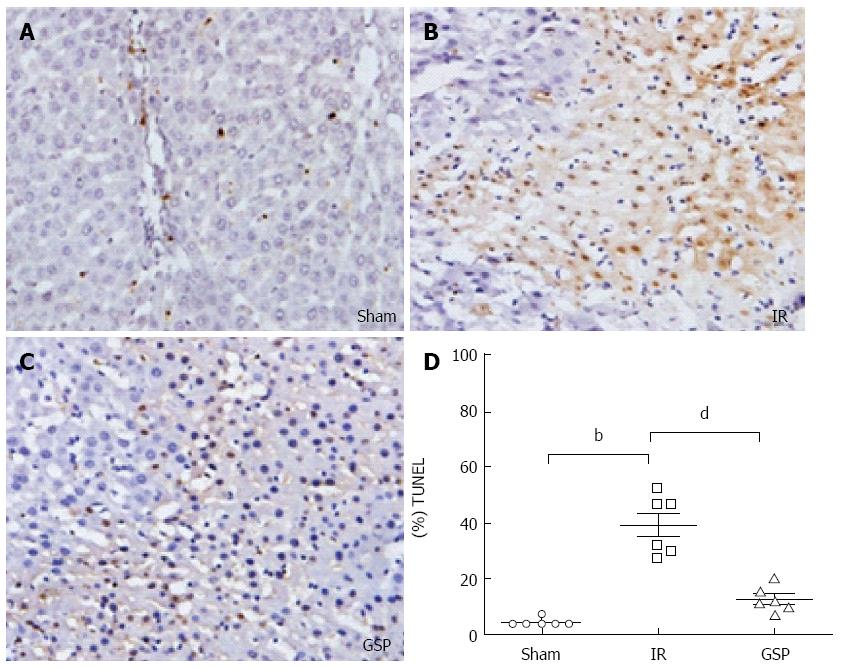
The result of TUNEL staining is shown in Figure 4. TUNEL+ cells were barely observed in the GSP group (Figure 4C), but were abundantly observed in the IR group (Figure 4B). Figure 4D shows the decrease in the number of apoptotic cells in the GSP group compared with the IR group. Protein procaspase-12 (Figure 5D), one of the apoptosis-regulated proteins, increased abundantly in the IR group compared with the GSP group as detected by Western blot.
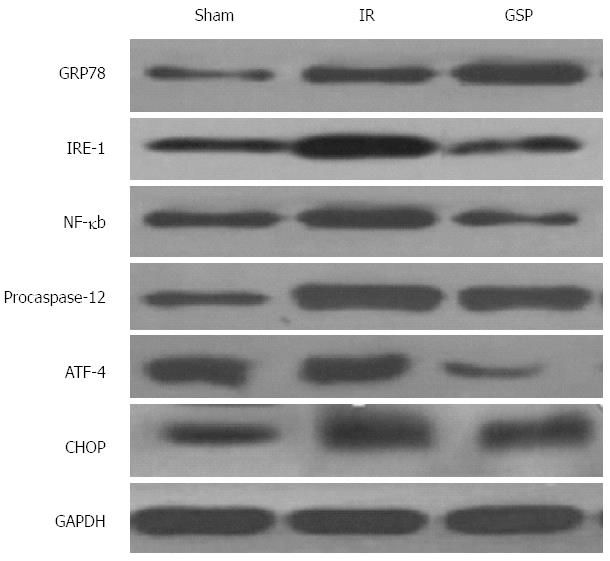
GRP78, IRE-1, and ATF-4 are markedly expressed proteins when severe ER stress occurs. As shown in Figure 5, the expression of ER stress protein GRP78 was increased in the IR group compared with the sham group, whereas the rats treated with GSP showed a much higher GRP78 expression compared with the IR group. However, the proteins IRE-1 and ATF-4 exhibited reversed results: the IR group showed a higher expression level than the GSP group. This phenomenon indicates that ER stress was successfully induced in the animal models, and GSP treatment could moderately increased the GRP78 expression and decreased the expression of IRE-1 and ATF-4.
NF-κb is a classical factor involved in the generation of the inflammatory response. NF-κb can be regulated by the IRE1-NF-κb signaling pathway[2]. The expression of NF-κb and IRE-1 was increased in the IR group compared with the sham group (Figure 5). However, this was attenuated by GSP treatment.
Previous studies reported that ATF-4-CHOP[2,23] is one of the signaling pathways of ER stress. Thus, ATF-4 expression in the liver tissues was analyzed by Western blot. The expression of ATF-4 and CHOP were increased with IR, whereas the rats treated with GSP showed comparatively lower levels (Figure 5).
This study shows that GSP protects the liver against IR injury with anti-inflammatory, antioxidative, and antiapoptotic effects by alleviating ER stress.
The increased ALT and AST coupled with the pathologic changes in the IR group revealed the severe damage induced by IR. Reduced ALT and AST levels and the minor pathologic changes in the GSP group confirmed the protective effect of GSP on IR. Meanwhile, the high level of ER stress proteins (e.g., GRP78, IRE-1, and ATF-4) indicate that ER stress was successfully induced in the animal models in the IR group compared with the sham group. GSP treatment effectively decreased the expression of those proteins, indicating that ER stress may be relieved by GSP treatment. The relationship between the subdued ER stress and the IR injury is unknown.
The inhibition of inflammation to alleviate IR injury was also investigated. While IL-6 and TNF-α decreased in the GSP group, TGF-β1 and IL-10 increased. TGF-β1 and IL-10 inhibit the production of proinflammatory cytokines, such as IL-1, IL-6, IL-8, and TNF-α[24]. Thus, we speculate that GSP relieves the inflammation response by decreasing proinflammatory factors and increasing anti-inflammatory factors. Another study indicated that GSP inhibits the production of TNF-α and IL-17a from T cells by inhibiting NF-κb[25]. The ROS-NF-κb signaling pathway is one of the downstream pathways involved in ER stress. Antioxidants reduce NF-κb activation[26]; in the present study, GSP treatment decreased MDA and NF-κb and increased SOD. Thus, GSP likely inhibited inflammation through the ROS-NF-κb signaling pathway. However, NF-κb could also be relieved by GSP through the IRE-1-NF-κb signaling pathway, as IRE-1-mediated TNF receptor-associated factor 2 can promote NF-κb-mediated inflammation[8]. In the present study, IRE-1 and NF-κb were significantly decreased as detected by Western blot. In summary, GSP relieved the inflammation induced by ER stress in the IR rats.
The antiapoptotic role of GSP in IR rats via relief of ER stress was explored. In the GSP group, the antiapoptotic effect of GSP was affirmed by decreasing procaspase-12 expression and reduced numbers of apoptotic cells compared with the IR group. The mechanism involved may include the following. First, GSP can affect caspase-12-dependent cell apoptosis induced by ER stress. When ER stress occurs, procaspase-12 is activated after its dissociation from the ER membrane[27], thus initiating downstream apoptotic pathways[27]. Caspase-12-deficient mice are resistant to ER stress-induced apoptosis[28], which supports this finding. The current results demonstrate that the level of procaspase-12 is downregulated with GSP treatment, which inevitably alleviates activation of the pathway. Second, the proapoptotic transcription factor CHOP, downstream of the ATF-4 pathway in UPR[29], is vital in ER stress-induced apoptosis[30]. A component of this pathway, P-eIF2, plays a key role in cell death signaling and results in increased activation of ATF-4 and CHOP[31] when ER stress is initiated. The transcription factor ATF-4 induces CHOP transcription through the branch signal mediated by p-eIF2[32]. The results of the present study show that the expression of ATF-4 and CHOP is significantly decreased in the GSP group compared with the IR group. Therefore, downregulation of the ATF-4-CHOP signaling pathway may be one of the protective mechanisms. Moreover, the higher increase of GRP78 protein in the GSP group than in the IR group suggests that GSP may effectively relieve ER stress by increasing GRP78. The increase in proper protein folding by upregulation of GRP78 was shown in a experiment where tunicamycin treatment easily induced ER stress in GRP78-depleted cells[26,33]. Upregulation of GRP78 reduced the expression of CHOP and apoptosis during the ER stress[30]. Nevertheless, pathologic development in the liver after IR involves a variety of complicated mechanisms, though the primary cause has not yet been completely clarified. Thus, the relationship between the effects of GSP (such as antiapoptotic, anti-inflammatory, and antioxidative effects) and the attenuation of ER stress should be further explored. In addition, further research is needed to identify an effective drug within the ER-stress pathway that hastens the fight against liver and other systemic diseases.
In conclusion, this study demonstrates that GSP possesses antioxidative, anti-inflammatory, and antiapoptotic effects by relieving ER stress to achieve a protection against liver IR. This protective mechanism may be a result of the botanical ingredients that comprise GSP. This finding may serve as a guide to prevent the damage induced by IR and possesses important clinical significance.
Liver ischemia/reperfusion (IR) injury induces the occurrence of endoplasmic reticulum (ER) stress. Numerous studies report that excessive ER stress aggravates IR injury. Grape seed proanthocyanidin (GSP) is an effective protector in IR injury. However, the detailed protective mechanisms remain unclear. Therefore, this study explored the modulation effect of GSP on protection against liver IR injury and the alleviation of ER stress.
Previous studies have demonstrated that GSP possess antibacterial, antiviral, anticarcinogenic, antimutagenic, anti-inflammatory, and antiallergic effects. Furthermore, they showed that GSP relieves the IR injury via its botanical effects. However, the relative mechanism needs to be explored.
The authors explored the mechanism that GSP protects the liver against IR injury and provided a new finding of the occurrence of IR injury. This finding may serve as a guide to prevent the damage induced by IR.
This study shows that GSP possesses antioxidative, anti-inflammatory, and antiapoptotic effects by relieving ER stress to achieve protection in the liver against IR. This finding may serve as a guide to prevent the damage induced by IR and possesses important clinical significance.
The ER regulates protein folding, calcium storage, and the biosynthesis of macromolecules such as steroids, lipids, and carbohydrates. Various stimuli increase the accumulation of unfolded proteins in the lumen of ER, leading to ER stress. GSP is abundant in phenolic compounds and exerts antibacterial, antiviral, anticarcinogenic, antimutagenic, anti-inflammatory, antiallergic, and vasodilatory effects.
This is a good descriptive study in which the authors suggest that GSP can protect rat liver from IR injury by its anti-inflammatory, antioxidative, and antiapoptotic effects and attenuating liver ER stress. The authors present GSP as a potent reagent.
P- Reviewer: Chen LZ, He JY, Liu EQ S- Editor: Qi Y L- Editor: AmEditor E- Editor: Liu XM
| 1. | Amersi F, Nelson SK, Shen XD, Kato H, Melinek J, Kupiec-Weglinski JW, Horwitz LD, Busuttil RW, Horwitz MA. Bucillamine, a thiol antioxidant, prevents transplantation-associated reperfusion injury. Proc Natl Acad Sci USA. 2002;99:8915-8920. [PubMed] |
| 2. | Bagchi D, Bagchi M, Stohs Sj, Ray SD, Sen CK, Preuss HG. Cellular protection with proanthocyanidins derived from grape seeds. Ann N Y Acad Sci. 2002;957:260-270. [PubMed] |
| 3. | Bailly-Maitre B, Fondevila C, Kaldas F, Droin N, Luciano F, Ricci JE, Croxton R, Krajewska M, Zapata JM, Kupiec-Weglinski JW. Cytoprotective gene bi-1 is required for intrinsic protection from endoplasmic reticulum stress and ischemia-reperfusion injury. Proc Natl Acad Sci USA. 2006;103:2809-2814. [PubMed] |
| 4. | Liu G, Sun Y, Li Z, Song T, Wang H, Zhang Y, Ge Z. Apoptosis induced by endoplasmic reticulum stress involved in diabetic kidney disease. Biochem Biophys Res Commun. 2008;370:651-656. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 138] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 5. | Schröder M, Kaufman RJ. The mammalian unfolded protein response. Annu Rev Biochem. 2005;74:739-789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2260] [Cited by in RCA: 2362] [Article Influence: 118.1] [Reference Citation Analysis (0)] |
| 6. | Szegezdi E, Logue SE, Gorman AM, Samali A. Mediators of endoplasmic reticulum stress-induced apoptosis. EMBO Rep. 2006;7:880-885. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1670] [Cited by in RCA: 1908] [Article Influence: 100.4] [Reference Citation Analysis (0)] |
| 7. | Puthalakath H, O’Reilly LA, Gunn P, Lee L, Kelly PN, Huntington ND, Hughes PD, Michalak EM, McKimm-Breschkin J, Motoyama N. ER stress triggers apoptosis by activating BH3-only protein Bim. Cell. 2007;129:1337-1349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1038] [Cited by in RCA: 1128] [Article Influence: 62.7] [Reference Citation Analysis (0)] |
| 8. | Zinszner H, Kuroda M, Wang X, Batchvarova N, Lightfoot RT, Remotti H, Stevens JL, Ron D. CHOP is implicated in programmed cell death in response to impaired function of the endoplasmic reticulum. Genes Dev. 1998;12:982-995. [PubMed] |
| 9. | Bozkurt M, Kapi E, Kulahci Y, Gedik E, Ozekinci S, Isik FB, Celik Y, Selcuk CT, Kuvat SV. Antioxidant support in composite musculo-adipose-fasciocutaneous flap applications: an experimental study. J Plast Surg Hand Surg. 2014;48:44-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 10. | Cho ML, Heo YJ, Park MK, Oh HJ, Park JS, Woo YJ, Ju JH, Park SH, Kim HY, Min JK. Grape seed proanthocyanidin extract (GSPE) attenuates collagen-induced arthritis. Immunol Lett. 2009;124:102-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 55] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 11. | DeGracia DJ, Montie HL. Cerebral ischemia and the unfolded protein response. J Neurochem. 2004;91:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 192] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 12. | Ferri KF, Kroemer G. Organelle-specific initiation of cell death pathways. Nat Cell Biol. 2001;3:E255-E263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1110] [Cited by in RCA: 1097] [Article Influence: 45.7] [Reference Citation Analysis (0)] |
| 13. | Meng F, Liu R, Gao M, Wang Y, Yu X, Xuan Z, Sun J, Yang F, Wu C, Du G. Pinocembrin attenuates blood-brain barrier injury induced by global cerebral ischemia-reperfusion in rats. Brain Res. 2011;1391:93-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 62] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 14. | Fawcett TW, Martindale JL, Guyton KZ, Hai T, Holbrook NJ. Complexes containing activating transcription factor (ATF)/cAMP-responsive-element-binding protein (CREB) interact with the CCAAT/enhancer-binding protein (C/EBP)-ATF composite site to regulate Gadd153 expression during the stress response. Biochem J. 1999;339:135-141. [PubMed] |
| 15. | Harding HP, Zhang Y, Bertolotti A, Zeng H, Ron D. Perk is essential for translational regulation and cell survival during the unfolded protein response. Mol Cell. 2000;5:897-904. [PubMed] |
| 16. | Kim TH, Jeon EJ, Cheung DY, Kim CW, Kim SS, Park SH, Han SW, Kim MJ, Lee YS, Cho ML. Gastroprotective Effects of Grape Seed Proanthocyanidin Extracts against Nonsteroid Anti-Inflammatory Drug-Induced Gastric Injury in Rats. Gut Liver. 2013;7:282-289. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 17. | Kozutsumi Y, Segal M, Normington K, Gething MJ, Sambrook J. The presence of malfolded proteins in the endoplasmic reticulum signals the induction of glucose-regulated proteins. Nature. 1988;332:462-464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 930] [Cited by in RCA: 989] [Article Influence: 26.7] [Reference Citation Analysis (0)] |
| 18. | Ulusoy S, Ozkan G, Yucesan FB, Ersöz Ş, Orem A, Alkanat M, Yuluğ E, Kaynar K, Al S. Anti-apoptotic and anti-oxidant effects of grape seed proanthocyanidin extract in preventing cyclosporine A-induced nephropathy. Nephrology (Carlton). 2012;17:372-379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 19. | Suzuki S, Nakamura S, Koizumi T, Sakaguchi S, Baba S, Muro H, Fujise Y. The beneficial effect of a prostaglandin I2 analog on ischemic rat liver. Transplantation. 1991;52:979-983. [PubMed] |
| 20. | Shi J, Shao W, Yang D, Zhao L, Deng L, Wang X, Sun B. Hydrodynamics-based transfection of plasmid encoding receptor activator for nuclear factor kappa B-Fc protects against hepatic ischemia/reperfusion injury in mice. Liver Transpl. 2010;16:611-620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 21. | Lin JH, Walter P, Yen TS. Endoplasmic reticulum stress in disease pathogenesis. Annu Rev Pathol. 2008;3:399-425. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 666] [Cited by in RCA: 635] [Article Influence: 37.4] [Reference Citation Analysis (0)] |
| 22. | Ke B, Shen XD, Ji H, Kamo N, Gao F, Freitas MC, Busuttil RW, Kupiec-Weglinski JW. HO-1-STAT3 axis in mouse liver ischemia/reperfusion injury: regulation of TLR4 innate responses through PI3K/PTEN signaling. J Hepatol. 2012;56:359-366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 92] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 23. | Bagchi D, Sen CK, Ray SD, Das DK, Bagchi M, Preuss HG, Vinson JA. Molecular mechanisms of cardioprotection by a novel grape seed proanthocyanidin extract. Mutat Res. 2003;523-524:87-97. [PubMed] |
| 24. | Yuan Y, Guo Q, Ye Z, Pingping X, Wang N, Song Z. Ischemic postconditioning protects brain from ischemia/reperfusion injury by attenuating endoplasmic reticulum stress-induced apoptosis through PI3K-Akt pathway. Brain Res. 2011;1367:85-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 120] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 25. | Zhang K, Kaufman RJ. From endoplasmic-reticulum stress to the inflammatory response. Nature. 2008;454:455-462. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1641] [Cited by in RCA: 1582] [Article Influence: 93.1] [Reference Citation Analysis (0)] |
| 26. | Zhang XY, Li WG, Wu YJ, Bai DC, Liu NF. Proanthocyanidin from grape seeds enhances doxorubicin-induced antitumor effect and reverses drug resistance in doxorubicin-resistant K562/DOX cells. Can J Physiol Pharmacol. 2005;83:309-318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 28] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 27. | Wu CX, Liu R, Gao M, Zhao G, Wu S, Wu CF, Du GH. Pinocembrin protects brain against ischemia/reperfusion injury by attenuating endoplasmic reticulum stress induced apoptosis. Neurosci Lett. 2013;546:57-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 66] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 28. | Yu Z, Luo H, Fu W, Mattson MP. The endoplasmic reticulum stress-responsive protein GRP78 protects neurons against excitotoxicity and apoptosis: suppression of oxidative stress and stabilization of calcium homeostasis. Exp Neurol. 1999;155:302-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 362] [Cited by in RCA: 353] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 29. | Marklund S, Marklund G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem. 1974;47:469-474. [PubMed] |
| 30. | Ma Y, Brewer JW, Diehl JA, Hendershot LM. Two distinct stress signaling pathways converge upon the CHOP promoter during the mammalian unfolded protein response. J Mol Biol. 2002;318:1351-1365. [PubMed] |
| 31. | Penna C, Mancardi D, Rastaldo R, Pagliaro P. Cardioprotection: a radical view Free radicals in pre and postconditioning. Biochim Biophys Acta. 2009;1787:781-793. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 165] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 32. | Shakhov AN, Collart MA, Vassalli P, Nedospasov SA, Jongeneel CV. Kappa B-type enhancers are involved in lipopolysaccharide-mediated transcriptional activation of the tumor necrosis factor alpha gene in primary macrophages. J Exp Med. 1990;171:35-47. [PubMed] |
| 33. | Luo S, Baumeister P, Yang S, Abcouwer SF, Lee AS. Induction of Grp78/BiP by translational block: activation of the Grp78 promoter by ATF4 through and upstream ATF/CRE site independent of the endoplasmic reticulum stress elements. J Biol Chem. 2003;278:37375-37385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 234] [Article Influence: 10.6] [Reference Citation Analysis (0)] |