Published online Jun 21, 2015. doi: 10.3748/wjg.v21.i23.7254
Peer-review started: December 8, 2014
First decision: January 27, 2015
Revised: February 6, 2015
Accepted: March 19, 2015
Article in press: March 19, 2015
Published online: June 21, 2015
Processing time: 196 Days and 1.9 Hours
AIM: To determine the cut-off value of intercellular adhesion molecule-1 (ICAM-1) and assess the correlation of ICAM-1 with clinicopathological features and the prognosis of hepatocellular carcinoma (HCC) patients who underwent surgical resection.
METHODS: We prospectively collected clinicopathological data from 236 HCC patients who had undergone successful hepatectomy. Receiver operating characteristic curve analysis was performed to determine the optimal cut-off value of ICAM-1. Enzyme-linked immunosorbent assay was used to measure the concentration of ICAM-1 in 236 serum samples isolated from HCC patients and the stratified analysis was used to compare the serum level of ICAM-1 in different HCC subgroups. Immunohistochemistry was performed to test the expression level of the ICAM-1 protein in 76 cases of HCC tissues and their adjacent normal liver tissues (ANLT). The survival probability of HCC patients was estimated using Kaplan-Meier plots and differences between the groups were obtained using the log-rank test. Furthermore, independent indicators of the prognosis were acquired using a stepwise Cox proportional hazard model to analyze a series of predictors that were associated with disease-free survival (DFS) and overall survival (OS) in HCC patients.
RESULTS: Our findings suggested that ICAM-1 promotes HCC metastasis and high serum ICAM-1 is significantly associated with alpha-fetoprotein (AFP) (P = 0.022), clinical tumor-node-metastasis stage (P < 0.001), portal vein tumor thrombus (P = 0.005), distant metastasis (P = 0.016) and recurrence (P = 0.034). We further detected the ICAM-1 protein in HCC specimens and found that 56 of 76 (73.7%) HCC tissues had ICAM-1 positive staining while only 23 of 76 (30.3%) ANLT were positively stained (P < 0.0001). Survival analysis indicated that HCC patients with increased ICAM-1 concentrations had significantly shorter DFS and OS after resection. A multivariate analysis showed that ICAM-1 > 684 ng/mL was an independent factor for DFS (HR = 1.643; 95%CI: 1.125-2.401; P = 0.010) and OS (HR = 1.692; 95%CI: 1.152-2.486; P = 0.007).
CONCLUSION: ICAM-1 may be a promising serological biomarker for HCC diagnosis and an independent predictor of DFS and OS after surgical resection and may provide a useful reference for the prediction of intra- and extrahepatic metastasis.
Core tip: Our previous research and other studies found that intercellular adhesion molecule-1 (ICAM-1) is overexpressed in hepatocellular carcinoma (HCC) and might be a biomarker for HCC diagnosis. However, the correlation between ICAM-1 and clinicopathological features and its prognostic significance for HCC have not been explored. In this paper, we validate that ICAM-1 promotes HCC metastasis and that high serum ICAM-1 is significantly associated with alpha-fetoprotein, clinical tumor-node-metastasis stage, portal vein tumor thrombus, distant metastasis, recurrence, disease-free survival and overall survival. ICAM-1 may be a promising serological biomarker for HCC diagnosis and prognosis and may provide a useful reference for the prediction of intra- and extrahepatic metastasis.
- Citation: Zhu PP, Yuan SG, Liao Y, Qin LL, Liao WJ. High level of intercellular adhesion molecule-1 affects prognosis of patients with hepatocellular carcinoma. World J Gastroenterol 2015; 21(23): 7254-7263
- URL: https://www.wjgnet.com/1007-9327/full/v21/i23/7254.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i23.7254
Hepatocellular carcinoma (HCC) is the sixth most common malignant tumor worldwide[1]. Recurrence and intra- and extrahepatic metastases contribute predominantly to the high mortality rate of HCC patients after curative resection[2,3]. Despite some well-known improvements in HCC, including epidemiology, etiology, fundamental biology, chemotherapy, radiofrequency, transarterial therapy and surgical resection, there is still a majority of patients with HCC who have an inferior prognosis[4]. Therefore, it is urgent for us to search for novel biomarkers that are useful to evaluate the survival of HCC patients.
Mounting evidence has demonstrated that the pathogenesis of HCC is partially based on systemic inflammation, as factors based on systemic inflammation can predict the outcome of various malignancies including HCC[5,6]. A number of studies have shown that systemic inflammation can be measured by widely available inflammatory cytokines, such as intercellular adhesion molecule-1 (ICAM-1)[7,8], C-reactive protein[9] and neutrophil-to-lymphocyte ratio (NLR)[10]. Our previous research and other studies found that ICAM-1 is overexpressed in malignant diseases, such as HCC[7,8], bladder cancer[11], gastric cancer, lung cancer[12] and renal cancer[13]. The expression of ICAM-1 has been reported to mediate the interaction of cells with one another and with their microenvironment, which plays an important role in cell differentiation and movement. ICAM-1 has also been shown to be positively correlated with tumor generation, metastasis and recurrence in HCC[14]. Elevated circulating ICAM-1 concentration may proportionally relate to a poor prognosis in patients with HCC. However, the cut-off value of ICAM-1 has not been confirmed at present, which is a major clinical problem. Therefore, it is of great importance to ascertain the cut-off value of ICAM-1, which can help clinicians to make the most use of ICAM-1 to evaluate the prognosis of patients with HCC. The aim of our study is to determine the cut-off value of ICAM-1 and to assess the correlation of ICAM-1 with the clinicopathological features and prognosis of HCC patients who have undergone surgical resection.
A total of 236 HCC patients who underwent surgical resection from December 1997 to July 2006 at the Affiliated Hospital of Guilin Medical University in China were analyzed retrospectively. Clinical and pathological evaluations of all patients were assessed using the Primary Liver Cancer Clinical Diagnosis and Staging Criteria[15] and patients were diagnosed by clinical symptoms, laboratory tests, ultrasonography (US), computed tomography (CT) scans and magnetic resonance imaging (MRI). The clinical and biochemical data of the examined patients are listed in Table 1 and Table 2, including age, gender, family history, alcohol abuse, cirrhosis, hepatitis B surface antigen (HBsAg), ICAM-1, alpha-fetoprotein (AFP), median size, tumor number, clinical tumor-node-metastasis (TNM) stage, portal vein tumor thrombus (PVTT), distant metastasis, recurrence, white blood cell (WBC), platelets, albumin, total bilirubin (TBIL), alanine aminotransferase (ALT), aspartate aminotransferase (AST), NLR and γ-glutamyl transpeptidase (γ-GT). All of the surgical samples that used for immunohistochemistry assay were fixed with formalin and then embedded with paraffin. In each case, blood samples were collected and the serum was separated and stored at -80 °C until use. All 236 HCC patients met the inclusion criteria and provided complete clinical background information for our study.
| Parameter | mean ± SD |
| Age (yr) | 49.38 ± 12.01 |
| Gender, female/male, n | 29/207 |
| Alcohol abuse, yes/no, n | 133/103 |
| Cirrhosis, yes/no, n | 211/25 |
| HBsAg, negative/positive, n | 34/202 |
| ICAM-1 | 984.32 ± 370.48 |
| AFP (ng/mL) | 1809.97 ± 5533.23 |
| WBC (109/L) | 6.35 ± 2.37 |
| Platelets (109/L) | 175.65 ± 78.02 |
| Albumin (g/L) | 40.67 ± 4.61 |
| TBIL(μmol/L) | 18.52 ± 24.98 |
| ALT (U/L) | 50.27 ± 47.91 |
| AST (U/L) | 63.78 ± 86.73 |
| NLR | 2.83 ± 2.01 |
| γ-GT (U/L) | 117.55 ± 108.79 |
| Clinical character | Variable | Number of patients | ICAM-1 level | χ2 | P value | |
| Low1 | High2 | |||||
| Age (yr) | ≤ 55 | 161 | 37 (23.0) | 124 (77.0) | 0.265 | 0.607 |
| > 55 | 75 | 15 (20.0) | 60 (80.0) | |||
| Gender | Male | 207 | 44 (21.3) | 163 (78.7) | 0.593 | 0.441 |
| Female | 29 | 8 (27.6) | 21 (72.4) | |||
| Family history | No | 203 | 42 (20.7) | 161 (79.3) | 1.527 | 0.217 |
| Yes | 33 | 10 (30.3) | 23 (69.7) | |||
| HBsAg | Negative | 34 | 7 (20.6) | 27 (79.4) | 0.048 | 0.826 |
| Positive | 202 | 45 (22.3) | 157 (77.7) | |||
| AFP (ng/mL) | ≤ 100 | 99 | 29 (29.3) | 70 (70.7) | 5.231 | 0.022 |
| > 100 | 137 | 23 (16.8) | 114 (83.2) | |||
| Median size (cm) | ≤ 5 | 32 | 9 (28.1) | 23 (71.9) | 0.800 | 0.371 |
| > 5 | 204 | 43 (21.1) | 161 (78.9) | |||
| Cirrhosis | No | 25 | 2 (8.0) | 23 (92.0) | 3.206 | 0.073 |
| Yes | 211 | 50 (23.7) | 161 (76.3) | |||
| Tumor number | Single | 149 | 37 (24.8) | 112 (75.2) | 1.842 | 0.175 |
| Multiple | 87 | 15 (17.2) | 72 (82.8) | |||
| NLR | ≤ 2.31 | 130 | 31 (23.8) | 99 (76.2) | 0.553 | 0.457 |
| > 2.31 | 106 | 21 (19.8) | 85 (80.2) | |||
| TNM stage | I-II | 108 | 37 (34.3) | 71 (65.7) | 17.324 | < 0.001 |
| III-IV | 128 | 15 (11.7) | 113 (88.3) | |||
| PVTT | No | 184 | 48 (26.1) | 136 (73.9) | 7.985 | 0.005 |
| Yes | 52 | 4 (7.7) | 48 (92.3) | |||
| Distant metastasis | No | 217 | 52 (24.0) | 165 (76.0) | 5.840 | 0.016 |
| Yes | 19 | 0 (0) | 19 (100) | |||
| Recurrence | No | 139 | 24 (17.3) | 115 (82.7) | 4.475 | 0.034 |
| Yes | 97 | 28 (28.9) | 69 (71.1) | |||
Patients were regularly followed-up from the date of operation; we inspected their serum AFP and performed US every 2 mo and chest radiography every 6 mo during the first two postoperative years and every 3-6 mo thereafter. Patients with abnormal AFP or suspected US examination underwent further examinations and recurrence was confirmed by contrast ultrasonography, magnetic resonance imaging and computerized tomography. The mean postoperative follow-up period was 39.6 mo (median, 25.0 mo; range, 2.0 to 120.0 mo). Disease-free survival (DFS) was measured from the date of surgery to the date of recurrence, metastasis, death or last follow-up. Overall survival (OS) was measured from the date of surgery to the date of death or last follow-up. The study was approved by the Ethical Committee of Affiliated Hospital of Guilin Medical University, which was in accordance with the Helsinki Declaration of 1975. Written informed consent was provided by all examined patients or their guardians.
The cutoff score for ICAM-1 in the serum of patients with HCC was analyzed using MedCalc analysis. We defined the optimal cutoff value for ICAM-1, which was closest to the key point with not only maximum sensitivity but also specificity. In addition, we adopted a dichotomy to categorize the rest of the clinicopathological features and then analyzed the correlation between circulating ICAM-1 concentrations and the prognostic significance of patients with HCC.
All serum samples in the study were assembled into anticoagulant-containing tubes during their preoperative inspection, after centrifuging, we separated the supernatants and stored them at -80 °C until use. The serum level of ICAM-1 was measured using commercially available ICAM-1 ELISA kits (Biosource Europe, Fleurus, Belgium) by two independent researchers according to the manufacturer's instructions. The testing details of ICAM-1 were described previously[8]; briefly, standard and experimental samples were added to 96-well flexible microtiter plates and pre-coated with anti-human ICAM-1 monoclonal antibodies for 2 h at 37 °C. Subsequently, the wells were washed four times with washing buffer and further incubated with biotinylated antibody for 1 h at 37 °C. After washing away unbound biotinylated antibody, streptavidin-HRP solution was added for 1 h at 37 °C. Lastly, color developing agents A and B were performed and the reaction was blocked with stop buffer. The OD value of all wells was read at 450nm. The concentration of ICAM-1 of each well was calculated by standard curves.
The 76 pairs of tissue specimens were deparaffinized with xylene, rehydrated using graded alcohols and pressure cooked for 3 min in citrate buffer (pH = 6.0) for antigen retrieval. Phosphate buffered saline (PBS) was used to wash the slides, followed by treatment with 3% hydrogen peroxide for 20 min to quench endogenous peroxidase activity. Then, the samples were preincubated with 10% goat serum at room temperature for 30 min to prevent nonspecific staining. Additionally, the cancerous foci and non-tumorous samples were incubated with rabbit polyclonal anti ICAM-1 antibody (catalog 25464, BioVision Company, 1:200 dilution) overnight in a humidified container at 4 °C; the next day, they were washed with PBS and the tissue slides were treated with a biotinylated horseradish-peroxidase detection system according to the manufacturer’s instructions and stained with 3,3-diaminobenzidine tetrahydrochloride. Lastly, the sections were counterstained with Mayer’s hematoxylin, dehydrated and mounted. We replaced the primary antibody with normal goat serum to obtain a negative control. The semi-quantitative immunohistochemistry results were evaluated by two independent pathologists who were blinded to the patients’ clinical and biochemical information and the stained tissue sections were evaluated using a 4 point scale as follows: the percentage of positive cells, grades 0-3 (0: no positive cells; 1: < 25% positive cells; 2: 25-50% positive cells; 3: > 50% positive cells).
SPSS 13.0 (SPSS Inc., Chicago, IL) and MedCalc statistical software version 11.3.0.0 (MedCalc Software, Broekstraat 52 Mariakerke, Belgium) were used to analyze the statistical data. The χ2 test was used for the correlation analysis. Quantitative variables were analyzed by the independent t test. Statistical significance was considered when P value < 0.05. Survival curves for the HCC patients were determined by the Kaplan-Meier method and the differences between groups were estimated by the log-rank test. Independent prognostic indicators for DFS and OS were assessed in the univariate and multivariate analyses using Cox’s proportional hazard model.
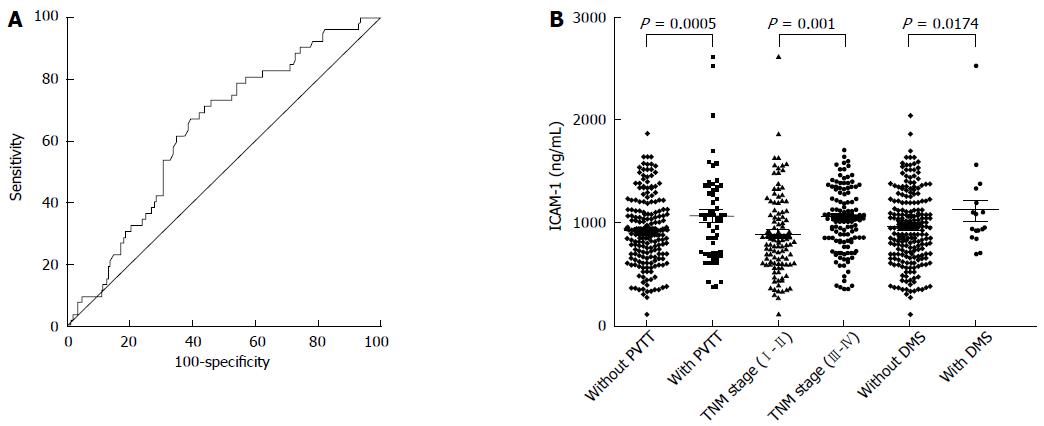
Receiver operating characteristic (ROC) curves were plotted to determine the optimal cut-off value of ICAM-1. As a result, a cutoff value of 684 ng/mL was found to have a relatively high specificity. The area under the ROC curves (AUC) was 0.636 with a 95%CI of 0.571 to 0.698, a sensitivity of 60.2% and a specificity of 77.3% (Figure 1A).
We compared the serum level of ICAM-1 in two groups of HCC patients with a series of different clinical features, including PVTT, TNM stage, distant metastasis, recurrence and AFP. We found that the level of ICAM-1 in serum was significantly associated with the presence of PVTT (P = 0.0005), TNM stage (P = 0.001) and distant metastasis (P = 0.0174) (Figure 1B). Therefore, we supposed that the overexpression of ICAM-1 may be associated with cellular invasion and venous permeation and may have contributed to tumor metastasis in HCC. It is therefore possible to use the serum ICAM-1 level to evaluate the curative effectiveness of patients with HCC.
The data from the two different groups are shown separately. The median ICAM-1 concentration in patients with PVTT was significantly higher than that in patients without PVTT, and that in the TNM stage III-IV group was significantly higher than that in the TNM stage I-II group. Furthermore, the median ICAM-1 concentration in patients with distant metastasis was significantly higher than that in patients without distant metastasis (Figure 1B).
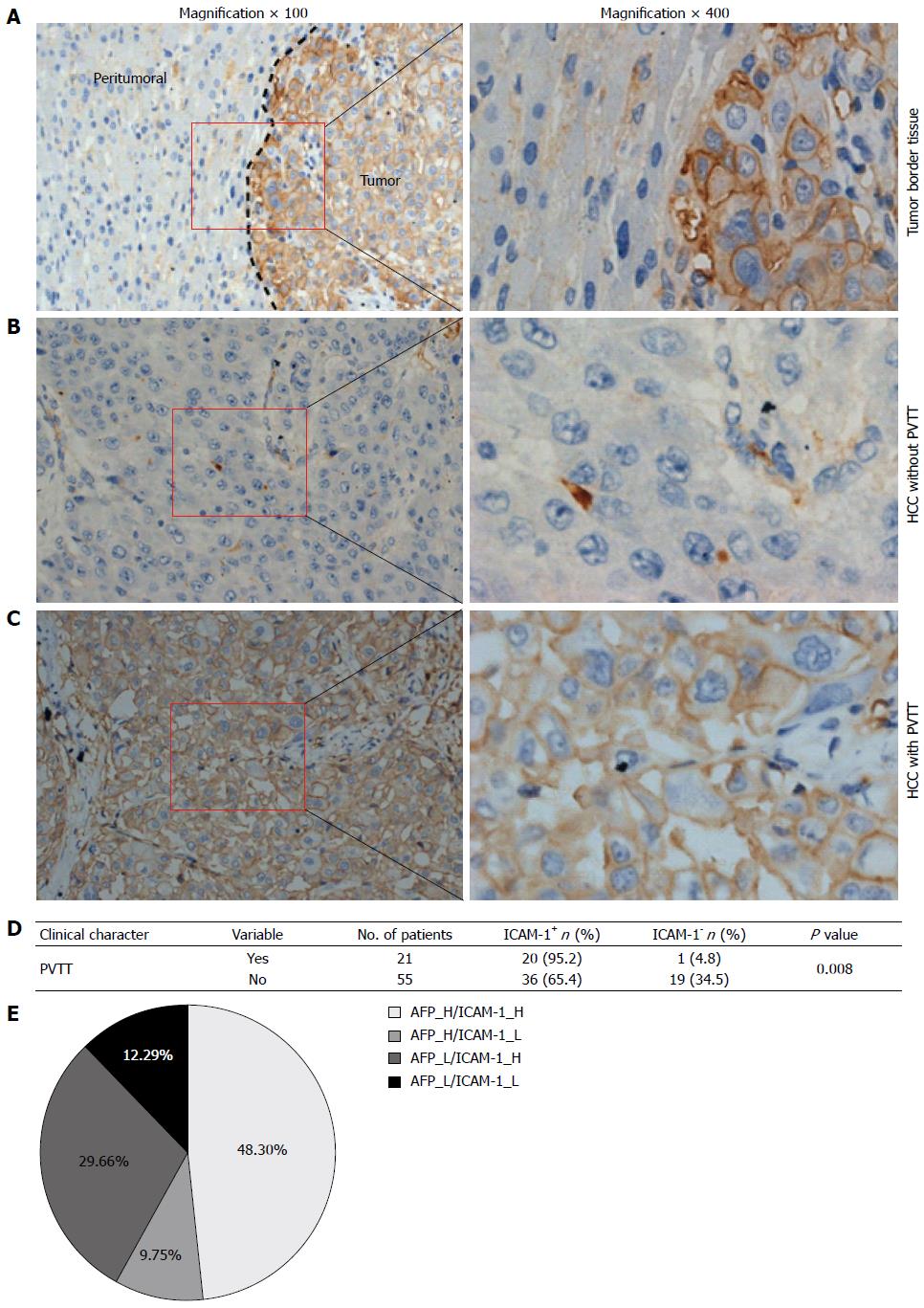
To further confirm that the concentration of ICAM-1 can be used as a tumor marker in HCC, we next detected ICAM-1 expression in HCC tissues by using immunohistochemical assays and found that 56 of 76 (73.7%) HCC tissues had ICAM-1 positive staining, while only 23 of 76 (30.3%) peritumoral tissues were positively stained (P < 0.0001; Figure 2A-C), suggesting that ICAM-1 may maintain the extracellular microenvironment for tumor cell growth. In addition, we showed positive staining in 20 of 21 (95.2%) HCC specimens with PVTT, when compared with 30 of 50 (60%) in those without PVTT (P = 0.008; Figure 2D), suggesting that the overexpression of ICAM-1 may be a key factor that promotes tumor cell proliferation, invasion and metastasis in the tumor microenvironment.
On the basis of the above findings, we next considered whether serum ICAM-1 correlates well with clinicopathological characteristics. Two hundred and thirty-six patients with HCC were divided into two groups according to their preoperative serum level of ICAM-1. Patients were classified into either the high ICAM-1 group (> 684 ng/mL, n = 184) or the low ICAM-1 group (≤ 684 ng/mL, n = 52). We found that the serum level of ICAM-1 was significantly associated with AFP (χ2 = 5.231; P = 0.022), clinical TNM stage (χ2 = 17.324; P < 0.001), PVTT (χ2 = 7.985; P = 0.005), distant metastasis (χ2 = 5.840; P = 0.016) and recurrence (χ2 = 4.475; P = 0.034). However, the serum level of ICAM-1 was not significantly associated with age, gender, tumor family history, HBsAg, median size, liver cirrhosis, the number of tumors or NLR (all P > 0.05), as shown in Table 2.
Interestingly, from the results of serological examination, we found that the increase in ICAM-1 (> 684 ng/mL) and AFP (> 100 ng/mL) in sera from HCC patients did not happen concurrently. Both increased in 114 cases (48.3%). Beyond that, the rise in ICAM-1 was not noticeable in 23 cases (9.75%) in which AFP alone increased; however, ICAM-1, but not AFP, increased in 70 cases (29.66%) (Figure 2E), demonstrating that it is valuable to link serum ICAM-1 and AFP for the final confirmatory diagnosis for HCC, which effectively guides the therapeutic schedule. When combining serum AFP and ICAM-1, the HCC diagnosis rate reached more than 87% in our study.
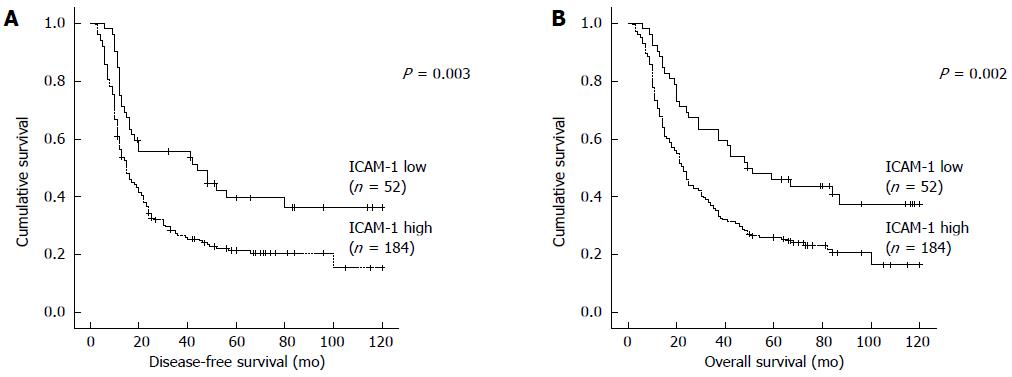
To further investigate the correlations between ICAM-1 concentration and survival of patients with HCC, Kaplan Meier analyses were performed. As shown in Figure 3, the median DFS time in patients with ICAM-1 > 684 ng/mL was 36.96 mo (95%CI: 30.51-43.40), which was remarkably shorter than that of ICAM-1 ≤ 684 ng/mL (58.18 mo, 95%CI: 44.77-71.58; P = 0.003; Figure 3A). Furthermore, the mean OS in patients with ICAM-1 > 684 ng/mL was 42.75 (36.48-49.01) mo, significantly shorter than that of the ICAM-1 ≤ 684 ng/mL group (64.57 mo, 95%CI: 52.14-77.01; P = 0.002; Figure 3B). Univariate analysis revealed an obvious association between clinical parameters and both DFS and OS (Table 3). In addition to the high ICAM-1 (> 684 ng/mL) group, the size of tumor > 5 cm, multiple tumors, NLR > 2.31, III-IV of TNM stage and PVTT were associated with a shorter DFS and OS (Table 3).
| Clinical character | Category | Number of patients | Disease-free survival (mo) | Overall survival (mo) | ||||
| mean | 95%CI | P value | mean | 95%CI | P value | |||
| ICAM-1 (ng/mL) | ≤ 684 | 52 | 58.18 | 44.77-71.58 | 0.003 | 64.57 | 52.14-77.01 | 0.002 |
| > 684 | 184 | 36.96 | 30.51-43.40 | 42.75 | 36.48-49.01 | |||
| Age (yr) | ≤ 55 | 161 | 40.37 | 33.32-47.43 | 0.325 | 45.89 | 39.05-52.74 | 0.262 |
| > 55 | 75 | 45.38 | 34.62-56.14 | 51.57 | 41.37-61.77 | |||
| Gender | Female | 29 | 57.84 | 38.90-76.78 | 0.066 | 62.96 | 43.72-82.19 | 0.076 |
| Male | 207 | 39.73 | 33.60-45.86 | 44.66 | 38.58-50.74 | |||
| Family history | No | 203 | 39.83 | 33.62-46.05 | 0.077 | 45.69 | 39.68-51.69 | 0.092 |
| Yes | 33 | 53.83 | 37.30-70.36 | 59.63 | 43.90-75.36 | |||
| HBsAg | Negative | 34 | 47.54 | 30.65-64.43 | 0.501 | 52.62 | 36.64-68.60 | 0.462 |
| Positive | 202 | 40.74 | 34.47-47.01 | 46.72 | 40.65-52.79 | |||
| AFP (ng/mL) | ≤ 100 | 99 | 46.01 | 36.52-55.49 | 0.286 | 52.18 | 43.07-61.28 | 0.214 |
| > 100 | 137 | 39.00 | 31.53-46.47 | 44.75 | 37.51-51.99 | |||
| Tumor size (cm) | ≤ 5 | 32 | 70.51 | 53.82-87.19 | < 0.001 | 77.42 | 62.56-92.28 | < 0.001 |
| > 5 | 204 | 37.57 | 31.49-43.66 | 43.36 | 37.44-49.28 | |||
| Cirrhosis | No | 25 | 34.60 | 17.57-51.64 | 0.190 | 39.16 | 22.83-55.49 | 0.237 |
| Yes | 211 | 42.81 | 36.53-49.09 | 48.83 | 42.77-54.89 | |||
| Tumor number | Single | 149 | 50.25 | 42.28-58.21 | < 0.001 | 55.27 | 47.66-62.87 | < 0.001 |
| Multiple | 87 | 27.72 | 20.32-35.12 | 34.92 | 27.37-42.48 | |||
| NLR | ≤ 2.31 | 130 | 53.58 | 45.00-62.17 | < 0.001 | 59.00 | 50.88-67.12 | < 0.001 |
| > 2.31 | 106 | 27.50 | 20.58-34.43 | 34.23 | 27.17-41.29 | |||
| TNM stage | I-II | 108 | 59.65 | 50.13-69.18 | < 0.001 | 66.86 | 58.17-75.54 | < 0.001 |
| III-IV | 128 | 27.17 | 20.87-33.46 | 31.88 | 25.56-38.21 | |||
| PVTT | No | 184 | 47.62 | 40.59-54.64 | < 0.001 | 54.25 | 47.61-60.90 | < 0.001 |
| Yes | 52 | 22.06 | 13.88-30.24 | 24.73 | 16.55-32.91 | |||
| Distant metastasis | No | 217 | 42.85 | 36.62-49.08 | 0.313 | 48.89 | 42.90-54.88 | 0.166 |
| Yes | 19 | 26.79 | 14.76-38.82 | 31.21 | 18.29-44.13 | |||
| Recurrence | No | 44.59 | 36.73-52.46 | 0.051 | ||||
| Yes | 51.58 | 43.77-59.39 | ||||||
To identify the association between clinicopathological factors and DFS/OS in HCC patients with surgical resection, multivariate analyses were conducted by using the Cox proportional hazards mode. Six factors (high ICAM-1 level, multiple tumor number, III-IV of TNM stage, PVTT, size of tumor > 5 cm and high NLR) were analyzed with the stepwise multivariate Cox proportional hazard model for both DFS and OS. The results showed that high ICAM-1 level (HR = 1.643; 95%CI: 1.125-2.401; P = 0.010), PVTT (HR = 1.397; 95%CI: 1.016-1.920; P = 0.040) and high NLR (HR = 1.578; 95%CI: 1.156-2.153; P = 0.004) were independent predictors for DFS (Table 4). High ICAM-1 level (HR = 1.692; 95%CI: 1.152-2.486; P = 0.007), III-IV of TNM stage (HR = 1.468; 95%CI: 1.074-2.015; P = 0.016), PVTT (HR = 1.514; 95%CI: 1.031-2.225; P = 0.035) and high NLR (HR = 1.485; 95%CI: 1.086-2.030; P = 0.013) were independent predictors for OS (Table 4).
| Variable | Disease-free survival | Overall survival | ||
| HR (95%CI) | P value | HR (95%CI) | P value | |
| ICAM-1 (ng/mL) (> 684 vs ≤ 684) | 1.643 (1.125-2.401) | 0.010 | 1.692 (1.152-2.486) | 0.007 |
| Tumor number (multiple vs single) | 1.324 (0.902-1.944) | 0.152 | 1.273 (0.924-1.755) | 0.140 |
| TNM stage (III-IV vs I-II) | 1.390 (0.931-2.077) | 0.108 | 1.468 (1.074-2.015) | 0.016 |
| PVTT (yes vs no) | 1.397 (1.016-1.920) | 0.040 | 1.514 (1.031-2.225) | 0.035 |
| Tumor size (cm) (> 5 vs ≤ 5) | 0.666 (0.389-1.149) | 0.144 | 1.551 (0.899-2.656) | 0.115 |
| NLR (> 2.31 vs ≤ 2.31) | 1.578 (1.156-2.153) | 0.004 | 1.485 (1.086-2.030) | 0.013 |
During the last few decades, the postoperative survival rate of HCC patients has improved. However, due to the difficulty in early diagnosis and the presence of tumor invasiveness, metastasis and recurrence of HCC, the prognosis of HCC is still not satisfactory, which is demonstrated by the low DFS rate in HCC patients within 5 years after surgical resection. Although the serum AFP level has been widely used for the diagnosis of HCC, the sensitivity and specificity of AFP for HCC were demonstrated to be limited. To improve the prognosis in HCC patients, seeking more effective biomarkers in diagnosis of HCC at very early stages and monitoring tumor recurrence are very important.
It has been shown that a series of hematological and biochemical examinations, including AFP[16], albumin[17], TBIL, ALT[18], AST[19], NLR[10] and γ-GT, were proposed as effective aids to the early diagnosis and prognosis of HCC. However, the diagnostic specificity and sensitivity were still not accurate. Our previous research and other studies found that ICAM-1 is overexpressed in HCC. Other research has shown that serum ICAM-1 is produced and secreted by tumor cells[14], ICAM-1 has the capability to induce the adhesion between cancer cells and the vascular endothelium, and the expression of ICAM-1 is a dominating step that may be involved in the metastatic process of HCC[20,21], suggesting that ICAM-1 may be able to serve as a new marker to predict the progression and prognosis of patients with HCC.
Our previous research showed that the mRNA and protein levels of ICAM-1 in patients with HCC were significantly higher than those in patients with chronic hepatitis B virus and healthy subjects[8] and that the malignant degree of the tumor was related to the ICAM-1 level, indicating that ICAM-1 contributed to the progression of HCC. In the present study, using the ROC curve, we identified 684 ng/mL as the optimal cutoff value of ICAM-1; the patients were divided into the low ICAM-1 group (ICAM-1 ≤ 684 ng/mL) and the high ICAM-1 group (ICAM-1 > 684 ng/mL). From the results of the correlation analysis, we also found that high levels of ICAM-1 were significantly associated with AFP, clinical TNM stage, PVTT, distant metastasis and recurrence in patients with HCC. In the present study, our data show that the combination of serum ICAM-1 and AFP significantly increased the positive diagnostic ratio for HCC.
PVTT, the main form of intrahepatic metastasis of HCC, affects the prognosis of HCC patients. It was reported that a total of 34% to 50% of patients with advanced HCC may suffer from PVTT and may be predisposed to metastasis and recurrence[22,23]. In this study, we also found that the ICAM-1 level was significantly higher in the HCC specimens with PVTT than in those without PVTT (Figure 2B-D). Although no substantive progress of finding effective indicators for PVTT has been made, the ICAM-1 protein level had the ability to forecast the incidence rate of PVTT, which merits a further large sample prospective study and which indicates the diagnostic value of ICAM-1 for further clinical practice. In addition, we compared serum ICAM-1 in two subgroups of HCC patients with different clinical features, including PVTT, TNM stage and distant metastasis, and found that the level of serum ICAM-1 was significantly associated with the presence of PVTT, the TNM stage and distant metastasis, which provided evidence that ICAM-1 overexpression is associated with cellular invasion, venous permeation and perhaps even metastasis in HCC.
From the results of the univariate analysis, we observed that ICAM-1 > 684 ng/mL, size of tumor > 5 cm, multiple tumor number, NLR > 2.31, III-IV of TNM stage together with PVTT were associated with a shorter DFS and OS. These observations are consistent with previous studies that multiple tumors are a useful prognostic factor for the recurrence of HCC[24]. Additionally, our previous studies found that elevated NLR (> 2.31) effectively reflected systemic inflammation, tumor invasion and even metastasis of HCC[10].
We also found that except III-IV of TNM stage was independent predictor for OS, and high ICAM-1 level, PVTT and high NLR were independent predictors for both DFS and OS. An elevated NLR may suppress antitumor immunity with the help of peritumoral macrophages[25,26] and myeloid-derived suppressor cells[27,28]. In addition, an elevated NLR may promote the production of vascular endothelial growth factor[29] and matrix metalloproteinases[30] and thus promote tumor angiogenesis and conductive tumor growth and metastasis.
In short, the value of 684 ng/mL as a cut-off point for ICAM-1 in this retrospective study has a substantial sensitivity and specificity, which is of great importance for the diagnosis of HCC. The serum ICAM-1 test is not only a cost-effective examination but also an optimal choice for HCC screening and it helps clinicians to make clinical decisions. We believe that ICAM-1 is a valuable marker for the early diagnosis of HCC, recurrence monitoring and the prediction of intrahepatic (PVTT) and extrahepatic metastasis for HCC. However, our results need to be further validated in a large cohort and prospective studies are warranted to verify the effectiveness of our results.
Hepatocellular carcinoma (HCC) is the sixth most common malignant tumor that is associated with recurrence and intra- and extrahepatic metastases. Elevated circulating intercellular adhesion molecule-1 (ICAM-1) concentration may proportionally relate to a poor prognosis in patients with HCC. However, the cut-off value of ICAM-1 and its clinical significance has not been further investigated.
ICAM-1 mediated the interaction of cells with one another and with their microenvironment and is involved in cell differentiation and movement. Its overexpression is greatly associated with tumor initiation, metastasis and recurrence in many human cancers. The cut-off value of ICAM-1 has also not been confirmed and the correlations of ICAM-1 with clinicopathological features and prognosis of HCC patients are still unavailable. All these questions are hot topics in the present research fields.
This is the first study to demonstrate that 684 ng/mL may serve as a cut-off point of ICAM-1 for the diagnosis of HCC patients. We believe that serum ICAM-1 may provide a useful reference for the prediction of intra- and extrahepatic metastasis.
Serum ICAM-1 can be used to predict the diagnosis and prognosis of patients with HCC, which may serve as a therapeutic target for the purpose of improving clinical treatment.
ICAM-1 is an important member of the immunoglobulin superfamily; it mediates the interaction of cells with one another and with their microenvironment and is involved in tumor initiation, metastasis and recurrence in many human cancers.
In this study, the authors confirmed that 684 ng/mL may serve as a cut-off point for ICAM-1 in improving the diagnosis and prognosis of patients with HCC. These findings are informative and suggest that ICAM-1 could be a potential biomarker for therapy for HCC. The results deserve publication in the journal.
P- Reviewer: Shen F, Xu Z S- Editor: Yu J L- Editor: Roemmele A E- Editor: Zhang DN
| 1. | Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9215] [Cited by in RCA: 9856] [Article Influence: 821.3] [Reference Citation Analysis (4)] |
| 2. | Forner A, Llovet JM, Bruix J. Hepatocellular carcinoma. Lancet. 2012;379:1245-1255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3249] [Cited by in RCA: 3594] [Article Influence: 276.5] [Reference Citation Analysis (4)] |
| 3. | Aihara A, Huang CK, Olsen MJ, Lin Q, Chung W, Tang Q, Dong X, Wands JR. A cell-surface β-hydroxylase is a biomarker and therapeutic target for hepatocellular carcinoma. Hepatology. 2014;60:1302-1313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 69] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 4. | Liao W, Huang G, Liao Y, Yang J, Chen Q, Xiao S, Jin J, He S, Wang C. High KIF18A expression correlates with unfavorable prognosis in primary hepatocellular carcinoma. Oncotarget. 2014;5:10271-10279. [PubMed] |
| 5. | Roxburgh CS, McMillan DC. Role of systemic inflammatory response in predicting survival in patients with primary operable cancer. Future Oncol. 2010;6:149-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 607] [Cited by in RCA: 733] [Article Influence: 48.9] [Reference Citation Analysis (1)] |
| 6. | Argilés JM, Busquets S, Toledo M, López-Soriano FJ. The role of cytokines in cancer cachexia. Curr Opin Support Palliat Care. 2009;3:263-268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 152] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 7. | Liu S, Li N, Yu X, Xiao X, Cheng K, Hu J, Wang J, Zhang D, Cheng S, Liu S. Expression of intercellular adhesion molecule 1 by hepatocellular carcinoma stem cells and circulating tumor cells. Gastroenterology. 2013;144:1031-1041.e10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 148] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 8. | Mei MH, Xu J, Shi QF, Yang JH, Chen Q, Qin LL. Clinical significance of serum intercellular adhesion molecule-1 detection in patients with hepatocellular carcinoma. World J Gastroenterol. 2000;6:408-410. [PubMed] |
| 9. | Oh BS, Jang JW, Kwon JH, You CR, Chung KW, Kay CS, Jung HS, Lee S. Prognostic value of C-reactive protein and neutrophil-to-lymphocyte ratio in patients with hepatocellular carcinoma. BMC Cancer. 2013;13:78. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 81] [Cited by in RCA: 91] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 10. | Liao W, Zhang J, Zhu Q, Qin L, Yao W, Lei B, Shi W, Yuan S, Tahir SA, Jin J. Preoperative Neutrophil-to-Lymphocyte Ratio as a New Prognostic Marker in Hepatocellular Carcinoma after Curative Resection. Transl Oncol. 2014;7:248-255. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 76] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 11. | Laurent VM, Duperray A, Sundar Rajan V, Verdier C. Atomic force microscopy reveals a role for endothelial cell ICAM-1 expression in bladder cancer cell adherence. PLoS One. 2014;9:e98034. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 12. | Yu JA, Sadaria MR, Meng X, Mitra S, Ao L, Fullerton DA, Weyant MJ. Lung cancer cell invasion and expression of intercellular adhesion molecule-1 (ICAM-1) are attenuated by secretory phospholipase A2 inhibition. J Thorac Cardiovasc Surg. 2012;143:405-411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 13. | Chaves KC, Peron JP, Chammas R, Turaça LT, Pesquero JB, Braga MS, Foguer K, Schor N, Bellini MH. Endostatin gene therapy stimulates upregulation of ICAM-1 and VCAM-1 in a metastatic renal cell carcinoma model. Cancer Gene Ther. 2012;19:558-565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 14. | Zhu XW, Gong JP. Expression and role of icam-1 in the occurrence and development of hepatocellular carcinoma. Asian Pac J Cancer Prev. 2013;14:1579-1583. [PubMed] |
| 15. | Verslype C, Rosmorduc O, Rougier P. Hepatocellular carcinoma: ESMO-ESDO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2012;23 Suppl 7:vii41-vii48. [PubMed] |
| 16. | Tamura Y, Suda T, Arii S, Sata M, Moriyasu F, Imamura H, Kawasaki S, Izumi N, Takayama T, Kokudo N. Value of highly sensitive fucosylated fraction of alpha-fetoprotein for prediction of hepatocellular carcinoma recurrence after curative treatment. Dig Dis Sci. 2013;58:2406-2412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 17. | Teng M, Pirrie S, Ward DG, Assi LK, Hughes RG, Stocken D, Johnson PJ. Diagnostic and mechanistic implications of serum free light chains, albumin and alpha-fetoprotein in hepatocellular carcinoma. Br J Cancer. 2014;110:2277-2282. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 18. | Tai DI, Lin SM, Sheen IS, Chu CM, Lin DY, Liaw YF. Long-term outcome of hepatitis B e antigen-negative hepatitis B surface antigen carriers in relation to changes of alanine aminotransferase levels over time. Hepatology. 2009;49:1859-1867. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 138] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 19. | Shen SL, Fu SJ, Chen B, Kuang M, Li SQ, Hua YP, Liang LJ, Guo P, Hao Y, Peng BG. Preoperative aspartate aminotransferase to platelet ratio is an independent prognostic factor for hepatitis B-induced hepatocellular carcinoma after hepatic resection. Ann Surg Oncol. 2014;21:3802-3809. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 89] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 20. | Sun JJ, Zhou XD, Liu YK, Tang ZY, Feng JX, Zhou G, Xue Q, Chen J. Invasion and metastasis of liver cancer: expression of intercellular adhesion molecule 1. J Cancer Res Clin Oncol. 1999;125:28-34. [PubMed] |
| 21. | Yoong KF, McNab G, Hübscher SG, Adams DH. Vascular adhesion protein-1 and ICAM-1 support the adhesion of tumor-infiltrating lymphocytes to tumor endothelium in human hepatocellular carcinoma. J Immunol. 1998;160:3978-3988. [PubMed] |
| 22. | Carr BI, Pancoska P, Branch RA. Tumor and liver determinants of prognosis in unresectable hepatocellular carcinoma: a large case cohort study. Hepatol Int. 2010;4:396-405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 29] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 23. | Llovet JM, Bustamante J, Castells A, Vilana R, Ayuso Mdel C, Sala M, Brú C, Rodés J, Bruix J. Natural history of untreated nonsurgical hepatocellular carcinoma: rationale for the design and evaluation of therapeutic trials. Hepatology. 1999;29:62-67. [PubMed] |
| 24. | Chan KM, Chou HS, Wu TJ, Lee CF, Yu MC, Lee WC. Characterization of hepatocellular carcinoma recurrence after liver transplantation: perioperative prognostic factors, patterns, and outcome. Asian J Surg. 2011;34:128-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 28] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 25. | Mano Y, Shirabe K, Yamashita Y, Harimoto N, Tsujita E, Takeishi K, Aishima S, Ikegami T, Yoshizumi T, Yamanaka T. Preoperative neutrophil-to-lymphocyte ratio is a predictor of survival after hepatectomy for hepatocellular carcinoma: a retrospective analysis. Ann Surg. 2013;258:301-305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 274] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 26. | Zhou J, Ding T, Pan W, Zhu LY, Li L, Zheng L. Increased intratumoral regulatory T cells are related to intratumoral macrophages and poor prognosis in hepatocellular carcinoma patients. Int J Cancer. 2009;125:1640-1648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 193] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 27. | Ohki S, Shibata M, Gonda K, Machida T, Shimura T, Nakamura I, Ohtake T, Koyama Y, Suzuki S, Ohto H. Circulating myeloid-derived suppressor cells are increased and correlate to immune suppression, inflammation and hypoproteinemia in patients with cancer. Oncol Rep. 2012;28:453-458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 94] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 28. | Vincent J, Mignot G, Chalmin F, Ladoire S, Bruchard M, Chevriaux A, Martin F, Apetoh L, Rébé C, Ghiringhelli F. 5-Fluorouracil selectively kills tumor-associated myeloid-derived suppressor cells resulting in enhanced T cell-dependent antitumor immunity. Cancer Res. 2010;70:3052-3061. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 793] [Cited by in RCA: 961] [Article Influence: 64.1] [Reference Citation Analysis (0)] |
| 29. | Kusumanto YH, Dam WA, Hospers GA, Meijer C, Mulder NH. Platelets and granulocytes, in particular the neutrophils, form important compartments for circulating vascular endothelial growth factor. Angiogenesis. 2003;6:283-287. [PubMed] |
| 30. | Kuang DM, Zhao Q, Wu Y, Peng C, Wang J, Xu Z, Yin XY, Zheng L. Peritumoral neutrophils link inflammatory response to disease progression by fostering angiogenesis in hepatocellular carcinoma. J Hepatol. 2011;54:948-955. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 346] [Cited by in RCA: 389] [Article Influence: 27.8] [Reference Citation Analysis (0)] |