Published online Jun 21, 2015. doi: 10.3748/wjg.v21.i23.7197
Peer-review started: October 15, 2014
First decision: October 18, 2014
Revised: December 11, 2014
Accepted: February 12, 2015
Article in press: February 13, 2015
Published online: June 21, 2015
Processing time: 247 Days and 21.6 Hours
AIM: To examine the potential anti-tumor activity of paeoniflorin in the human gastric carcinoma cell line MGC-803.
METHODS: Cell viability and cytotoxic effects in MGC-803 cells were analyzed using a 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide and lactate dehydrogenase assay, respectively. Cell apoptosis of MGC-803 cells was measured using flow cytometry, DAPI staining assay and caspase-3 activity assay. Quantitative reverse transcription-polymerase chain reaction (RT-PCR) was used to measure the expression of microRNA-124 (miR-124) in response to paeoniflorin. The expression of phosphatidylinositol 3-kinase (PI3K), protein kinase B (Akt), phospho-Akt (p-Akt) and phospho-signal transducer and activator of transcription 3 (p-STAT3) were also measured by quantitative RT-PCR and Western blot analysis in normal, miR-124 and anti-miR-124 over-expressing MGC-803 cells, treated with paeoniflorin.
RESULTS: Paeoniflorin was found to inhibit MGC-803 cell viability in a dose-dependent manner. Paeoniflorin treatment was associated with the induction of apoptosis and caspase-3 activity in MGC-803 cells. Paeoniflorin treatment significantly increased miR-124 levels and inhibited the expression of PI3K, Akt, p-Akt and p-STAT3 in MGC-803 cells. Interestingly, the over-expression of miR-124 inhibits PI3K/Akt and phospho-STAT3 expressions in MGC-803 cells. PI3K agonist (IGF-1, 1 μg/10 μL) or over-expression of STAT3 reversed the effect of paeoniflorin on the proliferation of MGC-803 cells. Over-expression of anti-miR-124 in MGC-803 cells reversed paeoniflorin-induced up-regulation.
CONCLUSION: In summary, the in vitro data suggest that paeoniflorin is a potential novel therapeutic agent against gastric carcinoma, which inhibits cell viability and induces apoptosis through the up-regulation of miR-124 and suppression of PI3K/Akt and STAT3 signaling.
Core tip: The in vitro data suggest that paeoniflorin is a potential novel therapeutic agent against gastric carcinoma, which inhibits cell viability and induces apoptosis through the up-regulation of microRNA-124 and suppression of phosphatidylinositol 3-kinase/Akt signaling.
- Citation: Zheng YB, Xiao GC, Tong SL, Ding Y, Wang QS, Li SB, Hao ZN. Paeoniflorin inhibits human gastric carcinoma cell proliferation through up-regulation of microRNA-124 and suppression of PI3K/Akt and STAT3 signaling. World J Gastroenterol 2015; 21(23): 7197-7207
- URL: https://www.wjgnet.com/1007-9327/full/v21/i23/7197.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i23.7197
Gastric cancer is one of the most common malignant tumors in China. According to the International Agency for Research on Cancer, there were approximately 989000 new cases of gastric cancer worldwide in 2008. Of this population, the number of new cases in China was 463000, accounting for 46.8% of the global incidence of gastric cancer[1,2]. Similarly, the number of deaths due to gastric cancer worldwide was approximately 737000. China accounted for 47.8% of global gastric cancer deaths. Gastric cancer ranked third in cancer deaths, after lung cancer and liver cancer, in 2004-2005. Despite this, the mortality of gastric cancer appears to be declining from its leading position as the number one cause of cancer deaths in China in 1973-1975 and 1990-1992[3,4].
Micro-RNA is a small non-coding sequence of RNA that may function as an oncogene or tumor-suppressor gene, as well as regulating cell proliferation, apoptosis and differentiation[5]. Micro-RNA may also play an important role within tumorigenesis and development[6,7]. Studies show that expression of microRNA-124 (miR-124) is down-regulated in liver and cervical cancer[8,9]. In gastric cancer, the expression of miR-124 is down-regulated and is associated with the clinical disease stage, the degree of differentiation and lymph node metastasis. MiR-124 can also inhibit the cell growth and invasion of medulloblastoma cells by targeting CDK6. As a result, targeting miR-124 expression may be a novel therapeutic strategy against gastric cancer[10,11].
Embryo prototype mutations of phosphatase and tension homolog (PTEN) are prevalent in many diseases. A substrate for PTEN is a lipid, generated by phosphatidylinositol 3-kinase (PI3K), and is necessary for the activation of protein kinase B (Akt). PTEN regulates the activity of Akt via activated phosphatidylinositol triphosphate (PIP3). Therefore, a mutation of PTEN uncouples Akt regulation, resulting in unchecked cell proliferation and tumorigenesis. PTEN can dephosphorylate PIP3, reduce the concentration of PIP3 within the cells and inhibit the activation of Akt[5]. Protein phosphatase activity is also closely associated with tumors. Yu et al[12] reported that the induction of apoptosis was accompanied by the inactivation of the PI3K/Akt signaling pathway. Torkinib suppresses cell proliferation of gastric cancer through inhibition of the PI3K/Akt pathway.
STAT3 is an important member of signal transducers and activators of transcription (STAT), defined as an oncogene currently[13]. STAT3 plays an important role in promoting tumor cell proliferation, inhibiting tumor cell apoptosis, and promoting tumor invasion and metastasis as well as immune escape, and the continuous activation of its signal transduction pathway is closely related to the occurrence and development of tumor[14].
Paeoniflorin is the main active monomer ingredient of Paeonia lactiflora and is reported to have anti-inflammatory, immunomodulatory, liver and nerve protective activity[15-17]. Recent in vitro studies report and confirm that the anti-cancer effect of paeoniflorin inhibits human lung cancer cell proliferation[18,19], human hepatocellular carcinoma cells[7], colorectal carcinoma[8], etc. However, the mechanisms underlying the anti-cancer effects of paeoniflorin on gastric cancer are still indeterminate. Therefore, we aimed to investigate the potential anti-proliferative effects of paeoniflorin on gastric cancer. We also explored the associated changes in the miR-124, PI3K/Akt, STAT3 signaling axis with paeoniflorin-mediated antitumor activity.
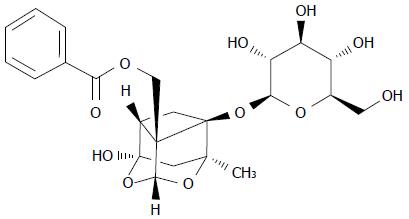
Paeoniflorin (Sigma, with a purity > 97%) was dissolved in physiological saline according to the manufacturer’s instructions and the chemical structure of it is indicated in Figure 1. Dulbecco’s modified Eagle’s medium (DMEM) and fetal bovine serum (FBS) was from Gibco (Grand Island, NY, United States). 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay was purchased from Sigma (Japan). The Annexin V fluorescein isothiocyanate (FITC)-propidium iodide (PI) apoptosis kit and Bicinchoninic Acid (BCA) Protein Assay kit were purchased from Sangon Biotech (Shanghai, China). Caspase-3 colorimetric assay kit and a Nuclear and Cytoplasmic Protein Extraction Kit were purchased from Beyotime (Nanjing, China). Trizol was purchase from Tiangen (Beijing, China). High Capacity cDNA Reverse Transcription kit and ABI 7500 were purchased from TAKARA (Japan).
Human gastric carcinoma MGC-803 cells and human normal gastric mucosa GES-1 cell lines were obtained from Academy of Military Medical Sciences (Beijing, China) and cultured in DMEM supplemented with 10% FBS with 100 U/mL penicillin and 100 mg/mL streptomycin under a humidified atmosphere of 5% CO2 and 95% air at 37 °C. MGC-803 cells were treated with paeoniflorin in complete DMEM medium.
MGC-803 cells (5000 cells per well) and GES-1 cells (5000 cells per well) were seeded in 96-well plates. Cell proliferation of MGC-803 cells and GES-1 cells was analyzed using an MTT (Sigma, Japan) assay after treatment with paeoniflorin (0, 5, 10 and 20 μmol/L) for 0, 24, 48 and 72 h. Then, 10 μL MTT (5 mg/mL) was added into every well and incubated for 4 h under a humidified atmosphere of 5% CO2 and 95% air at 37 °C. Afterwards, 150 μL dimethyl sulfoxide was added to each well and shaken for 10-20 min in a table concentrator. The absorbance was determined with an ELISA reader at 450 nm.
MGC-803 cells (5000 cells per well) were seeded in 96-well plates. Cytotoxic effects on MGC-803 cells were measured using an LDH assay after treatment with paeoniflorin (0, 5, 10 and 20 μmol/L) for 0, 24, 48 and 72 h. Then, 100 μL LDH solution was added to each well and incubated for 30 min at room temperature. The absorbance was read at 490 nm using a multiwell spectrophotometer (BioTek, XL-828, United States).
MGC-803 cells (1 × 105 cells per well) were seeded in 6-well plates. Cell apoptosis was detected using an Annexin V FITC-PI kit assay after treatment with paeoniflorin (0, 5, 10 and 20 μmol/L) for 48 h. The cells were collected and washed twice with PBS according to the manufacturer’s instructions (Sangon Biotech, Shanghai, China). The cells were treated with 5 μL VFITC and incubated for 30 min in darkness; 10 μL PI was added to the cells in darkness. The stained cells were immediately detected using FACSCalibur flow cytometry (BD Biosciences, San Jose, CA) and analyzed with Cell-Quest software.
MGC-803 cells (1 × 105 cells per well) were seeded in 6-well plates. MGC-803 cells were washed twice with PBS. Then, 0.5 mL (4%) paraformaldehyde was added to each well and incubated for 30 min at 4 °C. MGC-803 cells were washed twice with PBS, sodium citrate (0.1%) containing 0.1% Triton X-100 was added and they were incubated for 5 min at 4 °C. Then, DAPI was added to each well and incubated for 20 min at 4 °C in the dark. Apoptotic cells were excitated by ultraviolet light. MGC-803 cells were observed and photographed under florescence microscopy at 340 nm.
MGC-803 cells (5000 cells per well) were seeded in 96-well plates. The activity of caspase-3 was analyzed using the caspase-3 colorimetric assay kit (Beyotime, Nanjing, China) according to the manufacturer’s instructions, after treatment with paeoniflorin (0, 5, 10 and 20 μmol/L) for 48 h. Cells were collected and washed twice with ice-cold PBS. Then, cells (1 × 105 cells/50 mL) were resuspended with BD Cytofix/Cytoperm solution. Next, cells were incubated for 30 min on ice and then washed twice using Perm/Wash buffer at room temperature. The resuspended cells (1 × 106 cells/20 mL) were incubated for 30-60 min at room temperature. Finally, each test was washed with 1.0 mL Perm/Wash buffer and then resuspended with 0.5 mL Perm/Wash buffer. The caspase-3 activity was analyzed at the wavelength of 405 nm.
Total RNA was extracted from MGC-803 cells using Trizol (Tiangen, Beijing, China). MiR-124 analysis was performed using the High Capacity cDNA Reverse Transcription kit (TAKARA, Japan) with the ABI 7500 (TAKARA, Japan) quantitative PCR system according to the manufacturer’s instructions. All primers used were purchased from Sangon Biotech (Shanghai, China). Primer sequences were as follows: miR-124 forward, 5′-GCGGCCGTGTTCACAGCGGACC-3′ and miR-124 reverse, 5′-GTGCAGGGTCCGAGGT-3′; U6 forward, 5’-CGCTTCGGCAGCA CATATACTA-3’ and U6 reverse, 5’-CGCTTCACGAATTTGCGTGTCA.
MGC-803 cells (1 × 105 cells per well) were seeded in 6-well plates. The expressions of PI3K and Akt protein were detected using Western blot assay after treatment with paeoniflorin (0, 5, 10 and 20 μmol/L) for 48 h. Cells were collected and washed twice with ice-cold PBS. In accordance with the protocol described by the manufacturer (Beyotime, Haimen, China), cells were lysed using a Nuclear and Cytoplasmic Protein Extraction Kit. Smudge cells were centrifuged at 15000 ×g for 10 min at 4 °C. Then, protein concentrations were determined with the Bicinchoninic Acid (BCA) Protein Assay kit (Sangon Biotech, Shanghai, China). Protein samples were analyzed with SDS-polyacrylamide gel electrophoresis (PAGE) and then transferred to PVDF membrane (Millipore, Bedford, MA). After blocking with 5% (v/v) nonfat milk for 2 h in TBST buffer, membranes were incubated with anti-PI3K, anti-phospho-Akt (p-Akt), anti-Akt, anti-STAT3, anti-phospho-STAT3 (p-STAT3) and anti-β-actin from Abcam (Boster Biological, Wuhan, China) overnight at 4 °C. Then, membranes were incubated with the peroxidase-linked secondary antibodies for 2 h at room temperature. Proteins were developed using the enhanced chemiluminescence detection system (Amersham Biosciences, Piscataway, NJ, United States).
MiR-124, anti-miR124 and STAT3 plasmids were designed and structured by Gene Pharma (Shanghai, China). MGC-803 cells (1 × 105 cells per well) were seeded in 6-well plates. When the MGC-803 cells reached 70%-80% confluence, miR-124 and anti-miR124 plasmids were transfected into cells using lipofectamine 2000 (Invitrogen, United States) according to the manufacturer’s protocol. After 6 h, culture medium was removed, complete medium was changed and cells were cultured under a humidified atmosphere of 5% CO2 and 95% air at 37 °C.
The data were analyzed statistically using SPSS software 17 and shown as mean ± SD. Analysis was performed using one-way ANOVA followed by Dunnett’s post-hoc test. A P≤ 0.05 was considered indicative of statistical significance.
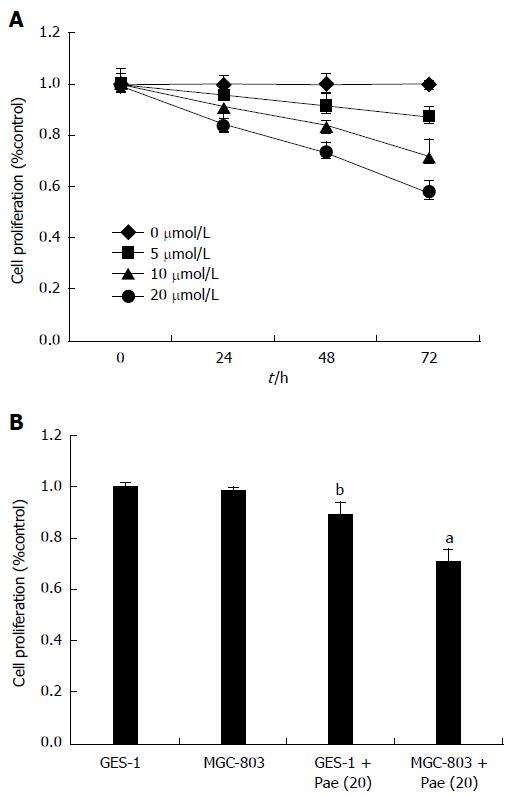
Cell viability was evaluated using MTT assays. Figure 2A shows a dose- and time-dependent decrease in cell viability; paeoniflorin (20 μmol/L) could observably reduce cell viability for 48 and 72 h, and paeoniflorin (10 μmol/L) could also effectively reduce cell viability for 72 h. Hence, 20 μmol/L was determined as the optimal dose for the effect of paeoniflorin on MGC-803 cells. Then, we checked whether the effect of paeoniflorin (20 μmol/L) influenced normal gastric mucosa cell lines (GES-1). Figure 2B shows that cell proliferation of GES-1 was increased after the treatment with paeoniflorin (20 μmol/L) for 48 h, compared to the MGC-803 cells + paeoniflorin (20 μmol/L) group.
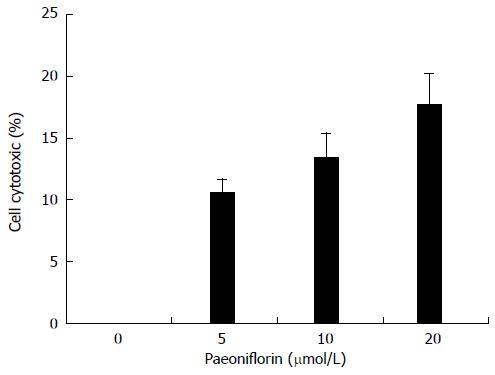
Cytotoxicity of MGC-803 cells was measured using an LDH assay. Figure 3 shows that paeoniflorin increased cell cytotoxicity in a dose-dependent manner. There is significantly elevated cell cytotoxicity after the treatment with paeoniflorin (20 μmol/L) for 48 h (Figure 3).
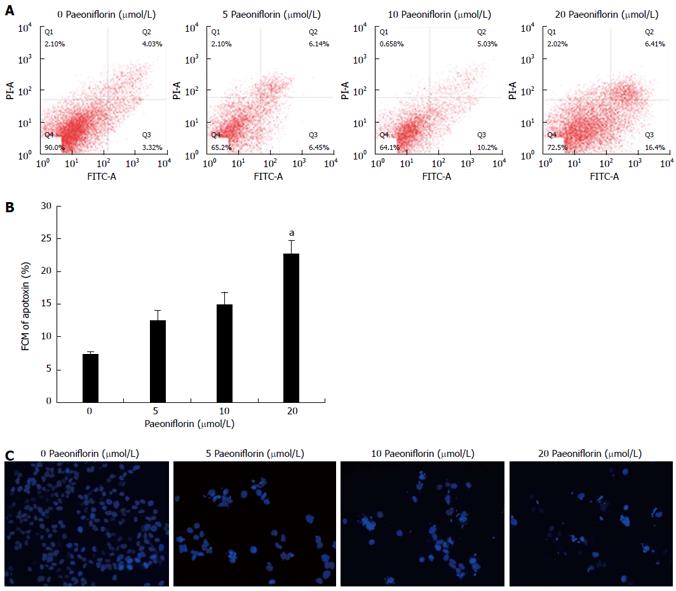
To investigate whether paeoniflorin can induce apoptosis, MGC-803 cells were incubated with paeoniflorin (10 and 20 μmol/L) for 48 h. Cells were then stained with Annexin VFITC/PI, and the apoptosis of MGC-803 cells was determined with flow cytometry. Figure 4A and B show a significant increase in the apoptosis rate when the MGC-803 cells were exposed to 20 μmol/L paeoniflorin for 48 h. Moreover, the results of DAPI staining show that different concentrations of paeoniflorin (5, 10 and 20 μmol/L) markedly increased the apoptosis of MGC-803 cells (Figure 4C).
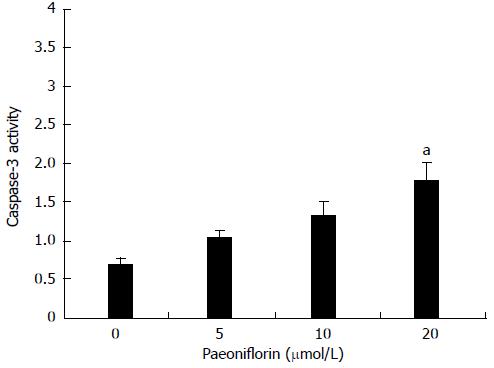
To further research whether paeoniflorin induces caspase-3 activity, MGC-803 cells were incubated with paeoniflorin (10 and 20 μmol/L) for 48 h. When the MGC-803 cells were treated with different concentrations of paeoniflorin (5, 10 and 20 μmol/L), the caspase-3 activity of MGC-803 cells was dose-dependent. The 20 μmol/L dose of paeoniflorin could effectively increase the activity of caspase-3 in MGC-803 cells (Figure 5).
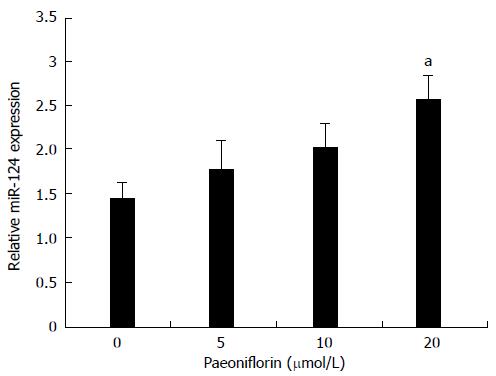
To determine whether paeoniflorin activates the expression of miR-124 in MGC-803 cells, the miR-124 expression was detected by qPCR. Figure 5 shows treatment with paeoniflorin (5, 10 and 20 μmol/L) resulted in a dose-dependent increase of the miR-124 expression (Figure 6). Moreover, the levels of miR-124 were significantly increased in the paeoniflorin (20 μmol/L)-treated cells for 48 h.
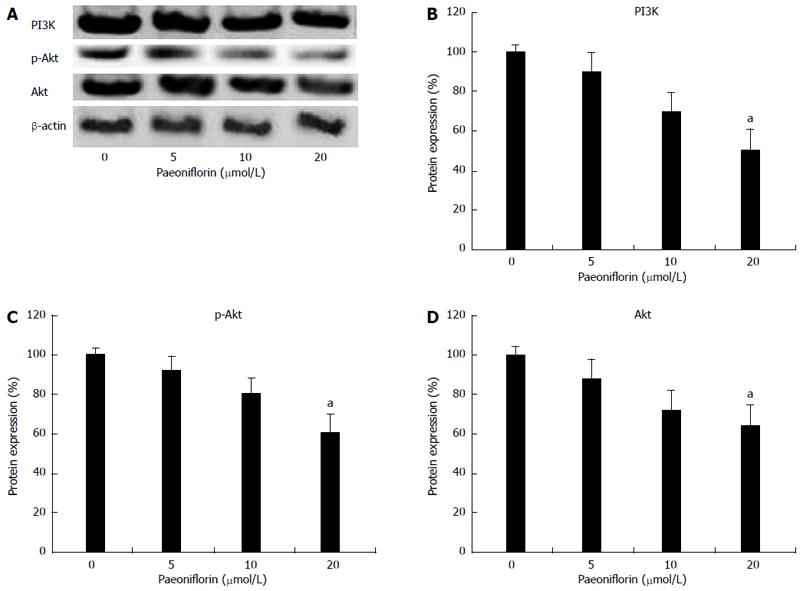
To clarify the anticancer mechanisms of paeoniflorin on MGC-803 cells, we examined the expression levels of PI3K, p-Akt and Akt in MGC-803 cells. Figures 7A and 7B show treatment of MGC-803 cells with different concentrations of paeoniflorin (5, 10 and 20 μmol/L) for 48 h induced a decrease in the expressions of PI3K, p-Akt and Akt proteins. Indeed, paeoniflorin (20 μmol/L) could observably lessen the expression of PI3K, p-Akt and Akt in MGC-803 cells (Figures 7A and 7B).
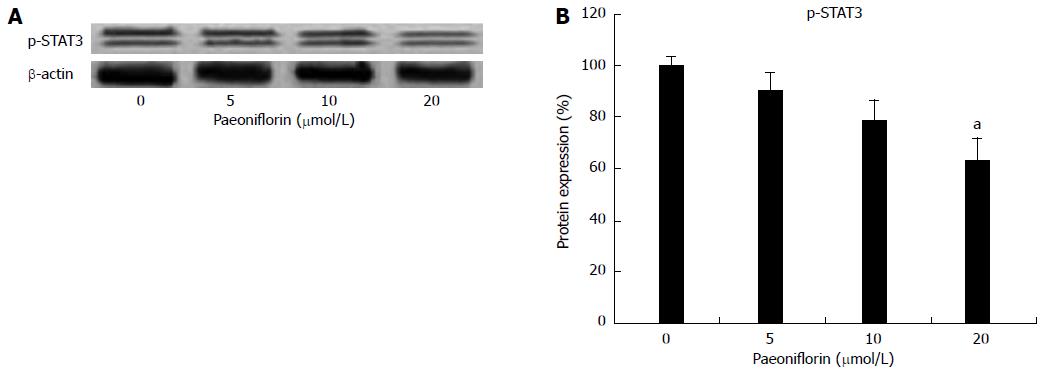
To explore the anticancer mechanisms of paeoniflorin on MGC-803 cells, p-STAT3 and STAT3 protein expression levels were checked in MGC-803 cells. Figures 8A and 8B show that p-STAT3 protein expression levels were reduced by paeoniflorin administration (Figures 8A and 8B).
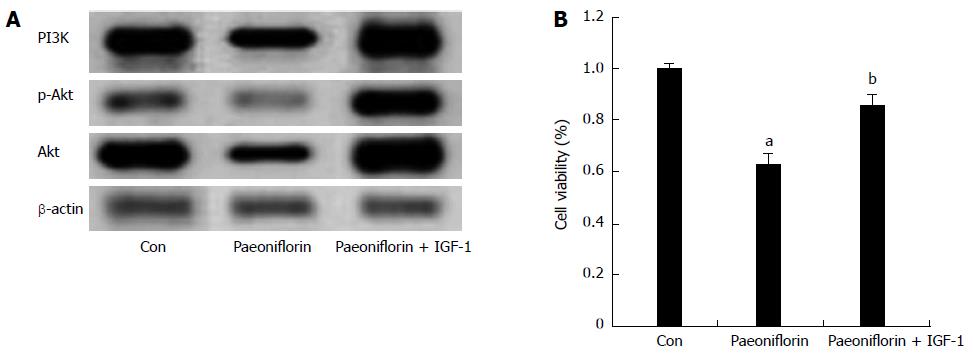
To further research whether a PI3K agonist inhibits the effect of paeoniflorin on cell proliferation, the expression of PI3K/Akt and the cell proliferation of MGC-803 cells were detected. First, after treatment with paeoniflorin (20 μmol/L) for 72 h, the expressions of PI3K, p-Akt and Akt proteins in MGC-803 cells were measured. We observed that the expressions of PI3K, p-Akt and Akt proteins in the paeoniflorin treatment group were remarkably increased (Figure 9A). However, a PI3K agonist (IGF-1, 1 μg/10 μL) inhibited the effect of paeoniflorin on the PI3K, p-Akt and Akt protein expressions in MGC-803 cells (Figure 9A). Meanwhile, IGF-1 reversed the effect of paeoniflorin on the cell proliferation of MGC-803 cells (Figure 9B).
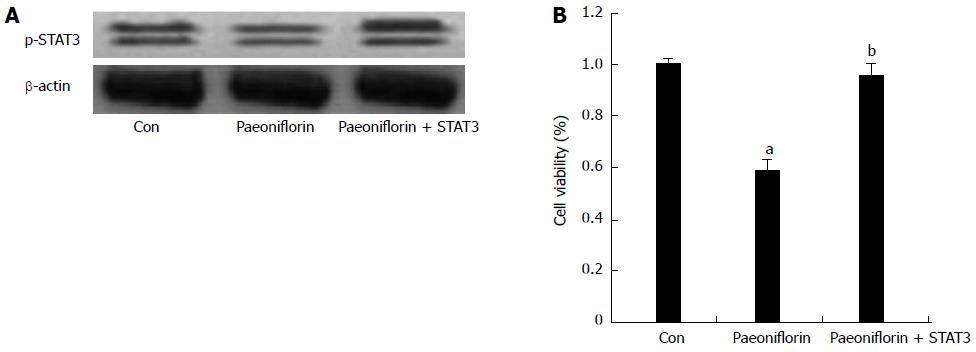
To further detect whether overexpression of STAT3 inhibits the effect of paeoniflorin on cell proliferation, p-STAT3 protein expression levels and the proliferation of MGC-803 cells were ascertained. p-STAT3 protein expression levels were markedly boosted (Figure 10A). Overexpression of STAT3 definitely reversed the effect of paeoniflorin on the proliferation of MGC-803 cells (Figure 10B).
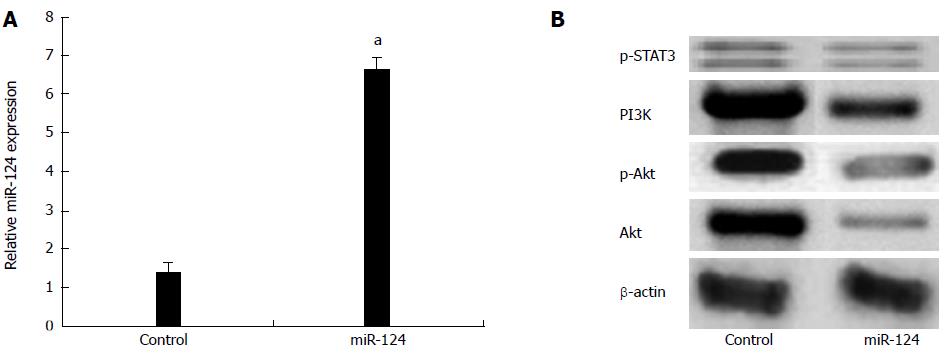
To determine whether miR-124 is a crucial transcription factor for regulating the expression of PI3K, p-Akt, Akt and p-STAT3, a miR-124 plasmid was transfected into MGC-803 cells. Figure 11A shows that the miR-124 plasmid could effectively augment the level of expression of miR-124 in MGC-803 cells. Meanwhile, overexpression of miR-124 could also control the PI3K, p-Akt, Akt (Figure 11B), p-STAT3 protein expression levels (Figure 11C) in MGC-803 cells.
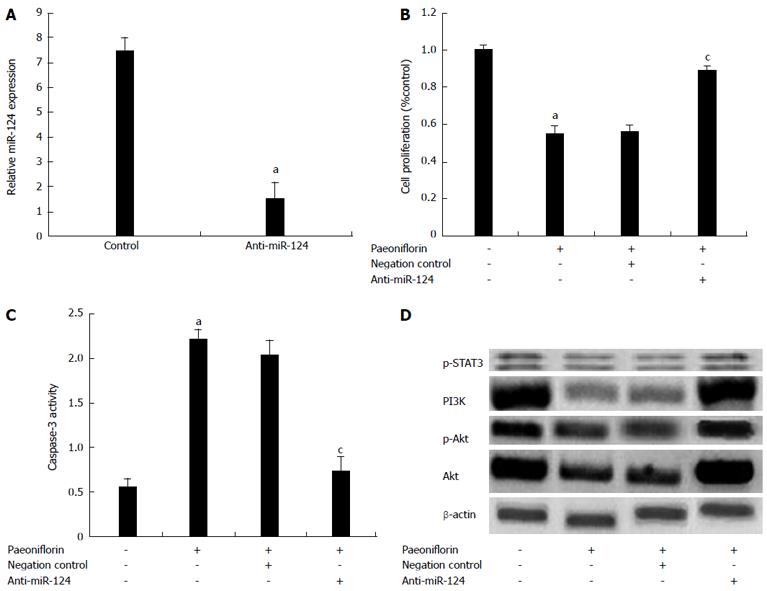
To further investigate the correlations of miR-124 expression level with the effect of paeoniflorin, paeoniflorin (20 μmol/L) treatment of MGC-803 cells was performed for 72 h. Our results showed that transfection of anti-miR-124 plasmids into MGC-803 cells significantly reduced the expression of miR-124 (Figure 12A). Meanwhile, anti-miR-124 plasmids could significantly reduce the effect of paeoniflorin on cell proliferation (Figure 12B) and the apoptotic effect on MGC-803 cells (Figure 12C). Meanwhile, anti-miR-124 plasmids could promote the levels of PI3K, p-Akt, Akt and p-STAT3 in MGC-803 cells (Figure 12D).
Age-specific incidence and mortality curves show that gastric cancer is an important malignant tumor in the elderly population, and the regional differences of morbidity and mortality indicate in China that pathogenic factors and susceptibility to gastric cancer are varied[20]. The incidence of gastric cancer in urban areas of China showed no clear upward trend in 2009, while in rural areas, incidence of gastric cancer seemed to increase with time[21]. However, the change in the standardized rate has stabilized; change in mortality of gastric cancer is similar to the incidence in the same period. Our most significant finding was that paeoniflorin could markedly inhibit the cell viability of MGC-803 gastric carcinoma cells[22]. Other studies have shown paeoniflorin could attenuate the viability, and increase apoptosis induced by Aβ25-35 in SH-SY5Y cells. In this study, paeoniflorin effectively suppressed cell proliferation, increased cell cytotoxicity, induced apoptosis and accelerated the activity of caspase-3 in MGC-803 cells. Wang et al[23] reported that paeoniflorin significantly ameliorated glutamate-induced reduction of cell viability, and facilitated apoptotic alteration in PC12 cells. Paeoniflorin was also found to have a pro-apoptotic and anti-proliferative effect in human colorectal carcinoma HT-29 cells, as shown by the activation of caspase-3 and caspase-9 in vitro and in vivo[8].
Oncogenic microRNA promotes cell proliferation, inhibits apoptosis, inhibits immune cell development and regulates the cell cycle. Conversely, tumor suppressive microRNA inhibits cell growth and promotes apoptosis[24]. Several microRNA genes are reported to show oncogenic or tumor suppressive activity related to tumor development. Studies show highly-expressed miR-124 influences the proliferation of MKN-45 cells[25]. The results show that the invasion speed of MKN-45 cells, transfected with miR-124, declines significantly after 24 h compared with that of scramble sequence control. This indicates that miR-124 can inhibit the invasion of gastric cancer cell motility, which may play an important role in the gastric tumorigenesis and development[26]. In our study, paeoniflorin treatment could significantly increase the levels of miR-124 in MGC-803 gastric carcinoma cells.
The variance of expression of PI3K and Akt was not statistically significant in cancer-adjacent tissues and normal gastric mucosa. This indicates that the appearance of PI3K and Akt in gastric cancer may be a late molecular event[27]. Mammary epithelial cells from mice inhibit AKT1 expression, delay epithelial cell differentiation and accelerate apoptosis and recovery of cells during pregnancy, lactation and recovery periods. The inhibition of AKT2 results in the opposite effect, suggesting that different subtypes of AKT have different functions[28,29].
Our results suggest that paeoniflorin could markedly inhibit the expression of PI3K, Akt and phospho-Akt in MGC-803 gastric carcinoma cells. Xu et al[30] reported that paeoniflorin attenuates lipopolysaccharide-induced permeability of endothelial cells through the suppression of the phosphorylation of PI3K/Akt. Wu et al[31] demonstrated that paeoniflorin-mediated neural stem/progenitor cell protection from hydrogen peroxide injury is dependent on the activation of the PI3K/Akt-1 pathway. In our study, we found that miR-124 regulates and controls the expression of PI3K, Akt and phospho-Akt in MGC-803 cells.
STAT3 regulates target gene expression downstream to accelerate tumor cell proliferation, prevent apoptosis and promote tumor angiogenesis. In vivo blocking or inhibiting the STAT3 signaling pathway of certain tumor cells can inhibit cell proliferation and survival, and induce apoptosis[32]. Therefore, the application of negative regulatory proteins to interfere with the STAT3 signaling pathway has become an important strategy for cancer intervention, and STAT3 is considered to be a new target for cancer therapy. Results of study show that the expression of STAT3 protein in gastric carcinoma tissues is significantly higher than that in normal gastric tissues, and its expression is not associated with patient gender and tissue type, but only with lymph node metastasis, differentiation degree and clinical stage[33]. The activation of the STAT3 signaling pathway promotes cell proliferation and inhibits apoptosis, and this relationship may lead to malignant cell transformation and abnormal proliferation, thus promoting the occurrence and development of gastric cancer[34]. In this study, the results show for the first time that paeoniflorin can suppress p-STAT3 protein expression in MGC-803 cells. Meanwhile, we found that overexpression of STAT3 inhibited the effect of paeoniflorin on proliferation of MGC-803 cells. Recent studies show that miR-124 functions as a tumor suppressor of p-STAT3 in the human endometrial carcinoma cell line HEC-1B[35] and colorectal cancer[36].
In summary, this study demonstrates that paeoniflorin possesses antitumor activity in gastric cancer cells by inhibiting cell proliferation and stimulating apoptosis via the up-regulation of miR-124 and suppression of PI3K/Akt signaling. Follow up studies are required to evaluate the efficacy of this drug within cancer-bearing animal models.
Gastric cancer is one of the most common malignant tumors in China. According to the International Agency for Research on Cancer, there were approximately 989000 new cases of gastric cancer worldwide in 2008. The mechanisms underlying the anti-cancer effects of paeoniflorin on gastric cancer are still indeterminate.
The authors aimed to investigate the potential anti-proliferative effects of paeoniflorin on gastric cancer. They also explored the associated changes in the miR-124, PI3K/Akt, STAT3 signaling axis with paeoniflorin-mediated antitumor activity.
The authors found that the in vitro data suggest that paeoniflorin is a potential novel therapeutic agent against gastric carcinoma, which inhibits cell viability and induces apoptosis through the up-regulation of miR-124 and suppression of PI3K/Akt and STAT3 signaling.
In this work authors presented data suggesting that paeoniflorin is a potential novel therapeutic agent against gastric carcinoma, which inhibits cell viability and induces apoptosis through the up-regulation of miR-124 and suppression of PI3K/Akt signaling. They carried out in vitro assays with the gastric carcinoma cell line MGC-803. It is an interesting study, with a lot of work.
P- Reviewer: Chiurillo MA, He XS, Tu LL S- Editor: Yu J L- Editor: Logan S E- Editor: Zhang DN
| 1. | Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893-2917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11128] [Cited by in RCA: 11831] [Article Influence: 845.1] [Reference Citation Analysis (4)] |
| 2. | Chajès V, Jenab M, Romieu I, Ferrari P, Dahm CC, Overvad K, Egeberg R, Tjønneland A, Clavel-Chapelon F, Boutron-Ruault MC. Plasma phospholipid fatty acid concentrations and risk of gastric adenocarcinomas in the European Prospective Investigation into Cancer and Nutrition (EPIC-EURGAST). Am J Clin Nutr. 2011;94:1304-1313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 33] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 3. | Yan S, Li B, Bai ZZ, Wu JQ, Xie DW, Ma YC, Ma XX, Zhao JH, Guo XJ. Clinical epidemiology of gastric cancer in Hehuang valley of China: a 10-year epidemiological study of gastric cancer. World J Gastroenterol. 2014;20:10486-10494. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 40] [Cited by in RCA: 40] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 4. | Zou XN, Duan JJ, Huangfu XM, Chen WQ, Zhao P. [Analysis of stomach cancer mortality in the national retrospective sampling survey of death causes in China, 2004-2005]. Zhonghua Yufang Yixue Zazhi. 2010;44:390-397. |
| 5. | Munroe ME, Businga TR, Kline JN, Bishop GA. Anti-inflammatory effects of the neurotransmitter agonist Honokiol in a mouse model of allergic asthma. J Immunol. 2010;185:5586-5597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 59] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 6. | Chen YJ, Tsai KS, Chan DC, Lan KC, Chen CF, Yang RS, Liu SH. Honokiol, a low molecular weight natural product, prevents inflammatory response and cartilage matrix degradation in human osteoarthritis chondrocytes. J Orthop Res. 2014;32:573-580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 62] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 7. | Lu JT, He W, Song SS, Wei W. Paeoniflorin inhibited the tumor invasion and metastasis in human hepatocellular carcinoma cells. Bratisl Lek Listy. 2014;115:427-433. [PubMed] |
| 8. | Wang H, Zhou H, Wang CX, Li YS, Xie HY, Luo JD, Zhou Y. Paeoniflorin inhibits growth of human colorectal carcinoma HT 29 cells in vitro and in vivo. Food Chem Toxicol. 2012;50:1560-1567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 40] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 9. | Li KK, Pang JC, Ching AK, Wong CK, Kong X, Wang Y, Zhou L, Chen Z, Ng HK. miR-124 is frequently down-regulated in medulloblastoma and is a negative regulator of SLC16A1. Hum Pathol. 2009;40:1234-1243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 132] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 10. | Tang X, Yao K, Zhang L, Yang Y, Yao H. Honokiol inhibits H(2)O(2)-induced apoptosis in human lens epithelial cells via inhibition of the mitogen-activated protein kinase and Akt pathways. Eur J Pharmacol. 2011;650:72-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 27] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 11. | Weng TI, Wu HY, Chen BL, Liu SH. Honokiol attenuates the severity of acute pancreatitis and associated lung injury via acceleration of acinar cell apoptosis. Shock. 2012;37:478-484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 12. | Yu HY, Kim SO, Jin CY, Kim GY, Kim WJ, Yoo YH, Choi YH. β-lapachone-Induced Apoptosis of Human Gastric Carcinoma AGS Cells Is Caspase-Dependent and Regulated by the PI3K/Akt Pathway. Biomol Ther (Seoul). 2014;22:184-192. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 34] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 13. | Wu LJ, Li HX, Luo XT, Lu RZ, Ma YF, Wang R, Zhang J, Yang DQ, Yu H, Liu J. STAT3 activation in tumor cell-free lymph nodes predicts a poor prognosis for gastric cancer. Int J Clin Exp Pathol. 2014;7:1140-1146. [PubMed] |
| 14. | Wang YX, Cai H, Jiang G, Zhou TB, Wu H. Silibinin inhibits proliferation, induces apoptosis and causes cell cycle arrest in human gastric cancer MGC803 cells via STAT3 pathway inhibition. Asian Pac J Cancer Prev. 2014;15:6791-6798. [PubMed] |
| 15. | Kim ID, Ha BJ. Paeoniflorin protects RAW 264.7 macrophages from LPS-induced cytotoxicity and genotoxicity. Toxicol In Vitro. 2009;23:1014-1019. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 64] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 16. | Liu DF, Wei W, Song LH. Protective effect of paeoniflorin on immunological liver injury induced by bacillus Calmette-Guerin plus lipopolysaccharide: modulation of tumour necrosis factor-alpha and interleukin-6 MRNA. Clin Exp Pharmacol Physiol. 2006;33:332-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 57] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 17. | Zhong SZ, Ge QH, Li Q, Qu R, Ma SP. Peoniflorin attentuates Abeta((1-42))-mediated neurotoxicity by regulating calcium homeostasis and ameliorating oxidative stress in hippocampus of rats. J Neurol Sci. 2009;280:71-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 94] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 18. | Li CR, Zhou Z, Zhu D, Sun YN, Dai JM, Wang SQ. Protective effect of paeoniflorin on irradiation-induced cell damage involved in modulation of reactive oxygen species and the mitogen-activated protein kinases. Int J Biochem Cell Biol. 2007;39:426-438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 47] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 19. | Hung JY, Yang CJ, Tsai YM, Huang HW, Huang MS. Antiproliferative activity of paeoniflorin is through cell cycle arrest and the Fas/Fas ligand-mediated apoptotic pathway in human non-small cell lung cancer A549 cells. Clin Exp Pharmacol Physiol. 2008;35:141-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 20. | Dong CX, Fu JF, Ye XY, Li XF, Zhong X, Yuan Y. Surgical resection of advanced gastric cancer following trastuzumab/oxaliplatin/capecitabine combination therapy. World J Gastroenterol. 2014;20:12355-12358. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 11] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 21. | Zhu F, Loh M, Hill J, Lee S, Koh KX, Lai KW, Salto-Tellez M, Iacopetta B, Yeoh KG, Soong R. Genetic factors associated with intestinal metaplasia in a high risk Singapore-Chinese population: a cohort study. BMC Gastroenterol. 2009;9:76. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 22. | Wang K, Zhu L, Zhu X, Zhang K, Huang B, Zhang J, Zhang Y, Zhu L, Zhou B, Zhou F. Protective effect of paeoniflorin on Aβ25-35-induced SH-SY5Y cell injury by preventing mitochondrial dysfunction. Cell Mol Neurobiol. 2014;34:227-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 87] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 23. | Wang D, Tan QR, Zhang ZJ. Neuroprotective effects of paeoniflorin, but not the isomer albiflorin, are associated with the suppression of intracellular calcium and calcium/calmodulin protein kinase II in PC12 cells. J Mol Neurosci. 2013;51:581-590. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 44] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 24. | Phuah NH, Nagoor NH. Regulation of microRNAs by natural agents: new strategies in cancer therapies. Biomed Res Int. 2014;2014:804510. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 94] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 25. | Li H, Xie S, Liu M, Chen Z, Liu X, Wang L, Li D, Zhou Y. The clinical significance of downregulation of mir-124-3p, mir-146a-5p, mir-155-5p and mir-335-5p in gastric cancer tumorigenesis. Int J Oncol. 2014;45:197-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 78] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 26. | Yang B, Jing C, Wang J, Guo X, Chen Y, Xu R, Peng L, Liu J, Li L. Identification of microRNAs associated with lymphangiogenesis in human gastric cancer. Clin Transl Oncol. 2014;16:374-379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 49] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 27. | Hu Y, Li L, Yin W, Shen L, You B, Gao H. Protective effect of proanthocyanidins on anoxia-reoxygenation injury of myocardial cells mediated by the PI3K/Akt/GSK-3β pathway and mitochondrial ATP-sensitive potassium channel. Mol Med Rep. 2014;10:2051-2058. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 28. | Lian JH, Wang WH, Wang JQ, Zhang YH, Li Y. MicroRNA-122 promotes proliferation, invasion and migration of renal cell carcinoma cells through the PI3K/Akt signaling pathway. Asian Pac J Cancer Prev. 2013;14:5017-5021. [PubMed] |
| 29. | Sun HW, Tong SL, He J, Wang Q, Zou L, Ma SJ, Tan HY, Luo JF, Wu HX. RhoA and RhoC -siRNA inhibit the proliferation and invasiveness activity of human gastric carcinoma by Rho/PI3K/Akt pathway. World J Gastroenterol. 2007;13:3517-3522. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 39] [Cited by in RCA: 38] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 30. | Xu H, Song J, Gao X, Xu Z, Xu X, Xia Y, Dai Y. Paeoniflorin attenuates lipopolysaccharide-induced permeability of endothelial cells: involvements of F-actin expression and phosphorylations of PI3K/Akt and PKC. Inflammation. 2013;36:216-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 32] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 31. | Wu YM, Jin R, Yang L, Zhang J, Yang Q, Guo YY, Li XB, Liu SB, Luo XX, Zhao MG. Phosphatidylinositol 3 kinase/protein kinase B is responsible for the protection of paeoniflorin upon H₂O₂-induced neural progenitor cell injury. Neuroscience. 2013;240:54-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 40] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 32. | Yang G, Ma F, Zhong M, Fang L, Peng Y, Xin X, Zhong J, Zhu W, Zhang Y. Interleukin-11 induces the expression of matrix metalloproteinase 13 in gastric cancer SCH cells partly via the PI3K-AKT and JAK-STAT3 pathways. Mol Med Rep. 2014;9:1371-1375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 33. | Huang S, Chen M, Ding X, Zhang X, Zou X. Proton pump inhibitor selectively suppresses proliferation and restores the chemosensitivity of gastric cancer cells by inhibiting STAT3 signaling pathway. Int Immunopharmacol. 2013;17:585-592. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 34. | Wang Z, Si X, Xu A, Meng X, Gao S, Qi Y, Zhu L, Li T, Li W, Dong L. Activation of STAT3 in human gastric cancer cells via interleukin (IL)-6-type cytokine signaling correlates with clinical implications. PLoS One. 2013;8:e75788. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 55] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 35. | Li Y, Zhang Z, Liu X, Huang T, He W, Shen Y, Liu X, Hong K, Cao Q. miR-124 functions as a tumor suppressor in the endometrial carcinoma cell line HEC-1B partly by suppressing STAT3. Mol Cell Biochem. 2014;388:219-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 32] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 36. | Zhang J, Lu Y, Yue X, Li H, Luo X, Wang Y, Wang K, Wan J. MiR-124 suppresses growth of human colorectal cancer by inhibiting STAT3. PLoS One. 2013;8:e70300. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 74] [Cited by in RCA: 94] [Article Influence: 7.8] [Reference Citation Analysis (0)] |