Published online Jun 14, 2015. doi: 10.3748/wjg.v21.i22.7052
Peer-review started: January 9, 2015
First decision: January 22, 2015
Revised: February 22, 2015
Accepted: April 3, 2015
Article in press: April 3, 2015
Published online: June 14, 2015
Processing time: 160 Days and 19.9 Hours
A 52-year-old man was referred for further investigation of a gastric submucosal tumor on the greater curvature of the antrum. Endoscopic ultrasonography demonstrated a hypoechoic solid mass, which was primarily connected to the muscular layer of the stomach. We performed endoscopic ultrasound-guided fine-needle aspiration biopsy. The pathological examination showed proliferation of oval-shaped cells with nest formation, which stained strongly positive for muscle actin, and negative for c-kit, CD34, CD56, desmin, S-100, chromogranin, and neuron-specific enolase. Therefore, we performed laparoscopy and endoscopy cooperative surgery based on the preoperative diagnosis of glomus tumor of the stomach. The final histological diagnosis confirmed the preoperative diagnosis. Although preoperative diagnosis of glomus tumor of the stomach is difficult with conventional images and endoscopic biopsy, endoscopic ultrasound-guided fine-needle aspiration biopsy is an essential tool to gain histological evidence of glomus tumor of the stomach for early diagnosis.
Core tip: Preoperative diagnosis of glomus tumor of the stomach is difficult as its exhibits a similar clinical appearance on conventional images (computed tomography, magnetic resonance imaging, endoscopic ultrasonography) to other submucosal tumors of the stomach. Furthermore, pathological evidence for diagnosis is difficult to obtain by conventional endoscopic biopsy. Endoscopic ultrasound-guided fine-needle aspiration biopsy (EUS-FNA) is an essential and useful diagnostic tool for glomus tumor of the stomach to obtain pathological evidence including immunohistochemical staining which is critically important to diagnose pathologically. There are only eight literatures of gastric glomus tumors which were diagnosed by FNA. This is a first report to review these literatures.
- Citation: Kato S, Kikuchi K, Chinen K, Murakami T, Kunishima F. Diagnostic utility of endoscopic ultrasound-guided fine-needle aspiration biopsy for glomus tumor of the stomach. World J Gastroenterol 2015; 21(22): 7052-7058
- URL: https://www.wjgnet.com/1007-9327/full/v21/i22/7052.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i22.7052
Glomus tumor is a rare benign neoplastic proliferation of modified smooth muscle cells arising from the neuroarterial structure called glomus body[1]. These tumors generally appear as skin lesions. Although glomus tumors are generally benign, several malignant cases have been reported[2-5].
Preoperative diagnosis of glomus tumor of the stomach is difficult, since glomus tumors and other typical submucosal tumors of the stomach, such as gastrointestinal stromal tumors (GISTs) and leiomyomas, exhibit a similar clinical appearance on conventional images. Furthermore, pathological evidence for diagnosis is difficult to obtain by conventional endoscopic biopsy as these tumors originate from submucosal lesions. Therefore, almost all reported cases of glomus tumor of the stomach are diagnosed from resected specimens.
Herein, we report a case of glomus tumor of the stomach that was preoperatively diagnosed by endoscopic ultrasound-guided fine-needle aspiration biopsy (EUS-FNA) and resected.
A 52-year-old asymptomatic Japanese male with a past medical history of polycystic kidney was referred to our hospital for further investigation of a gastric submucosal tumor (SMT) on the greater curvature of the antrum, which was detected at an annual health check. There was no significant finding on physical examination. Initial laboratory data were within the normal range including the tumor markers, carcinoembryonic antigen, and carbohydrate antigen 19-9.
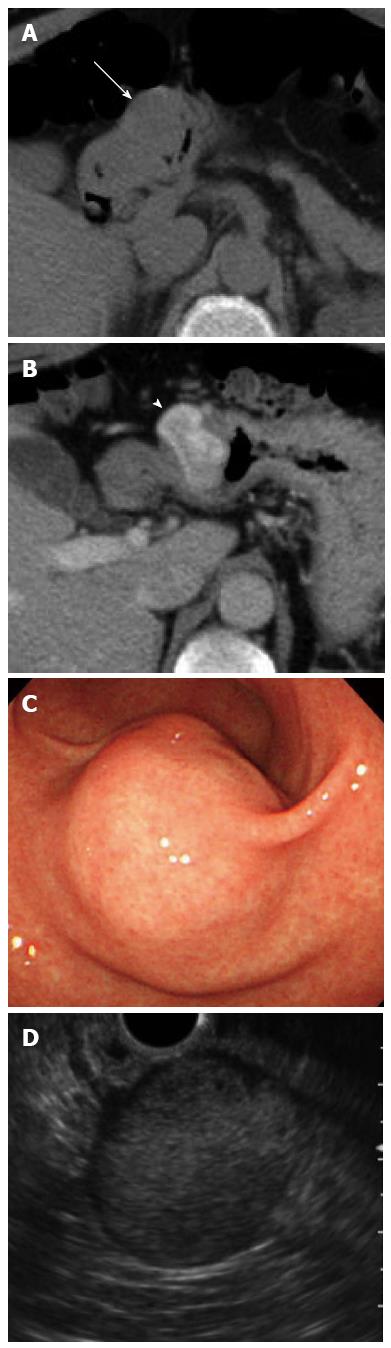
Plain computed tomography (CT) showed an approximately 3.0 cm round shaped mass lesion on the gastric antrum. A contrast-enhanced CT identified an enhanced mass without cystic change or calcification (Figure 1A and B). Esophagogastroduodenoscopy (EGD) revealed a 30 mm SMT on the greater curvature of the antrum without cushion sign or dell (Figure 1C). Endoscopic ultrasonography (EUS) showed a hypoechoic lesion with a small anechoic component, which was primarily connected to the muscular layer of the stomach (Figure 1D).
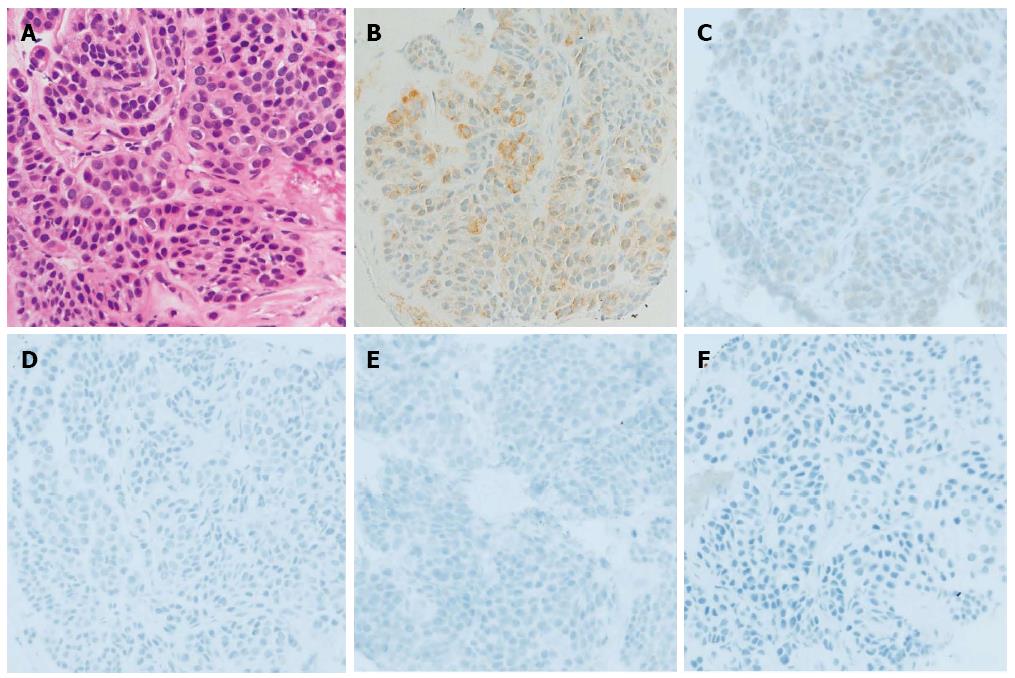
We performed EUS-FNA (UCT-240; Olympus Medical Systems, Tokyo, Japan) using a 22-gauge needle (Echotip; Wilson-Cook, NC, United States) while taking care to avoid needle penetration and puncture of the anechoic component of the mass to prevent tumor seeding. The obtained specimen revealed the proliferation of oval-shaped cells with a small nest formation and high nucleo-cytoplasmic ratio (Figure 2).
Immunohistochemical (IHC) staining revealed that the tumor cells were strongly and focally positive for muscle actin, slightly positive for synaptophysin, and negative for c-kit, CD34, CD56, desmin, S-100, chromogranin, and neuron-specific enolase (Figure 2).
The patients underwent laparoscopy and endoscopy cooperative surgery based on the preoperative diagnosis of glomus tumor.
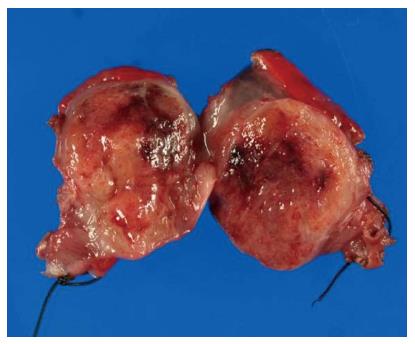
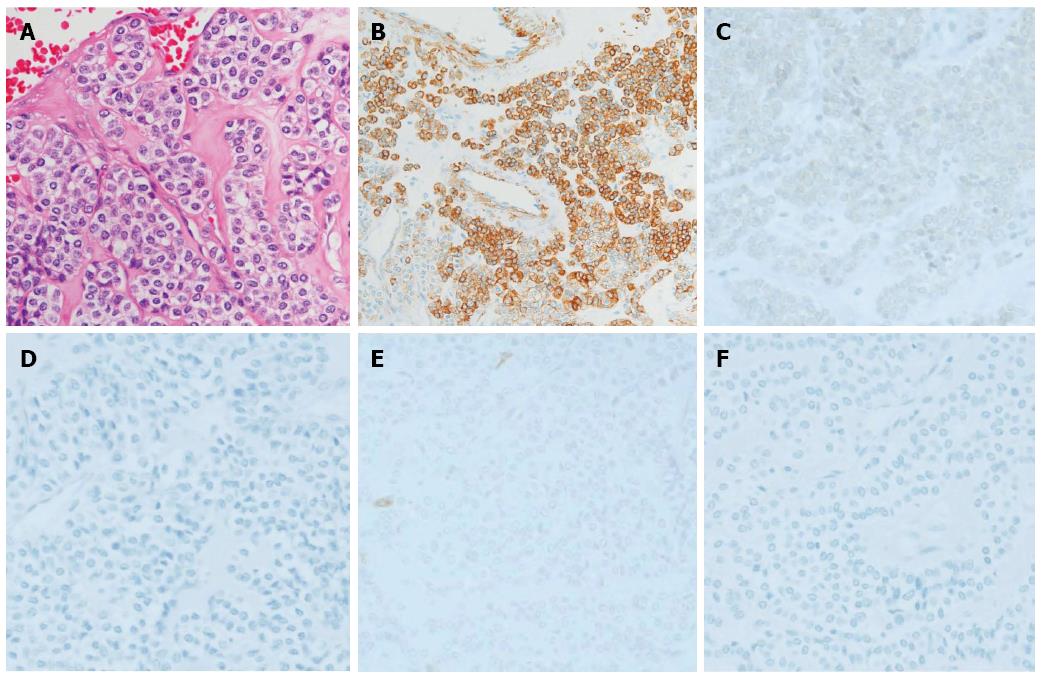
The surgical specimen showed a well-demarcated solid mass lesion located on the resected wall of the gastric antrum, measuring up to 3.5 cm in greatest diameter. The mass was homogeneous without necrosis or cystic change (Figure 3). Histologically, oval-shaped cell with a high nucleo-cytoplasmic ratio proliferated on the proper muscle layer forming small solid nests. IHC analysis was consistent with the EUS-FNA pathology results (Figure 4). Mitotic activity was absent. This patient did not receive any adjuvant therapy after surgery, as no evidence of malignancy was found in the resected specimen. Regular clinical follow up with EGD was performed, and the patient shows no signs of recurrence at 36 mo after surgery.
Glomus tumor is generally a benign neoplasm. These tumors commonly appear under the fingernails and arise from the arterial portion of the glomus body[1]. Glomus tumors of the stomach, which were first reported by De Bussacher in 1948[6], are extremely rare, accounting for 1% of the occurrence of GISTs[7].
Based on the histological characteristics, glomus tumors are now considered mesenchymal tumors with malignant potential. Although almost all glomus tumors are benign, some malignant cases have been reported[2-5]. Folpe et al[8] proposed the following criteria for malignant glomus tumor: deep location, ≥ 2 cm in size, atypical mitotic figures, moderate to high nuclear grade, and ≥ 5 mitotic figures/50 high-power fields. They found that metastasis was observed in 38% of glomus tumors fulfilling the criteria for malignancy. Therefore, complete resection based on the collect preoperative diagnosis is necessary. In our case, the EUS image showed that the tumor was located on the muscle layer and was over 2 cm in size. Although mitosis was not seen, the possibility of malignancy could not be ruled out in the preoperative diagnosis.
Preoperative diagnosis of glomus tumor of the stomach is difficult with conventional images, such as CT and magnetic resonance imaging (MRI). The typical CT image shows dense homogeneous enhancement in the arterial phase and continuous enhancement in the delayed phase[9-12]. These findings are key in distinguishing glomus tumors from other hypovascular submucosal lesions, such as leiomyomas, lipomas and ectopic pancreas. However, glomus tumors, GISTs, and neuro-endocrine tumors exhibit similar findings on CT, making differential diagnosis difficult. Moreover, some cases of glomus tumor of the stomach do not demonstrate the typical dense homogeneous and continuous enhancement pattern. In our case, the tumor showed mainly peripheral, not homogeneous, enhancement. Therefore, no conventional images, including CT and MRI, have the ability to replace histological diagnosis.
On EUS, gastric glomus tumors appear as circumscribed low echoic masses in the third and/or fourth layer. The mass components are mostly homogeneous, but sometimes described as heterogeneous echo mixed with high echo spot[13]. These findings are similar to those of GISTs or other gastrointestinal mesenchymal tumors[14-16]. Thus, it is rather difficult to distinguish glomus tumors from other mesenchymal tumors by EUS images.
EUS-FNA is an effective method to obtain pathological specimens of gastric submucosal neoplasms. Mekky et al[17] reported that adequate specimens were obtained in 83% of gastric submucosal neoplasm cases by EUS-FNA. Furthermore, the diagnostic accuracy rate was 95.6%. In cases of small SMT, it is rather difficult to obtain specimens by EUS-FNA, and the procedure carries the risks of needle penetration and malignant cells seeding. However, Akahoshi et al[18] reported the safety of EUS-FNA for gastric SMTs smaller than 2 cm after performing EUS-FNA in 90 cases without complication. EUS-FNA for small SMT (< 2.0 cm) should be performed carefully to prevent needle penetration and seeding, especially in cases exhibiting some malignant characteristics (e.g., necrotic change in the tumor, and rapid growth).
Pathological diagnosis of glomus tumor of the stomach using only hematoxylin and eosin (HE) staining is difficult because of the similarities between glomus tumors and neuroendocrine tumors. EUS-FNA allows for the collection of sufficient specimen not only for HE staining, but also for IHC analysis of SMT lesions. Therefore, it is now considered an essential tool for the preoperative diagnosis of glomus tumor of the stomach.
In the eight reported cases of gastric glomus tumor, FNA (EUS-FNA in seven cases, percutaneous FNA in one case) was performed preoperatively for pathological diagnosis[15,16,18-23]. Table 1 shows the clinical characteristics of these eight cases as well as our case. In seven cases a correct preoperative diagnosis was achieved from EUS-FNA specimens, whereas two cases were misdiagnosed as a neuroendocrine tumor or GIST. In the misdiagnosed cases, FNA specimens were not subjected to IHC analysis[19,23]. All the cases that performed IHC analysis were able to achieve correct preoperative diagnosis. It is important to perform IHC analysis to ensure an accurate preoperative diagnosis. No case was able to attain a preoperative diagnosis using only conventional images (CT, EUS). The IHC analysis of our case revealed positivity for both muscle actin and synaptophysin. Synaptophysin positivity is occasionally found in specimens from glomus tumors of the stomach, whereas other neuroendocrine markers, including chromogranin A, are generally negative[24]. Therefore, we consider these IHC results to be consistent with a glomus tumor.
| Vinette-Leduc et al[19] | Gu et al[16] | Debol et al[15] | Jones et al[20] | Minoda et al[21] | Mohanty et al[22] | Matevossian et al[23] | Akahoshi et al[18] | Our case | |
| Age and sex | 72, female | 32 , female | 62, female | 47, female | 50, female | 51, male | 44 | N.D. | 52, male |
| Tumor location size (cm) | Antrum | Body (LC) | Antrum | ND | Angle | Antrum | Antrum | Body | Antrum |
| 2 | 2.3 × 1.6 | 2.8 × 2.5 × 1.7 | 2.5 × 1.5 | 1.5 | 2.4 × 2.0 | 5 | 1.2 | 3 | |
| Enhanced CT | ND | ND | ND | ND | ND | ND | ND | N.D. | Enhanced in peripheral lesion, not homogeneously. |
| EUS | ND | Irregular shaped heterogeneous tumor arising from muscularis propria | Hypoechoic mass arise from the muscularis propria | Heterogeneous rounded lesion arising from muscularis propria | Homogeneous, hypoechoic tumor with continuity to the muscle layer | Hypoechoic submucosal lesion | Poorly reflective, non-homogeneous submucosal, solid tumor. | N.D. | Hypoechoic mass primarily connected to muscular layer |
| Diagnosis by images | ND | GIST | ND | GIST | GIMT including GIST | ND | GIST | N.D. | GIMT including GIST |
| FNA-procedure | Percutaneous FNA | EUS-FNA | EUS-FNA | EUS-FNA | EUS-FNA (25-gauge needle) | EUS-FNA | EUS-FNA (19-gauge needle) | EUS-FNA (22 or 25-gauge) | EUS-FNA (22-gauge needle) |
| FNA cytology (HE) | Well-demarcated nests of small, round to polygonal cells. | Small, uniform, round, epithelioid cells with round nuclei and scanty, amphophilic cytoplasm | Well differentiated small blue cell neoplasm like carcinoid tumor. | Epitheloid tumor cells. | Proliferation of oval shaped cells with eosinophilic cytoplasm arrange in nests. | Uniform round cells with ill-defined cytoplasmic borders and scanty amphophillic cytoplasm. | Hemorrhagic biopsy sample without representative cells. | N.D. | proliferation of oval-shaped cell with small nest formation . |
| FNA cytology (IHC staining) | Not performed. | c-kit, CD34, desmin, chromogranin, synaptophisin,desmin (-), SMA, vimentin (+) | CD34, synaptophisin,chromogranin, s-100,Desmin, CD117 (-), SMA, vimentin (+) | CD34, 56, c-kit, chromogranin (-) synaptophisin (±), SMA (+) | CD34, 56, c-kit, desmin, S-100, chromogranin, synaptophisin (-), SMA, vimentin (+) | CD34, 117, c-kit, desmin, chromogranin, Synaptophisin , Pancytokeratin (-), SMA, vimentin (+) | Not performed | N.D. | CD34, 56, c-kit, desmin,S-100, chromogranin(-), synaptophisin (±), SMA (+) |
| Preoperative diagnosis | Neuroendocrine tumor | Glomus tumor | Glomus tumor | Glomus tumor | Glomus tumor | Glomus tumor | GIST | Glomus tumor | Glomus tumor |
| Pathology in resected specimen (HE) | Highly vascular, tumor nests were separated by fascicles of smooth muscle. Uniform, small and round tumor cells. | Non encapsulated, with convoluted boundaries, confined to muscularis propria. Round and uniform tumor cells | Circumscribed, highly vascular, and contained nests of monomorphic, polygonal cells. | Confirmed the preoperative diagnosis of a glomus tumor. | Same as FNA pathology. | The pathological diagnosis was confirmed on resection | ND | N.D. | Oval shaped cells with high N/C ratio proliferated on proper muscle layer performing solid small nests. |
| IHC staining in resected specimen | Desmin, chromogranin (-), SMA, Vimentin (+) | Similar to those performed FNA | CD34, CD117, chromogranin (-), SMA, Vimentin (+) | ND | Same as FNA staining. | The pathological diagnosis was confirmed on resection | CD117 (-), Vimentin/actin (+) | N.D. | Same as FNA staining |
It remains controversial whether a 22G or 25G needle can adequately obtain a specimen from SMT lesions. Although we selected a 22G needle in this case, 25G needles were used to obtain sufficient specimens in other reported cases. Further analysis regarding needle gauge selection is expected to resolve this issue.
EUS-FNA is an essential and useful tool for the preoperative diagnosis of glomus tumor of the stomach. Preoperative diagnosis by EUS-FNA allows for early and minimal resection.
A 52-year-old man was referred for further investigation of a gastric submucosal tumor on the greater curvature of the antrum without particular symptoms.
There was no significant finding on physical examination which led to the clinical diagnosis.
Gastrointestinal mesenchymal tumor, such as gastrointestinal stromal tumors, leiomyoma.
This patient had no remarkable findings for the laboratory tests including tumor markers.
Plain computed tomography showed a round shaped mass lesion on the gastric antrum. Esophagogastroduodenoscopy revealed a 30 mm submucosal tumor without cushion sign on the antrum. Endoscopic ultrasonography (EUS) showed a hypoechoic lesion with a small anechoic component, which was primarily connected to the muscular layer of the stomach.
The specimen obtained by endoscopic ultrasound-guided fine-needle aspiration biopsy (EUS-FNA) revealed the proliferation of oval-shaped cells with a small nest formation and high nucleo-cytoplasmic ratio. Immunohistochemical staining revealed that the tumor cells were strongly and focally positive for muscle actin, and negative for c-kit, CD34, CD56, desmin, S-100, chromogranin, and neuron-specific enolase. These results were compatible with glomus tumor.
The patients underwent laparoscopy and endoscopy cooperative surgery based on the preoperative diagnosis of glomus tumor of the stomach.
In the only eight reported cases of gastric glomus tumor, FNA was performed preoperatively for pathological diagnosis. Six cases of them were diagnosed correctly by immunohistochemical staining. In the two misdiagnosed cases, FNA specimens were not subjected to immunohistochemical analysis.
Glomus tumor is now considered mesenchymal tumors with malignant potential. Glomus tumors of the stomach are extremely rare, accounting for 1% of the occurrence of GIMTs.
Preoperative pathological diagnosis of glomus tumor of the stomach is difficult with conventional endoscopic biopsy. Therefore, EUS-FNA is an essential tool to gain histological evidence of glomus tumor of the stomach. It allows for the collection of sufficient specimen not only for HE staining, but also for Immunohistochemical analysis which is necessary for correct diagnosis of glomus tumor.
The authors have described a case of glomus tumor of the stomach which was correctly preoperatively diagnosed by EUS-FNA biopsy. The authors reviewed eight former reports of gastric glomus tumor and suggested the utility of EUS-FNA for diagnosis. The article provided a quite useful method for early preoperative diagnosis of glomus tumor of the stomach.
P- Reviewer: Jang IS, Kondo N, Li LW, Surlin VM S- Editor: Qi Y L- Editor: A E- Editor: Ma S
| 1. | Enzinger F, Weiss S. Soft Tissue Tumors. 3rd ed. St Louis: Mosby 1995; . |
| 2. | Aiba M, Hirayama A, Kuramochi S. Glomangiosarcoma in a glomus tumor. An immunohistochemical and ultrastructural study. Cancer. 1988;61:1467-1471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 3. | Brathwaite CD, Poppiti RJ. Malignant glomus tumor. A case report of widespread metastases in a patient with multiple glomus body hamartomas. Am J Surg Pathol. 1996;20:233-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 68] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 4. | Gould EW, Manivel JC, Albores-Saavedra J, Monforte H. Locally infiltrative glomus tumors and glomangiosarcomas. A clinical, ultrastructural, and immunohistochemical study. Cancer. 1990;65:310-318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 5. | Hiruta N, Kameda N, Tokudome T, Tsuchiya K, Nonaka H, Hatori T, Akima M, Miura M. Malignant glomus tumor: a case report and review of the literature. Am J Surg Pathol. 1997;21:1096-1103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 50] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 6. | DeBusscher G. Etude morphologique et consideration physiologique sur la vascularization de l’estomac. Acta Gastroenterol Belg. 1948;11:333-351. |
| 7. | Miettinen M, Paal E, Lasota J, Sobin LH. Gastrointestinal glomus tumors: a clinicopathologic, immunohistochemical, and molecular genetic study of 32 cases. Am J Surg Pathol. 2002;26:301-311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 8. | Folpe AL, Fanburg-Smith JC, Miettinen M, Weiss SW. Atypical and malignant glomus tumors: analysis of 52 cases, with a proposal for the reclassification of glomus tumors. Am J Surg Pathol. 2001;25:1-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 9. | Cha SH, Cho SB, Kim YW, Park CM. Helical CT appearance of glomus tumor of the stomach. Eur Radiol. 2000;10:671-673. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 10. | Kim JK, Won JH, Cho YK, Kim MW, Joo HJ, Suh JH. Glomus tumor of the stomach: CT findings. Abdom Imaging. 2011;26:303-305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 26] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 11. | Tang M, Hou J, Wu D, Han XY, Zeng MS, Yao XZ. Glomus tumor in the stomach: computed tomography and endoscopic ultrasound findings. World J Gastroenterol. 2013;19:1327-1329. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 17] [Cited by in RCA: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 12. | Patel TH, Horton KM, Hruban RH, Fishman EK. Glomus Tumor of the Stomach: Depiction by Multidetector CT and Three-Dimensional Volume Rendering Imaging. Case Rep Med. 2010;2010:126095. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 13. | Imamura A, Tochihara M, Natsui K, Murashima Y, Suga T, Yaosaka T, Fujinaga A, Koito K, Miyakawa H, Higashino K. Glomus tumor of the stomach: endoscopic ultrasonographic findings. Am J Gastroenterol. 1994;89:271-272. [PubMed] |
| 14. | Akahoshi K, Sumida Y, Matsui N, Oya M, Akinaga R, Kubokawa M, Motomura Y, Honda K, Watanabe M, Nagaie T. Preoperative diagnosis of gastrointestinal stromal tumor by endoscopic ultrasound-guided fine needle aspiration. World J Gastroenterol. 2007;13:2077-2082. [PubMed] |
| 15. | Debol SM, Stanley MW, Mallery S, Sawinski E, Bardales RH. Glomus tumor of the stomach: cytologic diagnosis by endoscopic ultrasound-guided fine-needle aspiration. Diagn Cytopathol. 2003;28:316-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 46] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 16. | Gu M, Nguyen PT, Cao S, Lin F. Diagnosis of gastric glomus tumor by endoscopic ultrasound-guided fine needle aspiration biopsy. A case report with cytologic, histologic and immunohistochemical studies. Acta Cytol. 2002;46:560-566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 17. | Mekky MA, Yamao K, Sawaki A, Mizuno N, Hara K, Nafeh MA, Osman AM, Koshikawa T, Yatabe Y, Bhatia V. Diagnostic utility of EUS-guided FNA in patients with gastric submucosal tumors. Gastrointest Endosc. 2010;71:913-919. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 177] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 18. | Akahoshi K, Oya M, Koga T, Koga H, Motomura Y, Kubokawa M, Gibo J, Nakamura K. Clinical usefulness of endoscopic ultrasound-guided fine needle aspiration for gastric subepithelial lesions smaller than 2 cm. J Gastrointestin Liver Dis. 2014;23:405-412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 81] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 19. | Vinette-Leduc D, Yazdi HM. Fine-needle aspiration biopsy of a glomus tumor of the stomach. Diagn Cytopathol. 2001;24:340-342. [PubMed] |
| 20. | Jones J, Cichowitz A, Crosthwaite GL. Endoscopic ultrasound-guided fine needle aspiration as a diagnostic tool for gastric glomus tumours. ANZ J Surg. 2012;82:94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 21. | Minoda Y, Akahoshi K, Oya M, Kubokawa M, Motomura Y, Nakamura K. Gastric glomus tumor diagnosed by endoscopic ultrasound-guided fine-needle aspiration biopsy: report of a case. Fukuoka Igaku Zasshi. 2014;105:105-109. [PubMed] |
| 22. | Mohanty SK, Pradhan D, Stavropoulos S, Donovan V, Gupta M. Diagnosis of gastric glomus tumour by endoscopic ultrasound-guided fine needle aspiration cytology: a case report. Cytopathology. 2014;25:205-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 23. | Matevossian E, Brücher BL, Nährig J, Feußner H, Hüser N. Glomus tumor of the stomach simulating a gastrointestinal stromal tumor: a case report and review of literature. Case Rep Gastroenterol. 2008;2:1-5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 14] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 24. | Wang ZB, Yuan J, Shi HY. Features of gastric glomus tumor: a clinicopathologic, immunohistochemical and molecular retrospective study. Int J Clin Exp Pathol. 2014;7:1438-1448. [PubMed] |