Published online Jun 14, 2015. doi: 10.3748/wjg.v21.i22.7047
Peer-review started: August 20, 2014
First decision: November 14, 2014
Revised: December 29, 2014
Accepted: February 5, 2015
Article in press: February 5, 2015
Published online: June 14, 2015
Processing time: 303 Days and 20.6 Hours
Serous cystadenoma (SC) is a benign pancreatic cystic tumor. Surgical resection is recommended for symptomatic forms, but laparoscopic fenestration of large symptomatic macrocystic SC was not yet described in the literature. In this study, 3 female patients underwent laparoscopic fenestration for macrocystic SC (12-14 cm). Diagnosis was established via magnetic resonance imaging and endoscopic ultrasound, with intra-cystic dosage of tumors markers (ACE and CA19-9) in 2 patients. All patients were symptomatic and operated on 15-60 mo after diagnosis. Radiological evaluation showed constant cyst growth. Patients were informed about this new surgical modality that can avoid pancreatic resection. The mean operative time was 103 min (70-150 min) with one conversion. The post-operative course was marked by a grade A pancreatic fistula in one patient and was uneventful in the other two. The hospital stay was 3, 10, and 18 d, respectively. The diagnosis of macrocystic SC was histologically-confirmed in all cases. At the last follow-up (13-26 mo), all patients were symptom-free, and radiological evaluation showed complete disappearance of the cyst. Laparoscopic fenestration, as opposed to resection, should be considered for large symptomatic macrocystic SC, thereby avoiding pancreatic resection morbidity and mortality.
Core tip: Although surgical resection is the classical modality for treating symptomatic serous cystadenoma (SC), laparoscopic fenestration for large macrocystic SC was not yet described in the literature. In this study, 3 female patients underwent laparoscopic fenestration of symptomatic macrocystic SC. Conversion was needed in one patient for bile duct injury, while another patient developed a grade A pancreatic fistula. Histology confirmed the diagnosis in all patients and, after a follow-up of 13-26 mo, all patients are asymptomatic. Radiological evaluation showed complete disappearance of SC. This mini-invasive approach avoids the high mortality and morbidity encountered with pancreatic resection.
- Citation: Dokmak S, Aussilhou B, Rasoaherinomenjanahary F, Sauvanet A, Vullierme MP, Rebours V, Lévy P. Laparoscopic fenestration of pancreatic serous cystadenoma: Minimally invasive approach for symptomatic benign disease. World J Gastroenterol 2015; 21(22): 7047-7051
- URL: https://www.wjgnet.com/1007-9327/full/v21/i22/7047.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i22.7047
With the widespread use of cross-sectional imaging studies, pancreatic cysts are more frequently discovered, and a 2.6%-20% prevalence has been reported in patients undergoing computed tomography (CT) scans or magnetic resonance imaging (MRI) for non-related pancreatic disorders[1]. These lesions are represented mainly by intraductal papillary mucinous neoplasia (IPMN), mucinous cystadenoma (MC), and serous cystadenoma (SC)[2]. SC is mainly observed in females with a mean age of 60 years. Overall tumor size ranges from less than 1 cm to more than 10 cm. The diameter of cystic components inside the tumor may also vary from a few micrometers to several centimeters[3,4], and can be unilocular or pseudo-solid[4,5]. The diagnosis of SC can be accurately established on a CT scan[6], MRI[7], and coupled, if needed, with endoscopic ultrasound plus fine needle aspiration and intra-cystic dosage of tumors markers[8-11]. SC is a benign pancreatic lesion with a slow rate of size increase, a very low risk of complications, and malignant evolution. When the diagnosis is certain, no treatment is required in asymptomatic patients[12]. Although the main indications of surgical resection are symptomatic SC, there are doubtful cases that cannot be differentiated from malignant or low potential malignant diseases[13-15]. Many cases are operated because they are falsely considered to be symptomatic (i.e., non-specific abdominal pain and irritable bowel syndrome) or are doubtful cases (neuro-endocrine tumor, MC, and IPMN) after an incomplete diagnostic evaluation. With the increased prevalence of this disease and unclear data about management, recent studies have focused mainly on the long-term risk of growth in order to select the subgroup of patients who might benefit from surgical resection[16]. Symptomatic or misdiagnosed lesions are usually treated by open or laparoscopic pancreatic resection, with the inherent risk of morbidity, mortality, and long-term pancreatic insufficiency related to these procedures[17]. The aim of this short series is to show that some symptomatic SC can also be treated by a much more conservative surgical approach consisting only of cyst fenestration, without any pancreatic resection. We describe 3 cases of large symptomatic SC of the pancreatic head treated by laparoscopic fenestration.
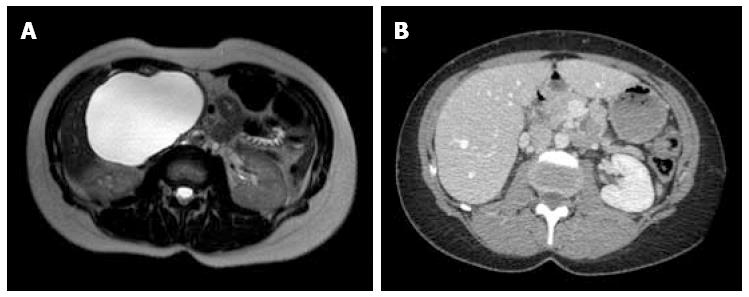
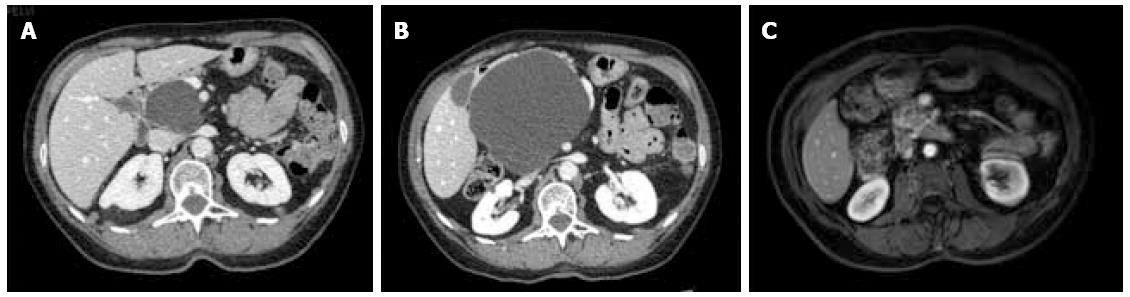
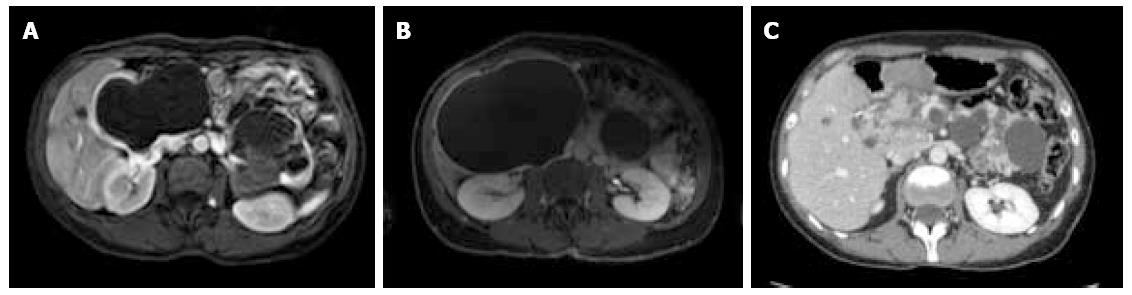
Between September 2012 and June 2013, three female patients (33-66 years old) were operated for large symptomatic SC in the pancreatic head. SC had been diagnosed 15, 40, and 60 mo before surgery. The main symptoms were pain and fullness in the right subcostal area (n = 3), palpable mass (n = 3), signs of gastric outlet obstruction (n = 1), and moderate cholestasis without jaundice (n = 1). All patients underwent MRI, with the aspect of the cyst being typical in 2. Endoscopic ultrasound with intra-cystic ACE level measurement was performed in 2 patients, and showed 2 and 0.2 μg/L, respectively. One patient had multiple SC, and the diagnosis of Von Hippel Lindau disease was ruled out by genetic screening. Cyst growth was observed in all patients (Figure 1, Figure 2 and Figure 3), and the size increased from 4, 9, and 10 cm at diagnosis to 12, 13, and 14 cm just before surgery, respectively.
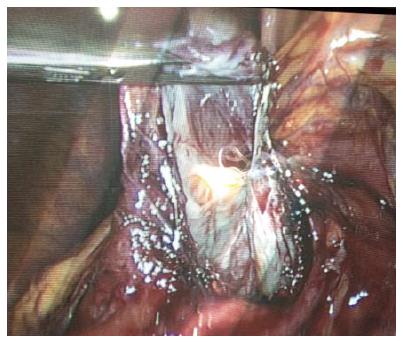
The patient was installed in the supine position under general anesthesia, with legs spread apart and the monitor to the patient’s left. Open coelioscopy was performed through the umbilicus; a total of 4 trocars were necessary for this procedure. The trocar placement was done in order to avoid crossing the hands of the surgeon and assistant. The 30-degree optic trocar was installed in the right hypochondrium, the 10 mm operator trocar in the umbilicus, and another two 5 mm trocars in the left hypochondrium and right subcostal area for apprehension. Harmonic shears (Harmonic; Ethicon, Issy les Moulineaux, France) and a bipolar cautery coagulation device were needed. Once exploration was complete, the right gastrocolic ligament was largely divided in order to expose the anterior surface of the pancreatic gland and the area of the cyst to be opened. The cyst was freed from some collateral circulation that can be encountered related to venous compression. The cyst was opened, the content was aspirated, liquid sampling for tumors markers dosages was taken, and as large as possible (5-10 cm) fenestration was performed (Figure 4). The specimen was removed in a surgical bag by the trocar incision for pathological examination. A small suction drain was left in the cyst cavity.
The mean operative time was 103 min (70-150 min). Conversion was needed in one patient for bile duct injury, which was treated by end-to-end biliary anastomosis with a biliary drain. SC was fenestrated anteriorly behind the anterior aspect of the pancreatic head (n = 2) and laterally posterior to the duodenum and hepatic pedicle in one patient. One patient needed pancreatectomy in order to have access to the cyst. In one patient, the great omentum was inserted in the cystic cavity.
The post-operative course was marked by a grade A pancreatic fistula in the one patient who had undergone pancreatectomy, while the other two had an uneventful post-operative course. Hospital stay was 3, 10, and 18 d, respectively. The definitive diagnosis of macrocystic SC was confirmed on histology in all patients, who showed a glycogen-rich epithelium, sometimes with abrasion, without any mucinous secretion or ovarian stroma.
At the last follow-up (13, 21, and 26 mo), all 3 patients were symptom-free, and radiological evaluation showed complete disappearance of the cyst (Figures 1, 2 and 3).
To the best of our knowledge, laparoscopic fenestration of pancreatic SC was not yet described in the literature. Only one case using an open approach of fenestration indicated for jaundice was published, and had an excellent long-term result[18].
This small series suggests that laparoscopic fenestration of large symptomatic SC is safe and effective. However, a larger series with longer post-operative follow-up is warranted to ascertain the fate of SC after such treatment.
Some cases of malignant transformation (< 1%) to lymph node invasion or liver metastases[19,20] had been described. However, this risk appears extremely low, if it exists at all, and has to be compared to the mortality risk of surgical pancreatic resection. Therefore, surgical treatment should be indicated only for symptoms clearly related to SC, and for cases remaining doubtful after a complete workup. Small SC without any local compression are less likely to be responsible for any symptoms. On the other hand, large SC might be symptomatic, and compression on the nearby structures might be observed either clinically (pain, abdominal mass, jaundice, or gastric outlet obstruction), radiologically (dilated main pancreatic or bile ducts) or biochemically (cholestasis). The impact of the observed symptoms on patient quality of life should be balanced against the risk of pancreatic surgical resection and its sequelae.
Some authors have described very large SC with local invasion of vessels, bile duct, stomach, and duodenum. In a recent study on 257 resected SC, it was shown that local invasion was mainly observed in large lesions (> 10 cm) located in the pancreatic head[3]. Two of our patients had gastric outlet obstruction and bile duct dilatation with mild cholestasis, respectively. Slow growth rate is observed in some SC. In a recent study on 145 patients who underwent MRI, it was shown that the growth rate of SC was 0.1 cm/year in the first 7 years, and 0.6 cm/year between 7 and 10 years from the baseline evaluation. This growth was mainly observed in oligocystic lesions, in patients with other malignant history, and in those of an advanced age[4]. Of course, the progression in size should not be an indication for surgery per se. Regular radiological follow-up should be the main option in asymptomatic patients, regardless of the overall size of the SC.
Pancreatic resection is considered the only surgical modality for symptomatic lesions. However, pancreatic resection, even for benign disease, is still associated with very high morbidity and long-term endocrine and exocrine insufficiency risks[21]. This mortality, even reduced to its minimum rate, should be considered very poor for such a benign lesion. Even if the risk of pancreatic insufficiency is much lower after atypical pancreatic resections (enucleation or central pancreatectomy), the risk of surgical morbidity or morality remains high. To avoid this morbidity, indications for surgical resection should be refined, and other treatment modalities should be discussed. In our opinion, the subgroup of patients with large symptomatic SC might safely benefit from cyst fenestration. A large cyst can modify the anatomy of the pancreatic head, and the area to be fenestrated should be selected on the pre-operative CT scan in order to avoid injury of surrounded structures. All our patients were operated by laparoscopic approach, but even the open approach can be considered for anatomical reasons. We should mention that fenestration with incomplete resection for SC is justified by the fact that the risk of malignant transformation is extremely low, and was probably not encountered with these SC due to their being purely cystic without any solid component. Histology of the cyst wall should be obtained to rule out malignancy.
Theoretically, there is a risk of recurrence, since a great part of the cyst and its epithelium are not resected or destroyed. This risk needs to be evaluated by a longer follow-up of our patients. In another paper, we described a case of calcic involution even after endoscopic ultrasound and complete aspiration of SC[22].
In conclusion, laparoscopic fenestration of large pancreatic macrocystic SC appears to be safe and very effective. It can be added to the less aggressive surgical tools for symptomatic SC.
Three female patients presented with epigastric and right hypochondrium pain.
An abdominal mass was noted on the clinical exam.
The differential diagnosis was mainly serous cystadenoma, mucinous cystadenoma, and pseudocyst.
Intracystic ACE level was low, which was suggestive of a diagnosis of serous cystadenoma.
Magnetic resonance imaging showed large macrocystic lesions in the pancreatic head compatible with the diagnosis of serous cystadenoma. Multiple cysts were present in one patient.
After partial resection, the diagnosis of macrocystic serous cystadenoma was confirmed in all patients.
Laparoscopic fenestration was performed in all 3 patients.
This treatment was not yet described in the literature, and can be a method of avoiding classical treatment by pancreatic resection.
Fenestration is incomplete resection, with only a part of the cyst being removed.
Laparoscopic fenestration, as opposed to resection, should be offered to any patient with symptomatic large macrocystic serous cystadenoma.
This treatment can be proposed because the risk of malignancy is exceptional. Histology should be obtained during fenestration.
P- Reviewer: Zhou YM S- Editor: Yu J L- Editor: Rutherford A E- Editor: Wang CH
| 1. | Laffan TA, Horton KM, Klein AP, Berlanstein B, Siegelman SS, Kawamoto S, Johnson PT, Fishman EK, Hruban RH. Prevalence of unsuspected pancreatic cysts on MDCT. AJR Am J Roentgenol. 2008;191:802-807. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 724] [Cited by in RCA: 658] [Article Influence: 38.7] [Reference Citation Analysis (0)] |
| 2. | Kim SY, Lee JM, Kim SH, Shin KS, Kim YJ, An SK, Han CJ, Han JK, Choi BI. Macrocystic neoplasms of the pancreas: CT differentiation of serous oligocystic adenoma from mucinous cystadenoma and intraductal papillary mucinous tumor. AJR Am J Roentgenol. 2006;187:1192-1198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 102] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 3. | Khashab MA, Shin EJ, Amateau S, Canto MI, Hruban RH, Fishman EK, Cameron JL, Edil BH, Wolfgang CL, Schulick RD. Tumor size and location correlate with behavior of pancreatic serous cystic neoplasms. Am J Gastroenterol. 2011;106:1521-1526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 78] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 4. | Malleo G, Bassi C, Rossini R, Manfredi R, Butturini G, Massignani M, Paini M, Pederzoli P, Salvia R. Growth pattern of serous cystic neoplasms of the pancreas: observational study with long-term magnetic resonance surveillance and recommendations for treatment. Gut. 2012;61:746-751. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 66] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 5. | Chatelain D, Hammel P, O’Toole D, Terris B, Vilgrain V, Palazzo L, Belghiti J, Lévy P, Ruszniewski P, Fléjou JF. Macrocystic form of serous pancreatic cystadenoma. Am J Gastroenterol. 2002;97:2566-2571. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 52] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 6. | Lee JH, Kim JK, Kim TH, Park MS, Yu JS, Choi JY, Kim JH, Kim YB, Kim KW. MRI features of serous oligocystic adenoma of the pancreas: differentiation from mucinous cystic neoplasm of the pancreas. Br J Radiol. 2012;85:571-576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 20] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 7. | Lee SE, Kwon Y, Jang JY, Kim YH, Hwang DW, Kim MA, Kim SH, Kim SW. The morphological classification of a serous cystic tumor (SCT) of the pancreas and evaluation of the preoperative diagnostic accuracy of computed tomography. Ann Surg Oncol. 2008;15:2089-2095. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 28] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 8. | Hammel P, Levy P, Voitot H, Levy M, Vilgrain V, Zins M, Flejou JF, Molas G, Ruszniewski P, Bernades P. Preoperative cyst fluid analysis is useful for the differential diagnosis of cystic lesions of the pancreas. Gastroenterology. 1995;108:1230-1235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 166] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 9. | O’Toole D, Palazzo L, Hammel P, Ben Yaghlene L, Couvelard A, Felce-Dachez M, Fabre M, Dancour A, Aubert A, Sauvanet A. Macrocystic pancreatic cystadenoma: The role of EUS and cyst fluid analysis in distinguishing mucinous and serous lesions. Gastrointest Endosc. 2004;59:823-829. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 99] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 10. | Linder JD, Geenen JE, Catalano MF. Cyst fluid analysis obtained by EUS-guided FNA in the evaluation of discrete cystic neoplasms of the pancreas: a prospective single-center experience. Gastrointest Endosc. 2006;64:697-702. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 128] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 11. | Allen PJ, Qin LX, Tang L, Klimstra D, Brennan MF, Lokshin A. Pancreatic cyst fluid protein expression profiling for discriminating between serous cystadenoma and intraductal papillary mucinous neoplasm. Ann Surg. 2009;250:754-760. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 43] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 12. | Tseng JF, Warshaw AL, Sahani DV, Lauwers GY, Rattner DW, Fernandez-del Castillo C. Serous cystadenoma of the pancreas: tumor growth rates and recommendations for treatment. Ann Surg. 2005;242:413-419; discussion 419-421. [PubMed] |
| 13. | Galanis C, Zamani A, Cameron JL, Campbell KA, Lillemoe KD, Caparrelli D, Chang D, Hruban RH, Yeo CJ. Resected serous cystic neoplasms of the pancreas: a review of 158 patients with recommendations for treatment. J Gastrointest Surg. 2007;11:820-826. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 128] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 14. | Kimura W, Moriya T, Hirai I, Hanada K, Abe H, Yanagisawa A, Fukushima N, Ohike N, Shimizu M, Hatori T. Multicenter study of serous cystic neoplasm of the Japan pancreas society. Pancreas. 2012;41:380-387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 94] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 15. | Valsangkar NP, Morales-Oyarvide V, Thayer SP, Ferrone CR, Wargo JA, Warshaw AL, Fernández-del Castillo C. 851 resected cystic tumors of the pancreas: a 33-year experience at the Massachusetts General Hospital. Surgery. 2012;152:S4-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 257] [Cited by in RCA: 278] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 16. | El-Hayek KM, Brown N, O’Rourke C, Falk G, Morris-Stiff G, Walsh RM. Rate of growth of pancreatic serous cystadenoma as an indication for resection. Surgery. 2013;154:794-800; discussion 800-802. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 36] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 17. | Hwang HK, Kim H, Kang CM, Lee WJ. Serous cyst adenoma of the pancreas: appraisal of active surgical strategy before it causes problems. Surg Endosc. 2012;26:1560-1565. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 18. | Watanabe H, Ohtsubo K, Yamaguchi Y, Mouri H, Motoo Y, Noto M, Kitagawa H, Kayahara M, Ohta T, Gabata T. Successful cystic fenestration for a macrocystic serous cystadenoma of the pancreas causing obstructive jaundice: report of a case. Surg Today. 2006;36:89-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 19. | King JC, Ng TT, White SC, Cortina G, Reber HA, Hines OJ. Pancreatic serous cystadenocarcinoma: a case report and review of the literature. J Gastrointest Surg. 2009;13:1864-1868. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 54] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 20. | Strobel O, Z’graggen K, Schmitz-Winnenthal FH, Friess H, Kappeler A, Zimmermann A, Uhl W, Büchler MW. Risk of malignancy in serous cystic neoplasms of the pancreas. Digestion. 2003;68:24-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 105] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 21. | Falconi M, Mantovani W, Crippa S, Mascetta G, Salvia R, Pederzoli P. Pancreatic insufficiency after different resections for benign tumours. Br J Surg. 2008;95:85-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 178] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 22. | Charpignon C, Corcos O, Vullierme MP, Hammel P, Ruszniewski P, Lévy P. [Calcic involution of a serous cystadenoma]. Gastroenterol Clin Biol. 2006;30:923-924. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |