Published online Jun 14, 2015. doi: 10.3748/wjg.v21.i22.6952
Peer-review started: January 4, 2015
First decision: February 10, 2015
Revised: March 1, 2015
Accepted: April 9, 2015
Article in press: April 9, 2015
Published online: June 14, 2015
Processing time: 168 Days and 20.9 Hours
AIM: To assess the prevalence and stability of different antiphospholipid antibodies (APLAs) and their association with disease phenotype and progression in inflammatory bowel diseases (IBD) patients.
METHODS: About 458 consecutive patients [Crohn’s disease (CD): 271 and ulcerative colitis (UC): 187] were enrolled into a follow-up cohort study in a tertiary IBD referral center in Hungary. Detailed clinical phenotypes were determined at enrollment by reviewing the patients’ medical charts. Disease activity, medical treatment and data about evolvement of complications or surgical interventions were determined prospectively during the follow-up. Disease course (development f complicated disease phenotype and need for surgery), occurrence of thrombotic events, actual state of disease activity according to clinical, laboratory and endoscopic scores and accurate treatment regime were recorded during the follow-up, (median, 57.4 and 61.6 mo for CD and UC). Sera of IBD patients and 103 healthy controls (HC) were tested on individual anti-β2-Glycoprotein-I (anti-β2-GPI IgA/M/G), anti-cardiolipin (ACA IgA/M/G) and anti-phosphatidylserine/prothrombin (anti-PS/PT IgA/M/G) antibodies and also anti-Saccharomyces cerevisiae antibodies (ASCA IgA/G) by enzyme-linked immunosorbent assay (ELISA). In a subgroup of CD (n = 198) and UC patients (n = 103), obtaining consecutive samples over various arbitrary time-points during the disease course, we evaluated the intraindividual stability of the APLA status. Additionally, we provide an overview of studies, performed so far, in which significance of APLAs in IBD were assessed.
RESULTS: Patients with CD had significantly higher prevalence of both ACA (23.4%) and anti-PS/PT (20.4%) antibodies than UC (4.8%, P < 0.0001 and 10.2%, P = 0.004) and HC (2.9%, P < 0.0001 and 15.5%, P = NS). No difference was found for the prevalence of anti-β2-GPI between different groups (7.2%-9.7%). In CD, no association was found between APLA and ASCA status of the patients. Occurrence of anti-β2-GPI, ACA and anti-PS/PT was not different between the group of patients with active vs inactive disease state according to appropriate clinical, laboratory and endoscopic scores in CD as well as in UC patients. All subtypes of anti-β2-GPI and ACA IgM status were found to be very stable over time, in contrast ACA IgG and even more ACA IgA status showed significant intraindividual changes. Changes in antibody status were more remarkable in CD than UC (ACA IgA: 49.9% vs 23.3% and ACA IgG: 21.2% vs 5.8%). Interestingly, 59.1% and 30.1% of CD patients who received anti-TNF therapy showed significant negative to positive changes in ACA IgA and IgG antibody status respectively. APLA status was not associated with the clinical phenotype at diagnosis or during follow-up, medical therapy, or thrombotic events and it was not associated with the probability of developing complicated disease phenotype or surgery in a Kaplan-Meier analysis.
CONCLUSION: The present study demonstrated enhanced formation of APLAs in CD patients. However, presence of different APLAs were not associated with the clinical phenotype or disease course.
Core tip: Enhanced serological antibody formation is a well-known feature of inflammatory bowel diseases. Antiphospholipid antibodies (APLAs) are a prothrombotic group of antibodies acquired in various inflammatory diseases. However their association with clinical phenotype and disease progression is still unclear in inflammatory bowel diseases (IBD). In the present study we report enhanced formation of APLAs in patients with Crohn’s disease, which was not associated with clinical phenotype or disease course during follow-up in a tertiary referral IBD center from Hungary.
- Citation: Sipeki N, Davida L, Palyu E, Altorjay I, Harsfalvi J, Antal Szalmas P, Szabo Z, Veres G, Shums Z, Norman GL, Lakatos PL, Papp M. Prevalence, significance and predictive value of antiphospholipid antibodies in Crohn’s disease. World J Gastroenterol 2015; 21(22): 6952-6964
- URL: https://www.wjgnet.com/1007-9327/full/v21/i22/6952.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i22.6952
Enhanced serological antibody formation is a well-known feature of inflammatory bowel diseases (IBD). A wide range of anti-microbial and autoantibodies have been reported to be associated with either Crohn’s disease (CD) or ulcerative colitis (UC)[1] as well as with complicated disease course. Anti-microbial antibodies are formed against different surface carbohydrate (anti-glycans[2]) or protein antigens of various gut microbes[3]. The first and still most relevant anti-microbial antibody is the ASCA (anti-Saccharomyces cerevisiae antibody). Autoantibodies are directed against various host proteins. Based on recent findings, their existence might also be related to enhanced microbial challenge to the gut[4,5] due to a disturbed gut innate immune system and may trigger an exaggerated adaptive immune response. Furthermore, these serological antibodies may also be actively involved in the pathophysiology of inflammation in IBD[6,7].
Antiphospholipid antibodies (APLAs) are a prothrombotic group of autoantibodies and established as the serological hallmark of antiphospholipid syndrome (APS)[8]. These antibodies comprise anti-cardiolipin (ACA), anti-β2-Glycoprotein-I (anti-β2-GPI), and anti- phosphatidylserine/prothrombin antibodies (anti-PS/PT). APLAs, however, are also found in a variety of disorders (chronic inflammatory diseases[9-12] or post infectious conditions[13-16]) not necessarily exhibiting prothrombotic activity. Even if non-prothrombotic, they may have certain pathogenetic roles in several diseases as well[17,18].
In IBD, available cross-sectional, mainly single-time point studies assessing different aspects of APLAs, came to discrepant conclusions regarding formation, prevalence, and stability of these antibodies. Their clinical significance, including association to thrombotic events in IBD is also unclear[19-33] (Table 1). Thus a comprehensive evaluation of the primary APLAs in a large prospectively followed-up IBD cohort is required.
| Ref. | Study design | Group | n | ACA prevalence (%) | Anti-β2-GPI prevalence (%) | ACA | Anti-β2-GPI | ||||||||||||||||
| Total | IgG | IgM | IgA | Total | IgG | IgM | IgA | Disease activity | Association with disease phenotype | TE event | IS therapy | Disease activity | Association with disease phenotype | TE event | IS therapy | ||||||||
| Location | Complicated disease behaviour | Need for surgery | Location | Complicated disease behaviour | Need for surgery | ||||||||||||||||||
| [19] | R | HC | 136 | 4.0 | |||||||||||||||||||
| CD | 73 | 20.0 | No | No | No | No | No | ||||||||||||||||
| UC | 63 | 10.0 | No | No | No | No | No | ||||||||||||||||
| [20] | P | AS | 19 | 5.3 | 5.3 | 0 | 0 | 0 | |||||||||||||||
| CD | 63 | 8.0 | 3.0 | 0 | 0 | 0 | No | No | |||||||||||||||
| [21] | CS | CD | 22 | 36.0 | No | No | |||||||||||||||||
| UC | 30 | 20.0 | No | No | |||||||||||||||||||
| [22] | CS | CD | 50 | 22.0 | 10.0 | 6.0 | 6 | No | No | No | No | ||||||||||||
| [23] | R | HC | 40 | 5.0 | |||||||||||||||||||
| CD | 41 | 26.8 | No | No | |||||||||||||||||||
| UC | 19 | 26.3 | No | No | |||||||||||||||||||
| [24] | CS | HC | 261 | 1.2 | 1.2 | ||||||||||||||||||
| UC | 80 | 10.0 | 17.5 | No | No | No | No | ||||||||||||||||
| [25] | R&CS | HC | 100 | 3.0 | 2.0 | 1.0 | 0 | 0 | 0 | ||||||||||||||
| CD | 45 | 15.6 | 8.9 | 6.7 | 4.4 | 2.2 | 2.2 | No | No | No | No | No | No | No | No | No | No | ||||||
| UC | 83 | 18.1 | 13.3 | 4.8 | 10.8 | 8.4 | 2.4 | + | No | No | No | No | No | No | No | ||||||||
| [26] | CS | HC | 118 | 2.5 | |||||||||||||||||||
| CD | 138 | 11.0 | No | No | No | ||||||||||||||||||
| [27] | CS | HC | 40 | ||||||||||||||||||||
| CD | 7 | 3.9 | 0 | ||||||||||||||||||||
| UC | 19 | ||||||||||||||||||||||
| [28] | P | CD | 4 | 0 | No | ||||||||||||||||||
| UC | 8 | 12.5 | |||||||||||||||||||||
| [29] | R | HC | 102 | ||||||||||||||||||||
| CD | 24 | 6.6 | No | ||||||||||||||||||||
| UC | 21 | ||||||||||||||||||||||
| [30] | R | HC | 53 | 0 | 0 | ||||||||||||||||||
| CD | 29 | 9.4 | 6.9 | ||||||||||||||||||||
| UC | 24 | 12.5 | |||||||||||||||||||||
| [31] | R | HC | 100 | ||||||||||||||||||||
| CD | 26 | 0 | 0 | ||||||||||||||||||||
| UC | 75 | 1.3 | 1.3 | ||||||||||||||||||||
| [32] | CS | IBD | 20 | 30.0 | |||||||||||||||||||
| [33] | CS | HC | 27 | 0 | 0 | ||||||||||||||||||
| CD | 5 | 7.4 | 3.7 | ||||||||||||||||||||
| UC | 22 | ||||||||||||||||||||||
Current advances may add a new spark to the investigation of the role of anti-β2-GPI antibodies in the pathomechanism of IBD. The presence of cross-reactive epitopes on Saccharomyces cerevisiae and β2-GPI[34] has been reported in APS and raises the possibility that ASCA alone or by cross-reactivity with anti-β2-GPI exaggerate the pathologic intestinal microvascular processes in IBD and interfering with the inhibitory effect of β2-GPI on von Willebrand factor-dependent platelet adhesion and aggregation. Inflammation and coagulation are closely linked, interdependent processes in the microvasculature. Coagulation abnormalities at the mucosal level result in microthrombi formation, which are well-known features of CD and thought to be involved in the disease pathogenesis and progression[35]. Theoretically, impairment in the function of β2-GPI due to the presence of anti-β2-GPI may deteriorate certain innate immune functions as well. A novel function of the β2-GPI protein with important relevance to innate immunity, is its ability to bind and scavenge lipopolysaccharide (LPS) through a direct interaction between domain 5 (D5) of β2-GPI and LPS[36,37].
The aims of this study were to investigate in a large IBD cohort: (1) the prevalence and type of APLAs; (2) associations between the presence of APLAs and clinical phenotype of the disease or its activity; and (3) whether the presence of APLAs is associated with the disease course or development of thrombosis during prospective follow-up. Additionally, we also provide an overview of studies, performed over the last 24 years, in which significance of APLAs in IBD were assessed.
We performed a cohort study among adult CD and UC patients in a Hungarian tertiary IBD referral center (Division of Gastroenterology, Department of Internal Medicine, University of Debrecen). In all, 458 well-characterized, unrelated, consecutive IBD patients with a complete clinical follow-up CD: 271 (male/female: 120/140, age at presentation: 27.7 ± 11.6 years, disease duration: 6.0 ± 6.7 years) and UC: 187 (male/female: 86/101, age at presentation: 34.0 ± 13.2 years, disease duration: 7.4 ± 8.6 years) seen at our outpatient clinic were included between January 1, 2005 and June 1, 2010. Serum samples were obtained at enrollment from each patient and frozen at -80C until testing. The clinical characteristics of the patients at time of inclusion and sample procurement are presented in Table 2.
| CD | UC | ||
| (n = 271) | (n = 187) | ||
| Male/female (n) | 115/156 | 86/101 | |
| Age (yr)1 | 31 (24.0-41.0) | 40 (29.0-52.0) | |
| Age at presentation (yr)1 | 25 (19.0-33.0) | 33 (23.0-43.0) | |
| Duration (yr)1 | 4 (1.0-9.0) | 4 (1.0-11.0) | |
| Familial IBD | 12 (4.4) | 6 (3.2) | |
| Location | |||
| L1 | 46 (17.0) | Proctitis | 30 (16.0) |
| L2 | 67 (24.7) | Left-sided | 104 (55.6) |
| L3 | 157 (57.9) | Extensive | 53 (28.3) |
| L4 only | 1 (0.4) | ||
| All L4 | 18 | ||
| Behavior | |||
| B1 | 154 (56.5) | Remitting | 174 (93.0) |
| B2 | 56 (20.7) | Continuous | 12 (6.4) |
| B3 | 61 (22.5) | Prolonged remission | 1 (0.5) |
| Perianal disease | 76 (27.5) | - | |
| Arthritis | 54 (19.9) | 26 (13.9) | |
| Ocular manifestations | 65 (24.0) | 12 (6.4) | |
| Cutaneous manifestation | 35 (12.9) | 16 (8.6) | |
| PSC | 9 (3.3) | 8 (4.3) | |
| Steroid use/refractory | 242 (89.3)/32 (13.2) | 144 (77.0)/11 (7.6) | |
| Azathioprine use | 196 (72.3) | 66 (35.3) | |
| Surgery/multiple in CD | 54 (19.6)/19 (7.0) | 7 (3.7) | |
| Biological use | 106 (39.1) | 25 (13.4) | |
| Smoking habits | |||
| never | 219 (80.8) | 167 (89.3) | |
| yes | 47 (17.3) | 18 (9.6) | |
| previous | 5 (1.8) | 2 (1.1) | |
| Disease activity | |||
| Inactive HBI ≤ 4 | 199 (73.4) | Inactive partial Mayo ≤ 3 | 135 (72.2) |
| Active partial Mayo > 4 | |||
| Active HBI ≥ 5 | 72 (26.6) | 52 (27.8) |
Diagnosis of IBD was based on the Lennard-Jones criteria[38]. The disease phenotype (age at onset, duration, location, and behaviour) was determined according to the Montreal Classification[39]. Clinical disease activity was calculated according to the Harvey-Bradshaw Index (HBI)[40] in CD and the partial Mayo score in UC[41]. In this study we followed the European Crohn’s and Colitis Organisation guidelines[42] and defined HBI ≤ 4 as a state of remission and ≥ 5 as a state of active disease. In case of UC, ≤ 3 was defined as a state of remission and > 4 as a state of active disease. Endoscopic activity was determined according to the Simple Endoscopic Score for Crohn’s Disease (SES-CD) in CD[43] and the endoscopic component of the Mayo score in UC[44]. SES-CD defines endoscopic activity as ≥ 3 points and inactive disease ≤ 2 in CD, meanwhile in UC a state of active disease was defined as ≥ 1 points according to invasive partial Mayo score. Detailed clinical phenotypes were determined by thorough review of patients’ medical records, which had been collected in a uniform format. Medical records that documented the presence of extraintestinal manifestations [for example, arthritis: peripheral and axial; ocular manifestations: conjunctivitis, uveitis, iridocyclitis; skin lesions: erythema nodosum, pyoderma gangrenosum; arterial (AT) and venous thrombosis (VT) or pregnancy loss; and hepatic manifestations: primary sclerosing cholangitis (PSC)], frequency of flare-ups (frequent flare-up: > 1 clinical relapse/year)[45], medication use (e.g., steroid, immunosuppressive and/or biological use at any time), need for surgery (resection in CD and colectomy in UC), the presence of familial IBD, smoking habits, and perianal involvement were retrospectively analyzed for the period prior to the prospective follow-up.
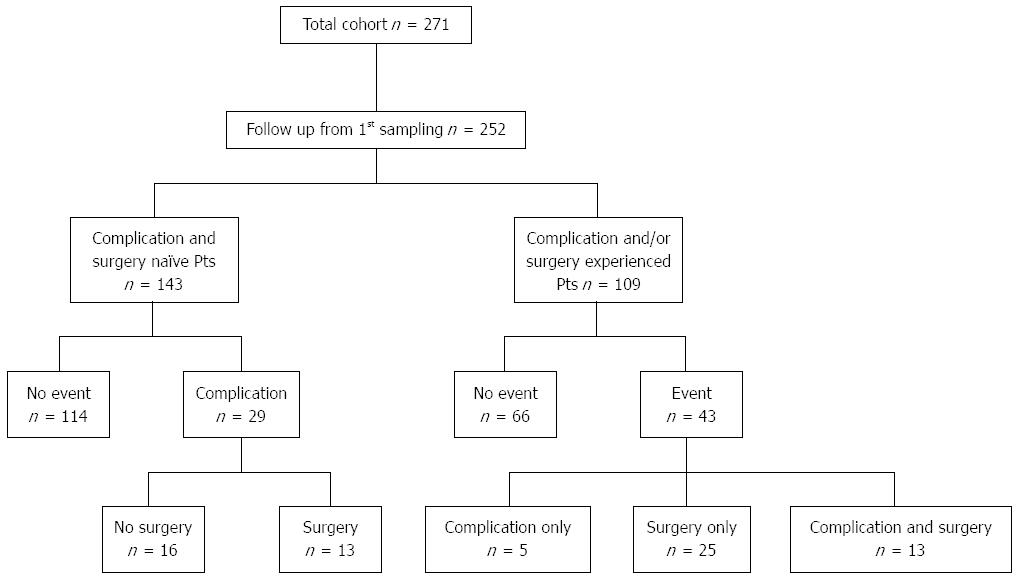
252 of 271 CD patients and 173 of 187 UC patients were available to be enrolled into a prospective follow-up study, where the treating IBD physicians registered laboratory data, endoscopic and imaging findings, disease activity, medical treatment, date and type of complications, surgery and thrombosis during regular and extraordinary outpatient follow-up visits and inpatient stays. In Hungary, a follow-up visit is usually scheduled for every 6 mo at a specialized gastroenterology center (the actual interval varies between 3-6 mo). Collected data were transferred and stored in a database for analysis. In October 1, 2013, all patients’ charts and database were reviewed and updated for the data points mentioned above. Follow-up for a particular patient was terminated if there was no further record available. Median follow-up was 57.4 mo (IQR: 40.9-80.1) for CD and 61.6 mo (IQR: 46.5-81.3) for UC patients. In CD, complicated disease behavior was defined as the occurrence of stenosis or internal penetration. Perianal fistulazing disease was distinguished from internal penetrating disease and evaluated separately. Need for surgery was defined as CD-related abdominal surgery (resection). Figure 1 summarizes flow chart of the patients with CD in the cohort study. In UC, complicated disease behavior was defined as progression of the disease extent or need for colectomy.
The control group consisted of 103 age- and gender- matched healthy blood donors (male/female: 46/57, age: 35.3 ±12.6 years old). The control subjects did not have any gastrointestinal and/or liver disease and were selected from consecutive blood donors in Debrecen.
Anti-β2-GPI, ACA and anti-PS/PT levels in serum samples were tested using the semiquantitative QUANTA LiteTM aβ2-GPI, ACAIII and aPS/PT IgA, IgG and IgM kits (INOVA Diagnostics, San Diego, California). These enzyme-linked immunosorbent assay (ELISA) kits detect IgA, IgG and IgM antibodies against β2-GPI, cardiolipin and the PS/PT complex in human serum. Plastic microwell plate wells are coated with purified β2-GPI, cardiolipin or PS/PT complex. Upon incubation, serum β2-GPI, cardiolipin and PS/PT IgA, IgG or IgM antibodies bind to β2-GPI, cardiolipin or the PS/PT complex. Unbound protein is removed by washing, while bound antibodies are detected by human IgA, IgG or IgM horseradish peroxidase-labelled conjugate. A peroxidase substrate is then added. The presence of anti-β2-GPI, ACA and aPS/PT antibodies is determined spectrophotometrically by measuring the signal intensity of each sample compared to a five-point calibration curve. All assays were performed according to the manufacturer’s instructions and were considered positive when titers were above the manufacturer’s pre-established cut-off points (for anti-β2-GPI and ACA assays, ≥ 20 units for all the IgA, IgG and IgM, and for anti-PS/PT assays ≥ 30 units for both the IgG and IgM). In case of anti-PS/PT IgA the results are presented as OD due to lack of established calibrators. Values above the OD cut-off 0.795 were considered positive for anti-PS/PT IgA. This cut-off OD value represented the mean ± SD values of the healthy controls. The results were documented in absolute OD values and in frequency of positivity. Of the 458 IBD samples obtained at enrollment, serologic analysis was technically successful in 451 of the 458 IBD cases.
ASCA antibody evaluation in CD patients was performed in our previous study[46] by ELISA (QUANTA LiteTM, INOVA Diagnostics, San Diego, CA) according to the manufacturers’ instructions. The results are presented as arbitrary units, and values above the cut-off of 25 units were considered as positive. The results were documented in absolute values and in frequency of positivity.
All the serological assays were performed in a blinded fashion without prior knowledge of the patients’ diagnosis or other clinical information.
NOD2/CARD15 SNP8, SNP12, and SNP13 genotypes were performed previously[46] in CD patients (n = 235), but not in UC patients. NOD2/CARD15 variants were detected by denaturing high-performance liquid chromatography (dHPLC, Wave DNA Fragment Analysis System, Transgenomic Limited, United Kingdom). Sequence variation, observed in the dHPLC profile, was sequenced on both strands to confirm the alteration. Sequencing reactions were performed with the ABI BigDye Terminator Cycle Sequencing Kit v1.1 (Applied Biosystems, Foster City, CA) and samples were sequenced on an ABI Prism 310 Genetic Analyzer (Applied Biosystems, Foster City, CA). All investigated polymorphisms were in Hardy-Weinberg equilibrium (data not shown).
We performed a systematic review of studies reporting on APLAs in IBD. Papers were eligible if they presented original research in adult IBD patients and reported occurrence of APLAs and their possible association with disease activity, medication, clinical phenotype of the disease, need for surgery and thromboembolic events. Case series or case reports were not included. Studies had to have been published in peer-reviewed journals. We started searching PubMed using the following search terms: “antiphospholipid antibodies” OR “anticardiolipin antibodies” OR “anti-β2-Glycoprotein-I antibodies” OR “phosphatidylserine-dependent anti-prothrombin antibodies” AND “inflammatory bowel disease” OR “Crohn’s disease” OR “ulcerative colitis”. Limits were human and time ranging from 1991 until 2014 (30th of November). This search revealed 15 articles. In Table 1 we summarize the prevalence and clinical significance of APLAs in IBD based on findings in relevant literature.
The study protocol was approved by the regional and national committee for research ethics. Each patient was informed of the nature of the study and signed an informed consent form.
Variables were tested for normality using Shapiro Wilk’s W test. Continuous variables were summarized as mean ± SD or as medians (IQR) according to their homogeneity. To evaluate differences between IBD and healthy control group, as well as within subgroups of patients with IBD the following statistical methods were used. Categorical variables were compared with the Fisher’s exact test or χ2-test with Yates correction, as appropriate. Continuous variables were compared with Student’s t test, one-way analysis of variance (ANOVA), or Mann-Whitney’s U test. Kaplan-Meier survival curves were plotted for analyzing the association between categorical clinical variables or APLAs and complicated disease outcomes during follow-up with LogRank and Breslow tests. Associations are given as OR and HR with a 95%CI. A 2-sided probability value < 0.05 was considered to be significant. For statistical analysis, GraphPad Prism 6 (San Diego, CA) and SPSS 15.0 (SPSS Inc, Chicago, IL) programs were used. The statistical methods of this study were reviewed by Elek Dinya from Semmelweis University, Institute of Health Informatics, Development and Further Training.
The prevalence rates of anti-β2-GPI, ACA and anti-PS/PT antibodies are presented in Table 3. ACA positivity was associated with increased risk for CD compared to the controls (ORACA = 10.18, 95%CI: 3.12-33.24). Of the different isotypes, ACA IgA (ORACA IgA = 49.70, 95%CI: 3.04-813.8, χ2-test with Yates correction) had the highest association to CD. ACA positivity was also significantly different between CD and UC. While the prevalence of anti-PS/PT was significantly different between CD and UC, there was not a significant difference between CD and the controls. No difference was found for the prevalence of anti-β2-GPI in different groups.
| CD (n = 265) | UC (n = 186) | HC (n = 103) | |
| Anti-β2-GPI IgG | 5 (1.9) | 4 (2.2) | 2 (1.9) |
| Anti-β2-GPI IgM | 8 (3.0) | 12 (6.5) | 3 (2.9) |
| Anti-β2-GPI IgA | 7 (2.6) | 7 (3.8) | 3 (2.9) |
| Any anti-β2-GPI | 19 (7.2) | 18 (9.7) | 8 (7.8) |
| ACA IgG | 27 (10.2)bd | 4 (2.2)d | 2 (1.9)b |
| ACA IgM | 8 (3.0) | 4 (2.2) | 1 (1.0) |
| ACA IgA | 51 (19.2)bd | 1 (0.5)d | 0 (0.0)b |
| Any ACA | 62 (23.4)bd | 9 (4.8)d | 3 (2.9)b |
| Anti-PS/PT IgG | 20 (7.5) | 9 (4.8) | 9 (8.7) |
| Anti-PS/PT IgM | 25 (9.4)bc | 8 (4.3)c | 1 (1.0)b |
| Anti-PS/PT IgA | 24 (9.1)c | 7 (3.8)c | 9 (8.7) |
| Any anti-PS/PT | 54 (20.4)d | 19 (10.2)d | 16 (15.5) |
In CD, no association was found between APLA and ASCA status of the patients. Neither ACA IgA nor IgG positivity differed significantly according to presence or absence of ASCA IgA and ASCA IgG. Similarly, the prevalence of ACA was not associated with the presence of major NOD2/CARD15 mutations. NOD2/CARD15 genotypes were available in 235 CD patients. The prevalence of any ACA was not different between patients with or without NOD2/CARD15 mutations (16.1% and 26.1%, P = NS, χ2-test with Yates correction).
At the time of enrollment, 26.6% of CD patients and 27.8% of UC patients had active disease according to clinical activity scores (Table 1). Occurrence of anti-β2-GPI, ACA and anti-PS/PT was not different between the group of patients with active (2.8%, 29.2% and 18.1%) and inactive disease state (8.8%, 21.2% and 21.2%, respectively) signified by HBI ≥ 5. Similarly, there was no correlation between the disease activity determined by partial Mayo score > 4 and APLA status in UC (data not shown).
Furthermore, the prevalence of any ACA was similar between CD patients with C-reactive protein (CRP) level > 10 mg/L and those with ≤ 10 mg/L (29.0% and 21.1%, P = NS, χ2-test with Yates correction).
A total of 87 CD patients had ileocolonoscopy at enrollment. The prevalence of any ACA was not different according to endoscopic disease activity denoted by a SES-CD cut-off value of ≥ 3 (inactive vs active: 25.6% and 29.5%). Likewise, ACA IgA and ACA IgG level was not associated with CRP level, actual HBI or SES-CD score in patients with CD applying Spearman correlation analysis (data not shown).
20.3% of the CD patients showed frequent relapse during the follow-up. The ACA prevalence was not significantly different between patients with or without frequent relapse (ACA IgA 23.5% vs 16.7%, ACA IgG 3.9% vs 10.1% and ACA IgM 2.0% vs 3.5%, P = NS for all).
Lastly, we investigated the association between disease duration and both the presence and magnitude of serologic response in CD. The rate of any ACA positivity was the same in all four disease duration quartile groups (Q1: 26.9%, Q2: 22.7%, Q3: 22.4% and Q4: 23.0%, P = NS, χ2-test with Yates correction). The level of ACA IgA and IgG was also not associated with disease duration (Kruskal-Wallis test).
A total of 154 (56.5%) CD patients had non-stricturing and non-penetrating disease (B1) according to Montreal classification at time of sampling. 143 patients were eligible for a prospective follow-up study. The median follow-up was 53.4 mo (IQR: 38.0-79.3). Among these complication and surgery naïve patients, 20.3% (29/143) experienced a complication during follow-up (31.03% developed strictures, 44.83% internal penetration and 24.14% perianal perforation only). The median time to complication was 21.4 mo (IQR: 8.1-43.1). In all, 9.1% (13/143) had to undergo CD-related abdominal surgery (resection) during the follow-up period (Figure 1). Two patients had surgical intervention without previous complication due to the development of colorectal cancer. In the remaining patients the reason for surgery was the occurrence of a complication (23.1% stenosis, 46.2% internal penetration, 15.4% both). The median time to surgery was 50.0 mo (IQR: 30.5-54.5).
In patients classified as B1, the progression of the disease to a first event defined as stenosis, internal and/or perianal penetration or CD-related surgery (Table 4) was not associated to presence or absence of APLA positivity. Furthermore in Kaplan-Meier analysis the likelihood for earlier progression to a disease event was similar in patients with or without APLA positivity. Of the clinical factors, disease location and smoking were those that associated with time to development of first internal penetrating and/or stricturing complication and frequent relapses to development of perianal penetrating disease (Table 5).
| Factor | Follow-up of CD B1 at first sampling total cohort (n = 143) | |||||
| CD B1 total | No complication | Perianal change only (B1p) | Behaviour change to B2 or B3 | No surgery | Surgery | |
| (n = 143) | (n = 114) | (n = 7) | (n = 22) | (n = 130) | (n = 13) | |
| Male/female (n) | 60/83 | 45/69 | 5/2 | 10/12 | 55/75 | 5/8 |
| Age at presentation (yr)1 | 25 (19.0-34.0) | 26 (20.0-34.3) | 17 (14.0-31.0) | 25 (18.8-34.8) | 25 (19.0-34.0) | 31 (18.0-35.5) |
| Duration (yr)1 | 3 (1.0-6.0) | 2 (1.0-7.0) | 3 (1.0-5.0) | 3 (1.0-5.0) | 2 (1.0-6.0) | 3 (1.0-10.0) |
| Location | ||||||
| L1 | 30 (21.0) | 24 (21.1) | 0 (0.0) | 6 (27.3) | 26 (20.0) | 4 (30.8) |
| L2 | 51 (35.7) | 44 (38.6) | 3 (42.9) | 4 (18.2) | 48 (36.9) | 3 (23.1) |
| L3 | 62 (43.3) | 46 (40.4) | 4 (57.1) | 12 (54.5) | 56 (43.1) | 6 (46.2) |
| L4 only | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Frequent relapse | 27 (18.9) | 18 (15.8)2 | 4 (57.1)2 | 5 (22.7) | 23 (17.7) | 4 (30.8) |
| Smoking habit yes | 21 (16.2) | 14 (12.3)2 | 2 (28.6) | 8 (36.4)2 | 21 (16.2) | 3 (23.1) |
| Follow up time from first sampling, mo1 | 53.4 (38.0-79.3) | 51.7 (36.9-73.9) | 79.8 (42.9-91.4) | 64.7 (45.6-85.0) | 50.93 (37.25-74.2) | 84.03 (60.0-91.7) |
| Positive markers | ||||||
| Anti-β2-GPI IgG | 1.4 | 1.8 | 0 | 0 | 1.6 | 0 |
| Anti-β2-GPI IgM | 5.0 | 5.3 | 16.7 | 0 | 5.5 | 0 |
| Anti-β2-GPI IgA | 2.8 | 1.8 | 16.7 | 4.5 | 3.1 | 0 |
| Any anti-β2-GPI | 8.5 | 8.8 | 16.7 | 4.5 | 9.4 | 0 |
| ACA IgG | 9.2 | 11.5 | 0 | 0 | 10.2 | 0 |
| ACA IgM | 2.8 | 3.5 | 0 | 0 | 3.1 | 0 |
| ACA IgA | 17.0 | 18.6 | 33.3 | 4.5 | 18.8 | 0 |
| Any ACA | 19.9 | 22.1 | 33.3 | 4.5 | 21.9 | 0 |
| Anti-PS/PT IgG | 8.5 | 8.0 | 0 | 13.6 | 7.8 | 15.4 |
| Anti-PS/PT IgM | 7.8 | 8.8 | 16.7 | 0 | 7.8 | 7.7 |
| Anti-PS/PT IgA | 9.9 | 9.7 | 16.7 | 9.1 | 10.9 | 0 |
| Any anti-PS/PT | 20.6 | 19.5 | 33.3 | 22.7 | 20.3 | 23.1 |
| Variable | Internal penetrating and/or stricturing complication | Perianal penetrating complication | CD-related abdominal surgery | |||
| HR (95%CI) | PLogRank-value | HR (95%CI) | PLogRank-value | HR (95%CI) | PLogRank-value | |
| Gender - male | 1.10 (0.47-2.55) | 0.83 | 2.69 (0.80-9.03) | 0.11 | 0.91 (0.33-2.55) | 0.86 |
| Smoking | 3.72 (1.23-11.21)1 | 0.021 | 1.61 (0.36-7.19) | 0.53 | 1.64 (0.45-5.97) | 0.45 |
| Location (colon only) | 0.41 (0.17-0.96)1 | 0.041 | 1.00 (0.29-3.43) | 0.99 | 0.61 (0.22-1.74) | 0.36 |
| Frequent relapse | 1.10 (0.39-3.07) | 0.85 | 7.29 (1.72-30.99)1 | 0.0071 | 1.64 (0.44-6.10) | 0.46 |
| APLA at all | 1.36 (0.59-3.16) | 0.47 | 1.30 (0.40-4.27) | 0.66 | 1.37 (0.49-3.80) | 0.55 |
| Anti-β2-GPI at all | 0.56 (0.13-2.32) | 0.42 | 0.99 (0.13-7.70) | 0.99 | 0.33 (0.06-1.81) | 0.20 |
| ACA at all | 0.41 (0.13-1.26) | 0.12 | 1.12 (0.23-5.49) | 0.89 | 0.30 (0.07-1.27) | 0.10 |
| Anti-PS/PT at all | 1.07 (0.39-2.97) | 0.89 | 1.74 (0.38-8.03) | 0.48 | 1.11 (0.29-4.18) | 0.88 |
In UC patient group association between APLAs and clinical phenotype or progression of the disease was not evaluated due to the lack of increased prevalence of any APLAs in UC population.
In total, 5.1% (23/452) of IBD patients had at least one thromboembolic event (14 CD and 9 UC patients). In CD, 18 events of VT, 1 event of PE and 3 events of AT were diagnosed. In UC, 8 events of VT and 1 events of AT were diagnosed. 7 (1.6%) patients had a TE also prior to diagnosis of IBD. 4 (1.5%) CD patients had recurrent TE. In women, pregnancy loss occurred in 6.4% (10/156) of CD and 6.9% (7/101) of UC patients.
CD patients presenting with VT were significantly older than those without (median age: 30.0 years vs 38.5 years, P = 0.003). Of the investigated clinical factors and laboratory markers, previous VT event (RR = 23.0, 95%CI: 10.6-50.1) and factor V Leiden mutation (RR = 8.3, 95%CI: 2.2-31.3) were associated with the risk of VT events. At the same time, in patients with UC, frequent relapse was associated with higher risk of VT event (RR = 6.4, 95%CI: 1.7-24.1). However, none of the APLA markers were associated with increased risk of VT events in IBD.
Table 6 summarizes the patient characteristics, prevalence of APLAs and the known genetic and serologic markers of thrombophilia according to presence and type of thrombotic events in CD patients. We further investigated the probability for thromboembolic events as a function of positivity for a certain amount of APLAs out of the whole panel. However, neither CD and nor UC patients positive for multiple APLAs showed a higher probability for the development of thromboembolic events.
| Factor | Follow-up of CD Total Cohort from diagnosis (n = 265) | ||||
| No thrombosis | Arterial thrombosis | Venous thrombosis | No pregnancy loss | Pregnancy loss | |
| (n = 251) | (n = 3) | (n = 11) | (n = 146) | (n = 10) | |
| Male/female | 104/147 | 1/2 | 7/4 | 146/0 | 10/0 |
| Age at presentation (yr)1 | 25.0 (19.0-33.0) | 40.0 (28.0-42.0) | 29.5 (23.3-39.3) | 26.0 (20.0-35.0) | 25.5 (21.0-35.0) |
| Frequent relapse | 48 (20.2) | 0 (0.0) | 4 (36.4) | 33 (24.1) | 2 (20.0) |
| Previous thrombosis | 1 (0.4)3 | 0 (0.0) | 4 (36.4)3 | 2 (1.4) | 0 (0.0) |
| Smoking habits yes | 48 (19.1) | 0 (0.0) | 3 (27.3) | 22 (15.1) | 2 (20.0) |
| Follow up time from diagnosis, mo1 | 102.2 (63.3-172.8)2 | 149.9 (130.8-219.8) | 186.3 (142.0-244.2)2 | 109.0 (61.8-184.6) | 136.5 (95.4-180.6) |
| Positive markers (%) | |||||
| Anti-β2-GPI IgG and/or IgM | 4.8 | 0 | 9.1 | 4.9 | 10 |
| Anti-β2-GPI IgA | 2.8 | 0 | 0 | 4.9 | 0 |
| ACA IgG and/or IgM | 12.1 | 0 | 27.3 | 13.9 | 10 |
| ACA IgA | 19.0 | 66.7 | 0 | 20.1 | 10 |
| Anti-PS/PT IgG and/or IgM | 14.6 | 50.0 | 36.4 | 18.3 | 40 |
| Anti-PS/PT IgA | 8.9 | 50.0 | 9.1 | 7.7 | 10 |
| At least 1 APLA pos | 48.0 | 66.7 | 54.5 | 52.8 | 60 |
| At least 2 APLA pos | 16.1 | 33.3 | 9.1 | 18.1 | 10 |
| At least 3 APLA pos | 3.2 | 0 | 9.1 | 4.9 | 0 |
| Thrombophilia markers3 (%) | |||||
| LA | 7.5 | 0 | 0 | 5.1 | 0 |
| PS deficiency (inherited and/or acquired) | 8.1 | 0 | 25.0 | 12.7 | 0 |
| ATIII deficiency (inherited and/or acquired) | 0 | 0 | 0 | 0 | 0 |
| PC deficiency (inherited and/or acquired) | 3.0 | 0 | 0 | 4.8 | 0 |
| FV Leiden | 5.94 | 0 | 42.94 | 7.8 | 0 |
| FII20210A | 5.9 | 0 | 16.7 | 7.8 | 0 |
In order to evaluate the stability in the APLA status (positive or negative for a respective antibody), we analyzed samples from same patients over various arbitrary time-points during the disease course. At least two serum samples were taken from a subgroup of CD patients (n = 198) and UC patients (n = 103). Median time between sample procurements were 13.5 mo (IQR: 6.8-22.9 mo) for CD and 12.1 mo (IQR: 5.7-20.4 mo) for UC patients. Interestingly, the anti-β2-GPI status was very stable over time with respect to all three Ig subtypes. Only 1.5%-5.8% of either CD or UC patients had a change in their anti-β2-GPI antibody status compared to the initial sample procurement. At the same time marked differences were found in case of ACA Ig subtypes. ACA IgM status, similar to anti-β2-GPI, was also stable. In contrast, ACA IgG and even more ACA IgA status showed significant changes over time, mainly from negative to positive. Changes in antibody status were more remarkable in CD than UC (ACA IgA: 49.9% vs 23.3% and ACA IgG: 21.2% vs 5.8%). Stability data are not available for anti-PS/PT antibodies since measurements were only preformed on the first available serum samples of the patients. APLA changes are summarized in Table 7.
| APLA marker | CD (n = 198) | UC (n = 103) |
| Anti-β2-GPI IgG | ||
| Stable negative | 194 (98.0) | 97 (94.2) |
| Stable positive | 1 (0.5) | 2 (1.9) |
| Neg. to pos. | 0 (0.0) | 3 (2.9) |
| Pos. to neg. | 3 (1.5) | 1 (1.0) |
| Anti-β2-GPI IgM | ||
| Stable negative | 186 (94.0) | 94 (91.3) |
| Stable positive | 4 (2.0) | 3 (2.9) |
| Neg. to pos. | 6 (3.0) | 5 (4.9) |
| Pos. to neg. | 2 (1.0) | 1 (0.9) |
| Anti-β2-GPI IgA | ||
| Stable negative | 190 (96.0) | 93 (90.3) |
| Stable positive | 1 (0.5) | 4 (3.9) |
| Neg. to pos. | 3 (1.5) | 5 (4.9) |
| Pos. to neg. | 4 (2.0) | 1 (0.9) |
| ACA IgG | ||
| Stable negative | 139 (70.2) | 95 (92.2) |
| Stable positive | 3 (1.5) | 1 (1.0) |
| Neg. to pos. | 42 (21.2) | 6 (5.8) |
| Pos. to neg. | 14 (7.1) | 1 (1.0) |
| ACA IgM | ||
| Stable negative | 173 (87.4) | 95 (92.2) |
| Stable positive | 3 (1.5) | 1 (1.0) |
| Neg. to pos. | 18 (9.1) | 7 (6.8) |
| Pos. to neg. | 4 (2.0) | 0 (0.0) |
| ACA IgA | ||
| Stable negative | 73 (36.9) | 78 (75.7) |
| Stable positive | 13 (6.6) | 0 (0.0) |
| Neg. to pos. | 93 (46.9) | 24 (23.3) |
| Pos. to neg. | 19 (9.6) | 1 (1.0) |
After availability of tumor necrosis factor (TNF) antagonist therapy through National Health reimbursement system in 2008, 43.7% (110/252) of our patients received either infliximab or adalimumab treatment. We assessed the impact of post-enrolment anti-TNF therapy in the induction of new ACA antibody formation. 59.1% (55/93) of patients who received anti-TNF therapy and had negative findings for ACA IgA at the baseline were found to have positive results later on (negative to positive change), which was significantly higher compared to the proportion of 35.6% (36/101) found among patients who did not receive anti-TNF therapy at all (P = 0.004). These ratios were 30.1% vs 12.9% (P = 0.033) for ACA IgG.
To our knowledge, this is the largest study to investigate prospectively the prevalence, type, and clinical significance of multiple APLAs simultaneously in a cohort of IBD patients to date. Three different antibodies were assessed by ELISA. Contrary to routine laboratory practice, APLAs were identified by anti-IgA secondary antibody in addition to anti-IgG and anti-IgM isotypes. Moreover, in the present study we also provided an overview of relevant APLA studies in IBD.
We demonstrated, for the first time, that enhanced ACA IgA formation is a feature of CD; the presence of ACA IgA was significantly higher as compared to UC and HC. Previously only one study[22], involving 50 CD patients, evaluated the occurrence of ACA IgA. However, the reported prevalence rate was much lower than in the present study (6.0% vs 19.2%). At the same time, occurrence of IgM and IgG class ACA was studied more extensively in IBD, but several studies provide only total prevalence rate for these two ACA subtypes. Reported prevalence of total ACA was widely varied both in CD (0.0%-36%)[21,22,25] and UC (1.3%-20.0%)[30,31] which was 12.6% and 4.3%, respectively in the present study. Studies evaluating separately these two subtypes of ACA revealed that the increased ACA prevalence is mainly due to IgG subtype. Except one study[30], occurrence of ACA IgG prevailed over ACA IgM[20,22,25]. This is concordant with our results in CD cohort (ACA IgG: 10.2 vs ACA IgM: 3.0%).
What can be the cause of enhanced ACA formation in CD? Animal models, immunization with lipid A and lipoteichoic acid have resulted in induction of ACA and/or lupus anticoagulant (LA) formation[47]. Moreover, appearance of ACA antibodies may be due to the sustained exposure to bacterial DNA, which is enriched in unmethylated CpG motifs. These motifs are expressed e.g. in Escherichia coli DNA[48]. Based on these literature findings, the role of bacterial translocation (BT) in the induction of ACA, similar to anti-microbial antibodies, seems plausible[1]. At the same time, we did not find any association between the presence of ACA and ASCA, even when assessing according to separate isotypes. This finding implies mechanisms other than BT in the formation of ACA. Moreover, in contrast to anti-microbial antibodies, APLA formation was not influenced by NOD2/CARD15 genotypes. The fact that neither the presence of ACA, nor the titers of the antibodies were affected by actual disease activity and were also not associated with the CD phenotype of frequent relapse contradict the involvement of BT in the formation mechanisms of ACA as well.
Former studies[19-26] extensively assessed the link between disease activity and APLA formation either by clinical activity or by CRP level, but found no association. Considering that clinical, laboratory and endoscopic activity are not inevitably congruent in all cases, in the present study, we re-evaluated this relationship in a complex way, applying all the three kinds of activity parameters simultaneously, but came to same conclusion.
The prevalence of anti-β2-GPI IgG/IgM in the present study was comparable to those reported in the study of Koutroubakis et al[25] (CD: 5.0% vs 4.4% and UC: 7.5% vs 10.8%). Anti-PS/PT antibodies were not assessed previously in IBD, however we did not find enhanced antibody formation in IBD as compared to HC.
Association of certain APLAs with clinical phenotype of IBD was mainly assessed in the cross-sectional single-time point studies summarized in Table 1. In most of these studies, these antibodies were neither linked to the disease location[19,21,22,25], behavior[25] and need for surgery[19] in CD patients, nor to the extent of the disease[19,21,24,25] and colectomy[19] in UC patients. Likewise, different APLAs were not associated with the risk of VTE events in either CD[19,22,23,25,26] or in UC[19,23-25]. In case of these single point cross sectional studies the serum samples were obtained at different times during the disease course. These approaches combine samples taken before, at the time of and after complications occur and only allow revealing associations, but do not have predictive capability. In the present study to enhance the potential clinical value of these markers, we applied a prospective study design, which enabled us to evaluate the potential predictive capabilities of APLAs in respect to complicated CD behavior and surgery. Predictive ability for serum markers might be beneficial at any time during the disease course but perform distinctly by chance. Thus we formed two separate groups for our CD cohort (1) complication and surgery naïve CD patients; and (2) complication and/or surgery experienced patients prior to sample procurement. Several observations suggest that the presence of perianal and internal penetrating disease signify distinct phenotypes in CD concerning all the clinical, serologic and genetic point of view[1,49]. Accordingly, we evaluated B1p complication separately from B3 complication. However, APLA did not proved as a predictive marker of the complicated disease in neither clinically setting of CD. A clear strength of our study was prospective follow-up design and the application of the widest panel of currently available APLAs.
Due to low frequency of different thromboembolic complications, we assessed the occurrence of these events even from the diagnosis involving the period prior to sample procurement as well. Development of VT, AT and pregnancy loss, however, did not vary according to APLA status.
At the same time, significant changes were found in ACA IgA and ACA IgG positivity over time in patients with CD. In rheumatology disorders, introduction of TNF antagonists (adalimumab, infliximab, and etanercept) was reported to induce production of various types of autoantibodies, such as APLAs[50]. However such data are scarce in IBD. In a study by Atzeni et al[20] involving 63 patients with CD, 5 (8%) had ACA antibodies (mainly IgM) at baseline and two additional patients developed these autoantibodies during infliximab treatment with no apparent clinical effect. Thus we investigated the possible role of the TNF antagonists therapy in our prospective CD cohort. Interestingly, the patients initially negative for ACA developed ACA IgA or IgG positivity significantly more frequently if they received anti-TNF therapy suggesting a causative association. In the non-anti-TNF treated subgroup of patients, the change of the ACA status is less well understood and needs further clarification. The pathogenic mechanism that changes the humoral response leading to development of autoimmunity during anti-TNF inhibitors therapy is unknown, but various hypothesis have been proposed[51]. One hypothesis is that binding of anti-TNF antagonists to the transmembrane and soluble TNF, rapidly lowering TNF level and enhancing apoptotic cell death, which triggers the development of autoantibodies. Further investigations are warranted to elucidate whether the new ACA appears associated with a worse clinical outcome.
In conclusion, the present study demonstrated enhanced formation of APLAs in CD patients. The presence of the APLAs however was not associated with the clinical phenotype, disease course, or risk of venous thrombotic events during the prospective follow-up.
Enhanced serological antibody formation is a well-known feature of inflammatory bowel diseases (IBD). A wide range of anti-microbial and autoantibodies have been reported to be associated with either Crohn’s disease (CD) or ulcerative colitis (UC) as well as with complicated disease course. Autoantibodies are directed against various host proteins. Based on recent findings, their existence might also be related to enhanced microbial challenge to the gut due to a disturbed gut innate immune system and may trigger an exaggerated adaptive immune response. Furthermore, these serological antibodies may also be actively involved in the pathophysiology of inflammation in IBD. Antiphospholipid antibodies (APLAs) are a prothrombotic group of antibodies acquired in various inflammatory diseases. These antibodies comprise anti-cardiolipin (ACA), anti-β2-Glycoprotein-I (anti-β2-GPI), and anti-phosphatidylserine/prothrombin antibodies (anti-PS/PT). Their association with clinical phenotype and disease progression is still unclear in IBD. Even if non-prothrombotic, they may have certain pathogenetic roles in several diseases as well.
In IBD, available cross-sectional, mainly single-time point studies assessing different aspects of APLAs, came to discrepant conclusions regarding formation, prevalence, and stability of these antibodies. Their clinical significance, including association to thrombotic events in IBD is also unclear. Thus a comprehensive evaluation of the primary APLAs in a large prospectively followed-up IBD cohort is required. Current advances may add a new spark to the investigation of the role of anti-β2-GPI antibodies in the pathomechanism of IBD.
This is the largest study to investigate prospectively the prevalence, type, and clinical significance of multiple APLAs simultaneously in a cohort of IBD patients to date. Three different antibodies were assessed by ELISA. Contrary to routine laboratory practice, APLAs were identified by anti-IgA secondary antibody in addition to anti-IgG and anti-IgM isotypes. Moreover, in the present study the authors also provided an overview of relevant APLA studies in IBD. Although they detected enhanced formation of APLAs in CD patients, the presence of the APLAs was not associated with the clinical phenotype, disease course, or risk of venous thrombotic events during the prospective follow-up. A clear strength of this study was prospective follow-up design and the application of the widest panel of currently available APLAs.
Previous studies investigating APLAs in IBD patients had certain shortcomings resulting in an inconclusive, contradictory and confusing viewpoint regarding their pathogenetic and clinical significance, as well as their predictive value. Based on these large scale and extensive evaluation of APLAs they were not proved as a predictive marker of the complicated disease course in neither clinically settings of CD. Thromboembolic events were also not associated to any individual or multiple APLA positivity. Therefore use of APLAs in everyday practice and decision making cannot be recommended.
APLAs are a prothrombotic group of antibodies acquired in various inflammatory diseases. These ACA, anti-β2-GPI, and anti-PS/PT. Bacterial translocation is defined as an enhanced passage of bacteria and/or bacterial products from the intestinal tract to systemic circulation.
This article relooks at the role of APLAs in IBD and provides a small systematic review of the currently available literature. The authors show the analysis of APLAs in patients with IBD (n = 458) using prospective cohort study. The manuscript has extensive information on APLAs. This is a well-designed and conducted study and gives clear conclusion that despite increased occurrence of APLAs in IBD, they are not associated with disease phenotype including the thrombosis and do not need further study unless a newer aspect comes in future.
P- Reviewer: Desai DC, Fujimori S, Lai JH S- Editor: Ma YJ L- Editor: A E- Editor: Ma S
| 1. | Papp M, Lakatos PL. Serological studies in inflammatory bowel disease: how important are they? Curr Opin Gastroenterol. 2014;30:359-364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 2. | Lakatos PL, Papp M, Rieder F. Serologic antiglycan antibodies in inflammatory bowel disease. Am J Gastroenterol. 2011;106:406-412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 28] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 3. | Rieder F, Kugathasan S. Circulating antibodies against bacterial wall products: are there arguments for early immunosuppression? Dig Dis. 2012;30 Suppl 3:55-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 4. | Terjung B, Söhne J, Lechtenberg B, Gottwein J, Muennich M, Herzog V, Mähler M, Sauerbruch T, Spengler U. p-ANCAs in autoimmune liver disorders recognise human beta-tubulin isotype 5 and cross-react with microbial protein FtsZ. Gut. 2010;59:808-816. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 107] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 5. | Papp M, Sipeki N, Vitalis Z, Tornai T, Altorjay I, Tornai I, Udvardy M, Fechner K, Jacobsen S, Teegen B. High prevalence of IgA class anti-neutrophil cytoplasmic antibodies (ANCA) is associated with increased risk of bacterial infection in patients with cirrhosis. J Hepatol. 2013;59:457-466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 34] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 6. | Pavlidis P, Romanidou O, Roggenbuck D, Mytilinaiou MG, Al-Sulttan F, Liaskos C, Smyk DS, Koutsoumpas AL, Rigopoulou EI, Conrad K. Ileal inflammation may trigger the development of GP2-specific pancreatic autoantibodies in patients with Crohn’s disease. Clin Dev Immunol. 2012;2012:640835. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 32] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 7. | Roggenbuck D, Reinhold D, Werner L, Schierack P, Bogdanos DP, Conrad K. Glycoprotein 2 antibodies in Crohn’s disease. Adv Clin Chem. 2013;60:187-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 32] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 8. | Ortel TL. Antiphospholipid syndrome: laboratory testing and diagnostic strategies. Am J Hematol. 2012;87 Suppl 1:S75-S81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 41] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 9. | Staub HL, Franck M, Ranzolin A, Norman GL, Iverson GM, von Mühlen CA. IgA antibodies to beta2-glycoprotein I and atherosclerosis. Autoimmun Rev. 2006;6:104-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 30] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 10. | Gabeta S, Norman GL, Gatselis N, Liaskos C, Papamichalis PA, Garagounis A, Zachou K, Rigopoulou EI, Dalekos GN. IgA anti-b2GPI antibodies in patients with autoimmune liver diseases. J Clin Immunol. 2008;28:501-511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 34] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 11. | Ramírez E, Serrano A, García F, Alfaro FJ, Pérez V, Paz-Artal E, Morales JM. Prospective study on autoantibodies against apolipoprotein H (beta2GPI) in several clinical parameters from patients with terminal renal failure and functioning renal transplants. Transplant Proc. 2009;41:2370-2372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 12. | Mankaï A, Achour A, Thabet Y, Manoubia W, Sakly W, Ghedira I. Anti-cardiolipin and anti-beta 2-glycoprotein I antibodies in celiac disease. Pathol Biol (Paris). 2012;60:291-295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 13. | Sène D, Piette JC, Cacoub P. Antiphospholipid antibodies, antiphospholipid syndrome and infections. Autoimmun Rev. 2008;7:272-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 131] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 14. | García-Carrasco M, Galarza-Maldonado C, Mendoza-Pinto C, Escarcega RO, Cervera R. Infections and the antiphospholipid syndrome. Clin Rev Allergy Immunol. 2009;36:104-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 15. | Frauenknecht K, Lackner K, von Landenberg P. Antiphospholipid antibodies in pediatric patients with prolonged activated partial thromboplastin time during infection. Immunobiology. 2005;210:799-805. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 24] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 16. | Shin JI, Lee JS, Kim HS. Lupus anticoagulant and IgM anti-phospholipid antibodies in Korean children with Henoch-Schonlein purpura. Scand J Rheumatol. 2009;38:73-74; author reply 74-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 17. | Horstman LL, Jy W, Bidot CJ, Ahn YS, Kelley RE, Zivadinov R, Maghzi AH, Etemadifar M, Mousavi SA, Minagar A. Antiphospholipid antibodies: paradigm in transition. J Neuroinflammation. 2009;6:3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 31] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 18. | Serrano A, García F, Serrano M, Ramírez E, Alfaro FJ, Lora D, de la Cámara AG, Paz-Artal E, Praga M, Morales JM. IgA antibodies against β2 glycoprotein I in hemodialysis patients are an independent risk factor for mortality. Kidney Int. 2012;81:1239-1244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 50] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 19. | Aichbichler BW, Petritsch W, Reicht GA, Wenzl HH, Eherer AJ, Hinterleitner TA, Auer-Grumbach P, Krejs GJ. Anti-cardiolipin antibodies in patients with inflammatory bowel disease. Dig Dis Sci. 1999;44:852-856. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 50] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 20. | Atzeni F, Ardizzone S, Sarzi-Puttini P, Colombo E, Maconi G, De Portu S, Carrabba M, Bianchi Porro G. Autoantibody profile during short-term infliximab treatment for Crohn’s disease: a prospective cohort study. Aliment Pharmacol Ther. 2005;22:453-461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 21. | Caccavo D, Greco B, Caradonna L, Leandro G, Bonomo L, Jirillo E. Antiphospholipid antibodies in patients with inflammatory bowel disease. Med Sci Res. 1996;24:711-713. |
| 22. | Chamouard P, Grunebaum L, Wiesel ML, Freyssinet JM, Duclos B, Cazenave JP, Baumann R. Prevalence and significance of anticardiolipin antibodies in Crohn’s disease. Dig Dis Sci. 1994;39:1501-1504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 35] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 23. | Chiarantini E, Valanzano R, Liotta AA, Cellai AP, Fedi S, Ilari I, Prisco D, Tonelli F, Abbate R. Hemostatic abnormalities in inflammatory bowel disease. Thromb Res. 1996;82:137-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 64] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 24. | Dalekos GN, Manoussakis MN, Goussia AC, Tsianos EV, Moutsopoulos HM. Soluble interleukin-2 receptors, antineutrophil cytoplasmic antibodies, and other autoantibodies in patients with ulcerative colitis. Gut. 1993;34:658-664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 41] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 25. | Koutroubakis IE, Petinaki E, Anagnostopoulou E, Kritikos H, Mouzas IA, Kouroumalis EA, Manousos ON. Anti-cardiolipin and anti-beta2-glycoprotein I antibodies in patients with inflammatory bowel disease. Dig Dis Sci. 1998;43:2507-2512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 64] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 26. | Lonjon I, Beaugerie L, Deschamps A, Barthet C, Carbonnel F, Ngô Y, Cosnes J, Abuaf N, Gendre JP. [Prevalence and role of anticardiolipin antibodies in Crohn disease]. Gastroenterol Clin Biol. 1996;20:633-637. [PubMed] |
| 27. | Maher MM, Soloma SH. Assessment of thrombophilic abnormalities during the active state of inflammatory bowel disease. Saudi J Gastroenterol. 2008;14:192-197. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 28. | Martinović Z, Perisić K, Pejnović N, Lukacević S, Rabrenović L, Petrović M. Antiphospholipid antibodies in inflammatory bowel diseases. Vojnosanit Pregl. 1998;55:47-49. [PubMed] |
| 29. | Oldenburg B, Van Tuyl BA, van der Griend R, Fijnheer R, van Berge Henegouwen GP. Risk factors for thromboembolic complications in inflammatory bowel disease: the role of hyperhomocysteinaemia. Dig Dis Sci. 2005;50:235-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 47] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 30. | Saibeni S, Vecchi M, Valsecchi C, Faioni EM, Razzari C, de Franchis R. Reduced free protein S levels in patients with inflammatory bowel disease: prevalence, clinical relevance, and role of anti-protein S antibodies. Dig Dis Sci. 2001;46:637-643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 31. | Souto JC, Borrell M, Fontcuberta J, Roca M. Antiphospholipid antibodies in inflammatory bowel disease. Dig Dis Sci. 1995;40:1524-1525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 32. | Vecchi M, Cattaneo M, de Franchis R, Mannucci PM. Risk of thromboembolic complications in patients with inflammatory bowel disease. Study of hemostasis measurements. Int J Clin Lab Res. 1991;21:165-170. [PubMed] |
| 33. | Yurekli BP, Aksoy DY, Aybar M, Egesel T, Gurgey A, Hascelik G, Kirazli S, Haznedaroglu IC, Arslan S. The search for a common thrombophilic state during the active state of inflammatory bowel disease. J Clin Gastroenterol. 2006;40:809-813. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 34. | Krause I, Blank M, Cervera R, Font J, Matthias T, Pfeiffer S, Wies I, Fraser A, Shoenfeld Y. Cross-reactive epitopes on beta2-glycoprotein-I and Saccharomyces cerevisiae in patients with the antiphospholipid syndrome. Ann N Y Acad Sci. 2007;1108:481-488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 35. | Deban L, Correale C, Vetrano S, Malesci A, Danese S. Multiple pathogenic roles of microvasculature in inflammatory bowel disease: a Jack of all trades. Am J Pathol. 2008;172:1457-1466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 117] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 36. | Agar C, de Groot PG, Mörgelin M, Monk SD, van Os G, Levels JH, de Laat B, Urbanus RT, Herwald H, van der Poll T. β2-glycoprotein I: a novel component of innate immunity. Blood. 2011;117:6939-6947. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 82] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 37. | Laplante P, Amireault P, Subang R, Dieudé M, Levine JS, Rauch J. Interaction of β2-glycoprotein I with lipopolysaccharide leads to Toll-like receptor 4 (TLR4)-dependent activation of macrophages. J Biol Chem. 2011;286:42494-42503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 38. | Lennard-Jones JE. Classification of inflammatory bowel disease. Scand J Gastroenterol Suppl. 1989;170:2-6; discussion 16-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1396] [Cited by in RCA: 1445] [Article Influence: 40.1] [Reference Citation Analysis (0)] |
| 39. | Silverberg MS, Satsangi J, Ahmad T, Arnott ID, Bernstein CN, Brant SR, Caprilli R, Colombel JF, Gasche C, Geboes K. Toward an integrated clinical, molecular and serological classification of inflammatory bowel disease: report of a Working Party of the 2005 Montreal World Congress of Gastroenterology. Can J Gastroenterol. 2005;19 Suppl A:5A-36A. [PubMed] |
| 40. | Vermeire S, Schreiber S, Sandborn WJ, Dubois C, Rutgeerts P. Correlation between the Crohn’s disease activity and Harvey-Bradshaw indices in assessing Crohn’s disease severity. Clin Gastroenterol Hepatol. 2010;8:357-363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 283] [Cited by in RCA: 332] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 41. | Lewis JD, Chuai S, Nessel L, Lichtenstein GR, Aberra FN, Ellenberg JH. Use of the noninvasive components of the Mayo score to assess clinical response in ulcerative colitis. Inflamm Bowel Dis. 2008;14:1660-1666. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 733] [Cited by in RCA: 711] [Article Influence: 41.8] [Reference Citation Analysis (0)] |
| 42. | Van Assche G, Dignass A, Panes J, Beaugerie L, Karagiannis J, Allez M, Ochsenkühn T, Orchard T, Rogler G, Louis E. The second European evidence-based Consensus on the diagnosis and management of Crohn’s disease: Definitions and diagnosis. J Crohns Colitis. 2010;4:7-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 818] [Cited by in RCA: 792] [Article Influence: 52.8] [Reference Citation Analysis (0)] |
| 43. | Daperno M, D’Haens G, Van Assche G, Baert F, Bulois P, Maunoury V, Sostegni R, Rocca R, Pera A, Gevers A. Development and validation of a new, simplified endoscopic activity score for Crohn’s disease: the SES-CD. Gastrointest Endosc. 2004;60:505-512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 999] [Cited by in RCA: 1319] [Article Influence: 62.8] [Reference Citation Analysis (0)] |
| 44. | Schroeder KW, Tremaine WJ, Ilstrup DM. Coated oral 5-aminosalicylic acid therapy for mildly to moderately active ulcerative colitis. A randomized study. N Engl J Med. 1987;317:1625-1629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1958] [Cited by in RCA: 2250] [Article Influence: 59.2] [Reference Citation Analysis (0)] |
| 45. | Stange EF, Travis SP, Vermeire S, Beglinger C, Kupcinkas L, Geboes K, Barakauskiene A, Villanacci V, Von Herbay A, Warren BF. European evidence based consensus on the diagnosis and management of Crohn’s disease: definitions and diagnosis. Gut. 2006;55 Suppl 1:i1-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 402] [Cited by in RCA: 381] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 46. | Papp M, Lakatos PL, Harsfalvi J, Farkas G, Palatka K, Udvardy M, Molnar T, Farkas K, Nagy F, Veres G. Mannose-binding lectin level and deficiency is not associated with inflammatory bowel diseases, disease phenotype, serology profile, and NOD2/CARD15 genotype in a large Hungarian cohort. Hum Immunol. 2010;71:407-413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 47. | Gotoh M, Matsuda J. Induction of anticardiolipin antibody and/or lupus anticoagulant in rabbits by immunization with lipoteichoic acid, lipopolysaccharide and lipid A. Lupus. 1996;5:593-597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 48. | Bauer M, Heeg K, Wagner H, Lipford GB. DNA activates human immune cells through a CpG sequence-dependent manner. Immunology. 1999;97:699-705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 122] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 49. | Tang LY, Rawsthorne P, Bernstein CN. Are perineal and luminal fistulas associated in Crohn’s disease? A population-based study. Clin Gastroenterol Hepatol. 2006;4:1130-1134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 114] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 50. | Atzeni F, Talotta R, Salaffi F, Cassinotti A, Varisco V, Battellino M, Ardizzone S, Pace F, Sarzi-Puttini P. Immunogenicity and autoimmunity during anti-TNF therapy. Autoimmun Rev. 2013;12:703-708. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 154] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 51. | Kolarz B, Majdan M, Darmochwal-Kolarz DA, Dryglewska M. Antiphospholipid antibodies during 6-month treatment with infliximab: a preliminary report. Med Sci Monit. 2014;20:1227-1231. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |