Published online Jun 14, 2015. doi: 10.3748/wjg.v21.i22.6835
Peer-review started: November 27, 2014
First decision: March 10, 2015
Revised: March 25, 2015
Accepted: April 16, 2015
Article in press: April 17, 2015
Published online: June 14, 2015
Processing time: 203 Days and 21.4 Hours
Diabetogenic traits in patients undergoing liver transplantation (LT) are exacerbated intraoperatively by exogenous causes, such as surgical stress, steroids, blood transfusions, and catecholamines, which lead to intraoperative hyperglycemia. In contrast to the strict glucose control performed in the intensive care unit, no systematic protocol has been developed for glucose management during LT. Intraoperative blood glucose concentrations typically exceed 200 mg/dL in LT, and extreme hyperglycemia (> 300 mg/dL) is common during the neohepatic phase. Only a few retrospective studies have examined the relationship between intraoperative hyperglycemia and post-transplant complications, with reports of infectious complications or mortality. However, no prospective studies have been conducted regarding the influence of intraoperative hyperglycemia in LT on post-transplant outcome. In addition to absolute blood glucose values, the temporal patterns in blood glucose levels during LT may serve as prognostic features. Persistent neohepatic hyperglycemia (without a decline) throughout LT is a useful indicator of early graft dysfunction. Moreover, intraoperative variability in glucose levels may predict the need for reoperation for hemorrhage after LT. Thus, there is an urgent need for guidelines for glucose control in these patients, as well as prospective studies on the impact of glucose control on various post-transplant complications. This report highlights some of the recent studies related to perioperative blood glucose management focused on LT and liver disease.
Core tip: Despite the fact that blood glucose control is essential in critically ill patients, glucose levels are typically not managed effectively in patients undergoing liver transplantation. Currently, there are insufficient data from clinical studies on intraoperative glucose in liver transplantation to establish guidelines for glucose management of these patients. Intraoperative features of blood glucose levels may be related to immediate and deleterious outcomes after liver transplantation. Identification of these associations will help to emphasize the prognostic role of intraoperative glucose, and stimulate the establishment of a standard protocol for intraoperative glucose management in liver transplantation.
- Citation: Park CS. Predictive roles of intraoperative blood glucose for post-transplant outcomes in liver transplantation. World J Gastroenterol 2015; 21(22): 6835-6841
- URL: https://www.wjgnet.com/1007-9327/full/v21/i22/6835.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i22.6835
Patients with end-stage liver disease have impaired glucose metabolism, which can manifest as glucose intolerance or full diabetes mellitus[1,2]. Blood glucose status in these patients often worsens considerably during liver transplantation (LT). Sudden increases in intraoperative blood glucose can result from intrinsic diabetogenic traits and from a variety of exogenous factors, such as surgical stress, corticosteroids, glucose-containing fluid solutions, blood transfusions, and catecholamine vasopressors[1,3,4].
Despite the strict guidelines used for blood glucose control in intensive care unit (ICU) patients[5-7], intraoperative criteria for blood glucose control during LT have not yet been established. Although more than 20 units of insulin are typically administered during LT[3,8], it is almost impossible to maintain blood glucose levels within a preoperatively sustained range. In fact, extreme hyperglycemia with blood glucose > 300 mg/dL is not uncommon during the neohepatic phase after reperfusion to the liver graft[1,9].
Hyperglycemia can increase morbidity and mortality in critically ill patients[10-12]. Clinical studies on the impact of perioperative blood glucose have been conducted in major surgical fields, particularly cardiac surgery[13-15]. However, severe hyperglycemia in LT has not been rigorously investigated in terms of post-transplant sequelae. Therefore, this review examines the associations of intraoperative blood glucose with clinical outcomes during the immediate period after LT in order to encourage clinicians to pay more attention to the importance of blood glucose management in LT.
Approximately 30%-60% of cirrhotic patients suffer from metabolic disorders related to blood glucose, known as “hepatogenic diabetes”[16]. Its pathophysiologic bases include insulin resistance in muscle, hepatic, and adipose tissues, as well as hyperinsulinemia. Whereas patients with liver cirrhosis show essentially normal hepatic production of glucose[17,18], hypoglycemia can develop in cases of acute decompensated liver failure[19]. Hypersecretion of glucagon can often compensate for this decrease in hepatic glucose production[17].
Insulin resistance is associated with endothelial dysfunction in patients with cirrhosis, which increases hepatic vascular resistance and promotes portal hypertension[20]. In addition, it contributes to the development of various complications, such as hepatic fibrosis, steatosis, hepatic carcinoma, and resistance to anti-viral treatments[21]. Chronic hepatitis C virus infection, which is the leading cause for LT in Western countries, can lead to insulin resistance via upregulation of inflammatory cytokines, such as tumor necrosis factor-α[22], phosphorylation of insulin-receptor substrate-1[23], upregulation of gluconeogenic genes, such as glucose-6-phosphatase and phosphoenolpyruvate carboxykinase 2[24], and the accumulation of lipid droplets[25]. Such insulin resistance results in hypersecretion of insulin in approximately 80% of patients with chronic liver disease, and oral glucose tolerance tests universally reveal high sensitivity for hyperinsulinemia in non-diabetic patients with nonalcoholic fatty liver disease[26].
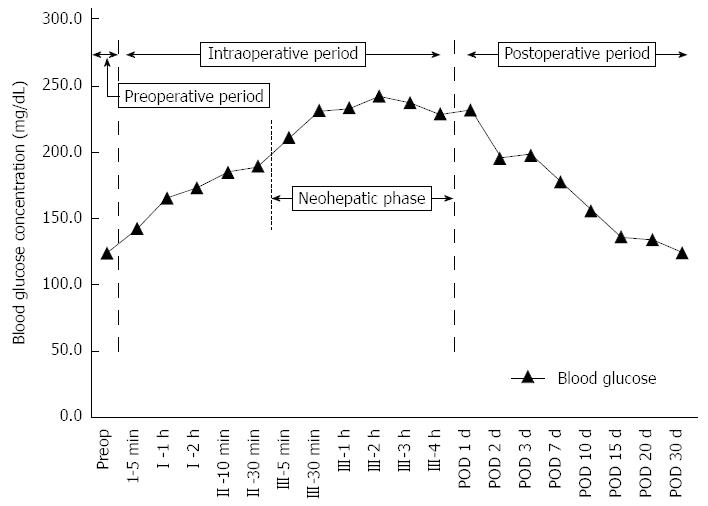
During orthotopic LT, blood glucose concentrations typically increase abruptly from 110 ± 46 mg/dL to 204 ± 60 mg/dL in the preanhepatic phase (phase I), followed by a further increase to 384 ± 72 mg/dL during the neohepatic phase (phase III)[27]. There are few data on blood glucose trends in living-donor LT. Figure 1 shows the perioperative blood glucose trend beginning from the preoperative day to post-transplant day 30 in patients who recently underwent living-donor LT at our center. Blood glucose concentrations peaked during the neohepatic phase, began to decline 3 h post-reperfusion near the end of LT, and then decreased abruptly two days post-transplantation.
Renal contributions: Although plasma insulin concentrations increase concomitantly with blood glucose, hyperinsulinemia does not effectively reduce hyperglycemia during the neohepatic phase[4]. This may be because post-reperfusion hyperglycemia is not mainly due to insulin hyposecretion, but to peripheral insulin resistance in glucose metabolism, which is exacerbated by the hepatectomy. As the kidney and gut are also involved in gluconeogenesis[28], the risk for hypoglycemia is increased with chronic renal failure, due to the suppression of renal glucose release and decreased glycogen storage[29]. Thus, patients with renal failure usually have hyperinsulinemia due to decreased renal clearance and the effects of uremic toxin on the liver[30]. Additionally, the subsequent malnutrition or muscle wasting may be more severe, which decreases their hepatic glycogen stores and gluconeogenic capacity[31]. Moreover, acidosis would limit the ability of the liver to compensate via hepatorenal reciprocity to maintain normoglycemia[32]. As a result, blood glucose must be monitored closely during the anhepatic phase in patients with severely damaged renal function undergoing LT because of the risk of hypoglycemia.
Exogenous contributions: Corticosteroid administration can exacerbate preexisting insulin resistance[33], and cause increased release of counterregulatory hormones, such as glucagon, adrenaline, noradrenaline, growth hormone, and cortisol. Therapeutic infusion of vasoactive drugs, such as epinephrine, norepinephrine, and dopamine, can also contribute to the increased blood glucose levels. Other sources include glucose within blood transfusions, and hepatic glucose released by the graft, which is accelerated during rewarming and after perfusion[34]. Indeed, the abrupt aggravation of hyperglycemia during the neohepatic phase is mainly caused by glucose influx from the grafted liver[4].
Until the early 2000s, strict blood glucose control (targeting 80-110 mg/dL) was recommended as standard practice in ICUs[5-7]. This protocol decreased patient morbidity and mortality compared to conventional management of blood glucose (targeting 180-200 mg/dL). However, the intensive insulin therapy was accompanied by a high risk of hypoglycemia[10]. Updated guidelines for regulating blood glucose now advise treating ICU patients to achieve levels ≤ 180 mg/dL[35,36]. This guideline was formulated based largely on the results of the multi-center NICE-SUGAR trial conducted in Australia, New Zealand, and Canada, which reported a lower incidence of hypoglycemia and hospital mortality compared to stricter blood glucose control[37].
Strict glucose control is not likely to be achieved during LT due to progressive hyperglycemia induced by exacerbated insulin resistance and exogenous intraoperative factors. Park et al[9] found that an intraoperative blood glucose criterion threshold of > 200 mg/dL was associated with post-transplant surgical site infection, whereas Ammori et al[3] used a blood glucose criterion of < 150 mg/dL, which appears to be a more reasonable goal[38]. However, due to the lack of any specialized standard protocol, blood glucose control in LT is maintained in accordance with inpatient glycemic management guidelines. The Consensus Statement by the American Association of Clinical Endocrinologists and the American Diabetes Association recommends initiating insulin infusions in critically ill patients at a blood glucose level no greater than 180 mg/dL[39]. The target glucose level is 140-180 mg/dL, and levels below 110 mg/dL should be avoided, even if a lower target may be beneficial in some patient groups.
Two retrospective studies have documented an association between intraoperative hyperglycemia during LT and post-transplant infectious complications[3,9]. In the study by Ammori et al[3] in 2007 that included 184 patients undergoing LT, the overall infection rate (including superficial skin infection, pneumonia, blood stream infections, peritonitis, urinary tract infection) during the first 30 d post-transplant was significantly higher (48%) in the group with poorly controlled hyperglycemia compared to those with well-controlled blood glucose (< 150 mg/dL; 30%). In the study of 680 LT patients by Park et al[9] in 2009, severe hyperglycemia (≥ 200 mg/dL) increased the risk of surgical site infection during the immediate post-transplant period by more than twofold (RR = 2.25, 95%CI: 1.26-4.03)[9].
Hyperglycemia influences major components of the immune system that combat infection. For example, early inflammatory responses to tissue injury are suppressed as a result of elevated expression of adhesion molecules, impaired complement activation, interference with the kininogen-bradykinin system, dysregulation of endothelial nitric oxide production[40-42], and increased levels of proinflammatory cytokines, such as interleukins 1β and 18 and tumor necrosis factor-α[43]. Hyperglycemia weakens macrophage phagocytosis, and reduces neutrophil adherence[44,45], chemotaxis, and reactive oxygen species production[46]. In addition, hyperglycemia interferes with the glycosylation of immune proteins and collagen[47].
Variability in blood glucose levels has been studied in association with mortality in the hospital or ICU[48,49]. However, there are few studies examining surgical consequences with respect to perioperative variability in serial blood glucose measurements. A recent single-center, prospective cohort study of 1461 patients undergoing cardiac surgery found that postoperative glycemic variability increases the risk of major adverse events, such as death, myocardial infarction, reoperation, sternal infection, cardiac tamponade, pneumonia, stroke, and renal failure (RR = 1.3, 95%CI: 1.1-1.5)[50]. Another retrospective study in 2013 revealed that large variability in preoperative blood glucose was associated with an increased rate of reoperation (RR = 4.14, 95%CI: 1.30-13.33)[51].
Only one study evaluated intraoperative glycemic variability in LT. In this retrospective study of 668 LT patients in 2010, intraoperative variability in blood glucose (SD ≥ 55.0 mg/dL) nearly doubled the risk of reoperation for hemorrhage (RR = 1.9, 95%CI: 1.2-3.0)[52]. However, the reason for this increased risk remains unclear. According to Hendriks et al[53], surgical re-intervention in LT patients is related to intraoperative blood loss. It is possible that the turnover in body fluids due to massive blood loss results in an instability in blood glucose concentrations. In vitro studies indicate that rapid, wide swings in glucose levels can adversely affect normal cellular defenses and coagulation/fibrinolytic systems[54,55].
As graft-related problems are the most important determinant of initial prognosis after LT[56], early detection of graft-related factors is important for improving post-transplant outcome. Since the early 1990s, blood glucose monitoring has been used as a sensitive indicator of early liver-graft function[57]. In animals receiving partial liver allografts, graft function was predicted by intraoperative balance of glucose production and utilization in the liver, measured as the difference between hepatic glucose inflow (at the portal vein and hepatic artery) and outflow (from the hepatic vein, to the liver)[58]. Impaired glucose uptake and continuous glycogenolysis causes persistent reperfusion hyperglycemia, which may be an early sign of impaired graft function[59]. Therefore, blood glucose levels during the neohepatic phase can be associated with post-transplant liver function. Moreover, a recent retrospective study found that hyperglycemia (> 200 mg/dL) maintained until the immediate post-transplant period was associated with liver allograft rejection within one year[60]. The decline in intraoperative blood glucose that we observed near the end of the neohepatic phase during LT (Figure 1) has not been previously described. Future study is needed to determine whether this decline is associated with functional recovery of the grafted liver.
Prior reports indicate that pronounced insulin insensitivity and hyperinsulinemia in liver failure are attributable to pancreatic hypersecretion and reduced hepatic insulin clearance, secondary to hyperglucagonemia[61]. These metabolic abnormalities disappear after successful LT[62]. Thus, resolution of hyperglycemia would be expected at the end and immediately following LT upon recovery of liver graft function.
The effect of intraoperative glucose management on mortality has primarily been examined in patients undergoing cardiac surgery. Such studies identified a benefit in patients with myocardial infarction who received intraoperative infusions of insulin and potassium[63,64]. Additional studies have evaluated the effects of intensive glycemic control on mortality following coronary artery bypass grafting[65-67]. The retrospective study by Ammori et al[3] also reported one-year mortality rates, which were higher in patients with poorly controlled hyperglycemia compared to those with well-controlled glucose levels (21.9% vs 8.8%). A prospective clinical study of the relationship between intraoperative blood glucose and post-transplant mortality is thus warranted.
Patients with end-stage liver disease exhibit hepatogenic diabetes, which manifests as peripheral insulin resistance and hyperinsulinemia. During LT, additional exogenous factors lead to intraoperative refractory hyperglycemia, with peak blood glucose levels occurring after reperfusion. As there are no specific guidelines, conventional methods from other clinical fields are used for the intraoperative management of hyperglycemia in these patients. Retrospective studies demonstrate that intraoperative hyperglycemia is associated with increased risk for infection and one-year mortality. Furthermore, the variability in blood glucose level during LT may predict post-transplant outcomes. Diabetogenic traits return after successful LT, so persistent neohepatic hyperglycemia in association with early indicators of graft dysfunction should be examined in future studies, including prospective clinical studies on the influence of intraoperative blood glucose on post-transplant outcomes.
P- Reviewer: Fulop T S- Editor: Qi Y L- Editor: A E- Editor: Ma S
| 1. | Shangraw RE. Metabolic issues in liver transplantation. Int Anesthesiol Clin. 2006;44:1-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 2. | Ahmadieh H, Azar ST. Liver disease and diabetes: association, pathophysiology, and management. Diabetes Res Clin Pract. 2014;104:53-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 71] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 3. | Ammori JB, Sigakis M, Englesbe MJ, O’Reilly M, Pelletier SJ. Effect of intraoperative hyperglycemia during liver transplantation. J Surg Res. 2007;140:227-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 85] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 4. | Shangraw RE, Hexem JG. Glucose and potassium metabolic responses to insulin during liver transplantation. Liver Transpl Surg. 1996;2:443-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 5. | Van den Berghe G, Wilmer A, Hermans G, Meersseman W, Wouters PJ, Milants I, Van Wijngaerden E, Bobbaers H, Bouillon R. Intensive insulin therapy in the medical ICU. N Engl J Med. 2006;354:449-461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2633] [Cited by in RCA: 2382] [Article Influence: 125.4] [Reference Citation Analysis (0)] |
| 6. | van den Berghe G, Wouters P, Weekers F, Verwaest C, Bruyninckx F, Schetz M, Vlasselaers D, Ferdinande P, Lauwers P, Bouillon R. Intensive insulin therapy in critically ill patients. N Engl J Med. 2001;345:1359-1367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7077] [Cited by in RCA: 6175] [Article Influence: 257.3] [Reference Citation Analysis (2)] |
| 7. | Van Herpe T, De Brabanter J, Beullens M, De Moor B, Van den Berghe G. Glycemic penalty index for adequately assessing and comparing different blood glucose control algorithms. Crit Care. 2008;12:R24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 39] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 8. | Xia VW, Obaidi R, Park C, Braunfeld M, Neelakanta G, Nourmand H, Hu KQ, Steadman RH. Insulin therapy in divided doses coupled with blood transfusion versus large bolus doses in patients at high risk for hyperkalemia during liver transplantation. J Cardiothorac Vasc Anesth. 2010;24:80-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 9. | Park C, Hsu C, Neelakanta G, Nourmand H, Braunfeld M, Wray C, Steadman RH, Hu KQ, Cheng RT, Xia VW. Severe intraoperative hyperglycemia is independently associated with surgical site infection after liver transplantation. Transplantation. 2009;87:1031-1036. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 98] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 10. | Vanhorebeek I, Langouche L, Van den Berghe G. Tight blood glucose control with insulin in the ICU: facts and controversies. Chest. 2007;132:268-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 109] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 11. | Van den Berghe G. How does blood glucose control with insulin save lives in intensive care? J Clin Invest. 2004;114:1187-1195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 362] [Cited by in RCA: 333] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 12. | Wintergerst KA, Foster MB, Sullivan JE, Woods CR. Association of hyperglycemia, glucocorticoids, and insulin use with morbidity and mortality in the pediatric intensive care unit. J Diabetes Sci Technol. 2012;6:5-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 13. | Duncan AE, Abd-Elsayed A, Maheshwari A, Xu M, Soltesz E, Koch CG. Role of intraoperative and postoperative blood glucose concentrations in predicting outcomes after cardiac surgery. Anesthesiology. 2010;112:860-871. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 14. | Ouattara A, Lecomte P, Le Manach Y, Landi M, Jacqueminet S, Platonov I, Bonnet N, Riou B, Coriat P. Poor intraoperative blood glucose control is associated with a worsened hospital outcome after cardiac surgery in diabetic patients. Anesthesiology. 2005;103:687-694. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 265] [Cited by in RCA: 226] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 15. | Song JW, Shim JK, Yoo KJ, Oh SY, Kwak YL. Impact of intraoperative hyperglycaemia on renal dysfunction after off-pump coronary artery bypass. Interact Cardiovasc Thorac Surg. 2013;17:473-478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 26] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 16. | García-Compean D, Jaquez-Quintana JO, Maldonado-Garza H. Hepatogenous diabetes. Current views of an ancient problem. Ann Hepatol. 2009;8:13-20. [PubMed] |
| 17. | Keller U, Sonnenberg GE, Burckhardt D, Perruchoud A. Evidence for an augmented glucagon dependence of hepatic glucose production in cirrhosis of the liver. J Clin Endocrinol Metab. 1982;54:961-968. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 34] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 18. | Shangraw RE, Jahoor F. Effect of liver disease and transplantation on urea synthesis in humans: relationship to acid-base status. Am J Physiol. 1999;276:G1145-G1152. [PubMed] |
| 19. | Pfortmueller CA, Wiemann C, Funk GC, Leichtle AB, Fiedler GM, Exadaktylos AK, Lindner G. Hypoglycemia is associated with increased mortality in patients with acute decompensated liver cirrhosis. J Crit Care. 2014;29:316.e7-316.12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 40] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 20. | Erice E, Llop E, Berzigotti A, Abraldes JG, Conget I, Seijo S, Reverter E, Albillos A, Bosch J, García-Pagán JC. Insulin resistance in patients with cirrhosis and portal hypertension. Am J Physiol Gastrointest Liver Physiol. 2012;302:G1458-G1465. [PubMed] |
| 21. | El-Zayadi AR, Anis M. Hepatitis C virus induced insulin resistance impairs response to anti viral therapy. World J Gastroenterol. 2012;18:212-224. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 37] [Cited by in RCA: 40] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 22. | Shintani Y, Fujie H, Miyoshi H, Tsutsumi T, Tsukamoto K, Kimura S, Moriya K, Koike K. Hepatitis C virus infection and diabetes: direct involvement of the virus in the development of insulin resistance. Gastroenterology. 2004;126:840-848. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 559] [Cited by in RCA: 543] [Article Influence: 25.9] [Reference Citation Analysis (0)] |
| 23. | Banerjee S, Saito K, Ait-Goughoulte M, Meyer K, Ray RB, Ray R. Hepatitis C virus core protein upregulates serine phosphorylation of insulin receptor substrate-1 and impairs the downstream akt/protein kinase B signaling pathway for insulin resistance. J Virol. 2008;82:2606-2612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 142] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 24. | Deng L, Shoji I, Ogawa W, Kaneda S, Soga T, Jiang DP, Ide YH, Hotta H. Hepatitis C virus infection promotes hepatic gluconeogenesis through an NS5A-mediated, FoxO1-dependent pathway. J Virol. 2011;85:8556-8568. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 83] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 25. | Kim KH, Hong SP, Kim K, Park MJ, Kim KJ, Cheong J. HCV core protein induces hepatic lipid accumulation by activating SREBP1 and PPARgamma. Biochem Biophys Res Commun. 2007;355:883-888. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 108] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 26. | Manchanayake J, Chitturi S, Nolan C, Farrell GC. Postprandial hyperinsulinemia is universal in non-diabetic patients with nonalcoholic fatty liver disease. J Gastroenterol Hepatol. 2011;26:510-516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 56] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 27. | Atchison SR, Rettke SR, Fromme GA, Janossy TA, Kunkel SE, Williamson KR, Perkins JD, Rakela J. Plasma glucose concentrations during liver transplantation. Mayo Clin Proc. 1989;64:241-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 28. | Stumvoll M, Meyer C, Mitrakou A, Gerich JE. Important role of the kidney in human carbohydrate metabolism. Med Hypotheses. 1999;52:363-366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 29. | Cano N. Inter-relationships between renal metabolism (both in physiology and renal dysfunction) and the liver. Curr Opin Clin Nutr Metab Care. 2001;4:279-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 30. | Mak RH, DeFronzo RA. Glucose and insulin metabolism in uremia. Nephron. 1992;61:377-382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 133] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 31. | Garber AJ, Bier DM, Cryer PE, Pagliara AS. Hypoglycemia in compensated chronic renal insufficiency. Substrate limitation of gluconeogenesis. Diabetes. 1974;23:982-986. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 73] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 32. | Woerle HJ, Meyer C, Popa EM, Cryer PE, Gerich JE. Renal compensation for impaired hepatic glucose release during hypoglycemia in type 2 diabetes: further evidence for hepatorenal reciprocity. Diabetes. 2003;52:1386-1392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 30] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 33. | Rizza RA, Mandarino LJ, Gerich JE. Cortisol-induced insulin resistance in man: impaired suppression of glucose production and stimulation of glucose utilization due to a postreceptor detect of insulin action. J Clin Endocrinol Metab. 1982;54:131-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 467] [Cited by in RCA: 464] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 34. | Nowak G, Ungerstedt J, Wernerman J, Ungerstedt U, Ericzon BG. Metabolic changes in the liver graft monitored continuously with microdialysis during liver transplantation in a pig model. Liver Transpl. 2002;8:424-432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 53] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 35. | Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, Sevransky JE, Sprung CL, Douglas IS, Jaeschke R. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock, 2012. Intensive Care Med. 2013;39:165-228. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3146] [Cited by in RCA: 3168] [Article Influence: 264.0] [Reference Citation Analysis (0)] |
| 36. | Orban JC, Scarlatti A, Lefrant JY, Molinari N, Leone M, Jaber S, Constantin JM, Allaouchiche B, Ichai C. [Management of glycemia: an audit in 66 ICUs]. Ann Fr Anesth Reanim. 2013;32:84-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 37. | Finfer S, Chittock DR, Su SY, Blair D, Foster D, Dhingra V, Bellomo R, Cook D, Dodek P, Henderson WR. Intensive versus conventional glucose control in critically ill patients. N Engl J Med. 2009;360:1283-1297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3409] [Cited by in RCA: 3182] [Article Influence: 198.9] [Reference Citation Analysis (0)] |
| 38. | Liu LL, Niemann CU. Intraoperative management of liver transplant patients. Transplant Rev (Orlando). 2011;25:124-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 39. | Moghissi ES, Korytkowski MT, DiNardo M, Einhorn D, Hellman R, Hirsch IB, Inzucchi SE, Ismail-Beigi F, Kirkman MS, Umpierrez GE; American Association of Clinical Endocrinologists; American Diabetes Association. American Association of Clinical Endocrinologists and American Diabetes Association consensus statement on inpatient glycemic control. Endocr Pract. 2009;15:353-369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 398] [Cited by in RCA: 378] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 40. | Turina M, Fry DE, Polk HC. Acute hyperglycemia and the innate immune system: clinical, cellular, and molecular aspects. Crit Care Med. 2005;33:1624-1633. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 312] [Cited by in RCA: 312] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 41. | Chen NG, Azhar S, Abbasi F, Carantoni M, Reaven GM. The relationship between plasma glucose and insulin responses to oral glucose, LDL oxidation, and soluble intercellular adhesion molecule-1 in healthy volunteers. Atherosclerosis. 2000;152:203-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 40] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 42. | Santilli F, Cipollone F, Mezzetti A, Chiarelli F. The role of nitric oxide in the development of diabetic angiopathy. Horm Metab Res. 2004;36:319-335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 61] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 43. | Pickup JC, Chusney GD, Thomas SM, Burt D. Plasma interleukin-6, tumour necrosis factor alpha and blood cytokine production in type 2 diabetes. Life Sci. 2000;67:291-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 388] [Cited by in RCA: 415] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 44. | Bagdade JD, Root RK, Bulger RJ. Impaired leukocyte function in patients with poorly controlled diabetes. Diabetes. 1974;23:9-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 396] [Cited by in RCA: 350] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 45. | Sima AA, O’Neill SJ, Naimark D, Yagihashi S, Klass D. Bacterial phagocytosis and intracellular killing by alveolar macrophages in BB rats. Diabetes. 1988;37:544-549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 36] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 46. | Alexiewicz JM, Kumar D, Smogorzewski M, Klin M, Massry SG. Polymorphonuclear leukocytes in non-insulin-dependent diabetes mellitus: abnormalities in metabolism and function. Ann Intern Med. 1995;123:919-924. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 142] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 47. | Black CT, Hennessey PJ, Andrassy RJ. Short-term hyperglycemia depresses immunity through nonenzymatic glycosylation of circulating immunoglobulin. J Trauma. 1990;30:830-832; discussion 832-833. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 88] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 48. | Egi M, Bellomo R, Stachowski E, French CJ, Hart G. Variability of blood glucose concentration and short-term mortality in critically ill patients. Anesthesiology. 2006;105:244-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 595] [Cited by in RCA: 590] [Article Influence: 31.1] [Reference Citation Analysis (0)] |
| 49. | Ali NA, O’Brien JM, Dungan K, Phillips G, Marsh CB, Lemeshow S, Connors AF, Preiser JC. Glucose variability and mortality in patients with sepsis. Crit Care Med. 2008;36:2316-2321. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 329] [Cited by in RCA: 309] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 50. | Subramaniam B, Lerner A, Novack V, Khabbaz K, Paryente-Wiesmann M, Hess P, Talmor D. Increased glycemic variability in patients with elevated preoperative HbA1C predicts adverse outcomes following coronary artery bypass grafting surgery. Anesth Analg. 2014;118:277-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 83] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 51. | Endara M, Masden D, Goldstein J, Gondek S, Steinberg J, Attinger C. The role of chronic and perioperative glucose management in high-risk surgical closures: a case for tighter glycemic control. Plast Reconstr Surg. 2013;132:996-1004. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 52. | Park C, Huh M, Steadman RH, Cheng R, Hu KQ, Farmer DG, Hong J, Duffy J, Busuttil RW, Xia VW. Extended criteria donor and severe intraoperative glucose variability: association with reoperation for hemorrhage in liver transplantation. Transplant Proc. 2010;42:1738-1743. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 53. | Hendriks HG, van der Meer J, de Wolf JT, Peeters PM, Porte RJ, de Jong K, Lip H, Post WJ, Slooff MJ. Intraoperative blood transfusion requirement is the main determinant of early surgical re-intervention after orthotopic liver transplantation. Transpl Int. 2005;17:673-679. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 97] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 54. | Quagliaro L, Piconi L, Assaloni R, Martinelli L, Motz E, Ceriello A. Intermittent high glucose enhances apoptosis related to oxidative stress in human umbilical vein endothelial cells: the role of protein kinase C and NAD(P)H-oxidase activation. Diabetes. 2003;52:2795-2804. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 572] [Cited by in RCA: 615] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 55. | Slaughter TF. Hemostasis and glycemic control in the cardiac surgical patient. Semin Cardiothorac Vasc Anesth. 2006;10:176-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 56. | Vogt DP, Henderson JM, Carey WD, Barnes D. The long-term survival and causes of death in patients who survive at least 1 year after liver transplantation. Surgery. 2002;132:775-780; discussion 780. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 66] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 57. | Steltzer H, Tüchy G, Hiesmayr M, Müller C, Germann P, Zimpfer M. Peri-operative liver graft function: monitoring using the relationship between blood glucose and oxygen consumption during anaesthesia. Anaesthesia. 1992;47:955-958. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 58. | Shiba H, Zhu X, Arakawa Y, Irefin S, Wang B, Trenti L, Sanchez IP, Fung JJ, Kelly DM. Glucose balance of porcine liver allograft is an important predictor of outcome. J Surg Res. 2011;171:851-858. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 59. | Mallett SV, Kang Y, Freeman JA, Aggarwal S, Gasior T, Fortunato FL. Prognostic significance of reperfusion hyperglycemia during liver transplantation. Anesth Analg. 1989;68:182-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 18] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 60. | Wallia A, Parikh ND, Molitch ME, Mahler E, Tian L, Huang JJ, Levitsky J. Posttransplant hyperglycemia is associated with increased risk of liver allograft rejection. Transplantation. 2010;89:222-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 69] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 61. | Vilstrup H, Iversen J, Tygstrup N. Glucoregulation in acute liver failure. Eur J Clin Invest. 1986;16:193-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 44] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 62. | Konrad T, Golling M, Vicini P, Toffolo G, Wittman M, Mahon A, Klar E, Cobelli C, Usadel K. Insulin sensitivity and beta-cell secretion after liver transplantation in patients with acute liver failure. Transplant Proc. 2001;33:2576-2579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 63. | Lazar HL, Philippides G, Fitzgerald C, Lancaster D, Shemin RJ, Apstein C. Glucose-insulin-potassium solutions enhance recovery after urgent coronary artery bypass grafting. J Thorac Cardiovasc Surg. 1997;113:354-360; discussion 360-362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 105] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 64. | Rao V, Christakis GT, Weisel RD, Ivanov J, Borger MA, Cohen G. The insulin cardioplegia trial: myocardial protection for urgent coronary artery bypass grafting. J Thorac Cardiovasc Surg. 2002;123:928-935. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 65. | Furnary AP, Gao G, Grunkemeier GL, Wu Y, Zerr KJ, Bookin SO, Floten HS, Starr A. Continuous insulin infusion reduces mortality in patients with diabetes undergoing coronary artery bypass grafting. J Thorac Cardiovasc Surg. 2003;125:1007-1021. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 824] [Cited by in RCA: 757] [Article Influence: 34.4] [Reference Citation Analysis (1)] |
| 66. | D'Alessandro C, Leprince P, Golmard JL, Ouattara A, Aubert S, Pavie A, Gandjbakhch I, Bonnet N. Strict glycemic control reduces EuroSCORE expected mortality in diabetic patients undergoing myocardial revascularization. J Thorac Cardiovasc Surg. 2007;134:29-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 51] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 67. | Lazar HL, Chipkin SR, Fitzgerald CA, Bao Y, Cabral H, Apstein CS. Tight glycemic control in diabetic coronary artery bypass graft patients improves perioperative outcomes and decreases recurrent ischemic events. Circulation. 2004;109:1497-1502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 520] [Cited by in RCA: 506] [Article Influence: 24.1] [Reference Citation Analysis (0)] |